Abstract
Mesoderm formation results from an inducing process that requires maternal and zygotic FGF/MAPK and TGFβ activities, while maternal activation of the Wnt/β-catenin pathway determines the anterior–dorsal axis. Here, we show a new role of Wnt/β-catenin signaling in mesoderm induction. We find that maternal β-catenin signaling is not only active dorsally but also all around the equatorial region, coinciding with the prospective mesoderm. Maternal β-catenin function is required both for expression of dorsal genes and for activation of MAPK and the mesodermal markers Xbra and eomesodermin. β-catenin acts in a non- cell-autonomous manner upstream of zygotic FGF and nodal signals. The Wnt/β-catenin activity in the equatorial region of the early embryo is the first example of a maternally provided mesoderm inducer restricted to the prospective mesoderm.
Keywords: embryonic development/FGF/MAPK/Wnt pathway/Xnr
Introduction
The basic subdivision of the vertebrate embryo into the three germ layers, ectoderm, mesoderm and endoderm, is established very early during development. In Xenopus, the three layers form along the primary animal–vegetal symmetry axis of the embryo: the pigmented animal pole becomes ectoderm, the equatorial region becomes mesoderm, and the yolk-rich vegetal pole becomes endoderm. A second axis is created at fertilization, which defines the dorso-ventral polarity, and the superimposition of these two patterns can account for the basic body plan of the embryo. Both patterns appear to rely on inductive signals, which are generated by pre-localized maternal determinants. The vegetal hemisphere seems to be the major source of such determinants: in particular, the vegetal pole emits a signal which can redirect the fate of animal cells from ectoderm to mesoderm and endoderm (Nieuwkoop, 1969). Another vegetally localized determinant is translocated on one side of the egg after fertilization and later induces the various dorsalizing signals, which constitute the activity of Spemann’s organizer (Harland and Gerhart, 1997).
Mesoderm–endoderm induction has been extensively studied, and the molecular nature of the signal has been at least partly elucidated (Harland and Gerhart, 1997; Zhang et al., 1998; Kimelman and Griffin, 2000). Experiments using dominant-negative receptor constructs have revealed an essential role for secreted growth factors of the FGF and TGFβ/activin family: FGF signaling was found to be necessary for induction of part of the mesodermal genes, while TGFβ/activin-like signaling is required for formation of all mesoderm and endoderm. Most of the recent studies have focused on the role of TFGβ-like factors of the Nodal sub-family, so-called Xenopus Nodal-related proteins (Xnrs). Xnrs are expressed zygotically under the control of a maternal, vegetally localized transcription factor, VegT (Clements et al., 1999). Knock down experiments have shown that depletion of maternal VegT has profound effects on early patterning: endoderm is not induced, mesoderm formation is delayed and, when some mesoderm eventually forms, it appears misplaced to the vegetal pole, and consequently gastrulation is strongly affected (Zhang et al., 1998). Such phenotype can largely be accounted for by the absence of early Xnr expression (Kofron et al., 1999; Xanthos et al., 2001). Current models thus propose that high levels of Xnrs in the vegetal hemisphere induce endoderm, while lower levels around the equator (so-called marginal zone) induce mesoderm.
While the VegT/Xnrs pathway undoubtedly plays a crucial role in germ layer specification, our knowledge of this process is clearly still incomplete. There is evidence that at least part of the mesoderm induction occurs very early, before the start of zygotic transcription, and thus before activation of the VegT/Xnrs pathway (Jones and Woodland, 1987; Ding et al., 1998). Candidates for such early induction are two maternal TGFβ-like factors, activin B and the vegetally localized Vg1, but a physiological role for these factors has not been yet established. The requirement for FGF signaling has also to be taken into account. Several FGFs have been identified, some of which are already maternally expressed, but their precise distribution at early stages is unclear. Indirect evidence suggests that FGFs might be active over the animal hemisphere, where they would act as a competence signal (Cornell and Kimelman, 1994; LaBonne and Whitman, 1997). The vegetal inducing signal(s) would reach the lower part of this competent region, i.e. the equatorial region where mesodermal genes would be induced (Kimelman et al., 1992; Cornell et al., 1995).
Recent data on the detection of activated pathways has also provided important spatial and temporal information on early patterning events. FGF and TGFβ signaling pathways induce phosphorylation and nuclear accumulation of their respective downstream transducers, MAP kinase (MAPK) and Smad2. Specific antibodies recognizing these phosphorylated forms can be used to localize these signaling activities directly (Christen and Slack, 1999; Curran and Grainger, 2000; Faure et al., 2000; Schohl and Fagotto, 2002). Studies on Smad2 activation have confirmed the existence of a strong TGFβ-like activity throughout the vegetal hemisphere of the late blastula–early gastrula (Faure et al., 2000; Schohl and Fagotto, 2002). On the other hand, the prospective mesoderm is quite precisely marked after the onset of zygotic transcription by a ring of activated, phosphorylated MAPK (P-MAPK) (Christen and Slack, 1999). Although MAPK can be activated by several tyrosine kinase receptors, its activation in early Xenopus embryos seems to be largely due to FGFs (Christen and Slack, 1999). The equatorial pattern of MAPK activation cannot be readily explained by the predicted animal source of FGF signaling and suggests that other mechanisms must localize MAPK activity to the marginal zone.
The dorsalizing activity present in the early embryo appears to be mediated by a third pathway involving β-catenin (Heasman et al., 1994; Harland and Gerhart, 1997; Heasman, 1997). β-catenin is a cytoplasmic protein with two distinct functions. It is constitutively present at the plasma membrane, where it interacts with the cadherin adhesion molecules and links them to the actin cytoskeleton (Yap et al., 1997). However, it is also a downstream component of the Wnt signaling pathway. Upon Wnt stimulation, a cytoplasmic pool of β-catenin is induced, which can accumulate in the nucleus, interact with the TCF/Lef-1 transcription factors and thus modulate gene expression (Cadigan and Nusse, 1997). In Xenopus, β-catenin signaling is activated maternally in the prospective dorsal side of the embryo (Schneider et al., 1996; Larabell et al., 1997). Dorsal maternal β-catenin then activates specific target genes, in particular the transcription factor Siamois, which in turn synergize with mesoderm-inducing signals to induce Spemann’s organizer (Moon and Kimelman, 1998).
We have recently examined in detail the pattern of nuclear β-catenin during Xenopus development (Schohl and Fagotto, 2002). At blastula stages, we found, to our surprise, that β-catenin was not only nuclear on the dorsal side. It was clearly detectable in a ring around the equator that overlapped strikingly with the pattern of P-MAPK. These observations suggested that it might have, in addition to its well-established function in dorso-ventral patterning, an unexpected role in formation of the mesoderm.
Here, we show that maternal β-catenin is also required for activation of MAPK along the marginal zone and for expression of early mesodermal genes.
