Abstract
Ticks introduce a variety of pharmacologically active molecules into their host during attachment and feeding in order to obtain a blood meal. People who are repeatedly exposed to ticks may develop an immune response to tick salivary proteins. Despite this response, people usually are unaware of having been bitten, especially if they are not repeatedly exposed to ticks. In order to develop a laboratory marker of tick exposure that would be useful in understanding the epidemiology of tick-borne infection and the immune response to tick bite, we developed an enzyme-linked immunosorbent assay (ELISA) to detect antibody to a recombinant form of calreticulin protein found in the salivary glands of Ixodes scapularis, a member of a complex of Ixodes ticks that serve as the vectors for Lyme disease, human babesiosis, and human granulocytic anaplasmosis. Using this assay, we tested sera obtained from C3H/HeN and BALB/c mice before and after experimental deer tick infestation. These mice developed antibody to Ixodes calreticulin antigen after infestation. We then used the same assay to test sera obtained from people before and after they experienced deer tick bite(s). People experiencing deer tick bite(s) developed Ixodes calreticulin-specific antibody responses that persisted for up to 17 months. This Ixodes recombinant calreticulin ELISA provides objective evidence of deer tick exposure in people.
Ticks introduce a variety of pharmacologically active molecules into their host during feeding in order to successfully obtain a blood meal (29). An array of proteins inhibit hemostasis, block pain and itch responses, reduce inflammation, and suppress or modulate innate and specific acquired immune defenses (5, 32). Tick-transmitted pathogens are transferred to their hosts during feeding, and the actions of tick salivary proteins are essential for both tick feeding and pathogen transmission (15, 17, 22, 30, 32). Hosts may develop an immune response to tick salivary proteins following repeated tick exposure that may impair tick and pathogen viability, including cutaneous inflammation that may result in itch and an increased awareness of infesting ticks (30, 32). Experiments with laboratory animals suggest that host immune reactivity against Ixodes scapularis (also known as Ixodes dammini) protects against transmission of Borrelia burgdorferi, the causative agent of Lyme disease (14, 36). Hypersensitivity against I. scapularis bites in people also may protect against the acquisition of Lyme disease (6).
Although the human response to tick bite may include intense cutaneous inflammation with accompanying histological changes, people often are unaware of having been bitten (1, 5, 9, 20, 24-26). Quantitative biologic markers of tick exposure are needed to better understand the epidemiology, pathogenesis, immunology, and clinical manifestations of the human tick bite response. One such marker may be host antibody directed against tick antigen. The frequency of exposure to Ixodes ticks can be determined using whole salivary gland extract derived from I. scapularis and a recombinant calreticulin antigen derived from Amblyomma americanum (20, 24-26). No previous studies have used an Ixodes recombinant antigen to test deer tick exposure or examined people whose antibody status could be measured before and more than a few months after tick exposure in order to determine antibody kinetics. Accordingly, we determined whether Ixodes recombinant calreticulin salivary protein in an enzyme-linked immunosorbent assay (ELISA) may serve as a useful marker of deer tick exposure. In particular, we used an ELISA for detecting human antibody against Ixodes recombinant calreticulin salivary protein in people with defined histories of exposure to deer ticks, including some whose sera were available prior to and more than a year following tick bite.
MATERIALS AND METHODS
I. scapularis infestation of C3H/HeN and BALB/c mice.
Pathogen-free I. scapularis ticks were obtained from a colony maintained at the University of Connecticut Health Center. Ticks were maintained at 22°C in 97% relative humidity and oversaturated potassium sulfate and with a 16-h:8-h light-dark cycle. Ticks were placed on female C3H/HeN or BALB/c mice. Larvae (200 to 300 per mouse) or nymphs (10 to 40 per mouse) were applied to the entire body of a mouse. Ticks were then left to feed to completion over 3 to 7 days, and the engorged ticks were collected (4). In some instances, a second infestation was performed after mice were housed for 14 days. Two to three months after the last infestation, mouse blood was collected by retro-orbital bleeding, allowed to clot, and centrifuged at 150 × g for 10 min at 4°C to collect sera prior to storage at −30°C for further use. All animal experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Connecticut Health Center.
Human study population.
The first study group consisted of residents of Block Island, Rhode Island, who developed Lyme disease, babesiosis, or human granulocytic anaplasmosis (HGA) and enrolled in our tick-borne illness study between 1995 and 2000 as previously described (8). These subjects agreed to a history and physical examination and submission of an acute-phase and a convalescent-phase blood sample. For the purposes of this study, we included only the 10 subjects who reported no tick bite prior to illness and who had enrolled in a biannual serosurvey on Block Island for determination of antibodies to the agents of Lyme disease, babesiosis, and HGA prior to development of tick-borne illness. Thus, we were able to test serum samples for antibody against tick salivary protein before, during, and after development of tick-borne illness in these subjects.
