Abstract
We recently reported that the posttraumatic spread of degeneration in the damaged optic nerve can be attenuated by the adoptive transfer of autoimmune T cells specific to myelin basic protein. However, it would be desirable to obtain immune neuroprotection free of any possible autoimmune disease. In an attempt to obtain disease-free immune neuroprotection, we used the synthetic four-amino acid polymer copolymer 1 (Cop-1), which is known not to be encephalitogenic despite its cross-reactivity with myelin basic protein. We show here that active immunization with Cop-1 administered in adjuvant, as well as adoptive transfer of T cells reactive to Cop-1, can inhibit the progression of secondary degeneration after crush injury of the rat optic nerve. These results have implications for the treatment of optic neuropathies.
Optic neuropathies are neurodegenerative diseases of the optic nerve in which degeneration starts at the optic nerve and ends with the death of the retinal ganglion cells (RGCs) (1, 2). In many cases of optic neuropathies, the primary risk factors may be removed but degeneration continues. We have suggested that the progression of damage may be attributable to the fact that at any given time fibers undergoing acute degeneration create a hostile environment for neighboring fibers that are still intact, thereby causing their eventual degeneration (3, 4). This process of “secondary degeneration” is like that seen after any traumatic injury to other areas of the central nervous system (CNS), such as the spinal cord and the brain. Hence neuroprotection should be a useful complement to the treatment of optic neuropathies.
We recently demonstrated that the adoptive transfer of T cells specific to proteins associated with CNS myelin, such as myelin basic protein (MBP), can reduce the posttraumatic secondary degeneration of the rat optic nerve and spinal cord (refs. 5, 6 and 8 and G. Moalem, A. Gdalyahu, Y. Shani, U. Otten, P. Lazarovici, I.R.C., and M. S., unpublished work). We further showed that this neuroprotective effect is not restricted to T cells directed against major encephalitogenic peptides, because T cells against cryptic peptides in the MBP molecule were similarly effective in reducing secondary degeneration (6). However, in the development of therapies based on autoimmune neuroprotection, it is important to seek “safe” antigenic epitopes that will not cause the induction of an autoimmune disease.
The question was whether we could induce a protective immune response with a nonself protein resembling or having a cross-recognition with the self protein, but without the danger of disease induction. A possible candidate for this role is the nonpathogenic polymer, copolymer 1 (Cop-1, trade name Copaxone). Cop-1 is a synthetic amino acid polymer (4.7−11 kDa) composed of l-alanine, l-lysine, l-glutamic acid, and l-tyrosine, in a molar ratio of 4.2:3.4:1.4:1.0 (9). It initially was designed to mimic MBP and induce experimental autoimmune encephalomyelitis (EAE), but was found to be nonencephalitogenic and to suppress MBP-induced EAE. Cop-1 also blocks chronic-relapsing EAE induced by mouse spinal cord homogenate or by the encephalitogenic peptides of proteolipid protein in a (SJL/J × BALB/c) F1 mouse model (10). Cop-1 binds to the relevant major histocompatibility complex proteins and leads to the activation of T suppressor cells, which are triggered by determinants common to Cop-1 and MBP (11). We reasoned that the existence of cross-reaction between Cop-1 and MBP or other components of myelin might enable Cop-1-specific T cells to recognize the damaged tissue, accumulate there, and become activated to induce neuroprotection. We also were interested in finding out whether, in addition to adoptive transfer, active immunization with Cop-1 could be used to reinforce immune neuroprotection.
In the present study we show that neuroprotection of crush-injured rat optic nerves can be obtained by active immunization with Cop-1 on the day of injury and by adoptive transfer of Cop-1 reactive T cells.
Materials and Methods
Animals.
Inbred female adult Lewis rats (8–12 weeks old) were supplied by the Animal Breeding Center of The Weizmann Institute of Science. The rats were housed in a light- and temperature-controlled room and matched for age in each experiment. Animals were handled according to the regulations formulated by the Institutional Animal Care and Use Committee.
Antigens.
MBP from the spinal cords of guinea pigs and ovalbumin (OVA) were purchased from Sigma. Cop-1 was purchased from Teva Pharmaceuticals (Petach-Tikva, Israel).
Antibodies.
Mouse mAbs specific to rat T cell receptor (TCR) were kindly provided by Boris Reizis, Israel. Cy-3-conjugated goat anti-mouse IgG (with minimal cross-reaction to rat, human, bovine, and horse serum proteins) was purchased from Jackson ImmunoResearch.