Results
β-catenin is required for early MAPK activation in the blastula
At mid- and late-blastula stages (stages 8.5–9.5), nuclear β-catenin accumulates all around the equatorial region, where it co-localizes with activated P-MAPK (Schohl and Fagotto, 2002). To test whether β-catenin signaling is required for activation of MAPK, we knocked down β-catenin using morpholino oligonucleotides (MO). MO specifically block translation of the targeted protein and show no toxicity even when used at high concentrations. Control MO or anti-β-catenin MO were injected around the equatorial region of early cleaving embryos, and the injected embryos were analyzed at stage 9–9.5 for nuclear β-catenin and P-MAPK by immunofluorescence on cryosections. Compared with embryos injected with control MO (Figure 1A), nuclear β-catenin was strongly reduced in embryos injected with anti-β-catenin MO (Figure 1B). The levels of β-catenin at the plasma membrane did not appear to be affected (Figure 1A and B), consistent with the observation that cadherin-bound β-catenin is rather stable and that oligonucleotide-mediated depletion mainly affects the soluble pool of β-catenin in early embryos (Kofron et al., 1997).
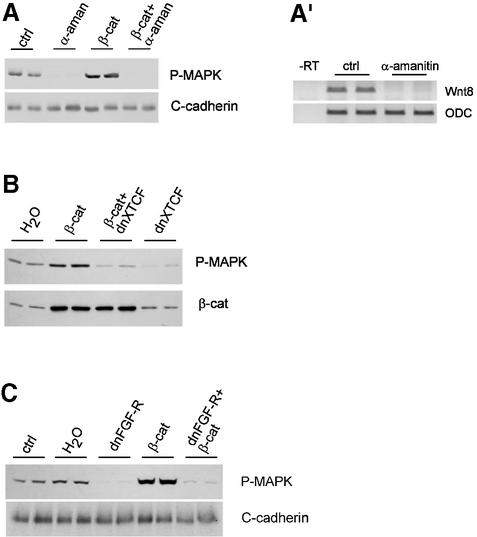
Fig. 1. β-catenin is required for activation of MAPK and early expression of pan-mesodermal genes. Embryos were injected four times equatorially at the four-cell stage and fixed or extracted at the indicated stages. (A and B) Double staining for β-catenin (A and B) and P-MAPK (A′ and B′) of cryosections from embryos injected with (A) control morpholino oligonucleotides (MO control, 4 × 5 ng) or (B) β-catenin MO (MO β-catenin, 4 × 5 ng). Small panels show details of β-catenin staining in the ventral and dorsal marginal zone. Brackets indicate marginal zone; arrows point to β-catenin and P-MAPK positive nuclei; an, animal pole; vg, vegetal pole; β-catenin MO depleted nuclear β-catenin on both the dorsal and the ventral side and reduced MAPK activation along the marginal zone. (C) Immunoblot of total extracts from embryos injected with control or β-catenin MO (4 × 5 ng) stained with anti-P-MAPK antibody or with anti-C-cadherin antibody used as loading control. β-catenin MO decreased the levels of activated P-MAPK. (D) RT–PCR analysis of pan-mesodermal (Xbra, eomesodermin), dorsal (gsc) and ventral (Wnt8) markers. Depletion of nuclear β-catenin (MOβcat, 4 × 5 ng) or downregulation of β-catenin signaling by injection of dominant-negative XTFC3 mRNA (dnXTCF, 4 × 2 ng) inhibited expression of the early mesodermal genes Xbra and eomesodermin and of gsc. β-catenin overexpression (4 × 2 ng mRNA) stimulated expression of Xbra, eomesodermin and gsc and inhibited expression of Wnt8. ODC was used as loading control. (E) dnXTCF inhibits Xbra and eomesodermin expression not only on the dorsal side, but also on the ventral side. Control embryos and embryos injected with dnXTCF (4 × 0.5 ng) were dissected into dorsal and ventral halves at stage 9.5 and analyzed by RT–PCR. Siamois, a dorsal marker directly dependent on β-catenin signaling, was used as control for proper dissection.
β-catenin depletion had a dramatic effect on activation of MAPK, as the nuclear staining for P-MAPK was strongly reduced, on both the dorsal and the ventral sides, in embryos injected with anti-β-catenin MO (Figure 1B′). A significant decrease of total P-MAPK levels could also be observed by immunoblot (Figure 1C). Conversely, β-catenin overexpression induced an increase in P-MAPK (Figure 2C; Schohl and Fagotto, 2002). Thus, activation of MAPK in the prospective marginal zone appears to be tightly dependent on β-catenin.
Fig. 2. Activation of MAPK by β-catenin is indirect and requires FGF signaling. P-MAPK immunoblots of embryo extracts, stage 9. Embryos were injected with β-catenin mRNA (4 × 2 ng), α-amanitin (4 × 50 pg), dominant-negative XTFC3 mRNA (dnXTCF, 4 × 2 ng) or dominant-negative FGF receptor (dnFGF-R, 4 × 2 ng). Control, uninjected; H2O, water injected. Both normal and β-catenin-induced P-MAPK activation were blocked by (A) α-amanitin, (B) dnXTCF and (C) dnFGF-R. β-catenin blot in (B): co-expression of dnXTCF does not affect the levels of β-catenin. (A′) RT–PCR showing complete block of transcription of the zygotic gene Wnt8 in embryos injected with α-amanitin.
β-catenin is required for early expression of pan-mesodermal markers
Activation of MAPK is a hallmark of FGF signaling in the prospective mesoderm. This pathway is known to activate and to be required for a set of mesodermal genes, including Xbra. The influence of β-catenin on activation of MAPK suggested that β-catenin might control the expression of these mesodermal markers. Embryos depleted of β-catenin (anti-β-catenin MO) or overexpressing β-catenin were analyzed at stage 9 by RT–PCR for the following markers (Figure 1D): goosecoid (gsc), a dorsal–anterior mesodermal marker; the ‘classical’ mesodermal marker Xbra, which is expressed in most of the mesoderm except the most dorsal anterior domain; eomesodermin, a very early pan-mesodermal marker; and Xwnt8, a gene expressed in the ventro-lateral mesoderm and endoderm.
In agreement with the well-documented role of dorsal maternal β-catenin in induction of dorsal genes, gsc expression was inhibited by anti-β-catenin MO and activated by β-catenin overexpression. β-catenin also appeared to regulate the expression of the pan-mesodermal markers Xbra and eomesodermin, which were reduced by anti-β-catenin MO and increased by β-catenin overexpression. Xwnt8 was not affected by anti-β-catenin MO, while β-catenin overexpression, which dorsalizes the embryo, inhibited expression of Xwnt8, as expected for a ventral marker. Note that, unlike Xbra, Xwnt8 expression is known to be independent of FGF signaling (Cornell and Kimelman, 1994).
The fact that β-catenin depletion prevented activation of MAPK not only on the dorsal, but also on the ventral side, suggested that β-catenin regulates mesoderm induction all around the marginal zone. To verify this possibility, we analyzed expression of pan-mesodermal markers in the dorsal and ventral sides of embryos expressing dominant-negative XTCF (dnXTCF), an inhibitor of β-catenin signaling that blocked expression of Xbra and eomesodermin very efficiently (Figure 1D; see below). The dorsal-specific gene Siamois was used to control for proper dorsal–ventral dissection. We found that Xbra and eomesodermin expression was inhibited on both sides (Figure 1E), showing that β-catenin signaling is required throughout the marginal zone.
We also examined a set of markers expressed during gastrulation (stage 11; see Supplementary data, available at The EMBO Journal Online). At this stage, Xbra expression appeared to have recovered to normal levels in embryos injected with anti-β-catenin MO. The mes-endodermal marker Mix1 and the endodermal marker sox17α were unaffected. However, myoD, a myogenic transcription factor expressed in the ventro-lateral mesoderm, was virtually absent from β-catenin-depleted embryos, while its expression was stimulated by β-catenin overexpression. In the case of myoD expression, both FGF signaling (Cornell and Kimelman, 1994) and zygotic Wnt signaling (Hoppler et al., 1996) are required.