The second study group consisted of 234 Block Island residents who enrolled in our 2004 serosurvey but did not experience symptomatic Lyme disease, babesiosis, or HGA. They were asked to complete a questionnaire that included information on tick bite within the previous year and tick-associated itch, an indication of the intensity of tick exposure, as well as provide a blood sample for antibody to tick salivary protein.
Finally, we enrolled seven subjects from the Mansfield Family Practice in Mansfield, Connecticut, who had experienced an I. scapularis tick bite within the previous 2 days of enrollment during the summer of 2005 and who submitted the tick that bit them for identification and estimate of engorgement level. They were asked to provide a history, undergo a physical examination, provide a blood sample to determine their antibody against tick salivary protein, and to return 4 to 6 weeks later for a clinical examination and for blood testing for antibody against tick salivary protein.
Positive control sera consisted of pooled acute-phase sera from Connecticut residents who had experienced Lyme disease within the previous 3 months and whose sera contained anti-B. burgdorferi antibody. Negative control sera were obtained from two residents of Iceland, where no vector ticks are found, and from three children between 1 to 2 years of age living in Connecticut whose sera were obtained for routine serum electrolyte testing. Sera were extracted immediately after blood drawing and maintained frozen at −80°C until testing. Negative control sera were individually tested in all experiments. Written informed consent was obtained from study participants in accordance with human experimentation guidelines approved by the institutional review boards at Connecticut Children's Medical Center and the Harvard School of Public Health.
Insect cell culture and media for preparation of recombinant I. scapularis calreticulin antigen.
Trichoplusia ni cells (BTI-TN-5B1-4, High 5; Invitrogen, Carlsbad, CA) used in protein expression were grown at 27°C in Express 5 serum-free medium (Invitrogen), supplemented with 17 mM l-glutamine, 10 μg/ml gentamicin (GIBCO, Carlsbad, CA), and 10 μg/ml blasticidin (Invitrogen). Cells were started as adherent cultures and then used to inoculate 65-ml to 125-ml spinner flask suspension cultures at an initial density of 1.0 × 106 cells/ml. Once a density of 6 × 106 to 7 × 106 cells/ml was reached, cells were harvested for processing.
RACE synthesis for preparation of recombinant Ixodes calreticulin antigen.
Prior to dissection, partially fed female I. scapularis ticks were washed with sterile 0.1 M phosphate-buffered saline (PBS; pH 7.4). Salivary glands were then removed, rinsed into 500 μl of sterile PBS containing Complete EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN), and transferred immediately into a 2.0-ml cryovial containing 1.0 ml RNALater RNA storage solution (Ambion, Austin, TX). The suspensions of glands were then stored at −80°C until use.
Poly(A)+ mRNA was isolated from tick salivary glands with the Oligotex Direct mRNA Micro kit (QIAGEN, Valencia, CA) following the manufacturer's protocol. First-strand cDNA was isolated from tick salivary gland poly(A)+ mRNA and used directly in 5′- and 3′-rapid amplification of cDNA ends (RACE) PCR, using the SMART RACE cDNA amplification kit (Clontech, Mountain View, CA) according to the manufacturer's instructions. Calreticulin-specific oligonucleotide primers were designed from the consensus sequence resulting after alignment of published calreticulin sequences from the ticks Amblyomma americanum (U07708) and Boophilus microplus (AF420211). Sequences were as follows: sense primer Calret3, 5′-ACTCGGGCTTGTCCCAGTCCTCGGG-3′; and antisense primer Calret5, 5′-AAGCACGAGCAGAACATCGACTGCG-3′. These primers created overlapping 5′- and 3′-RACE products, respectively, that were joined by restriction digestion and ligation using restriction site XhoI in the region of the overlap to create the full-length cDNA. The resulting 1,548-bp cDNA was then used as template to generate a PCR product containing the entire 5′ end of the gene and the 3′ end up to the stop codon. Oligonucleotide primers used for this purpose were CalretFFL (5′-GGCTTCTAATACGACTCACTATAGGG-3′) and CalretRFL (5′-CACAAGTTCCTCGTGGTCGTGCTTG-3′). The stop codon was eliminated in order to allow fusion of the C-terminal region of the expressed protein to a His6 tag to facilitate protein purification. The 1,349-bp PCR product was then cloned into the pIB/V5-His-TOPO expression vector (Invitrogen), which contains the blasticidin resistance gene for selection of cells that are stably transfected. Transcription of the calreticulin insert was driven by the baculovirus Orgyia pseudotsugata immediate-early 2 promoter (OpIE2) (28). The resulting construct, pIB/Calreticulin, was then sequenced in both directions using standard dideoxynucleotide sequencing procedures (19). After alignment of published calreticulin protein sequences from various Ixodes tick species (Ixodes woodi, Ixodes ricinus, Ixodes persulcatus, Ixodes pararicinus, Ixodes pacificus, Ixodes pavlovskyi, Ixodes ovatus, Ixodes nipponensis, Ixodes muris, Ixodes minor, Ixodes jellisoni, and Ixodes affinis) with the I. scapularis used in this study, we found that the difference in amino acid sequences between species was negligible, with an average percent identity of approximately 98% among all sequences. These data indicate that antibody response to recombinant Ixodes scapularis calreticulin antigen would be the same as that against any other recombinant Ixodes calreticulin antigen.