T Cell Lines.
T cell lines were generated from draining lymph node cells obtained from Lewis rats immunized with the above antigens (12). The antigen was dissolved in PBS (1 mg/ml) and emulsified with an equal volume of incomplete Freund's adjuvant (IFA) (Difco) supplemented with 4 mg/ml Mycobacterium tuberculosis (Difco). Ten days after the antigen was injected into the rats' hind foot pads in 0.1 ml of the emulsion, the rats were killed and their draining lymph nodes were surgically removed and dissociated. The cells were washed and activated with the antigen (10 μg/ml) in stimulation medium containing DMEM supplemented with l-glutamine (2 mM), 2-mercaptoethanol (5 × 10-5 M), sodium pyruvate (1 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), nonessential amino acids (1 ml/100 ml), and autologous serum 1% (vol/vol). After incubation for 72 h at 37°C, 98% relative humidity and 10% CO2, the cells were transferred to propagation medium consisting of DMEM, l-glutamine, 2-mercaptoethanol, sodium pyruvate, nonessential amino acids, and antibiotics in the same concentrations as above, with the addition of 10% FCS (vol/vol) and 10% T-cell growth factor derived from the supernatant of Con A-stimulated spleen cells (13). Cells were grown in propagation medium for 4−10 days before being restimulated with their antigen (10 μg/ml) in the presence of irradiated (2,000 rad) thymus cells (107 cells/ml) in stimulation medium. The T cell lines were expanded by repeated stimulation and propagation (14).
Crush Injury of Optic Nerve.
The optic nerve was subjected to crush injury as described (15). Briefly, rats were deeply anesthetized by i.p. injection of XYL-M 2% (xylazine, 10 mg/kg; Arendonk, Belgium) and Ketaset (ketamine, 50 mg/kg; Fort Dodge Laboratories, Fort Dodge, IA). Using a binocular operating microscope, lateral canthotomy was performed in the right eye, and the conjunctiva was incised lateral to the cornea. After separation of the retractor bulbi muscles, the optic nerve was exposed intraorbitally by blunt dissection. Using calibrated cross-action forceps; the optic nerve was subjected to a crush injury 1−2 mm from the eye. Mild and severe crush injuries were inflicted for short-term trials (2 weeks), as this time period was shown to be optimal for demonstrating secondary degeneration and its response to treatment (16). The uninjured contralateral nerve was left undisturbed.
Measurement of Secondary Degeneration by Retrograde Labeling of RGCs.
Secondary degeneration of the optic nerve axons and their attached RGCs was measured after postinjury application of the fluorescent lipophilic dye, 4-(4-(didecylamino)styryl)-N-methylpyridinium iodide (4-Di-10-Asp) (Molecular Probes), distally to the lesion site, 2 weeks after crush injury. Because only axons that are intact can transport the dye back to their cell bodies, application of the dye distally to the lesion site after 2 weeks ensures that only axons that survived both the primary damage and the secondary degeneration will be counted. This approach enabled us to differentiate between neurons that are still functionally intact and neurons in which the axons are injured but the cell bodies are still viable, because only those neurons whose fibers are morphologically intact can take up dye applied distally to the site of injury and transport it to their cell bodies. Using this method, the number of labeled RGCs reliably reflects the number of still-functioning neurons. Labeling and measurement were carried out as follows: the right optic nerve was exposed for the second time, again without damaging the retinal blood supply. Complete axotomy was performed 1−2 mm from the distal border of the injury site and solid crystals (0.2−0.4 mm diameter) of 4-Di-10-Asp were deposited at the site of the newly formed axotomy. Five days after dye application the rats were killed. The retina was detached from the eye, prepared as a flattened whole mount in 4% paraformaldehyde solution, and examined for labeled RGCs by fluorescence microscopy.
ELISA.