In summary, these data show that β-catenin is involved in activation of two types of early genes: the dorsal genes of Spemann’s organizer, such as gsc, and a specific set of mesodermal genes, which are all dependent on FGF signaling (Cornell and Kimelman, 1994; see below).
Activation of MAPK by β-catenin is indirect and requires FGF signaling
We next wanted to investigate the mechanism through which β-catenin regulates MAPK. It could affect the MAPK pathway directly, post-translationally, or could act more indirectly, through its TCF-dependent transcriptional activity. To discriminate between these possibilities, we examined the effect of the transcription inhibitor α-amanitin on P-MAPK levels, detected on western blots. α-amanitin effectively blocked transcription, as shown in Figure 2A′ for the zygotic gene Wnt8. Both endogenous MAPK activation and β-catenin-induced MAPK activation were completely inhibited by α-amanitin (Figure 2A), indicating that transcription is required. To determine whether the transcriptional activity required TCF, we tested the effect of dnXTCF (Figure 2B). dnXTCF is a trunctated TCF that can still bind DNA, but lacks the β-catenin binding site, and acts as a constitutive repressor. Injection of dnXTCF mRNA caused a strong decrease of P-MAPK compared with control embryos, and co-injection of dnXTCF mRNA blocked activation of MAPK by overexpressed β-catenin. We conclude that β-catenin acts on MAPK indirectly, through TCF-dependent transcription. Consistent with this observation, we found that expression of all markers that depended on β-catenin, i.e. gsc, eomesodermin, Xbra and myoD, were also sensitive to dnXTCF (Figure 1D and E; see Supplementary data).
We also tested the effect of blocking FGF signaling. In agreement with previous reports (Christen and Slack, 1999), endogenous activation of MAPK was completely inhibited by expression of a dominant-negative FGF receptor construct (dnFGF-R, Figure 2C). Furthermore, β-catenin-induced MAPK activation was also blocked by dnFGF-R. These results imply that FGF signaling is required for β-catenin-induced MAPK activation.
Activation of MAPK by β-catenin is non-cell autonomous
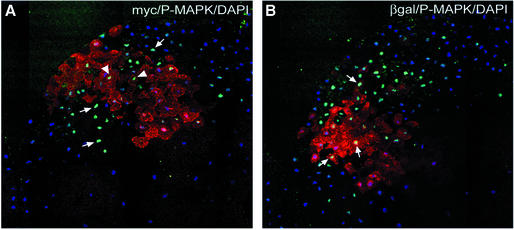
β-catenin could act on MAPK cell autonomously or non-cell autonomously. In the first case, β-catenin-induced transcriptional activity could for instance cause an increased sensitivity for FGF signals in the receiving cells. In the second case, β-catenin would induce production of an extracellular signal. We thus tested the range of action of β-catenin on activation of MAPK. We injected mRNA coding for myc-tagged β-catenin in a single ventral blastomere at the 16-cell stage and β-galactosidase mRNA in an adjacent blastomere. The blastomeres were chosen such that they had been already separated by at least two rounds of cleavages, so as to exclude any possible diffusion of mRNA between not yet fully separated sister cells. Equatorial sections were prepared from stage 9 embryos and stained for P-MAPK and either myc-β-catenin or β-galactosidase. In the ventral side, P-MAPK is normally weak at this stage, and the intense ectopic activation of MAPK generated at the site of β-catenin overexpression could thus be clearly detected. Note that not all nuclei showed high P-MAPK levels within this area. This heterogeneity is characteristic of MAPK activation in the early embryo (Schohl and Fagotto, 2002).
We found strong P-MAPK signal not only in the myc-labelled β-catenin-expressing cells, but also in adjacent cells (Figure 3A). The complementary staining showed clearly β-galactosidase-expressing cells strongly positive for P-MAPK in the proximity of β-catenin-expressing cells (Figure 3B). These results demonstrate that β-catenin-induced MAPK activation occurs in a non-cell-autonomous fashion, i.e. β-catenin induces the expression of a secreted factor. We also observed that TCF function was only required in the secreting cells, but not in the receiving cells, since β-catenin could also induce high levels of P-MAPK in neighboring cells expressing dnXTCF (data not shown).
Fig. 3. Activation of MAPK by β-catenin is non-cell autonomous. Stage 16-cell embryos were injected ventrally in one blastomere with myc-tagged β-catenin mRNA (500 pg) and in an adjacent blastomere with β-galactosidase mRNA (1 ng). Embryos were fixed at stage 9 and analyzed by immunofluorescence on equatorial sections. (A) Double staining for myc-β-catenin (anti-myc, red) and P-MAPK (green). (B) Double staining for β-galactosidase (red) and P-MAPK (green). (A) and (B) show consecutive sections. Nuclei were stained with DAPI (blue). P-MAPK signal was not only increased in β-catenin-overexpressing cells (arrowheads in A), but also in adjacent cells (arrows).
XFGF3 is a target of β-catenin
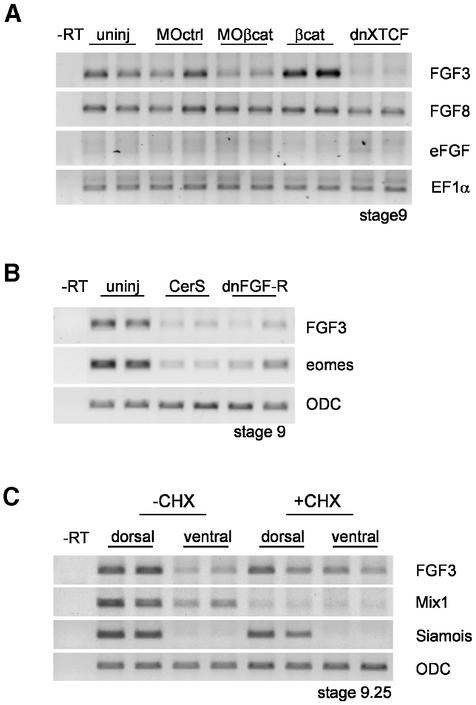
Since activation of MAPK by β-catenin is non-cell autonomous and inhibited by dnFGF-R, the simplest possible explanation would be that β-catenin signaling stimulates expression of an FGF-like factor. We thus examined three early zygotic FGF factors, eFGF, XFGF3 and XFGF8 (Isaacs, 1997; Kofron et al., 1999). We analyzed their expression at blastula stage (stage 9–9.5) under conditions where β-catenin signaling was either inhibited (injection of anti-β-catenin MO or dnXTFC3 mRNA) or activated (β-catenin overexpression) (Figure 4A). eFGF, which is involved in Xbra regulation during gastrulation, was barely detectable at this stage and is thus unlikely to play a role in these early events. XFGF8 was not affected by manipulating β-catenin signaling activity. However, XFGF3 expression was significantly decreased in embryos injected with anti-β-catenin MO and dnXTCF and strongly stimulated by β-catenin overexpression. These results confirm that β-catenin activates FGF signaling in the blastula and identify XFGF3 as a specific target of β-catenin. While there may be other yet unidentified FGFs under the control of β-catenin at this stage, XFGF3 appears to be a good candidate for the signal, or at least part of the signal, that mediates activation of MAPK by β-catenin.