Endotoxin-free pIB/Calreticulin recombinant plasmid was purified using the EndoFree plasmid maxi kit (QIAGEN) and transfected into High 5 cells according to protocols supplied by the manufacturer. Stably transfected cells were maintained routinely in medium containing blasticidin at a final concentration of 10 μg/ml. For medium collection, cells were grown to a density of greater than 6 7 × 106 to 7 × 106 cells/ml, at which point media were collected and centrifuged at 6,000 × g to remove cells and particulate matter. Cell culture supernatants were then buffer exchanged with PBS (pH 7.2) and concentrated at least 10× with a 250-ml stirred cell (Millipore, Billerica, MA) fitted with low-protein-binding regenerated cellulose membranes (10-kDa molecular mass cutoff).
For purification by immobilized-metal affinity chromatography, concentrates were loaded onto a Ni+2-nitriloacetic acid (QIAGEN) column that was preequilibrated and washed with 50 mM NaH2PO4, 500 mM NaCl, and 10 mM imidazole (pH 8.0). His-tagged protein was eluted with 50 mM NaH2PO4, 500 mM NaCl, and 250 mM imidazole (pH 8.0). Eluted fractions were dialyzed against PBS and concentrated using Amicon Ultra 15 concentrators (10-kDa molecular mass cutoff; Millipore). Proteins were quantified by means of the bicinchoninic acid assay (27), and purity was assessed by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10) and immunoblot analysis (3).
ELISA for detecting antibody against Ixodes recombinant calreticulin antigen.
The ELISA is a quantitative microtiter method for detecting immunoglobulin G (IgG) antibody to recombinant Ixodes calreticulin purified from High 5 cells. This ELISA method is a modification of that described by Magnarelli et al. (11). Recombinant calreticulin was added to alternate wells of flat-bottom microdilution plates at a final concentration of 5 μg per well. In the intervening wells, 50 μl of PBS (1.54 mM KH2PO4, 2.7 mM Na2HPO4 · 7H2O, 154 mM NaCl, pH 7.2) was added to test for nonspecific binding. The plates were blocked with PBS (200 μl) containing 5% horse serum (JR Scientific, Woodland, CA) and 0.01% dextran sulfate (Sigma) and washed five times with PBS-Tween 20. Sera from selected patients with a previous history of tick exposure (diluted from 1:40 to 1:320) were added to matching wells and incubated for 1 h at 37°C and washed as previously described. After incubation for 1 h with peroxidase-labeled anti-human IgG, plates were washed again as described above, and the reactions were visualized with 2,2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt/tetramethyl benzidine (ABTS/TMB) as a substrate. Optical density readings at 414 nm were taken on a μQuant plate reader (BioTek, Winooski, VT). The optical density of the positive control minus that for the nonspecific binding well was standardized to 1.0 for IgG. A sample was considered as reactive if the net absorbance (antigen well minus the nonspecific binding well) was 3 standard deviations or more than the mean absorbance of the PBS-containing comparison wells. A reactive serum was defined as one reacting at a dilution equal to or greater than 1:80 for IgG.
Western blot assays for detection of antibody against Ixodes recombinant calreticulin antigen.
For immunoblot analysis, tick salivary gland extract protein or purified recombinant calreticulin protein was separated, along with molecular mass standards (Bio-Rad, Hercules, CA), by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions using 12% gels (28) and transferred to nitrocellulose membranes using a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad). Human sera were diluted 1:2,000 in blocking buffer (10 mm Tris-HCl, 300 mM NaCl, pH 7.4, 5% nonfat dry milk) and incubated for 1 h at room temperature (11). Antigen binding was detected with horseradish peroxidase-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:10,000 in blocking buffer and reacted for 1 h at room temperature. Antigen-antibody complexes were visualized by chemiluminescence (Supersignal; Pierce, Rockford, IL) on radiography film (Kodak, Rochester, NY).
Statistical analysis.
Fisher's exact test was used to compare two proportions (2-by-2 contingency tables). SAS 9 for Windows (SAS Institute Inc., Cary, NC) was used for statistical analysis.
RESULTS
Antibody against Ixodes recombinant calreticulin protein using a mouse model.