Anti-MBP or anti-Cop-1 T cells were grown for a week in a propagation medium, then washed with PBS and resuspended in stimulation medium. The T cells (0.5 × 106 cells/ml) were incubated, in the presence of irradiated thymocytes (107 cells/ml), with Con A (1.25 μg/ml), MBP antigen (10 μg/ml), Cop-1 antigen (10 μg/ml), OVA antigen (10 μg/ml), or with no antigen, in stimulation medium at 37°C, 98% relative humidity and 10% CO2. In addition, irradiated thymocytes (107 cells/ml) alone were incubated in stimulation medium. After 48 h the cells were centrifuged and their supernatants were collected and sampled. Concentrations of neurotrophin (NT)-3, nerve growth factor, and NT-4/5 in the samples were determined by the use of sandwich ELISA kits (Promega) and comparison with a NT standard (absorbance measurement at 450 nm using an ELISA reader). Concentrations of brain-derived neurotrophic factor (BDNF) in the samples were determined with a sensitive sandwich ELISA. In brief, 96-well flat-bottomed plates were coated with a chicken anti-human BDNF antibody (Promega) in 0.025 M NaHCO3 and 0.025 M Na2CO3 (pH 8.2). Recombinant human BDNF (used as standard; Research Diagnostics, Flanders, NJ) was used in serial dilutions in blocking solution containing 3% BSA, 0.05% polyoxyethylene-sorbitan monolaurate (Tween-20), and 1% FCS in PBS (pH 8.2). Bound BDNF was detected by incubating the plates with a mouse anti-human BDNF antibody (Research Diagnostics) and then with peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) in blocking solution. The plates were developed by using a 3,3′,5,5′-tetramethyl-benzidine liquid substrate system (Sigma). The reaction was stopped by adding 1 M H3PO4, and the optical density was determined at 450 nm. Results for each experiment were calculated as the amount of secreted NT per 1 ml of sample, after subtraction of the background levels of the irradiated thymocytes incubated with the stimulation medium.
Immunization.
Lewis rats (8–12 weeks old) each were injected with a total of 100 μg of Cop-1 emulsified with an equal volume of complete Freund's adjuvant (CFA) containing 5 mg/ml of mycobacteria H37 RA (Difco). The emulsion, in a total volume of 0.2 ml, was injected into the rat's two hind footpads immediately after crush injury to the optic nerve. Beginning 24 h after the injury, each rat was fed 1-mg of Cop-1 every 24 h for 5 days.
Immunohistochemistry.
Longitudinal cryosections (10 μm thick) of the nerves were picked up onto gelatin-coated glass slides and frozen until preparation for fluorescence staining. The sections were fixed in ethanol for 10 min at room temperature, washed twice with double-distilled water, and incubated for 3 min in PBS containing 0.05% Tween-20. Sections then were incubated for 1 h at room temperature with mouse anti-rat mAbs to TCR (17) diluted in PBS containing 3% FCS and 2% BSA. The sections then were washed three times with PBS containing 0.05% Tween-20 and incubated with Cyô3-conjugated goat anti-mouse IgG (with minimal cross-reaction to rat, human, bovine, and horse serum proteins; Jackson ImmunoResearch) for 1 h at room temperature. The sections were washed with PBS containing Tween-20 and treated with glycerol containing 1,4-diazobicyclo-(2, 2, 2)octane to inhibit quenching of fluorescence. The sections were viewed with a Zeiss Universal fluorescence microscope.
Results
Adoptive Transfer of T Cells Reactive to Cop-1 Is Neuroprotective in the Injured Optic Nerve.
To examine whether T cells to Cop-1 display a neuroprotective effect in the rat optic nerve model, we injected 10 × 106 Cop-1-reactive T cells into rats immediately after a mild or severe injury of their optic nerves. After 2 weeks, the number of surviving fibers was determined by applying the dye 4-Di-10-Asp distally to the lesion site. Retinas were excised 5 days later and whole-mounted, and labeled RGCs were counted. Rats injected with Cop-1-reactive T cells on the day of a mild or severe crush injury to the optic nerve showed significantly less secondary degeneration than that seen in control rats injected with PBS (Fig. 1). None of the rats in these experiments developed EAE (data not shown). Representative fields from retinas treated with Cop-1-reactive T cells and from untreated retinas are shown in Fig. 2. It should be noted that rats injected with OVA-reactive T cells did not have any effect, as previously reported (6).
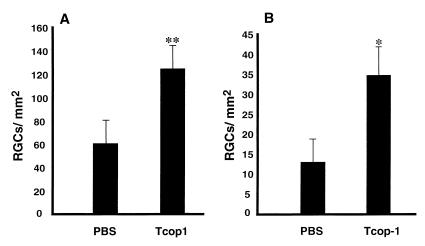
Figure 1.
