Abstract
The 81-kDa malate synthase (MS; Rv 1837c) and the 27-kDa MPT51 (Rv 3803c) of Mycobacterium tuberculosis are immunodominant antigens recognized by serum antibodies from ∼80% of human immunodeficiency virus-negative smear-positive tuberculosis patients from India. We now provide evidence that the use of the MS/MPT51-based serodiagnostic assay can serve as an adjunct to sputum microscopy in the rapid diagnosis of pulmonary tuberculosis.
Approximately 50% of the pulmonary tuberculosis (PTB) cases in the United States are smear negative for acid-fast bacilli (AFB), leading to a diagnostic delay of several weeks due to the low growth rate of Mycobacterium tuberculosis (2a). Delayed recognition of smear-negative PTB is estimated to be responsible for ∼20% of the cases of TB transmission (2). Nucleic acid amplification assays can detect ∼50% of these cases but are too expensive for routine screening for smear-negative PTB (1, 13, 20). Diagnostic tests that can enhance the early recognition of paucibacillary TB are therefore urgently required.
Our earlier studies have delineated a subset of ∼10 to 12 culture filtrate proteins of M. tuberculosis that are recognized by antibodies (Abs) in patients with human immunodeficiency virus-negative (HIV−) multibacillary as well as paucibacillary TB and in HIV-infected (HIV+) TB patients (19). Two of these, the 81-kDa protein malate synthase (MS; Rv 1837c) and the 27-kDa protein MPT51 (Rv3803c) are recognized by Abs in ∼80% of HIV−, smear-positive TB patients from India (21). Our studies have shown that paucibacillary TB patients have low titers of Abs compared to multibacillary patients (19). The goal of the present study was to determine the presence of Abs to MS and/or MPT51 in culture-confirmed patients from the United States, who tend to present at an early stage of TB, and to assess the ability of the serodiagnostic assay to serve as an adjunct to AFB smears for the rapid diagnosis of PTB.
Approval for human subject research was obtained from the institutional review boards of the New York University School of Medicine, Bellevue Hospital, and the Manhattan Veterans Administration, and written informed consent was obtained from all individuals prior to the drawing of blood samples and review of medical records. Patients clinically suspected of having PTB were enrolled, and their sera were frozen. Only specimens from patients later confirmed to be M. tuberculosis culture positive (TB+; n = 53) were included in the studies (Table 1). Subjects treated for TB for >14 days were excluded. Serum specimens from 55 healthy controls with no risk factors for HIV infection (36 with tuberculin skin test [TST]-positive results and 19 with TST-negative results) were also obtained. The TST-positive subjects had indurations of >10 mm and included volunteers who had been vaccinated with Mycobacterium bovis BCG and/or had a history of TB exposure. Sera from 40 asymptomatic HIV+ patients, all of whom were on highly active antiretroviral therapy, were also included; 36/40 (90%) had undetectable viral loads (<75 copies/ml), and 4/40 had viral loads between 77 and 1,233 copies/ml. The median absolute CD4 count was 401 cells/mm3 (range, 108 to 862 cells/mm3).
TABLE 1.
Characteristics of subjects with culture-confirmed PTB
| Subjects (n) | Smear
|
Median age (yr) (range) | No. of subjectsh
|
Median duration of symptomse (wk) | CD4 countf (range) | VLg (range) (103) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resulta | No. of subjects | Male | Foreign born | With cavitary lesionsb,c | With upper lobe infiltratesb,d | With other infiltratesb | With no infiltratesb | |||||
| HIV− (19) | − | 9 | 35 (25-66) | 7 | 4 | 1 | 7 | 1 | 0 | 3 (0-8) | NA | NA |
| + | 10 | 41 (20-56) | 7 | 6 | 3 | 4 | 3 | 0 | 6 (4-24) | NA | NA | |
| HIV+ (25) | − | 8 | 38 (29-50) | 8 | 2 | 0 | 4 | 3 | 1 | 3 (1-12) | 126 (14-483) | 147 (18-490) |
| + | 17 | 36 (27-54) | 17 | 3 | 3 | 4 | 5 | 5 | 4 (1-16) | 161 (10-397) | 265 (42-518) | |
| HIV? (9) | − | 3 | 58 (34-64) | 3 | 1 | 1 | 2 | 0 | 0 | 6 (2-12) | NA | NA |
| + | 6 | 40 (28-60) | 3 | 6 | 2 | 3 | 0 | 1 | 8 (4-16) | NA | NA | |
| Total | 53 | 47 (20-66) | 45 (85) | 22 (42) | 10 (19) | 24 (45) | 12 (23) | 7 (13) | 4 (0-24) | |||
−, first three sputum smears AFB negative; +, at least one of the first three sputum smears AFB positive.