C3H/HeN and BALB/c mice were exposed to deer ticks and tested for antibody against Ixodes recombinant calreticulin. Larval or nymphal I. scapularis ticks were allowed to feed to repletion on mice, and in some instances, the infestation was repeated 2 weeks later. Blood was obtained 2 to 3 months after the last infestation for determination of serum calreticulin antibody by ELISA. Sera from mice exposed to deer ticks contained anticalreticulin antibody at concentrations of 1:80 to 1:160, while sera from tick-naïve mice were nonreactive (Table 1). Mice exposed to deer ticks develop antibody against I. scapularis recombinant calreticulin.
TABLE 1.
Development of antibody against Ixodes recombinant calreticulin antigen in C3H/HeN and BALB/c mice following I. scapularis feeding
| Mouse | Tick stage | No. of ticks | Antibody titer |
|---|---|---|---|
| C3H/HeN | |||
| 1 | Nymph | 30-40 | 1:80 |
| 2 | Nymph | 30-40 | 1:160 |
| 3 | Nymph | 30-40 | 1:160 |
| 4 | Larva | 200-300 | 1:80 |
| 5 | Larva | 200-300 | 1:160 |
| 6 | Larva | 200-300 | 1:160 |
| 7 | Larva | 200-300 | 1:160 |
| 8 | Larva | 100-200 (×2)a | 1:80 |
| 9 | Larva | 100-200 (×2) | 1:160 |
| 10 | None | None | <1:80 |
| 11 | None | None | <1:80 |
| BALB/c | |||
| 1 | Nymph | 10 (×2) | 1:80 |
| 2 | Nymph | 10 (×2) | 1:80 |
| 3 | None | None | <1:80 |
| 4 | None | None | <1:80 |
×2, 2 weeks between double infestations.
Development of antibody against Ixodes calreticulin protein in people exposed to deer ticks who develop tick-borne infection.
We tested the sera of 10 Block Island subjects who had experienced Lyme disease, babesiosis, and/or HGA; reported no tick bite prior to infection; and submitted a serum sample before (as part of the biannual serosurvey), during, and within 3 months after the onset of illness. Seven of the 10 subjects had no detectable I. scapularis calreticulin ELISA antibody prior to development of infection (Table 2). All of these subjects seroconverted during or within 3 months of acute infection. Among the three subjects from whom long-term follow-up sera were available, calreticulin-specific antibody was detectable at 17 months but not at 29 months after infection in one person and present at 3 to 4 months but absent at 15 and 27 months after infection in the other two. Three of the 10 subjects had detectable calreticulin antibody prior to developing infection despite no recollection of a previous tick bite. As residents of Block Island, these subjects were very likely to have had previous tick bites. One of the three experienced a twofold rise in antibody during the interval before and after infection, while the titer did not change in the other two subjects. Positive control sera derived from pooled acute-phase sera of several patients experiencing Lyme disease were reactive against Ixodes recombinant calreticulin antigen, while no such antibodies were detected in the sera of two subjects who lived in Iceland, where no deer ticks are found, and in three children 1 to 2 years of age whose sera were obtained for serum electrolyte testing (Table 2). Antibody against Ixodes calreticulin is detectable by ELISA in people following a deer tick bite(s) but may fall to undetectable levels as early as 15 months following tick exposure.
TABLE 2.
Anti-Ixodes calreticulin ELISA antibody in subjects before and after Lyme disease, Lyme disease and babesiosis, or HGA
| Subject | Illness | Result:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Prior to illness
|
Following illness
|
||||||||
| Mo | Antibody titer | Mo | Antibody titer | Mo | Antibody titer | Mo | Antibody titer | ||
| 1 | Lyme disease | 26 | <1:40 | 1 | 1:40 | 4 | 1:160 | 27 | <1:40 |
| 2 | Lyme disease | 26 | <1:40 | 1 | 1:40 | 3 | 1:80 | 15 | <1:40 |
| 3 | Lyme disease | 31 | <1:40 | 1 | 1:40 | 17 | 1:80 | 29 | <1:40 |
| 4 | Lyme disease | 44 | <1:40 | 1 | 160 | 2 | 1:160 | ||
| 5 | Lyme disease | 21 | <1:40 | 1 | 1:40 | 2 | 1:40 | ||
| 6 | Lyme disease-babesiosis | 72 | <1:40 | 1 | 1:40 | 3 | 1:160 | ||
| 7 | Lyme disease-babesiosis | 3 | <1:40 | 1 | <1:40 | 3 | 1:80 | ||
| 8 | Lyme disease | 13 | 1:640 | 1 | 1:640 | 2 | 1:640 | ||
| 9 | Lyme disease-babesiosis | 11 | 1:160 | 1 | 1:160 | 7 | 1:160 | ||
| 10 | HGA | 13 | 1:320 | 1 | 1:640 | ||||
| Controls | |||||||||
| Positive | Lyme disease | 1:160 | |||||||
| Negative | None | <1:40 | |||||||
Confirmation that antibody detected by Ixodes recombinant calreticulin ELISA is directed at calreticulin.