T cells specific to Cop-1 protect neurons from secondary degeneration. Immediately after mild (A) or severe optic nerve injury (B), rats were injected with PBS or with Cop-1-specific T cells. For assessment of secondary degeneration, the neurotracer dye 4-Di-10-Asp was applied to the optic nerve distal to the site of injury, 2 weeks after the injury. After 5 days, the rats were killed and their retinas were excised and flat-mounted. Labeled (surviving) RGCs, from four fields located at approximately the same distance from the optic disk in each retina, were counted under a fluorescence microscope. The neuroprotective effect of Cop-1-reactive T cells compared with that of PBS was significant for both mild crush injury (P < 0.005, Student's t test) and severe crush injury (P < 0.05, Student's t test). The graph shows representative results of one experiment of the three that were performed with 6–10 animals in each group in each experiment.
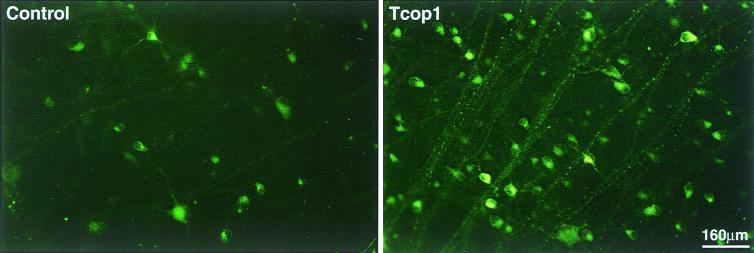
Figure 2.
Photomicrographs of retrogradely labeled retinas of injured optic nerves of rats. Immediately after unilateral crush injury of their optic nerves, rats were injected with Cop-1-reactive T cells or PBS. Two weeks later the neurotracer dye 4-Di-10-Asp was applied to the optic nerves distal to the site of injury, and 5 days later the retinas were excised and flat-mounted. Shown are representative fields with labeled RGCs, located at approximately the same distance from the optic disk in the retinas of the two groups.
Cop-1-Reactive T Cells Accumulate in Both Injured and Uninjured Neuronal Tissues.
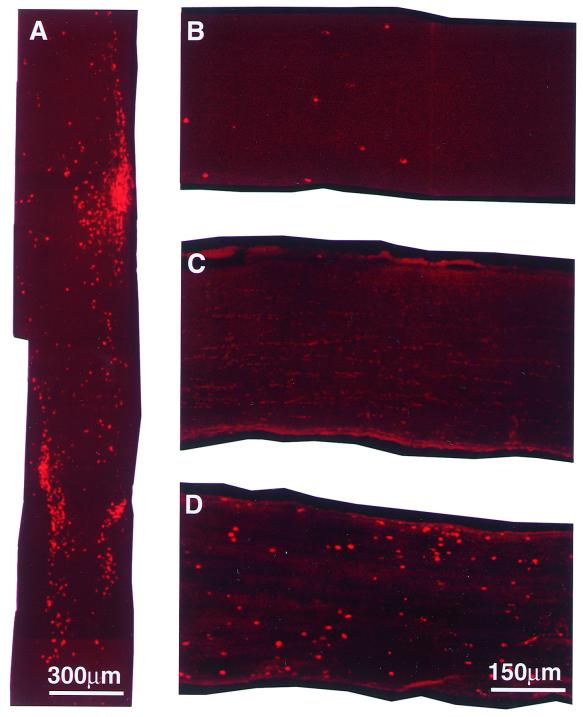
To investigate whether T cells to Cop-1 accumulate at the site of the injury, we injected rats with Cop-1 reactive T cells and excised the nerves 7 days later, at the time point previously identified as the peak of accumulation of T cells in a damaged optic nerve (18, 19). The adoptive transfer of Cop-1-reactive T cells in the present study also caused a significant accumulation of T cells at the site of injury relative to the accumulation of T cells in the PBS-treated injured rats. T cell accumulation in the Cop-1-treated rats was greatest on day 7 after the injection (Fig. 3A). These findings were in line with our earlier results showing that injection of T cell lines of different specificities results in T cell accumulation at the site of the lesion.
Figure 3.
In situ detection of immunostained T cells. (A) Injured nerve. (B–D) Uninjured nerves. (A) Seven days after crush injury and systemic injection of T cells reactive to Cop-1, cryosections (10 μm thick) of injured optic nerve were stained for TCR. Uninjured nerves were excised 7 days after injection with T cells specific to Cop-1 (B), OVA (C), or MBP (D), and then cryosectioned (10 μm) and stained for TCR. Representative slices are shown. At least four rats were tested in each experiment.
