Abstract
The undecapeptide substance P (SP) is a member of the tachykinin family of neurotransmitters, which has a pivotal role in the regulation of inflammatory and immune responses. One of the major barriers to the study of the in vivo role of SP in a number of immune disorders is the accurate measurement of SP in fluids. This is reflected in the variability of reported SP levels in serum and plasma of humans in both healthy and diseased states. This study was initiated in order to identify sources of variability by the comparative evaluation of the influences of sample preparation and analytical detection methods on the measurement of SP in plasma. The results indicate that sample preparation (peptide extraction versus no extraction) and the choice of analytical method for SP quantitation may yield significantly different values and may contribute to the variability in SP values reported in the literature. These results further emphasize the need for careful consideration in the selection of methods for SP quantitation, as well as caution in the interpretation and comparison of data reported in the literature.
The undecapeptide substance P (SP) is a member of the tachykinin family and was first sequenced by Chang et al. (8). SP was subsequently characterized as a neurotransmitter (31, 32, 33). Elevation of serum or plasma SP and/or its cell-associated receptor (NK-1R) has been observed by our laboratory and others in a variety of disorders, including inflammatory bowel disease (25); sickle cell crisis (29); depression and anxiety (4, 15, 20, 28, 37); rheumatological diseases (1, 26); and infectious diseases, such as AIDS and respiratory syncytial virus (12, 39), as well as in cancer (34, 38). More recently, the role of SP in neurotransmission has been expanded to include a major role in the regulation of the immune response (27, 40; reviewed by Ho and Douglas [17]). The influence of SP on the production of inflammatory and immune-regulatory cytokines is now well documented (5, 24). In addition, SP; its receptor, NK-1R; and the development of a number of NK-1R antagonists have received considerable attention as potential therapeutic agents for depression, pain, and emesis (5, 11, 20, 30). The evaluation of SP antagonists as potential therapeutic agents in the setting of clinical trials further emphasizes the need for accurate measurements of serum or plasma SP that have high levels of specificity, sensitivity, and reproducibility.
A review of the literature, however, reveals considerable variability in reported levels of SP in the sera or plasma from control subjects, as well as patients with diverse disease processes (9, 13, 16, 17, 18). A number of studies (Table 1) indicate control serum or plasma-derived SP levels ranging from 12.25 to 397 pg/ml (4, 7, 12, 14, 16, 21, 22, 23, 35) and have varied in their methods of both sample preparation and analysis. The variability in published SP levels may be attributed, in part, to the fact that SP (an undecapeptide with a molecular mass of 1,347 Da) is present in human plasma and serum in free and bound states. Using gel filtration and glutaraldehyde cross-linking of radiolabeled SP to plasma proteins, Corbally et al. (10) found that endogenous plasma-derived SP was reversibly and nonspecifically bound to both high-molecular-mass protein species (>400,000 Da) and intermediate-molecular-mass proteins (58,000 Da). In addition, SP was readily dissociated from these proteins by repeated gel filtration. These observations have led to methods for the extraction of SP from a variety of biological fluids (14, 36). However, the extraction of SP from fluids, particularly serum or plasma, should be considered carefully, since most extraction procedures have been designed for the enrichment of low-molecular-weight peptides and can potentially exclude peptides nonspecifically bound to high-molecular-weight plasma or serum proteins. Such preparative procedures could lead to underestimates of the total amount of SP present in these types of specimens (10).
TABLE 1.
Reported human control serum and plasma-derived substance P levels
| Specimen type | Specimen treatment | Analytical method | Substance P level (pg/ml) | Reference |
|---|---|---|---|---|
| Plasma | Unextracted | RIAa | 186 ± 14 | 7 |
| Plasma | Unextracted | RIA | 397 ± 84 | 35 |
| Plasma | Extracted | RIA | 21.94 ± 18 | 16 |
| Plasma | Unextracted | EIAb | 32.9 ± 1 | 23 |
| Plasma | Extracted | EIAb | 38 ± 35.7 | 14 |
| Plasma | Extracted | EIAb | 31.5 ± 9.7 | 12 |
| Plasma and serum | Extracted | EIAb | 12.25 ± 0.38 | 22 |
| Serum | Unextracted | EIAc | 216.87 ± 81.9 | 4 |
| Serum | Unextracted | EIAc | 261.7 ± 98.8 | 21 |
RIA, radioimmunoassay.