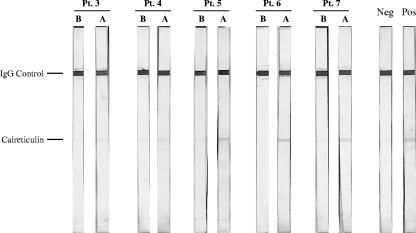
We used a Western blot assay with Ixodes recombinant calreticulin as the antigen to test the sera of five of the same Lyme disease subjects who had developed antibody against Ixodes calreticulin as measured by ELISA. In each of five Lyme disease serum pairs, a reactive calreticulin band was absent in sera collected before the development of Lyme disease and present in sera obtained after the development of Lyme disease (Fig. 1). A sample of positive control sera from pooled acute-phase sera of several patients experiencing Lyme disease also was reactive against the Ixodes calreticulin protein band. In contrast, sera obtained from a control subject with no history of tick bites who lived in Iceland failed to react to the Ixodes calreticulin protein band.
FIG. 1.
Western blots of sera against Ixodes recombinant calreticulin (10 μg/strip) from people before (lanes B) and within 3 months after (lanes A) development of Lyme disease. The ELISA antibody results of these subjects are shown in Table 2. The positive control consists of pooled acute-phase sera from patients experiencing Lyme disease. The negative control consists of serum from a resident of Iceland.
Development of antibody against Ixodes calreticulin protein in people exposed to deer ticks who do not develop tick-borne infection.
We determined whether anticalreticulin antibody forms in people who were bitten by I. scapularis ticks but who removed these ticks before transmission of tick-borne infection could develop. Seven subjects who experienced a tick bite within the previous 2 days submitted the I. scapularis tick that bit them and a blood sample. The sera from each of these subjects reacted against Ixodes calreticulin antigen within 1 to 3 days after exposure. Serum samples were obtained from four of these people 4 weeks after exposure, and all were reactive, although only one increased above the previous titer (Table 3). The same positive and negative control sera used in previous experiments (as depicted in Table 2) were reactive and nonreactive, respectively. People who experience a deer tick bite but no tick-borne infection develop antibody against Ixodes calreticulin antigen.
TABLE 3.
Anti-Ixodes calreticulin antibody in subjects 1 to 2 days and 4 weeks after I. scapularis tick bite
| Case | Description of attached tick
|
No. of bites in past year | Antibody titer at:
|
|||
|---|---|---|---|---|---|---|
| Stage | Feeding status | No. attached | 1-3 days | 4 wk | ||
| 1 | Larva | Unfed | ∼60 | 2 | 1:80 | 1:160 |
| 2 | Nymph | Fully fed | 1 | 2 | 1:80 | 1:80 |
| 3 | Nymph | Partially fed | 1 | 5 | 1:80 | 1:80 |
| 4 | Nymph | Partially fed | 3 | Uncertain | 1:80 | 1:80 |
| 5 | Nymph | Partially fed | 2 | 2 | 1:80 | |
| 6 | Larva | Fully fed | 1 | Uncertain | 1:160 | |
| 7 | Adult | Partially fed | 2 | 1 | 1:640 | |
| Controls | ||||||
| Positive | 1:160 | |||||
| Negative | <1:40 | |||||
Association between self-reports of tick bite and the presence of anti-Ixodes calreticulin antibody.
We tested the sera of 234 residents of Block Island who participated in the 2004 Block Island serosurvey for antibody against Ixodes recombinant calreticulin. We compared their antibody response with a history of tick bite within the previous year, tick bite-associated itch, and the presence of antibody against B. burgdorferi and Babesia microti (Table 4). None had experienced symptomatic tick-borne illness within the previous year, and most provided a complete history and sufficient sera for testing, although some residents did not respond to questions about tick bite or tick-associated itch. Only 6% (n = 14) had detectable antibody against Ixodes calreticulin. A significant association was found between seroreactivity to Ixodes recombinant calreticulin and the presence of antibody against B. burgdorferi and Babesia microti, but not with a history of tick bite or tick-associated itch. The sera from about half of the subjects whose sera contained antibody against B. burgdorferi and Babesia microti were nonreactive against calreticulin antigen, presumably because these subjects had experienced Lyme disease or babesiosis a year or more before their sera were tested for anticalreticulin antibody. The persistence of anticalreticulin antibody generally appears to be less than that for antibody directed against either the Lyme disease or babesial pathogens. The presence of detectable anti-Ixodes calreticulin antibody is associated with previous B. burgdorferi and B. microti infection but not with self-reports of previous tick bite or tick-associated itch.