Fig. 4. Regulation of FGF3 by early β-catenin, Xnr and FGF signals. (A) FGF3 is a target of β-catenin. FGF3, FGF8 and eFGF were analyzed by RT–PCR at stage 9. FGF3 expression was inhibited by β-catenin morpholino oligonucleotides (MOβcat, 4 × 5 ng) and dominant-negative XTFC3 mRNA (dnXTCF, 4 × 2 ng mRNA) and activated by β-catenin overexpression (4 × 2 ng mRNA). FGF8 was unaffected. eFGF was barely detectable at this early stage. EF1α was used as loading control. (B) FGF3 and eomesodermin expression at stage 9 were inhibited by cerberus short (CerS, 4 × 150 pg mRNA) and dominant-negative FGF receptor (dnFGF-R, 4 × 2 ng mRNA). (C) Early FGF3 expression is resistant to cycloheximide (CHX) treatment. Embryos were cut into dorsal and ventral halves at stage 7, and the halves were cultured in the presence or absence of 100 µg/ml CHX until stage 9.25. Siamois was used as a dorsal marker and Mix1 as positive control for the effectiveness of CHX.
XFGF3 is a direct target of very early signals
While we have shown that XFGF3 expression is regulated by β-catenin, XFGF3 has also been reported to be inducible by activin and FGF in animal caps (Tannahill et al., 1992). To determine whether, in addition to β-catenin, other pathways were also involved in regulation of early XFGF3 expression, we tested the effect of blocking Xnr and FGF signaling. To inhibit Xnr signals, we expressed in the marginal zone a fragment of cerberus, cerberus short (CerS), which is a strong and specific secreted inhibitor of Xnrs (Piccolo et al., 1999). XFGF3 expression was significantly reduced by CerS (Figure 4B), indicating that Xnr activity is involved in activation of XFGF3 expression. We also tested XFGF3 expression when FGF signaling is blocked by dnFGF-R and found that it was strongly inhibited by this treatment (Figure 4B). Thus, XFGF3 expression depends on three inputs: β-catenin, TGFβ/Xnr and FGF signaling.
Zygotic XFGF3 appears to be an early gene, as its expression is clearly detectable by stage 9. To test whether XFGF3 is directly induced by maternal signals, we examined the expression pattern in embryos in which protein synthesis had been blocked before mid-blastula transition (MBT), which is the onset of zygotic transcription (Figure 4C). We dissected embryos at stage 7 in dorsal and ventral halves and incubated them in the presence or absence of the protein synthesis inhibitor cycloheximide (CHX). Dissection had two purposes: it insured a better penetration of CHX and also allowed us to examine possible differences between dorsal and ventral regions. We verified that protein synthesis was completely blocked by CHX under these conditions by analyzing [35S]methionine incorporation (data not shown). We further verified that the CHX treatment was effectively inhibiting early zygotic processes by monitoring Mix1 expression (Figure 4C), a very early endodermal marker partially sensitive to CHX (Yasuo and Lemaire, 1999). The dorsal-specific gene Siamois was used as control for proper dorsal–ventral dissection. Consistent with Siamois being a direct target of maternal β-catenin, its expression was largely unaffected by CHX. Similar to Siamois, activation of XFGF3 was not inhibited by CHX, on neither the dorsal nor the ventral side (Figure 4C). On the contrary, FGF3 expression in the ventral side was reproducibly stronger in CHX-treated embryos. We conclude that early expression of XFGF3 does not require new protein synthesis and thus relies on signals already present before MBT.
Rescue of β-catenin-dependent mesoderm induction by XFGF3 and Xnr1
If β-catenin regulation of MAPK and mesodermal gene expression is mediated by FGF signals, FGF ligands should be able to rescue MAPK activity and eomesodermin expression when β-catenin signaling is blocked. We found that inhibition of P-MAPK signal by dnXTCF could be overcome by co-expression of FGF3, indicating that FGF3 acts downstream of β-catenin and upstream of MAPK (Figure 5A). However, FGF3 was not able to rescue eomesodermin expression (Figure 5B). β-catenin is also known to be required for early Xnr expression (Agius et al., 2000), and both FGF and Xnr signals may cooperate for activation of eomesodermin. We found that co-expression of FGF3 and Xnr1 could rescue normal levels of eomesodermin expression in dnXTCF-expressing embryos (Figure 5B). Xnr1 alone was capable of partial rescue, but this was completely blocked by dnFGF-R. We conclude that FGF3 is sufficient to mediate the action of β-catenin on MAPK. For eomesodermin expression, however, both FGF and Xnr signaling are required downstream of β-catenin.
Fig. 5. Rescue of MAPK activity and eomesodermin expression by FGF3 and Xnr1. (A) P-MAPK blot of stage 9.25 extracts: P-MAPK was decreased by dominant-negative XTFC3 mRNA (dnXTCF, 4 × 2 ng mRNA) but could be rescued by co-expression of FGF3 (4 × 10 pg mRNA). Control, uninjected; H2O, water injected. (B) RT–PCR: expression of eomesodermin at stage 9.25 was strongly inhibited by dnXTCF (4 × 2 ng mRNA). Co-expression of FGF3 (4 × 10 pg mRNA) and Xnr1 (4 × 5 pg mRNA) could rescue normal levels of eomesodermin. FGF3 alone had no effect. Partial rescue was observed with Xnr1 alone, which was completely blocked by dominant-negative FGF receptor (dnFGF-R, 4 × 2 ng mRNA).
β-catenin in the marginal zone is activated maternally
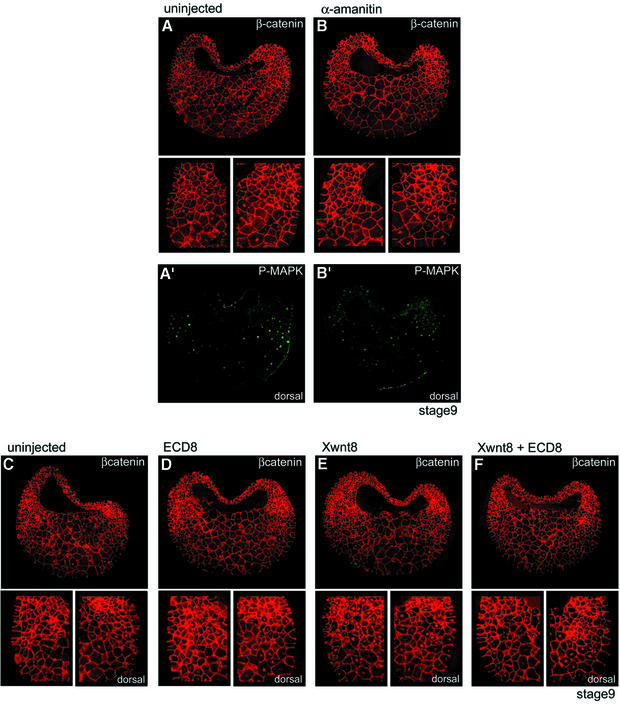
In our previous study, we had observed that nuclear β-catenin accumulates first on the dorsal side (Schohl and Fagotto, 2002). On this side, it is already detectable at stage 8, i.e. before the start of zygotic transcription, and has reached high levels by stage 8.5. In the ventro-lateral region, nuclear β-catenin accumulates later, starting at stage 8.5. It would thus be conceivable that the ring observed in late blastulae (stage 9–9.5) results from the overlap of two distinct patterns, due to the early, maternal dorsal activity and to a second zygotic activity emerging in the ventro-lateral equatorial region. One should then be able to distinguish the maternal and the zygotic activity using the transcriptional inhibitor α-amanitin. We found that α-amanitin, while blocking expression of Wnt8 (Figure 2A′), had no effect on nuclear β-catenin, on neither the dorsal nor the ventral side of stage 9 (Figure 6B) and stage 9.5 blastulae (data not shown). We conclude that most or all of the nuclear β-catenin signal detected at these stages, both in the dorsal and in the ventral side, is of maternal origin. Note that the signal for P-MAPK was strongly inhibited by α-amanitin in these experiments (Figure 6B′), consistent with our biochemical data (Figure 2A).