In contrast to the accumulation of activated T cells at the site of injury after injection of T cells of any specificity, only T cells specific to CNS self-antigens were found to accumulate in uninjured nerves (18, 19). To learn whether Cop-1-specific T cells might behave like these anti-self T cells, we injected naive rats with T cells to MBP, OVA, or Cop-1, and excised the optic nerves 3 days later. Injection of T cells to Cop-1, like the injection of T cells to MBP (Fig. 3D), resulted in accumulation of T cells in the uninjured nerve (Fig. 3B). In contrast, the injection of T cells to OVA did not result in any detectable T cell accumulation in the uninjured optic nerve (Fig. 3C). Although there was less accumulation of T cells, in uninjured nerve, after injection of T cells reactive to Cop-1 than of T cells specific to MBP, these findings support the conclusion that Cop-1-reactive T cells can recognize in vivo an antigen in the intact optic nerve, presumably myelin-associated.
Cytokine and NT Profiles of T Cells to MBP or Cop-1.
Anti-MBP T cells were stimulated in vitro with their specific antigen or Cop-1. The cultured media containing products secreted by these cells were collected and their cytokine contents were quantified by ELISA. The activated T cells secreted much larger amounts of cytokines than did the unstimulated T cells. The T helper 1-specific cytokine IFN-γ, was similarly expressed by both anti-MBP T cells and the anti-Cop-1 T cells, whereas the T helper 2-specific cytokine IL-10 was found to be secreted mainly by the anti-Cop-1 T cells. The largest amounts of secreted cytokines were detected in the supernatants of T cells stimulated with Con A (Table 1).
Table 1.
ELISA of secreted cytokines
| Cytokine, pgr/ml | Resting state
|
Stimulation with MBP
|
Stimulation with Cop-1
|
Stimulation with Con A
|
||||
|---|---|---|---|---|---|---|---|---|
| Tmbp | Tcop | Tmbp | Tcop | Tmbp | Tcop | Tmbp | Tcop | |
| IFN-γ | 725 | 6,645 | 15,692 | 925 | 7,242 | 11,825 | 22,758 | 22,525 |
| IL-10 | 41 | 382 | 1,941 | 13 | 365 | 7,244 | 3,565 | 6,503 |
Supernatants from unstimulated T cells or T cells stimulated for 48 h with Con A mitogen, MBP antigen, or Cop-1 antigen in stimulation medium were subjected to sandwich ELISA. The table shows the concentration of cytokines. The amounts of secreted T helper 2-related cytokines were significantly higher in supernatants of Cop-1-reactive T cells than in supernatants of T cells specific to MBP.
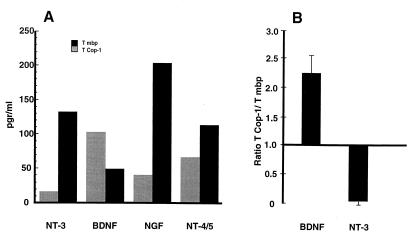
Up-regulation of neurotrophic expression and secretion by T cells activated with their specific antigens recently was demonstrated by our group (G. Moalem, A. Gdalyahu, Y. Shani, U. Otten, P. Lazarovici, I.R.C., and M. S., unpublished work and ref. 20). In an attempt to gain an insight into the mechanism underlying T cell-mediated neuroprotection, the T cell supernatants in the present study were subjected to ELISA to determine the NT profiles of T cells responsible for neuroprotection. The Cop-1-stimulated T cells secreted both nerve growth factor and NT-4/5, but in lower amounts than those secreted by the anti-MBP T cells. Relative to the production by anti-MBP T cells, the production of NT-3 by the Cop-1-stimulated T cells was insignificant; the production of BDNF, however, was massive (Fig. 4A). Thus, the Cop-1-stimulated T cells produced smaller amounts of all of the examined neurotrophic factors, with the notable exception of BDNF (Fig. 4A). Four independent determinations of the amounts of NT-3 and BDNF secreted by the differentially stimulated T cells yielded similar results. In each case, Cop-1-stimulated T cells produced about 2.5-fold more BDNF than anti-MBP T cells, and only 10% of the amounts of NT-3 (Fig. 4B).
Figure 4.
ELISA of secreted neurotrophic factors. Rat anti-MBP or anti-Cop-1 T cells were cultured for 48 h with their specific antigen in stimulation medium. The T-cell supernatants were collected and subjected to sandwich ELISA. The histogram shows the concentration of secreted NTs in each sample. The amounts of nerve growth factor (NGF) and NT-4/5 were higher in the anti-MBP T cells than in the anti-Cop-1 T cells. NT-3 was found almost exclusively in T cells specific to MBP, whereas the amounts of BDNF secreted in the supernatants of Cop-1-stimulated T cells were significantly higher than in the supernatants of anti-MBP T cells. Representative data are shown (A). The mean ratios ± SD (from five independent experiments) of the amounts of BDNF or NT-3 secreted by anti-Cop-1 T cells to the amounts secreted by anti-MBP T cells are shown in B.
