Abstract
In anticipation of the development of a vaccine against cytomegalovirus (CMV), we conducted a large, nationally representative serosurvey to examine the seroprevalence of CMV in Australia. Sera were collected opportunistically from laboratories around Australia. Age- and gender-representative samples were tested for CMV antibody. The population-weighted rate of CMV seropositivity in subjects between 1 and 59 years of age was 57% (95% confidence interval, 55.2 to 58.6%). An association between CMV seroprevalence and increasing age was recognized; however, little overall difference in seroprevalence between the sexes was found. The finding that high levels of CMV exposure occur in the first few years of life suggests that for a universal vaccination program to have maximal impact, the vaccine would need to be delivered to infants and have a long duration of protective efficacy. This is the first national serosurvey looking at cytomegalovirus in the Australian community. This study provides valuable information that can be used to examine the incidence of infection in the community and help focus the administration of a future CMV vaccine to appropriate target populations.
Cytomegalovirus (CMV) infections are common and usually asymptomatic in otherwise healthy children and adults (13). CMV has a worldwide distribution, infecting between 40% and 90% of adults, leading to lifelong latent infection (1).
However, for recipients of solid-organ or hematopoetic-cell allografts and individuals with advanced AIDS, CMV is a well-known cause of serious morbidity and sometimes fatal infections (14). Recurrent CMV disease has been reported to occur in 6 to 59% of solid-organ transplant recipients (5). The risk factors cited for the development of CMV disease in transplant recipients are graft rejection, antilymphocyte therapy, and high-dose corticosteroids (11). Patients who develop CMV infections incur costs associated with diagnostics, hospitalization, and multiple physician visits. For a transplant patient who has been diagnosed with severe CMV disease, these costs have been estimated at $14,000 (21).
Following the implementation of universal rubella immunization programs, CMV has become a common cause of intrauterine infection, with between 0.3 and 2.4% of neonates becoming infected in different countries (19). In some countries, congenital CMV infection may affect more children than do other, better-known childhood conditions, such as Down syndrome, fetal alcohol syndrome, and spina bifida (2, 17). Data from the United States of America have led to an estimate that 8,000 newborns have health problems each year as a result of congenital CMV infection (3). Analysis conducted in the early 1990s revealed that the estimated costs to the U.S. healthcare system associated with congenital CMV infection were approximately $1.9 billion annually (6), with a cost per affected child of over $300,000 (7).
In a recent review of the priorities for vaccine development, CMV was ranked in the highest of five tiers by the Institute of Medicine in the United States as a potentially cost-saving vaccine target (21). The ranking was based on the economic cost and years of disability that would be avoided and the years of life saved by an effective vaccine.
There are several promising candidate vaccines against CMV in various stages of preclinical and clinical testing, including recombinant vaccines, live attenuated vaccines, and chimeric vaccines (18). Progress in the development of vaccines against CMV encouraged us to examine the seroepidemiology of CMV in Australia.
This study therefore aimed to determine the seroprevalence of CMV in Australia in relation to age and sex. These results of this study will inform future vaccination strategies and public policy.
MATERIALS AND METHODS
Serum samples.
A total of 3,593 serum samples were tested for CMV seroprevalence as part of the National Centre for Immunization Research and Surveillance of Vaccine Preventable Diseases (NCIRS) serosurveillance program. Samples were collected in 2002 from 37 major diagnostic laboratories throughout Australia that agreed to participate in the program. These laboratories supplied remnant sera from samples that had been submitted for serological testing and would otherwise have been discarded. Sera from subjects who were either immunocompromised, had received multiple transfusions in the past 3 months, or were known to be infected with human immunodeficiency virus were excluded (8). Sera were identified at the referring laboratory by the sex of the subject, age or date of birth, residential postcode, date of collection, and a unique identifier to ensure that only one sample from any subject was tested. These identifiers were removed from the samples before testing, and the samples were coded by date of collection, state/territory of origin, and referring laboratory.
Study population.
Serum samples were collected from people between 1 and 59 years of age and stratified into the following age groups: 1 to 2, 3 to 4, 5 to 9, 10 to 14, 15 to 19, 20 to 24, 25 to 29, 30 to 39, 40 to 49, and 50 to 59 years. Serum samples were not available for infants less than 1 year of age. Approximately equal numbers of males and females were tested. Within each age group, states and territories were sampled in proportion to their population size. Prevalence was calculated separately for each age group and for Australia as a whole by weighting the prevalence estimates for each age group by the age distribution of the 2002 Australian population. The CMV prevalence for the group aged 60 years and above was assumed to be the same as for the group aged 50 to 59 years. Sample sizes were calculated to achieve a 95% confidence interval (CI) of approximately ±5% for each age group.