Fig. 6. Nuclear β-catenin in the marginal zone is maternal and independent of Wnt ligands. (A and B) Double staining for β-catenin (A and B) and P-MAPK (A′ and B′) of cryosections from (A) control embryos or (B) embryos injected with α-amanitin (stage 9, 4 × 50 pg). Small panels show enlargements of β-catenin staining in the ventral and dorsal marginal zone. α-amanitin strongly reduced the P-MAPK signal but had no effect on nuclear β-catenin, neither on the dorsal nor on the ventral side. (C–F) β-catenin staining for (C) control embryos or for embryos injected with mRNA coding for (D) the extracellular domain of Xenopus Frizzled 8 (ECD8, 4 × 500 pg), (E) Xwnt8 (4 × 10 pg) or (F) ECD8 and Xwnt8. Xwnt8 strongly increased nuclear β-catenin on the ventral side (E), and this increase was prevented by co-injection of ECD8 (F). However, ECD8 had no effect on the endogenous ventral and dorsal β-catenin signals (compare C and D).
It is thought that dorsal β-catenin activity may not be triggered by a Wnt ligand but activated intracellularly (Moon and Kimelman, 1998). Obviously, nothing is known about the origin of the rest of the early β-catenin pattern, i.e. the ventro-lateral signal. To start to address this issue, we tried to antagonize Wnt ligands by expressing large amounts of the extracellular domain of Xenopus Frizzled 8 (ECD8). ECD8 binds and strongly inhibits a wide range of Wnts (Itoh and Sokol, 1999). While EDC8 could efficiently block Wnt-induced β-catenin accumulation on the ventral side (Figure 6E and F), it had no effect on the endogenous β-catenin pattern at blastula stage (Figure 6C and D). This suggests that Wnt ligands are not involved in activation of maternal β-catenin, although one cannot exclude that the signal occurs so early that ECD8 is not produced fast enough to inhibit it.
We also tested the influence of TGFβ and FGF pathways on the β-catenin ring. Expression of CerS and dnFGF-R did not affect the pattern of nuclear β-catenin (data not shown). Furthermore, overexpression of activin and FGF failed to increase nuclear β-catenin in the animal pole cells (see Supplementary data). These results suggest that TGFβ and FGF signals are probably not responsible for patterning of maternal β-catenin to the prospective marginal zone.
Discussion
β-catenin and mesoderm induction
In the current models of Xenopus development, one precise function is assigned to maternal β-catenin signaling, i.e. to define the dorsal side, while the establishment of the three germ layers relies on FGF and TGFβ signaling.
We show here that β-catenin also plays a role in induction of the early mesoderm. In contrast to what had been generally assumed, β-catenin is not only nuclear in the dorsal cells, but also around the whole equatorial region. We found that β-catenin is required for activation of the FGF/MAPK pathway in the marginal zone and for expression of the early FGF-dependent mesodermal genes Xbra and eomesodermin. This role is perfectly consistent with the overlap of the patterns of β-catenin and MAPK activities in the marginal zone.
We have investigated the mechanism through which β-catenin regulates MAPK and found that it is indirect, transcription dependent and non-cell autonomous, which implies that β-catenin signaling activates the production of a diffusible signal. As activation of MAPK by β-catenin requires functional FGF signaling, we hypothesized that this signal is an FGF factor. We have identified FGF3 as a candidate for this signal: FGF3 is a target of β-catenin, and it can rescue inhibition of MAPK activation by dnXTCF. We do not know, however, whether the inductive signal activated by β-catenin is composed of one or several FGFs.
Vonica and Gumbiner (2002) have also recently reported regulation of Xbra by Wnt, through a different mechanism. They proposed that Xbra was activated cell autonomously and probably directly by zygotic Xwnts. Their analysis was performed at later stages (9.5–10.5) and probably deals with a subsequent step in mesoderm induction. While direct regulation of Xbra by β-catenin may also be active in the early phase, our present work shows that maternal β-catenin has a wider impact on early mesoderm induction by controlling FGF-MAPK signaling.
β-catenin controls simultaneously dorsalization and mesoderm induction
If β-catenin is active all around the embryo, what makes the difference between the dorsal and the ventral side? In other words, why are genes such as Siamois expressed only dorsally and not all around the marginal zone? We think that the answer probably resides in timing and signal intensity. Nuclear β-catenin accumulates faster on the dorsal side, where it reaches rapidly the high levels required for expression of Siamois and other dorsal-specific genes. On the ventral side, similar levels of β-catenin are reached only in the late blastula, and competence for Siamois expression may be lost by that stage (Darken and Wilson, 2001). MAPK, on the other hand, seems to be activated already by lower levels of β-catenin, as it also occurs early on the ventral side. We propose that mesoderm induction and dorsalization are responses to different levels of a single maternal β-catenin signal. Two nested domains of gene expression would thus be created, one in the most dorsal region (Siamois), the second spanning over most of the field of nuclear β-catenin (eomesodermin). This hypothesis should be tested in the future.
Maternal β-catenin activity is pre-localized in the equatorial region
Detection of the ring of maternal β-catenin activity raises a puzzle about how this nuclear pattern becomes locally activated. It is thought that maternal TGFβ and FGF signals may be located in the vegetal, respectively in the animal half, and the overlap of these two signals in the equatorial region could define the marginal zone (Kimelman et al., 1992). However, maternal nuclear β-catenin did not appear to be affected by inhibition or ectopic activation of these pathways. As inhibiting Wnts also had no effect, the simplest explanation is that β-catenin activation is independent of extracellular signals, as already proposed for the dorsal activity [Hoppler et al., 1996; Sokol, 1996; Moon and Kimelman, 1998; but see Sumanas et al. (2000) for contradictory results]. How, then, is the pattern established? One possibility is that the vegetal determinant responsible for β-catenin activation may not be entirely translocated to the dorsal side during the upward movement caused by cortical rotation, but part of it may also spread all around the equator. Another possibility would be that a second β-catenin-inducing activity is pre-localized in the equatorial region, through a distinct patterning mechanism.