Vaccination with Cop-1 Followed by Oral Administration of Cop-1 Protects Neurons from Secondary Degeneration.
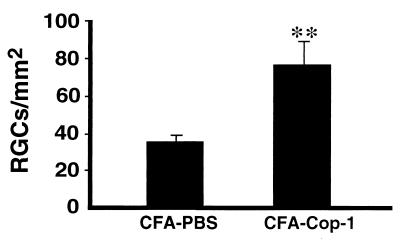
Oral administration of antigen in low doses is known to boost the immune response and switch it toward T helper 2 regulatory cells, but in high doses it may cause anergy or depletion (21, 22). We postulated that if rats with crush-injured optic nerves were vaccinated with Cop-1 in CFA and then fed with low-dose Cop-1, the immune response to the vaccination might be boosted. Anesthetized rats were subjected to mild crush injury of the optic nerve, immediately vaccinated, and then fed for 5 days with Cop-1 dissolved in PBS. After 2 weeks the RGCs were retrogradely labeled and 5 days later the retinas were excised. Rats vaccinated with Cop-1 and then fed with the antigen showed evidence of significant neuroprotection compared with that in control rats injected with PBS in CFA and then fed with PBS (Fig. 5).
Figure 5.
Neuroprotection by active immunization with Cop-1. Immunization with Cop-1 in CFA, boosted with oral administration of the antigen, leads to protection against secondary degeneration. Immediately after mild optic nerve injury, rats were immunized s.c. with PBS in CFA or Cop-1 in CFA. For assessment of secondary degeneration, the neurotracer dye 4-Di-10-Asp was applied to the optic nerve distal to the site of injury 2 weeks after crush injury, and 5 days later the rats were killed and their retinas were excised and flat-mounted. Labeled (surviving) RGCs, from four fields located at approximately the same distance from the optic disk in each retina, were counted under the fluorescence microscope. The neuroprotective effect of Cop-1 immunization compared with that of PBS injection was significant (P < 0.01, Student's t test). The results are the summary of two experiments, each carried out with 5–6 rats in each group.
Discussion
The results of this study demonstrate a possible neuroprotective effect of T cell immunity to Cop-1 in a crush-injured CNS nerve. In the rat model of partial optic nerve crush, adoptive administration of Cop-1-reactive T cells or vaccination with Cop-1 on the day of CNS injury had a marked preventive effect on the secondary degeneration of nerve fibers.
Cop-1 originally was designed to mimic MBP and to induce EAE, but was found to be nonencephalitogenic and even to suppress EAE induced by MBP (9), proteolipid protein (10), or myelin oligodendrocyte glycoprotein (23). The precise mechanisms by which Cop-1 prevents the development of EAE and ameliorates multiple sclerosis are not yet known. Nevertheless, some important immunological properties of this copolymer have emerged. Studies have demonstrated partial cross-reactivity of Cop-1 with MBP at both the T cell (24) and the antibody (25) level. Cop-1 can serve as an antagonist of the T-cell antigen receptor for the MBP immunodominant epitope (26). It also can bind to various major histocompatibility complex class II molecules and prevent them from binding to T cells with specific antigen-recognition properties (27). In rodents, Cop-1 induces regulatory cells that suppress the encephalitogenic T cells. Adoptive transfer of such T cells was found to prevent the development of EAE induced by MBP (28), proteolipid protein (10), or whole spinal cord homogenate (29).
T cells reactive to MBP were shown to be neuroprotective in rat models of partially crushed optic nerve (6) and spinal cord injury (8). The massive accumulation of exogenously administered T cells at the site of CNS injury suggests that the presence of T cells at the site of injury plays a prominent role in neuroprotection. It appears, however, that the accumulation, although a necessary condition, is not sufficient for conferring neuroprotection, as T cells specific to the non-self antigen OVA also accumulate at the site, but have no neuroprotective effect (18). As a feasible scenario, we suggest that T cells arriving at the site of injury recognize the antigen(s) presented there as major histocompatibility complex class II molecules (which are known to be up-regulated after CNS trauma) (19), and thus become activated, switching to a neuroprotective phenotype and producing factors that promote neuroprotection. It seems that recognition of TCR−major histocompatibility complex class II antigens at the site of injury is a precondition for activation of T cells and achievement of a neuroprotective effect.