TABLE 4.
Relationship between anti-Ixodes calreticulin antibody in 234 Block Island residents and a history of tick bite within the previous year, tick-associated itch, and concurrent antibody against B. burgdorferi and B. microti
| Indicator of tick exposure | No. of subjects with antibody status/no. with indicator
|
P value | |
|---|---|---|---|
| Anti-Ixodes calreticulin antibody | No anti-Ixodes calreticulin antibody | ||
| Report of tick bite | 2/11 | 48/200 | 1.000 |
| Report of tick-associated itch | 1/14 | 47/214 | 0.311 |
| B. burgdorferi antibody (total) | 8/14 | 64/220 | 0.029 |
| B. microti antibody | |||
| IgM | 5/14 | 13/220 | 0.002 |
| IgG | 5/14 | 0/220 | <0.001 |
DISCUSSION
We found that people experiencing a deer tick bite develop antibody against recombinant Ixodes calreticulin protein detectable by ELISA. Anti-Ixodes calreticulin antibody is present in the sera of some people within 2 days of tick exposure and may persist for as long as a year and a half. The development of antibody in some people after brief tick attachment suggests previous tick exposure and an amnestic immune response, consistent with the frequent tick exposure encountered by people living where deer ticks are highly endemic. Western blot analysis confirmed that antibody detected by ELISA is directed against calreticulin antigen. No antibody against recombinant Ixodes calreticulin was detected in three subjects from Iceland and two subjects less than 2 years of age who were very unlikely to have experienced tick bite. This Ixodes recombinant calreticulin ELISA appears to provide a reliable method for detecting IgG antibody against Ixodes calreticulin protein following recent Ixodes tick exposure.
In a previous study, antibodies to tick salivary gland sonicate were shown to be a potential biological marker of exposure to tick bites among outdoor workers (23). Rabbits experimentally infested with adult Amblyomma americanum or Dermacentor variabilis (50 females and 20 males) developed antibodies to recombinant calreticulin derived from cDNA prepared from partially fed A. americanum females; however, gerbils exposed to the bites of Aedes aegypti mosquitoes did not develop calreticulin-specific antibodies (21). Recombinant A. americanum salivary gland calreticulin was used to screen sera of military personnel stationed in an area where A. americanum is endemic. Personnel exposed to natural tick infestation developed antibodies to the salivary gland recombinant protein (21). Subjects with a recent history of exposure to bites of I. scapularis nymphs or adults developed increasing amounts of antibody to A. americanum salivary gland recombinant calreticulin over an approximately 6-week period after tick bite (20). A tick that became engorged was a risk factor for development of antibodies to A. americanum salivary gland recombinant calreticulin. These studies indicated that people exposed to I. scapularis ticks develop antibody to A. americanum calreticulin antigen. Calreticulin is a highly conserved protein among tick species (36). Unlike previous reports, we used Ixodes recombinant calreticulin antigen and studied a population whose tick-borne disease and tick exposure history were well defined and whose sera were available before and more than a year after tick bite exposure. Consequently, we were able to derive information about the kinetics of antibody persistence in the apparent absence of reexposure to ticks and to rule out any possible cross-reactivity between B. burgdorferi and Ixodes calreticulin antigen.
Variations in antibody responses to tick bite of participants in this study likely reflect the level of exposure to tick antigens and the balance between the ability of the subject to mount a response and tick modulation of those host responses. The number and frequency of tick bites determine the development of acquired resistance and cutaneous reactivity to tick feeding, which is mediated in part by circulating and homocytotropic antibodies and cell-mediated immune responses (5, 32, 33). Tick feeding has been shown to reduce the ability of the host to develop an antibody response (7, 13, 31). While it was not possible to document all previous arthropod bites that might have altered the calreticulin antibody status of our subjects, any such effect would have been mitigated by testing each subject following a well-defined tick exposure.
Although our Ixodes recombinant calreticulin ELISA appears to provide a reliable method for detection of recent exposure to deer ticks, the results of this assay do not correlate well with self-reported tick bite or tick-associated itch in residents living in areas where deer tick-borne disease is endemic. Several possible explanations may account for this apparent contradiction. Tick bites usually go unnoticed. Only about a third of people who experience Lyme disease report being bitten by a tick, in part because saliva of I. scapularis contains kininase activity and histamine binding proteins that reduce host pain and itch responses (16, 18, 35). This would weaken the observed association between anticalreticulin antibody and self-reports of tick bite due to misclassification and thus lead to a decreased chance of detecting such an association. The duration of deer tick attachment may be insufficient to allow for adequate amount of saliva to elicit a detectable antibody response, several tick exposures may be required in some people before the antibody response is strong enough to be detected by ELISA, or people may confuse the bite of non-Ixodes ticks or other arthropods with that of a deer tick and such bites might not elicit calreticulin antibody. Finally, our data indicate that serum antibody concentration decreases with time after tick bite and may become undetectable in people who have experienced a deer tick bite more than 2 years prior to testing. Any one of these events would weaken the association between self-reported tick bites and anticalreticulin titers.