Radiographic findings on chest X-ray and/or computer tomography.
Cavitary lesions with or without the presence of infiltrates.
Upper lobe infiltrates without the presence of cavitary lesions.
Symptoms included one or more of the following: fever, night sweats, weight loss, cough (with or without expectoration), hemoptysis, and shortness of breath.
Median absolute CD4 count (cells/mm3). NA, not applicable.
Median viral load, determined with the Bayer HIV-1 RNA 3.0 assay (bDNA) (Bayer Corporation, Tarrytown, NY). The lowest value detected by this assay is 75 copies/ml. NA, not applicable.
Values in parentheses indicate percentages of total number of subjects.
Purified recombinant MS and MPT51 (21, 22) were obtained via the Tuberculosis Research Materials and Vaccine Testing Contract (NIH) (http://www.cvmbs.colostate.edu/microbiology/tb/top.htm). The presence of serum immunoglobulin G (IgG) and IgA to MS and MPT51 were determined by an enzyme-linked immunosorbent assay (ELISA) described previously by a person not blinded to the clinical status of the subjects (21). The antigens were coated at 4 μg/ml (50 μl/well), and the serum samples were tested at a 1:50 dilution for MS or at a 1:25 dilution for the MPT51 protein.
No selection of control subjects was made, and the mean optical density (OD) at 490 nm of the 55 TST-positive and -negative healthy subjects plus 3 standard deviations (SD) was used as a cutoff to determine positive reactivity (upper-limit confidence interval [CI] of 99.7%). All 55 control sera were included in each assay. Each specimen was tested two or three times, and specimens that were positive two out of two or two out of three times were considered positive. The maximum variation for repeat analysis of the same serum sample was 15%. The result of the ELISA was considered positive if Ab reactivity to either MS and/or MPT51 was detected. Binomial 95% CIs for sensitivities and specificities were calculated (gold standard, positive culture for M. tuberculosis) (5), and differences in proportions were tested using the chi-square test with Yates' correction.
No Abs to MS and/or MPT51 were detected in 54/55 HIV-negative volunteers (specificity, 0.98; CI, 0.90 to 1.00). This level of specificity in healthy volunteers is in accordance with results for these proteins from other laboratories (3, 17). The specificity was further validated in asymptomatic HIV+ subjects, 39/40 of whom lacked Abs to MS/MPT51 (specificity, 0.98; CI, 0.87 to 1.00). The single Ab-positive healthy volunteer was TST positive, had been BCG vaccinated, had a history of TB exposure, and had not been treated for latent TB. The TST status of the asymptomatic HIV+ Ab-positive subject is unknown. Of the TB+ subjects, 31/53 (sensitivity, 0.58; CI, 0.44 to 0.72) had Abs to either one or both antigens. No difference in Ab reactivity between smear-positive (18/33; 55%) and smear-negative (13/20; 65%) TB+ subjects was observed. Antibody detection was significantly higher in the HIV+ TB+ subjects (20/25 [0.80; CI, 0.59 to 0.93]) than in the HIV− TB+ subjects (8/19 [0.42; CI, 0.20 to 0.67]) (P = 0.023). Of the 20 HIV+ TB+ Ab-positive subjects, the median absolute CD4 count was 137 cells/mm3 (range, 17 to 483 cells/mm3) and the median viral load was 147 × 103 copies/ml (range, 18 to 397 × 103 copies/ml). Of the TB+ subjects whose HIV status was unknown, three of nine (33%) tested Ab positive.