Serological testing.
Serum samples were tested for CMV-specific immunoglobulin G (IgG) using a CMV IgG enzyme-labeled antigen test (Medac, Hamburg, Germany), and the results were interpreted according to the manufacturer's instructions. Samples with optical densities 10% or more below the cutoff were recorded as negative, those with optical densities between 10% below and 10% above the cutoff were equivocal, and all others were positive. If the sample's absorbance was within 10% of the cutoff level, the sample was retested and classified according to the retest result.
Statistical methods and ethical approval.
The percentages of individuals with positive, negative, and equivocal results were determined for each age group and sex. Epi-Info version 3.3.2 was used for the analysis and comparison of serostatuses among age groups. Ninety-five-percent confidence intervals were calculated where appropriate, and P values of <0.05 were considered statistically significant. Ethics approval was obtained from the Human Research Ethics Committee of the Western Sydney Area Health Service.
RESULTS
Age-specific seroprevalence.
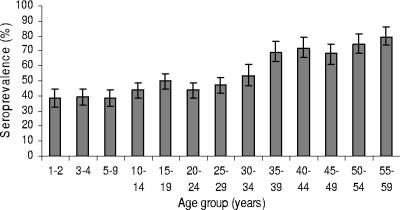
The seroprevalence of CMV in the population tested was 57% (95% CI, 55.2 to 58.6%), with an overall association identified between seroprevalence and increasing age (Table 1). For the groups of children aged 1 to 2 and 3 to 4 years, the recorded seroprevalences were 38% (95% CI, 32.2 to 44.3%) and 39% (95% CI, 34.0 to 44.5%), respectively. Seroprevalence continued to rise with age from 50% (95% CI, 48.5 to 58.7%) in 20- to 24-year-olds to 79% (95% CI, 72.7 to 84.7%) in 50- to 59-year-olds (Fig. 1).
TABLE 1.
CMV seroprevalence in Australia by age group
| Age group (yr) | No. of samples | % Positive | CI (95% ± 5%) |
|---|---|---|---|
| 1-2 | 251 | 38.2 | 32.2-44.3 |
| 3-4 | 331 | 39.1 | 34.0-44.5 |
| 5-9 | 330 | 38.8 | 33.5-44.3 |
| 10-14 | 369 | 43.6 | 38.5-48.8 |
| 15-19 | 387 | 49.9 | 44.7-54.9 |
| 20-24 | 384 | 53.6 | 48.5-58.7 |
| 25-29 | 384 | 47.1 | 42.0-52.3 |
| 30-34 | 192 | 53.6 | 46.3-60.8 |
| 35-39 | 194 | 69.1 | 62.0-75.5 |
| 40-44 | 192 | 71.8 | 64.9-78.1 |
| 45-49 | 194 | 68.0 | 61.7-75.2 |
| 50-54 | 193 | 74.1 | 67.3-80.1 |
| 55-59 | 192 | 79.2 | 72.7-84.7 |
FIG. 1.
CMV seroprevalence by age group in Australia. T bars represent 95% CIs.
Gender-specific seroprevalence.
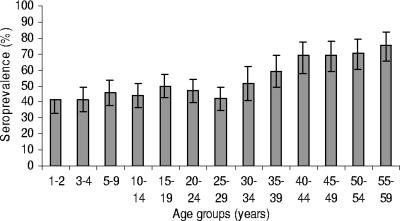
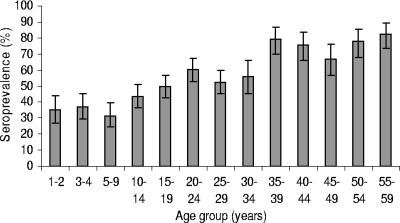
There was little overall difference in seroprevalence between males and females (51% and 54% seropositive, respectively; P = 0.09). Although the percentages that were seropositive did not differ between the sexes overall, they did differ by age group (Fig. 1). In the younger age groups, slightly greater percentages of males than females (Fig. 2) were seropositive, and in the adult age groups, the reverse was true. For females (Fig. 3), the levels of CMV seroprevalence increased substantially between the group aged 30 to 34 years and that aged 35 to 39 years (56% and 79% seropositive, respectively; P value = 0.0005).
FIG. 2.
CMV seroprevalence in males by age group in Australia. T bars represent 95% CIs.
FIG. 3.
CMV seroprevalence in females by age group in Australia. T bars represent 95% CIs.
DISCUSSION
The population-weighted rate of CMV seropositivity in subjects between 1 and 59 years of age was found to be 57%. This rate was found to increase with age, so that by 20 years of age, 53% of the tested population was seropositive. For women, a significant rise in CMV seroprevalence occurred between the group aged 30 to 34 years and that aged 35 to 39 years. This may be explained by motherhood and the fact that women in these age groups have close contact with young children, who may act as a vehicle for transmission.