The fact that T cells accumulate in the uninjured optic nerve supports the notion of recognition between the Cop-1-specific T cells and antigens presented by the optic nerve. It should be noted that the method used here to assess T cell accumulation involved the use of antibodies to the TCR. Using this approach, it is not possible to determine whether the accumulated T cells detected in the nerve are the ones that were injected. In our earlier studies we showed, however, that the injected activated T cells are indeed the ones that accumulate (18). Activated T cells can pass through the blood−brain barrier regardless of their specificity, but only those that are reactive to CNS antigens can accumulate in the uninjured nerve (30). Thus, our present findings demonstrate in vivo cross-recognition between Cop-1-reactive T cells and components of CNS myelin. This recognition probably serves as the trigger for T cell reactivation, causing the T cells to switch toward the protective phenotype. We found in this study that Cop-1-reactive T cells activated by their specific antigen secrete significant amounts of BDNF, an NT that plays a major role in neuronal survival (31, 32). It is possible that myelin proteins expressed at the lesion site reactivate the Cop-1-specific T cells to a similar extent.
Immunization with Cop-1, unlike immunization with MBP and other myelin-associated proteins, does not induce EAE, and the T cells evoked by Cop-1 in the absence of adjuvants are of a regulatory nature. In the present study, immunization with Cop-1 in CFA immediately after the injury, followed by five consecutive daily feedings, had a strongly neuroprotective effect. Such immunization is likely to evoke a cellular response that is large enough to exert at least some neuroprotective activity. It is possible that this response was somewhat delayed relative to the response obtained after adoptive transfer of T cells, but nevertheless it was still achieved within the time window needed for protection of nerve fibers that escaped the primary lesion. Previous studies in the rat optic nerve have shown that the loss of neurons resulting from secondary degeneration is about 25% a week after mild crush injury and about 55% 2 weeks after the injury (16). Thus, even if the T cell response took 1 week to reach the required strength, there would still be nerve fibers in need of protection at that time. A comparison of our results obtained after adoptive transfer of activated Cop-1-reactive T cells and after active immunization with Cop-1 shows that the extent of protection from secondary degeneration was almost the same in both. It is worth noticing that, although Cop-1 is known as an agent designed to suppress T cell autoimmunity, its effect in this study requires that an anti-myelin T cell autoimmunity be activated. Thus, Cop-1 is used here as a potentially “safe” antigen for activating an effective T cell response that can be cross-activated by myelin proteins.
In conclusion, earlier studies have proposed that axonal injury in the rat CNS awakens an autoimmune T cell response which is directed against myelin proteins, but is too weak to protect the nerve fibers from secondary degeneration (6, 33). Boosting of this immune response without risk of accompanying autoimmune disease was achieved in this study by using a copolymer, which is cross-recognized by the CNS but is not encephalitogenic. The effect of Cop-1 here is reminiscent of the effect obtained with a cryptic epitope of MBP (6). We suggest that the T cell immune response to the polymer, obtained either by adoptive transfer or immunization at the time of the injury, seems to provide an effective means of posttraumatic protection. It remains to be seen whether the activity of Cop-1 as a neuroprotectant rather than as a suppressor depends on the way of its administration. It is also essential to find out how the locally accumulated CNS-specific T cells or T cells specific to cross-reactive antigens such as Cop-1, mediate neuroprotection in the context of CNS injuries. The T cell-mediated neuroprotection demonstrated here might be applicable to both chronic and acute injuries of CNS nerves, in which neurons are vulnerable to degeneration and amenable to neuroprotection (7, 34).