Our observations reveal a suggestive analogy with patterning in prechordate embryos. In the sea urchin, β-catenin is activated maternally in the vegetal hemisphere, first in the most vegetal region (micromeres), then in the overlying cell layers (macromeres), and determines the mesendoderm (Angerer and Angerer, 2000). One could hypothesize that the Xenopus embryo has conserved this mechanism, with one modification: the most vegetal part of the signal has now been moved laterally by cortical rotation, while the second part of the signal is still located along the equator. Of course, in vertebrates, a large part of the mesendoderm induction process has been taken over by Nodal signaling, but the original β-catenin-based mechanism is still active.
The importance of maternal signals in the blastula embryo
Most of the information available on mesoderm formation and patterning comes from analysis of late blastula–early gastrula stages, or later stages, i.e. at least 3–4 h after the start of zygotic transcription. In this study, we have looked at the earliest events occuring at or just after MBT. Direct detection of activated pathways has been particularly useful to observe early signals and has revealed that significant patterning already takes place at these early stages.
One striking observation is the importance of maternal signals during blastula stages. The first known zygotic Wnt, Xwnt8, starts to be expressed in the ventral side soon after the mid-blastula (Christian et al., 1991). Unexpectedly, however, α-amanitin experiments show that all β-catenin signaling is maternal until late blastula and that zygotic influence on the β-catenin pattern is not significant before the beginning of gastrulation. Maternal β-catenin appears to control both FGF signaling and Xnrs in the blastula (Agius et al., 2000; the present study). Similar to FGF3, early Xnr1 expression (stage 9) requires β-catenin signaling (Agius et al., 2000), consistent with the fact that the initial patterns of Xnr expression (Agius et al., 2000; Takahashi et al., 2000) and P-Smad2 staining (Schohl and Fagotto, 2002) coincide with the dorsal field of strong nuclear β-catenin (Schohl and Fagotto, 2002). According to the P-Smad2 pattern (Schohl and Fagotto, 2002), the vegetal VegT-dependent Xnr pathway does not become significantly activated before the late blastula stage.
Multiple signals cooperate in the early embryo
Our data reveal that the blastula is a complex network of signals (Figure 7). Three signals, i.e. β-catenin, Xnr and FGF signaling, act together to induce expression of zygotic FGF3 and to activate the MAPK pathway in the marginal zone. β-catenin also controls Xnr expression, and both FGF and Xnr pathways then cooperate for expression of eomesodermin. This β-catenin-controlled circuitry is specific for early stages. During gastrulation, Xbra reappears even when β-catenin signaling is blocked (data not shown). The regulation of Xbra is then probably taken over by VegT-dependent signals.

Fig. 7. Model for early mesoderm induction. Maternal β-catenin activity appears to be pre localized along the equator. It is strongest and most widespread in the dorsal side, where it induces dorsal genes, such as Siamois. Both β-catenin and VegT induce Xnrs. FGF, Xnr and β-catenin signals cooperate to induce FGF3 and, perhaps, other FGFs along the marginal zone and thus activate MAPK. The combination of Xnr and FGF/MAPK signals then activates expression of mesodermal genes such as eomesodermin and Xbra.
While the early β-catenin pattern strikingly coincides with the marginal zone, β-catenin is clearly not the only spatial determinant that defines the field of prospective mesoderm. Treatment with LiCl, which strongly stimulates β-catenin activity in all cells of the embryo, reveals an underlying pattern: the MAPK activation induced by LiCl is also strong and widespread but does not reach the animal pole (Schohl and Fagotto, 2002). This is consistent with the fact that β-catenin is not sufficient for FGF3 expression, which also requires FGF and Xnr inputs. We do not know yet the precise nature of these two other signals. The resistance to CHX would imply that only maternal components are involved. There is indirect evidence for maternal FGF activity (Harland and Gerhart, 1997), although this activity must be weak, as α-amanitin treatment reduces P-MAPK levels close to the limit of detection (Figures 2 and 6B′). Xnrs, however, appear to be exclusively zygotic. It is possible that CerS may not be absolutely specific for Xnrs and may also inhibit endogenous maternal TGFβ-like Vg1 or activin when overexpressed. Alternatively, FGF3 could be under the control of a repressor that would normally be antagonized by zygotic Xnrs but that is also sensitive to CHX.
Kimelman et al. (1992) had suggested that patterning of the germ layers was achieved through the combination of differentially localized FGF and TGFβ signals. We can now add a third pattern of equatorial β-catenin to this model. We propose that all the signals considered here, maternal β-catenin, FGF, as well as VegT/Xnrs, contribute to the positioning of the prospective mesoderm (Figure 7). Cooperation of several overlapping patterns would thus ensure that the final pattern is always accurate, despite variations in the individual signals, which are likely to occur in the early embryo.
Conclusion
While it has been widely assumed that the position of the mesoderm was defined by vegetal and animal sources of signals, we show here that the marginal zone is already marked by a pre-localized maternal β-catenin activity. We further demonstrate that β-catenin is required, together with other signals, to activate early mesodermal genes. What the molecular mechanism of β-catenin activation is and how this activity is localized to the equator are exciting and challenging questions to be addressed in the future.
Materials and methods
Oligonucleotides and mRNAs
Control MO, 5′-CCTCTTACCTCAgTTACAATTTATA-3′ (human β-globin mutant sequence); β-catenin MO, 5′-TTTCAACCGTTTCCAA AGAACCAGG-3′. Oligonucleotides were resuspended in sterile 0.1× MBSH (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 10 mM HEPES, pH 7.4, 10 mg/ml benzylpenicillin, 10 mg/ml streptomycin) and injected in doses of 4 × 5 ng per embryo.
mRNA was synthesized from linearized plasmids containing the following subcloned cDNAs: β-catenin in pCS2+myc-tagged (Fagotto et al., 1996); dnXTFC in pT7Ts (Molenaar et al., 1996); β-galactosidase in pCS2+ (Rupp et al., 1994); CerS in pCS2+ (Piccolo et al., 1999); dnFGF-R in p64T (Amaya et al., 1991); FGF3 in pCS2+ (Lombardo et al., 1998); eFGF in pSP64 (Krain and Nordheim, 1999); ECD 8 in pCS2+ (Deardorff et al., 1998); Xwnt8 in psp64T (Christian et al., 1991); activin in pBSK (Thomsen et al., 1990); and Xnr1 in pCS2+ (Campione et al., 1999).
Embryo manipulations
Fertilized eggs were obtained as described by Kay and Peng (1991). They were dejellied in 2% cysteine hydrochloride (pH 8.0) and kept in 0.1× MBSH. For injections, embryos were transferred to 2% Ficoll (Sigma) in 1× MBSH. Embryos were injected at the four-cell stage in each blastomere in the equatorial region. Embryos were washed and kept in 0.1× MBSH until blastula stage. Staging was carried out according to Nieuwkoop and Faber (1967).
Immunofluorescence
Embryos were fixed in paraformaldehyde, postfixed in DMSO/methanol and embedded in fish gelatin, and 10 µm of serial cryosections were prepared as described previously (Fagotto, 1999). Sections were stained with the following antibodies: affinity-purified rabbit anti-β-catenin (Schneider et al., 1996) (1 µg/ml), mouse anti-P-ERK (1:250; Sigma), mouse anti-myc 9E10 and rabbit anti-β-galactosidase (1:1000; Cappel Oregon Technologies). Secondary and tertiary antibodies were donkey anti-mouse Alexa 488 (20 µg/ml; Molecular Probes), goat anti-donkey Alexa 488 (20 µg/ml; Molecular Probes) and donkey anti-rabbit Cy3 (15 µg/ml; Dianova). The nuclei were counterstained with DAPI and the yolk with Eriochrome Black (Sigma). Image acquisition and analysis were performed as described previously (Schohl and Fagotto, 2002).