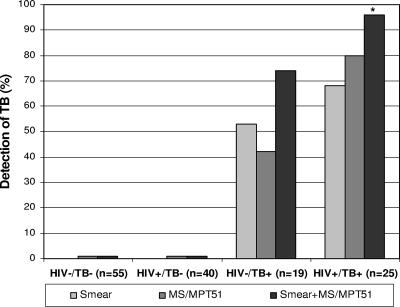
Together, the AFB smear and the serologic assay identified 14/19 (sensitivity, 0.74; CI, 0.49 to 0.91) HIV− TB+ subjects, compared to 10/19 (0.53) by smear and 8/19 (0.42) by ELISA alone. For the HIV+ TB+ subjects, both tests combined were able to identify 24/25 (0.96; CI, 0.80 to 1.00) subjects, compared to 17/25 (0.68) by smear and 20/25 (0.80) by ELISA alone (Fig. 1). Thus, in both the HIV− and HIV+ TB patients, the use of dual testing enhanced the sensitivity of rapid detection of TB over that of microscopy alone. This enhancement was statistically significant for the HIV+ TB patients (P = 0.027). The prevalence of TB in a high-risk population of patients who are suspected of having active TB has previously been estimated to be ∼25% (15). Based on this prevalence, the positive predictive value of the serologic test alone for HIV− TB+ patients would be 0.88 (CI, 0.49 to 1.00) and that for HIV+ TB+ patients would be 0.93 (CI, 0.74 to 1.00), varying both the specificity and sensitivity to the highest and lowest values of their 95% CIs.
FIG. 1.
Detection rate of active TB in U.S. subjects by either sputum smears for AFB (smear; with one or more of the initial three samples positive), serum Ab reactivity to MS and/or MPT51 (MS/MPT51), or both methods combined (smear+MS/MPT51). Control groups are healthy, HIV-negative volunteers (HIV−/TB−; 36 TST positive and 19 TST negative) and asymptomatic HIV-infected patients (HIV+/TB−). Patient groups with culture-confirmed PTB are either HIV negative (HIV−/TB+) or HIV infected (HIV+/TB+). *, P = 0.023 for comparison of sensitivity of smear+MS/MPT51 to that of smear alone.
The high Ab reactivities to the two antigens in the HIV+ TB+ subjects were seen at all levels of immunosuppression. This is important because ∼30% of new adult TB patients in the United States are coinfected with HIV (4), and diagnosis of PTB in these patients is especially challenging, since these patients are frequently sputum smear negative and have atypical chest X-ray findings (6-8, 10, 16). The higher level of Ab reactivity in HIV+ TB+ subjects may be attributed to increased antigenic stimulation due to high bacterial loads even in smear-negative subjects. This belief is supported by the similarly high sensitivity (∼80%) of our assay for the HIV− TB+ patients from India, who presented mostly with advanced stages of TB (22). It is also possible that the polyclonal B-lymphocyte activation frequently seen with HIV infection (12) also contributes to a higher level of Abs. Since the asymptomatic HIV+ subjects lack anti-MS and/or anti-MPT51 Abs (9, 18; present study), and since earlier studies have shown that HIV+ patients with Mycobacterium avium complex bacteremia lack Abs that react with culture filtrates of M. tuberculosis which contain both the proteins (11), the responses in the HIV+ TB+ patients are unlikely to be nonspecific. Although studies based on stored sera from TB subjects whose diagnoses were later confirmed by culture may cause selection bias, this approach was necessary for evaluating the value of the serodiagnostic test in a culture-confirmed population. Based on the results of this pilot study, prospective studies are planned wherein Ab responses in consecutive patients suspected of having TB will be evaluated and Ab responses of TB+ patients will be assessed during and after antituberculosis therapy.