Acknowledgments
We thank Shirley Smith for editorial assistance, Avital Shapira for animal maintenance, and Wolfgang Klinkert for advice on BDNF ELISA. The work is supported by grants from Proneuron Ltd, The Alan Brown Foundation for Spinal Cord Injury, and the Glaucoma Research Foundation, all awarded to M. Schwartz.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CFA
complete Freund's adjuvant
- CNS
central nervous system
- Cop-1
copolymer 1
- 4-Di-10-Asp
4-(4-(didecylamino)styryl)-N-methylpyridinium iodide
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- NT
neurotrophin
- OVA
ovalbumin
- RGC
retinal ganglion cells
- TCR
T cell receptor
References
- 1.Jonas J B, Budde W M. Prog Retin Eye Res. 2000;19:1–40. doi: 10.1016/s1350-9462(99)00002-6. [DOI] [PubMed] [Google Scholar]
- 2.Steinsapir K D. Curr Opin Ophthalmol. 1999;10:340–342. doi: 10.1097/00055735-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M, Belkin M, Yoles E, Solomon A. J Glaucoma. 1996;5:427–432. [PubMed] [Google Scholar]
- 4.Yoles E, Schwartz M. Surv Ophthalmol. 1998;42:367–372. doi: 10.1016/s0039-6257(97)00123-9. [DOI] [PubMed] [Google Scholar]
- 5.Moalem, G., Yoles, E., Leibowitz-Amit, R., Muller-Gilor, S., Mor, F., Cohen, I. R. & Schwartz, M. (2000) J. Neuroimmunol., in press. [DOI] [PubMed]
- 6.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen I R, Schwartz M. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz M, Yoles E. Curr Opin Ophthalmol. 2000;11:107–111. doi: 10.1097/00055735-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen I R, Schwartz M. Lancet. 2000;355:286–287. doi: 10.1016/s0140-6736(99)05140-5. [DOI] [PubMed] [Google Scholar]
- 9.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum D, Fridkis-Hareli M, Arnon R, Sela M. J Neuroimmunol. 1996;64:209–217. doi: 10.1016/0165-5728(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum D, Sela M, Arnon R. Isr J Med Sci. 1997;33:280–284. [PubMed] [Google Scholar]
- 12.Ben-Nun A, Wekerle H, Cohen I R. Eur J Immunol. 1981;11:195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- 13.Gillis S, Ferm M M, Ou W, Smith K A. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 14.Ben-Nun A, Cohen I R. J Immunol. 1982;129:303–308. [PubMed] [Google Scholar]
- 15.Duvdevani R, Rosner M, Belkin M, Sautter J, Sabel B A, Schwartz M. Restor Neurol Neurosci. 1990;2:31–38. doi: 10.3233/RNN-1990-2104. [DOI] [PubMed] [Google Scholar]
- 16.Yoles E, Schwartz M. Exp Neurol. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- 17.Hunig T, Wallny H J, Hartley J K, Lawetzky A, Tiefenthaler G. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschberg D L, Moalem G, He J, Mor F, Cohen I R, Schwartz M. J Neuroimmunol. 1998;89:88–96. doi: 10.1016/s0165-5728(98)00118-0. [DOI] [PubMed] [Google Scholar]
- 19.Moalem G, Monsonego A, Shani Y, Cohen I R, Schwartz M. FASEB J. 1999;13:1207–1217. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
- 20.Kerschensteiner M, Gallmeier E, Behrens L, Leal V V, Misgeld T, Klinkert W E, Kolbeck R, Hoppe E, Oropeza-Wekerle R L, Bartke I, et al. J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiner H L. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 22.Weiner H L. Annu Rev Med. 1997;48:341–351. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Nun A, Mendel I, Bakimer R, Fridkis-Hareli M, Teitelbaum D, Arnon R, Sela M, Kerlero de Rosbo N. J Neurol. 1996;243:S14–S22. doi: 10.1007/BF00873697. [DOI] [PubMed] [Google Scholar]
- 24.Webb C, Teitelbaum D, Arnon R, Sela M. Immunol Commun. 1973;2:185–192. doi: 10.3109/08820137309022791. [DOI] [PubMed] [Google Scholar]
- 25.Teitelbaum D, Aharoni R, Arnon R, Sela M. Proc Natl Acad Sci USA. 1988;85:9724–9728. doi: 10.1073/pnas.85.24.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aharoni R, Teitelbaum D, Sela M, Arnon R. J Neuroimmunol. 1998;91:135–146. doi: 10.1016/s0165-5728(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 27.Fridkis-Hareli M, Aharoni R, Teitelbaum D, Arnon R, Sela M, Strominger J L. Int Immunol. 1999;11:635–641. doi: 10.1093/intimm/11.5.635. [DOI] [PubMed] [Google Scholar]
- 28.Aharoni R, Teitelbaum D, Arnon R. Eur J Immunol. 1993;23:17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- 29.Aharoni R, Teitelbaum D, Sela M, Arnon R. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hickey W F, Hsu B L, Kimura H. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 31.Yan Q, Elliott J, Snider W D. Nature (London) 1992;360:753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 32.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde Y A. Nature (London) 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz M, Moalem G, Leibowitz-Amit R, Cohen I R. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz M, Yoles E. Invest Ophthalmol Visual Sci. 2000;41:349–351. [PubMed] [Google Scholar]