Western blot analysis
Embryos were extracted at stage 9 in 10 mM HEPES/NaOH pH 7.4, 150 mM NaCl, 2 mM EDTA pH 8.0, 1% NP-40, supplemented with a cocktail of protease inhibitors. Embryo (equivalent, 0.75 per lane) was loaded on SDS–PAGE. The gels were blotted and stained for P-ERK (1:5000; Sigma) β-catenin (1:1000; Schneider et al., 1996) and C-cadherin (1:50 000; a generous gift from B.Gumbiner).
RT–PCR analysis
Total RNA was prepared from embryos using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. RT was performed with 1 µg of total RNA using Revert Aid H Minus M-MuLV Reverse Transcriptase (MBI Fermentas). One-tenth (or 1/20 for ODC, eomesodermin, FGF8 and Siamois) of the RT mixture was used as templates in 25 µl of PCR reaction (Taq-Polymerase, MBI Fermentas). Sequences of primers used for PCR analysis with the number of cycles in brackets (upstream primer listed first): XmyoD, 5′-AACTGCTCCGATGGCATGATGGATTA-3′ and 5′-AATTGCTGGGAGAAGGGATGGTGATTA-3′ (25 cycles); Mix1, 5′-AATGTCTCAAGGCAG AGG-3′ and 5′-TGTCACTGACACCAGAA-3′ (25 cycles); XSox17α, 5′-GGACGAGTGCCAGATGATG-3′ and 5′-CTGGCAAGTACATCTGTCC-3′ (25 cycles); ODC, 5′-GTCAATGATGGAGTGTATGGATC-3′ and 5′-TCCATTCCGCTCTCCTGAGCAC-3′ (23 cycles); goosecoid, 5′-ACAACTGGAAGCACTGGA-3′ and 5′-TCTTATTCCAGAGGAACC-3′ (27 cycles); Xwnt8, 5′-AGATGACGGCATTCCAGA-3′ and 5′-TCTCCCGATATCTCAGGA-3′ (27 cycles); eomesodermin, 5′-GCAGAGGTTCTACCTATC-3′ and 5′-CATTGCTTGAGGTGCTAGG-3′ (27 cycles); FGF3, 5′-GTCATTTGTTTCCAGACTTC-3′ and 5′-TATCTGTAGGTGGTACTTAG-3′ (27 cycles); FGF8, 5′-CTGGTGACCGACCAACTAAG-3′ and 5′-ACCAGCCTTCGTACTTGACA-3′ (27 cycles); Xbra, 5′-CACAGTTCATAGCAGTGACCG-3′ and 5′-TTCTGTGAGTGTACGGACTGG-3′ (27 cycles); Siamois, 5′-CCATGATATTCATCCAACTGTGG-3′ and 5′-CAAGAGAAGGATCTAGACCATG-3′ (29 cycles); eFGF, 5′-TTACCGGACGGAAGGATA-3′ and 5′-CCTCGATTCGTAAGCGTT-3′ (27 cycles); and EF1α, 5′-CAGATTGGTGCTGGATATGC-3′ and 5′-ACTGCCTTGATGACTCCTAG-3′ (27 cycles). The annealing temperatures were 55°C, except for ODC and Xbra (65°C) and XmyoD (58°C). The products were then analyzed on 1% agarose gels.
Protein synthesis inhibition
Embryos were dissected at stage 7 into dorsal and ventral halves and cultered in low-calcium–magnesium Ringer’s solution (LCMR) (Stewart and Gerhart, 1990). Embryo halves were incubated with or without 100 µg/ml CHX and kept until blastula stage 9.25 and frozen for later analysis. To monitor inhibition of protein synthesis, halfed embryos were incubated in LCMR containing 500 µCi/ml [35S]methionine, with or without 100 µg/ml CHX. Embryos were extracted at stage 9 and 9.5 and analyzed by SDS–PAGE and fluorography (Amplify; Amersham Pharmacia Biotech).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Rudi Winklbauer, Herbert Steinbeisser and Wolfgang Reintsch for helpful discussions and critical reading of the manuscript.
References
- Agius E., Oelgeschlager,M., Wessely,O., Kemp,C. and De Robertis,E.M. (2000) Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development, 127, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E., Musci,T.J. and Kirschner,M.W. (1991) Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell, 66, 257–270. [DOI] [PubMed] [Google Scholar]
- Angerer L.M. and Angerer,R.C. (2000) Animal–vegetal axis patterning mechanisms in the early sea urchin embryo. Dev. Biol., 218, 1–12. [DOI] [PubMed] [Google Scholar]
- Cadigan K. and Nusse,R. (1997) Wnt signaling: a common theme in animal development. Genes Dev., 11, 3286–3305. [DOI] [PubMed] [Google Scholar]
- Campione M. et al. (1999) The homeobox gene Pitx2: mediator of asymmetric left–right signaling in vertebrate heart and gut looping. Development, 126, 1225–1234. [DOI] [PubMed] [Google Scholar]
- Christen B. and Slack,J. (1999) Spatial response to fibroblast growth factor signalling in Xenopus embryos. Development, 126, 119–125. [DOI] [PubMed] [Google Scholar]
- Christian J.L., McMahon,J.A., McMahon,A.P. and Moon,R.T. (1991) Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development, 111, 1045–1055. [DOI] [PubMed] [Google Scholar]
- Clements D., Friday,R.V. and Woodland,H.R. (1999) Mode of action of VegT in mesoderm and endoderm formation. Development, 126, 4903–4911. [DOI] [PubMed] [Google Scholar]
- Cornell R.A. and Kimelman,D. (1994) Activin-mediated mesoderm induction requires FGF. Development, 120, 453–462. [DOI] [PubMed] [Google Scholar]
- Cornell R.A., Musci,T.J. and Kimelman,D. (1995) FGF is a prospective competence factor for early activin-type signals in Xenopus mesoderm induction. Development, 121, 2429–2437. [DOI] [PubMed] [Google Scholar]
- Curran K.L. and Grainger,R.M. (2000) Expression of activated MAP kinase in Xenopus laevis embryos: evaluating the roles of FGF and other signaling pathways in early induction and patterning. Dev. Biol., 228, 41–56. [DOI] [PubMed] [Google Scholar]
- Darken R.S. and Wilson,P.A. (2001) Axis induction by Wnt signaling: target promoter responsiveness regulates competence. Dev. Biol., 234, 42–54. [DOI] [PubMed] [Google Scholar]
- Deardorff M.A., Tan,C., Conrad,L.J. and Klein,P.S. (1998) Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development, 125, 2687–2700. [DOI] [PubMed] [Google Scholar]
- Ding X., Hausen,P. and Steinbeisser,H. (1998) Pre-MBT patterning of early gene regulation in Xenopus: the role of the cortical rotation and mesoderm induction. Mech. Dev., 70, 15–24. [DOI] [PubMed] [Google Scholar]
- Fagotto F. (1999) The Wnt pathway in Xenopus development. In Guan,J.-L. (ed.), Signaling through Cell Adhesion. CRC Press, Boca Raton, FL, pp. 303–356. [Google Scholar]
- Fagotto F., Funayama,N., Gluck,U. and Gumbiner,B.M. (1996) Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. J. Cell Biol., 132, 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S., Lee,M.A., Keller,T., ten Dijke,P. and Whitman,M. (2000) Endogenous patterns of TGFβ superfamily signaling during early Xenopus development. Development, 127, 2917–2931. [DOI] [PubMed] [Google Scholar]
- Harland R. and Gerhart,J. (1997) Formation and function of Spemann’s organizer. Annu. Rev. Cell Dev. Biol., 13, 611–667. [DOI] [PubMed] [Google Scholar]
- Heasman J. (1997) Patterning the Xenopus blastula. Development, 124, 4179–4191. [DOI] [PubMed] [Google Scholar]