Although the sensitivity of the assay with these two proteins (58%) is higher than that of other serological tests for similar patient populations (1, 16a, 24), it is lower than the sensitivity for smear-positive TB patients from India (22). This difference is most likely due to differences in the stage at which TB is detected in the two settings; most U.S. patients had disease less advanced (43/53 [81%] noncavitary and 20/53 [38%] smear negative) than that of the Indian TB patients (all smear positive and cavitary). Identification of additional highly immunogenic antigens of M. tuberculosis are ongoing (14, 23, 25), and it is anticipated that the sensitivity of the immunoassay will be further increased by these antigens.
Using a serological test as an adjunct for rapid diagnosis of TB is attractive because the test is fast, cheap, and easy to perform. It does not require a specimen from the site of infection and potentially can be adapted to point-of-care formats. These studies demonstrate that the MS/MPT-based serodiagnostic assay can serve as an adjunct to sputum microscopy in the rapid diagnosis of PTB.
Acknowledgments
This study was supported by National Institute of Health grants AI-07382, RO1 AI-05627, NO1-AI-40091, MO1 00096, and HL 59832, by the Institute for Urban and Global Health, New York University School of Medicine, and by the Department of Veterans Affairs Research Enhancement Award Program, New York Harbor Healthcare System.
REFERENCES
- 1.Al Zahrani, K., H. Al Jahdali, L. Poirier, P. Rene, M. L. Gennaro, and D. Menzies. 2000. Accuracy and utility of commercially available amplification and serologic tests for the diagnosis of minimal pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 162:1323-1329. [DOI] [PubMed] [Google Scholar]
- 2.Behr, M. A., S. A. Warren, H. Salamon, P. C. Hopewell, A. Ponce de Leon, C. L. Daley, and P. M. Small. 1999. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353:444-449. [DOI] [PubMed] [Google Scholar]
- 2a.Centers for Disease Control and Prevention. September 2005, posting date. Reported tuberculosis in the United States, 2004. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Ga. [Online.] http://www.cdc.gov/nchstp/tb/surv/surv2004/default.htm.
- 3.Chaudhary, V. K., A. Kulshreshta, G. Gupta, N. Verma, S. Kumari, S. K. Sharma, A. Gupta, and A. K. Tyagi. 2005. Expression and purification of recombinant 38-kDa and Mtb81 antigens of Mycobacterium tuberculosis for application in serodiagnosis. Protein Expr. Purif. 40:169-176. [DOI] [PubMed] [Google Scholar]
- 4.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 5.Diem, K. (ed.).1962. Documenta Geigy scientific tables, 6th ed. Geigy Pharmaceuticals, Ardsley, N.Y.
- 6.Elliott, A. M., K. Namaambo, B. W. Allen, N. Luo, R. J. Hayes, J. O. Pobee, and K. P. McAdam. 1993. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber. Lung Dis. 74:191-194. [DOI] [PubMed] [Google Scholar]
- 7.Gold, J. A., W. N. Rom, and T. J. Harkin. 2002. Significance of abnormal chest radiograph findings in patients with HIV-1 infection without respiratory symptoms. Chest 121:1472-1477. [DOI] [PubMed] [Google Scholar]
- 8.Haramati, L. B., E. R. Jenny-Avital, and D. D. Alterman. 1997. Effect of HIV status on chest radiographic and CT findings in patients with tuberculosis. Clin. Radiol. 52:31-35. [DOI] [PubMed] [Google Scholar]
- 9.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of Mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karstaedt, A. S., N. Jones, M. Khoosal, and H. H. Crewe-Brown. 1998. The bacteriology of pulmonary tuberculosis in a population with high human immunodeficiency virus seroprevalence. Int. J. Tuberc. Lung Dis. 2:312-316. [PubMed] [Google Scholar]
- 11.Laal, S., K. M. Samanich, M. G. Sonnenberg, J. T. Belisle, J. O'Leary, M. S. Simberkoff, and S. Zolla-Pazner. 1997. Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secreted antigen of Mycobacterium tuberculosis. J. Infect. Dis. 176:133-143. [DOI] [PubMed] [Google Scholar]
- 12.Lane, H. C., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 13.Lim, T. K., A. Gough, N. K. Chin, and G. Kumarasinghe. 2000. Relationship between estimated pretest probability and accuracy of automated Mycobacterium tuberculosis assay in smear-negative pulmonary tuberculosis. Chest 118:641-647. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee, S., N. Daifalla, Y. Zhang, J. Douglass, L. Brooks, T. Vedvick, R. Houghton, S. G. Reed, and A. Campos-Neto. 2004. Potential serological use of a recombinant protein that is a replica of a Mycobacterium tuberculosis protein found in the urine of infected mice. Clin. Diagn. Lab. Immunol. 11:280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New York City Department of Health. 2005. Tuberculosis in New York City, 2004: information summary. Department of Health, New York, N.Y.