Antigen competition enzyme immunoassay; Cayman Chemical Co., Ann Arbor, MI.
Antigen competition enzyme immunoassay; Assay Designs, Inc., Ann Arbor, MI.
In addition to sample preparation for the measurement of SP, attention should be paid to methods used for the analytical and statistical evaluation of experimental data. More recently, enzyme immunoassays (EIA) have been the methods of choice for the measurement of SP. Further, due to the low molecular weight of SP, these assays have been formatted as antigen competition assays (Table 1). However, different studies employing similar EIA continue to report control human serum and plasma SP values that differ significantly. These assays vary considerably in their reported ranges of detection, and no studies have methodically compared their characteristics from the standpoint of accuracy and reproducibility. Thus, we compared the influences of sample extraction and direct measurement on the determination of SP levels in human and rhesus macaque (Macaca mulatta) plasmas using two commercially available EIA.
MATERIALS AND METHODS
Human and rhesus macaque plasmas.
EDTA-anti-coagulated plasmas obtained from healthy human subjects or rhesus macaques were stored at −70°C until they were evaluated in parallel for SP using two commercially available EIA kits.
SP extraction method.
The method used for the extraction of SP from plasma or serum represented a modification of that previously described (14, 36). Briefly, 0.5-ml aliquots of plasma or sera were acidified by the addition of 1.5 ml of 4% (vol/vol) acetic acid. Prior to use, C18 reverse-phase single-use columns (Bond Elute, Harbor City, CA) were activated by first applying under negative pressure 3 ml of acetonitrile, followed by the application of 3 ml of 1% trifluoroacetic acid three times. The acidified plasma or serum samples were then applied to the columns, which were subsequently washed three times with 3 ml of 1% trifluoroacetic acid. The bound peptides were eluted from the columns with two 1-ml applications of elution buffer (90 ml acetonitirile and 10 ml of 1% trifluoroacetic acid). The eluted samples were air dried under nitrogen at room temperature and reconstituted in 0.3 ml of appropriate EIA diluent.
Enzyme immunoassays.
Two commercially available antigen competition EIA, the Cayman SP Assay (Cayman Chemical Company, Ann Arbor, MI) and the Assay Designs SP Assay (Assay Designs, Inc., Ann Arbor, MI), were evaluated in parallel for the quantitation of SP in extracted and unextracted plasma or serum samples. The two assays are similar in that they both use immobilized antibody to capture a rabbit polyclonal antibody specific for SP. They differ in that the Cayman SP Assay uses an acetylcholinesterase-conjugated SP tracer while the Assay Designs SP Assay uses an alkaline phosphatase-conjugated SP tracer. The most striking difference between the two assays is their effective detection ranges. The Cayman SP Assay allows a detection range of 3.9 pg/ml to 500 pg/ml, while the Assay Designs SP Assay allows a detection range of 9.76 pg/ml to 10,000 pg/ml. This was of some concern, because the breadth of the analyte detection range can have a significant impact on the values interpolated from the lower limits of the standard curve. Therefore, we determined the performances of both assays in the parallel evaluation of specimens using both the manufacturer's recommended standard curve and the identical modified standard curves (15.63 pg/ml to 250 pg/ml) prepared with the two kits' SP standards. The measurable range of the modified standard curve was constructed to allow more data points within the range of values previously observed with unknown samples, the values of which were interpolated from the extreme lower end of the recommended standard curve. Four-parameter logistic fit was used to evaluate all data. Furthermore, the Cayman SP Assay requires an overnight incubation at 4°C for the capture of immune-complexed SP, while the Assay Designs SP Assay requires only a 2-h incubation at room temperature. All other steps of the two assays are similar. The Assay Designs EIA is reported by the manufacturer to have intra-assay and interassay coefficients of variation of 6.7 and 4.2%, respectively. The Cayman EIA is reported by the manufacturer to have intra-assay and interassay coefficients of variation of 10 to 15%. Assay performance for each kit was monitored by the evaluation of a previously validated human plasma pool that was included with each run. Our preliminary studies evaluating identical specimens in parallel using both assays, as well as previously reported studies (Table 1) that used either the Cayman or Assay Designs EIA in the evaluation of similar control samples, revealed significantly different SP values. Therefore, in addition to the evaluation of unknown samples in parallel using both kits, known concentrations of SP standards supplied with each kit were evaluated to determine possible qualitative and/or quantitative differences between the two standards used for constructing each kit's standard curve.