- Heasman J., Crawford,A., Goldstone,K., Garner-Hamrick,P., Gumbiner,B., McCrea,P., Kintner,C., Noro,C.Y. and Wylie,C. (1994) Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell, 79, 791–803. [DOI] [PubMed] [Google Scholar]
- Hoppler S., Brown,J.D. and Moon,R.T. (1996) Dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev., 10, 2805–2817. [DOI] [PubMed] [Google Scholar]
- Isaacs H.V. (1997) New perspectives on the role of the fibroblast growth factor family in amphibian development. Cell. Mol. Life Sci., 53, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K. and Sokol,S.Y. (1999) Axis determination by inhibition of Wnt signaling in Xenopus. Genes Dev., 13, 2328–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.A. and Woodland,H.R. (1987) The development of animal cap cells in Xenopus. A measure of start of animal cap competence to form mesoderm. Development, 101, 557–563. [Google Scholar]
- Kay B.K. and Peng,H.B. (1991) Xenopus laevis: practical uses in cell and molecular biology. Methods Cell Biol., Vol. 36. Academic Press, Inc., New York, NY. [PubMed] [Google Scholar]
- Kimelman D. and Griffin,K.J.P. (2000) Vertebrate mesendoderm induction and patterning. Curr. Opin. Genet. Dev., 10, 350–356. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Christian,J.L. and Moon,R.T. (1992) Synergistic principles of development: overlapping patterning systems in Xenopus mesoderm induction. Development, 116, 1–9. [DOI] [PubMed] [Google Scholar]
- Kofron M., Spagnuolo,A., Klymkowsky,M., Wylie,C. and Heasman,J. (1997) The roles of maternal α-catenin and plakoglobin in the early Xenopus embryo. Development, 124, 1553–1560. [DOI] [PubMed] [Google Scholar]
- Kofron M. et al. (1999) Mesoderm inudction in Xenopus is a zygotic event regulated by maternal VegT via TGFβ growth factors. Development, 126, 5759–5770. [DOI] [PubMed] [Google Scholar]
- Krain B. and Nordheim,A. (1999) Artefactual gene induction during preparation of Xenopus laevis animal cap explants. Int. J. Dev. Biol., 43, 563–566. [PubMed] [Google Scholar]
- LaBonne C. and Whitman,M. (1997) Localization of MAP kinase activity in early Xenopus embryos: implications for endogenous FGF signaling. Dev. Biol., 183, 9–20. [DOI] [PubMed] [Google Scholar]
- Larabell C.A., Torres,M., Rowning,B.A., Yost,C., Miller,J.R., Wu,M., Kimelman,D. and Moon,R.T. (1997) Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signaling pathway. J. Cell Biol., 136, 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo A., Isaacs,H.V. and Slack,J.M. (1998) Expression and functions of FGF-3 in Xenopus development. Int. J. Dev. Biol., 42, 1101–1107. [PubMed] [Google Scholar]
- Molenaar M., van de Wetering,M., Oosterwegel,M., Peterson-Maduro,J., Godsave,S., Korinek,V., Roose,J., Destrée,O. and Clevers,H. (1996) XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell, 86, 391–399. [DOI] [PubMed] [Google Scholar]
- Moon R.T. and Kimelman,D. (1998) From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. BioEssays, 20, 536–545. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D. (1969) The formation of mesoderm in Urodelean amphibians. I. Induction by the endoderm. Roux’s Arch. Entw. Mech. Org., 162, 341–373. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. and Faber,P. (1967) Normal Table of Xenopus laevis. North-Holland, Amsterdam, The Netherlands. [Google Scholar]
- Piccolo S., Agius,E., Leyns,L., Bhattacharyya,S., Grunz,H., Bouwmeester,T. and De Robertis,E.M. (1999) The head inducer Cerberus is a mutlifunctional antagonist of Nodal, BMP and Wnt signals. Nature, 397, 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R.A., Snider,L. and Weintraub,H. (1994) Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev., 8, 1311–1323. [DOI] [PubMed] [Google Scholar]
- Schneider S., Steinbeisser,H., Warga,R.M. and Hausen,P. (1996) β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev., 57, 191–198. [DOI] [PubMed] [Google Scholar]
- Schohl A. and Fagotto,F. (2002) β-catenin, MAPK and Smad signaling during early Xenopus development. Development, 129, 37–52. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. (1996) Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol., 6, 1456–1467. [DOI] [PubMed] [Google Scholar]
- Stewart R.M. and Gerhart,J.C. (1990) The anterior extent of dorsal development of the Xenopus embryonic axis depends on the quantity of organizer in the late blastula. Development, 109, 363–372. [DOI] [PubMed] [Google Scholar]
- Sumanas S., Strege,P., Heasman,J. and Ekker,S.C. (2000) The putative wnt receptor Xenopus frizzled-7 functions upstream of β-catenin in vertebrate dorsoventral mesoderm patterning. Development, 127, 1981–1990. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Yokota,C., Takano,K., Tanegashima,K., Onuma,Y., Goto,J.I. and Asashima,M. (2000) Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development, 127, 5319–5329. [DOI] [PubMed] [Google Scholar]
- Tannahill D., Isaacs,H.V., Close,M.J., Peters,G. and Slack,J.M.W. (1992) Developmental expression of the Xenopus int-2 (FGF-3) gene: activation by mesodermal and neural induction. Development, 115, 695–702. [DOI] [PubMed] [Google Scholar]
- Thomsen G., Woolf,T., Whitman,M., Sokol,S., Vaughan,J., Vale,W. and Melton,D.A. (1990) Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell, 63, 485–493. [DOI] [PubMed] [Google Scholar]
- Vonica A. and Gumbiner,B.M. (2002) Zygotic Wnt acitvity is required for Brachyury expression in the early Xenopus laevis embryo. Dev. Biol., 250, 112–127. [DOI] [PubMed] [Google Scholar]
- Xanthos J.B., Kofron,M., Wylie,C. and Heasman,J. (2001) Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development, 128, 167–180. [DOI] [PubMed] [Google Scholar]
- Yap A.S., Brieher,W.M. and Gumbiner,B.M. (1997) Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol., 13, 119–146. [DOI] [PubMed] [Google Scholar]
- Yasuo H. and Lemaire,P. (1999) A two-step model for the fate determination of presumptive endodermal blastomeres in Xenopus embryos. Curr. Biol., 9, 869–879. [DOI] [PubMed] [Google Scholar]
- Zhang J., Houston,D.W., King,M.L., Payne,C., Wylie,C. and Heasman,J. (1998) The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell, 94, 515–524. [DOI] [PubMed] [Google Scholar]