A study conducted in Australia by Kelly et al. showed that anonymous opportunistic testing of sera provides estimates of immunity that are comparable with those of random population-based surveys of some vaccine-preventable diseases (12). However, the lack of detailed information about participants in opportunistic serosurveys means that it is not possible to identify or control for various potential biases. A number of measures were implemented to ensure that selection bias was reduced. These included enrollment of major laboratories that represented both regional and rural areas and the use of sera that were collected from outpatients rather than hospitalized patients.
These are, to our knowledge, the first published results on the national seroepidemiology status of CMV in Australia. Previously published studies have examined only select subpopulations. The first study (10), published in 1968, examined stored sera from children and adults living in only one Australian state. The sera were collected from a range of sources, including hospitalized children and their parents, blood donors, and patients attending antenatal clinics. The study concluded that infection appeared to be acquired with increasing frequency early in adult life. However, as these findings were not published by age group, it was hard to make a distinction about when the increase occurred (10). A more recent Australian study (15) examined the baseline CMV IgG seroprevalence by the use of blood donor sera. The study again was limited to one state in Australia. This comparative study found that 35% of subjects less than 20 years of age were seropositive for CMV IgG, whereas our study identified rates of seroprevalence in children and teenagers ranging from 39% in 3- to 4-year-olds to 50% in 15- to 19-year-olds. For people over the age of 50 years, the comparative study found that two-thirds (72%) were seropositive, a finding that was consistent with our study results.
A review of CMV seroprevalence studies conducted around the world reveals that residents of developing countries have higher rates of CMV seropositivity than those of developed countries (4). In general, CMV is acquired earlier in life in developing countries and among the lower socioeconomic strata of developed countries (20). In some African nations, seropositivity rates reach 80 to 90% by 10 years of age (19); in comparison, in the United States and Great Britain, certain subgroups of children have CMV seropositivity rates below 20% at 15 years (19).
In contrast with the vaccination rates needed for the elimination of measles (93%), mumps (93%), and rubella (92%), studies have shown that CMV requires a much lower rate of vaccination (60%). This is related to the fact that CMV has a low force of infection and a relatively low basic reproductive number (R0) of 2.5 (9). R0 refers to the number of secondary infections caused by the introduction of a single infectious case into a completely susceptible population and is affected by several factors, including the duration of infectivity of affected patients, their contact with susceptible people, and the infectious nature of the organism.
The finding that the highest levels of CMV exposure occur in the first few years of life (Table 1) suggests that, for a universal vaccination program to have maximal impact, the vaccine would need to be delivered to infants and have a long duration of protective efficacy. If the duration was only brief, a vaccine given just before pregnancy would be advised, or, if the vaccine had a medium duration of efficacy (5 years), regular boosters could be given throughout childbearing years. Concerns are raised with strategies based on targeting women prior to or during pregnancy. These include the cost and time related to the establishment of screening programs to identify CMV-specific antibody in pregnant women. Also, past experience with targeted programs for pregnant women in Australia has shown low rates of vaccine uptake, even in situations of high risk such as that with carriers of hepatitis B (16). It should be noted that cohorts of immunized children would have to reach childbearing age before CMV disease could be completely brought under control. This is due to the persistent nature of CMV disease and its tendency to reactivate periodically (9). As with other childhood diseases, CMV has the potential to infect children prior to the scheduled age of vaccination; therefore, successive generations would need to be immunized before the disease could be eradicated (9).
The potential benefits of a CMV vaccine would include reduced transmission to pregnant women and less CMV disease due to primary infection or reactivation in organ transplant recipients and the immunosuppressed (9). Stratton et al. have suggested that if a vaccine program which had 100% efficacy and 100% uptake were implemented today, the annualized present value of the quality-adjusted life-years gained would be 70,000 (21). They went on to suggest that the vast majority of these quality-adjusted life-years would be attributed to the lack of the long-term sequelae now experienced by infants who acquire congenital CMV infections (21).
A decision to introduce vaccination will depend on the epidemiology of infection and on the safety of the vaccine. Studies such as this one provide valuable information that can be used to examine the epidemiology of infection in the community and to focus the administration of a future vaccine on populations at high risk of the disease.
Acknowledgments
NCIRS is supported by the Australian Government Department of Health and Ageing, the NSW Department of Health, and The Children's Hospital at Westmead. CIDM-Public Health is supported by an infrastructure grant from the New South Wales Health Department. The National Center for Immunization Research and Surveillance funded this study.