Statistical models.
Since the two EIA yielded significantly different results when evaluating unknown samples in parallel according to each manufacturer's specifications, samples containing known amounts of purified SP were evaluated in parallel according to both the manufacturer's specifications and a four-parameter logistic fit. For the four-parameter model, the parameters were the theoretical response at concentration, the measure of the slope of the curve at its inflection point, the value of the concentration at the inflection point, and the theoretical response at infinite concentration. These data were processed using KC4 v3.4 software (Bio-Tek Instruments, Inc., Winooski, VT).
Statistical methods.
The paired t test was used to compare differences in SP levels between Cayman and Assay Designs EIA in (i) macaque plasma (extracted) and (ii) human serum (unextracted), (iii) extracted and unextracted and (iv) 1:2 and 1:4 dilutions in macaque plasma using the Cayman EIA, and recommended and user-modified standard curves using (v) the Cayman EIA and (vi) the Assay Designs EIA. Agreement between methods (e.g., Cayman and Assay Designs) was assessed by Bland-Altman (2, 3) 95% confidence intervals (CI) for limits of agreement (LOA). The 95% CI for LOA indicates that 95% of the differences fall between these two limits. Precision estimates of the mean difference and the LOA are also provided. All statistical analyses and figures were done using SAS software.
RESULTS
Parallel EIA evaluations of human and macaque plasmas for SP using the manufacturer's recommendations. (i) Differences in SP levels obtained in the parallel evaluation in duplicate of five extracted rhesus macaque plasmas using the Cayman and Assay Designs SP EIA.
The mean SP difference and standard deviation (SD) between Cayman and Assay Designs assays were −115.2 ± 22.8 pg/ml (paired t test; P < 0.01). Similar results were obtained in the parallel evaluation of five unextracted human plasmas in which the mean SP difference and SD between Cayman and Assay Designs assays were −124.7 ± 49.7 pg/ml (P = 0.01).
(ii) Results of the parallel measurement of SP in extracted and unextracted macaque plasmas using the Cayman EIA.
Extracted and unextracted samples yielded a mean SP value difference ± SD of −56.5 ± 21.4 pg/ml (P < 0. 01). Higher values were obtained with the Assay Designs EIA, in which extracted and unextracted samples yielded a mean SP value difference ± SD of −154 ± 150.6 (P = 0.08). Samples of unextracted rhesus macaque plasma were diluted 1:2 and 1:4 prior to evaluation in the Cayman EIA. The mean SP value difference and SD between 1:2 and 1:4 dilutions was −26.8 ± 16.7 pg/ml (P < 0. 01).
Parallel EIA evaluation of unextracted human plasma for substance P using the manufacturer's recommended and user-modified standard curves.
The Cayman EIA yielded a mean SP level difference and SD of −8.4 ± 6.2 pg/ml between the manufacturer's suggested (3.9-pg/ml to 500-pg/ml) and user-modified (15.63-pg/ml to 250-pg/ml) standard curves and four-parameter analysis (P = 0.04). By comparison, the Assay Designs EIA yielded a mean SP difference and SD between the user-modified (15.63-pg/ml to 250-pg/ml) and manufacturer's suggested (9.76-pg/ml to 10,000-pg/ml) standard curves of −154.9 ± 51.5 pg/ml (P < 0.01).
Comparative analysis of Cayman and Assay Designs SP standards.
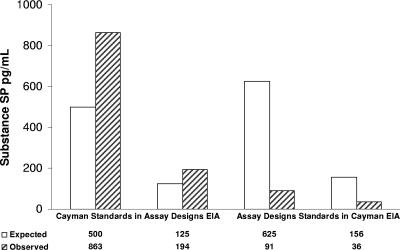
Two concentrations of the SP standards provided with each EIA kit for the construction of standard curves were evaluated with the other EIA. This was done to determine if the differences in SP values seen with the parallel evaluation of unknown plasma samples would also be found with the evaluation of purified SP from two different sources. The analysis of the Cayman SP standard in the Assay Designs EIA gave observed values (863 pg/ml and 194 pg/ml) significantly higher than the expected values (500 pg/ml and 125 pg/ml), while the analysis of the Assay Designs SP standard in the Cayman EIA gave observed values (91 pg/ml and 36 pg/ml) significantly lower than the expected values (625 pg/ml and 156 pg/ml) (see Fig. 2).