- 16.Pitchenik, A. E., and H. A. Rubinson. 1985. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am. Rev. Respir. Dis. 131:393-396. [DOI] [PubMed] [Google Scholar]
- 16a.Pottumurthy, S., V. C. Wells, and A. J. Morris. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramalingam, B., A. R. Baulard, C. Locht, P. R. Narayanan, and A. Raja. 2004. Cloning, expression, and purification of the 27 kDa (MPT51, Rv3803c) protein of Mycobacterium tuberculosis. Protein Expr. Purif. 36:53-60. [DOI] [PubMed] [Google Scholar]
- 18.Ramalingam, B., K. R. Uma Devi, and A. Raja. 2003. Isotype-specific anti-38 and 27 kDa (mpt 51) response in pulmonary tuberculosis with human immunodeficiency virus coinfection. Scand. J. Infect. Dis. 35:234-239. [DOI] [PubMed] [Google Scholar]
- 19.Samanich, K., J. T. Belisle, and S. Laal. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 69:4600-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schluger, N. W. 2004. Novel approaches to the rapid diagnosis of tuberculosis, p. 177-181. In W. N. Rom and S. M. Garay (ed.), Tuberculosis, 2nd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.15850755
- 21.Singh, K. K., Y. Dong, J. T. Belisle, J. Harder, V. K. Arora, and S. Laal. 2005. Antigens of Mycobacterium tuberculosis recognized by antibodies during incipient, subclinical tuberculosis. Clin. Diagn. Lab. Immunol. 12:354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh, K. K., Y. Dong, L. Hinds, M. A. Keen, J. T. Belisle, S. Zolla-Pazner, J. M. Achkar, A. J. Nadas, V. K. Arora, and S. Laal. 2003. Combined use of serum and urinary antibody for diagnosis of tuberculosis. J. Infect. Dis. 188:371-377. [DOI] [PubMed] [Google Scholar]
- 23.Singh, K. K., Y. Dong, S. A. Patibandla, D. N. McMurray, V. K. Arora, and S. Laal. 2005. Immunogenicity of the Mycobacterium tuberculosis PPE55 (Rv3347c) protein during incipient and clinical tuberculosis. Infect. Immun. 73:5004-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talbot, E. A., D. C. H. Burgess, N. M. Hone, M. F. Iademarco, M. J. Mwasekaga, H. J. Moffat, T. L. Moeti, R. A. Mwansa, P. Letsatsi, N. T. Gokhale, T. A. Kenyon, and C. D. Wells. 2004. Tuberculosis serodiagnosis in a predominantly HIV-infected population of hospitalized patients with cough, Botswana, 2002. Clin. Infect. Dis. 39:e1-e7. [DOI] [PubMed] [Google Scholar]
- 25.Weldingh, K., I. Rosenkrands, L. M. Okkels, T. M. Doherty, and P. Andersen. 2005. Assessing the serodiagnostic potential of 35 Mycobacterium tuberculosis proteins and identification of four novel serological antigens. J. Clin. Microbiol. 43:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]