The most important practical value of this Ixodes calreticulin ELISA is to confirm recent deer tick exposure. Anticalreticulin antibody may be detectable as early as a few days after tick bite in people who have been prexposed to ticks or as long as 2 to 3 months after tick exposure. Epidemiologic and clinical studies of tick-borne disease are complicated by the poor reliability of tick exposure history because tick bites generally are not noticed and people may mistake the bite of another arthropod as that of a tick. The Ixodes calreticulin ELISA also may increase our understanding of the human immune response to tick bite and help confirm whether hypersensitivity to Ixodes bites protects people against infection by the agent of Lyme disease. Feeding of ticks and other blood-feeding arthropods is facilitated by saliva that contains mixtures of pharmacologically active molecules capable of inhibiting host pain/itch responses, hemostasis, and immune defenses (5, 12, 22, 32). Tick pathogen transmission is enhanced because lack of awareness of tick attachment allows sufficient time for transmission to occur and perhaps because pathogen survival is improved by the immunosuppressive action of tick salivary proteins at the site of skin attachment (5). Repeated exposure to the bites of pathogen-free ticks in animals elicits host responses that protect against subsequent tick transmission of infectious agents (2, 34). These immune responses neutralize tick countermeasures against host defenses and lead to rejection of feeding ticks (5, 32). The Ixodes calreticulin ELISA appears to be a useful diagnostic indicator of exposure to Ixodes ticks and may be useful in developing a vaccine against tick-borne infections based on tick salivary protein.
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1 AI062735 to S.W.) and the University of Connecticut Health Center General Clinical Research Center (MO1RR06192 to P.J.K. and S.W.).
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Beaudouin, E., G. Kanny, B. Guerin, L. Guerin, F. Plenat, and D. A. Moneret-Vautrin. 1997. Unusual manifestations of hypersensitivity after tick bite: report of two cases. Ann. Allergy Asthma Immunol. 79:43-46. [DOI] [PubMed] [Google Scholar]
- 2.Bell, J. F., S. J. Stewart, and S. K. Wikel. 1979. Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity. Am. J. Trop. Med. Hyg. 28:876-880. [PubMed] [Google Scholar]
- 3.Bergman, D. K., M. J. Palmer, M. J. Caimano, J. D. Radolf, and S. K. Wikel. 2000. Isolation and molecular cloning of a secreted immunosuppressant protein from Dermacentor andersoni salivary gland. J. Parasitol. 86:516-525. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard, K. R., and S. K. Wikel. 2005. Care, maintenance, and experimental infestation of ticks in the laboratory setting, p. 705-711. In W.C. Marquart (ed.), Biology of disease vectors, 2nd ed. Elsevier Academic Press, San Diego, Calif.
- 5.Brossard, M., and S. K. Wikel. 2004. Tick immunobiology. Parasitology 129:S161-S176. [DOI] [PubMed] [Google Scholar]
- 6.Burke, G., S. K. Wikel, A. Spielman, S. R. Telford, K. McKay, P. J. Krause, et al. 2005. Hypersensitivity to ticks and Lyme disease risk. Emerg. Infect. Dis. 11:36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fivaz, B. H. 1989. Immune suppression induced by the brown ear tick Rhipicephalus appendiculatus. J. Parasitol. 75:946-952. [PubMed] [Google Scholar]
- 8.Krause, P. J., K. McKay, J. Gadbaw, D. Christianson, L. Closter, T. Lepore, S. A. Telford, V. Sikand, R. Ryan, D. Persing, J. D. Radolf, A. Spielman, et al. 2003. Increasing health burden of human babesiosis in endemic sites. Am. J. Trop. Med. Hyg. 68:431-436. [PubMed] [Google Scholar]
- 9.Krinsky, W. L. 1983. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int. J. Dermatol. 22:75-91. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Magnarelli, L. A., J. M. Meegan, J. F. Anderson, and W. A. Chappell. 1984. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J. Clin. Microbiol. 20:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall, J. 1967. Ticks and human skin. Dermatologica 135:60-65. [DOI] [PubMed] [Google Scholar]
- 13.Mejri, N., B. Rutti, and M. Brossard. 2002. Immunosuppressive effects of Ixodes ricinus tick saliva or salivary gland extracts on innate and acquired immune response of BALB/c mice. Parasitol. Res. 88:192-197. [DOI] [PubMed] [Google Scholar]