We thank the staffs of the laboratories who provided the sera and the laboratory staff at the Institute of Clinical Pathology and Medical Research (ICPMR) for their help in processing and testing the sera.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Boeckh, M., and P. Ljungman. 1998. Cytomegalovirus infection after bone marrow transplantation, p. 215-227. In R. Bowden, C. Paya, and P. Ljungman (ed.), Transplant infections. Lippincott-Raven, Philadelphia, Pa.
- 2.Cannon, M. J., and K. Davis. 2005. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobbins, J. G., J. A. Stewart, G. J. Demmler, et al. 1992. Surveillance of congenital cytomegalovirus disease, 1990-1991. Morb. Mortal. Wkly. Rep. 41(SS-2):35-39. [PubMed] [Google Scholar]
- 4.Enright, A. M., and C. G. Prober. 2004. Herpesviridae infections in newborns: varicella zoster virus, herpes simplex virus, and cytomegalovirus. Pediatr. Clin. N. Am. 51:889-908. [DOI] [PubMed] [Google Scholar]
- 5.Falagas, M. E., and D. R. Snydman. 1995. Recurrent cytomegalovirus in disease in solid organ transplant recipients. Transplant. Proc. 27:34-37. [PubMed] [Google Scholar]
- 6.Fowler, K., S. Stagno, R. Pass, W. Britt, T. Boll, and C. Alford. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326:663-667. [DOI] [PubMed] [Google Scholar]
- 7.Fowler, K. B., S. Stagno, and R. F. Pass. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008-1011. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert, G. L., R. G. Escott, H. F. Gidding, F. M. Turnball, T. C. Heath, P. B. McIntyre, and M. A. Burgess. 2001. Impact of the Australian measles control campaign on immunity to measles and rubella. Epidemiol. Infect. 127:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths, P. D., A. Mclean, and V. C. Emery. 2000. Encouraging prospects for immunisation against primary cytomegalovirus infection. Vaccine 19:1356-1362. [DOI] [PubMed] [Google Scholar]
- 10.Jack, I., and K. McAuliffe. 1968. Sero-epidemiology study of cytomegalovirus infections in Melbourne children and some adults. Med. J. Aust. 1:206-209. [DOI] [PubMed] [Google Scholar]
- 11.Jassal, S., J. Roscoe, J. Zaltzman, T. Mazzulli, M. Krajden, and M. Gadawski. 1998. Clinical practice guidelines: prevention of cytomegalovirus disease after renal tranplantation. J. Am. Soc. Nephrol. 9:1697-1708. [DOI] [PubMed] [Google Scholar]
- 12.Kelly, H., M. A. Riddell, H. F. Gidding, T. Nolan, and G. L. Gilbert.2002. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine 20:3130-3136. [DOI] [PubMed] [Google Scholar]
- 13.Landolfo, S., M. Gariglo, G. Gribaudo, and D. Lembo. 2003. The human cytomegalovirus. Pharmacol. Ther. 98:269-297. [DOI] [PubMed] [Google Scholar]
- 14.Lautenschlager, I. 2003. Cytomegalovirus and solid organ transplantation: an update. Curr. Opin. Organ Transplant. 8:269-275. [Google Scholar]
- 15.Munro, S. C., B. Hall, L. R. Whybin, L. Leader, P. Robertson, G. T. Maine, and W. D. Rawlinson. 2005. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J. Clin. Microbiol. 43:4713-4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oman, K. M., J. Carnie, and T. Ruff. 1997. Hepatitis B immunisation coverage of infants born to chronic carrier mothers in Victoria. Aust. N. Z. J. Public Health 21:731-734. [DOI] [PubMed] [Google Scholar]
- 17.Ross, D. S., S. C. Dollard, M. Victor, E. Sumartojo, and M. J. Cannon. 2006. The epidemiology and prevention of congenital cytomegalovirus infection and disease: activities of the Centers for Disease Control and Prevention workgroup. J. Women's Health 15:224-229. [DOI] [PubMed] [Google Scholar]
- 18.Schleiss, M. R., and T. C. Heineman. 2005. Progress toward an elusive goal: current status of cytomegalovirus vaccines. Expert Rev. Vaccines 4:381-406. [DOI] [PubMed] [Google Scholar]
- 19.Stagno, S. 1990. Cytomegalovirus, p. 240-281. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 4th ed. WB Saunders, Philadelphia, Pa.
- 20.Stagno, S., and A. C. Gretchen. 1990. Changes in the epidemiology of cytomegalovirus. Adv. Exp. Med. Biol. 278:93-104. [DOI] [PubMed] [Google Scholar]
- 21.Stratton, K. R., J. S. Durch, and R. S. Lawrence (ed.). 2000. Vaccines for the 21st century: a tool for decision making. Institute of Medicine, Washington, D.C. [PubMed]