- 14.Nazario, S., S. Das, A. M. de Silva, K. Deponte, N. Marcantonio, J. F. Anderson, D. Fish, E. Fikrig, and F. Kantor. 1998. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am. J. Trop. Med. Hyg. 58:780-785. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro, J. M. C. 1995. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 4:143-152. [PubMed] [Google Scholar]
- 16.Ribeiro, J. M. C., and T. N. Mather. 1998. Ixodes scapularis: salivary kininase activity is a metallo dipeptidyl carboxypeptidase. Exp. Parasitol. 89:213-221. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro, J. M. C., and I. M. B. Francischetti. 2003. Role of arthropod saliva in blood feeding. Sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73-88. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro, J. M. C., F., Alarcon-Chaidez, I. M. B. Francischetti, B. Mans, T. N. Mather, J. G. Valenzuela, and S. K. Wikel. 2006. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 36:111-129. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Sanders, M. L., G. E. Glass, R. B. Nadelman, G. Wormser, A. L. Scott, S. Raha, B. C. Ritchie, D. C. Jaworski, and B. S. Schwartz. 1999. Antibody levels to recombinant tick calreticulin increase after exposure to Ixodes scapularis (Say) and are correlated with tick engorgement indices. Am. J. Epidemiol. 149:777-784. [DOI] [PubMed] [Google Scholar]
- 21.Sanders, M. L., D. C. Jaworski, J. L. Sanchez, R. F. DeFraites, G. E. Glass, A. L. Scott, S. Raha, B. C. Ritchie, G. R. Needham, and B. S. Schwartz. 1998. Antibody to a cDNA-derived calreticulin protein from Amblyomma americanum as a biomarker of tick exposure in humans. Am. J. Trop. Med. Hyg. 59:279-285. [DOI] [PubMed] [Google Scholar]
- 22.Schoeler, G. B., and S. K. Wikel. 2001. Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 95:755-771. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz, B. S., D. P. Ford, J. E. Childs, N. Rothman, and R. J. Thomas. 1991. Anti-tick saliva antibody: a biologic marker of tick exposure that is a risk factor for Lyme disease seropositivity. Am. J. Epidemiol. 134:86-95. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz, B. S., M. D. Goldstein, and J. E. Childs. 1993. Antibodies to Borrelia burgdorferi and tick salivary gland proteins in New Jersey outdoor workers. Am. J. Public Health 83:1746-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz, B. S., R. B. Nadelman, D. Fish, J. E. Childs, G. Forseter, and G. Wormser. 1993. Entomologic and demographic correlates of anti-tick saliva antibody in a prospective study of tick bite subjects in Westchester County, New York. Am. J. Trop. Med. Hyg. 48:50-57. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz, B. S., J. M. C. Ribeiro, and M. D. Goldstein.1990. Anti-tick antibodies: an epidemiologic tool in Lyme disease research. Am. J. Epidemiol. 132:58-66. [DOI] [PubMed] [Google Scholar]
- 27.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 28.Theilmann, D. A., and S. Stewart. 1992. Molecular analysis of the transactivating IE-2 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology 187:84-96. [DOI] [PubMed] [Google Scholar]
- 29.Valenzuela, J. G. 2004. Exploring tick saliva: from biochemistry to ‘sialomes’ and functional genomics. Parasitology 129:S83-S94. [DOI] [PubMed] [Google Scholar]
- 30.Wikel, S. K. 1982. Immune responses to arthropods and their products. Annu. Rev. Entomol. 27:21-48. [DOI] [PubMed] [Google Scholar]
- 31.Wikel, S. K. 1985. Effects of tick infestation on the plaque-forming cell response to a thymic dependent antigen. Ann. Trop. Med. Parasitol. 79:195-198. [DOI] [PubMed] [Google Scholar]
- 32.Wikel, S. K. 1996. Host immunity to ticks. Annu. Rev. Entomol. 41:1-22. [DOI] [PubMed] [Google Scholar]
- 33.Wikel, S. K., and D. K. Bergman. 1997. Tick-host immunology: significant advances and challenging opportunities. Parasitol. Today 13:383-389. [DOI] [PubMed] [Google Scholar]
- 34.Wikel, S. K., R. N. Ramachandra, D. K. Bergman, T. R. Burkot, and J. Piesman. 1997. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect. Immun. 65:335-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams, C. L., B. Strobino, A. Lee, A. S. Curran, J. L. Benach, S. Inamdar, and R. Cristofaro. 1990. Lyme disease in childhood: clinical and epidemiologic features of ninety cases. Pediatr. Infect. Dis. J. 9:10-14. [PubMed] [Google Scholar]
- 36.Xu, G., Q. Q. Fang, Y. Sun, J. E. Keirans, and L. A. Durden. 2005. Hard tick calreticulin (CRT) gene coding regions have only one intron with conserved positions and variable sizes. J. Parasitol. 91:1326-1331. [DOI] [PubMed] [Google Scholar]