FIG. 2.
Evaluation of Cayman and Assay Designs SP standards in the other EIA using the manufacturer's recommended standard curves.
Analytical summary.
The results of the Bland-Altman analysis obtained in the parallel evaluation of macaque and human plasmas for SP using extracted and unextracted samples analyzed by the two commercially available antigen competition EIA are summarized in Tables 2, 3, and 4. This analysis confirms the variability between the assay comparison methods and suggests lack of agreement, indicating that they should not be used interchangeably.
TABLE 2.
Summary of influences of specimen preparation and method of analysis on measurement of substance P in paired rhesus macaque and human plasmas; comparison of Cayman and Assay Designs EIA
| Specimen | n | Substance SP level (pg/ml) (mean ± SD)
|
95% CI of LOA and biasa | ||
|---|---|---|---|---|---|
| Cayman EIA | Assay Designs EIA | Mean difference | |||
| Macaque plasma (extracted) | 5 | 17.0 ± 5.7 | 132.2 ± 27.1 | −115.2 ± 22.8 | Biasb (−160.0, −70.4) |
| LOAc (−143.6, −86.8) | |||||
| Lower LOAd (−209.1, −110.8) | |||||
| Upper LOAd (−119.6, −21.3) | |||||
| Human serum (unextracted) | 5 | 58.6 ± 3.1 | 183.2 ± 51.2 | −124.7 ± 49.7 | Bias (−222.2, −27.2) |
| LOA (−186.4, −62.9) | |||||
| Lower LOA (−329.1, −115.2) | |||||
| Upper LOA (−134.2, 79.7) | |||||
95% CI bias = mean difference ± t critical value on n − 1 degrees of freedom × standard error of mean difference (SE).
95% CI LOA = mean difference ± 1.96 × standard deviation.
95% CI lower LOA = (mean difference − 1.96 × SD) ± t critical value on n − 1 degrees of freedom × 1.732 × SE; 95% CI upper LOA = (mean difference − 1.96 × SD) ± t critical value on n − 1 degrees of freedom × 1.732 × SE.
TABLE 3.
Summary of influences of specimen preparation and method of analysis on measurement of substance P in paired rhesus macaque and human plasmas; comparison of specimen treatments and sample dilutions in rhesus macaque plasmas using Cayman EIA
| n | Substance SP level (pg/ml) (mean ± SD)
|
Mean difference | 95% CI of LOA and biasa | |||
|---|---|---|---|---|---|---|
| Extracted | Unextracted | 1:2 Dilution | 1:4 Dilution | |||
| 18 | 12.7 ± 1.8 | 69.3 ± 21.6 | −56.5 ± 21.4 | Biasb (−67.2, −45.9) | ||
| LOAc (−98.6, −14.6) | ||||||
| Lower LOAd (−117.0, −80.1) | ||||||
| Upper LOAd (−33.0, 3.9) | ||||||
| 18 | 42.5 ± 12.6 | 69.3 ± 21.6 | −26.8 ± 16.7 | Bias (−35.1, −18.5) | ||
| LOA (−59.6, 6.0) | ||||||
| Lower LOA (−74.0, −45.2) | ||||||
| Upper LOA (−8.4, 20.4) | ||||||
95% CI bias = mean difference ± t critical value on n − 1 degrees of freedom × standard error of mean difference (SE).
95% CI LOA = mean difference ± 1.96 × SD.
95% CI lower LOA = (mean difference − 1.96 × SD) ± t critical value on n − 1 degrees of freedom × 1.732 × SE; 95% CI upper LOA = (mean difference + 1.96 × SD) ± t critical value on n − 1 degrees of freedom × 1.732 × SE.
TABLE 4.
Summary of influences of specimen preparation and method of analysis on measurement of substance P in paired rhesus macaque and human plasmas; comparison of standard curve definitions (in human plasma)
| Assay | n | Substance SP level (pg/ml) (mean ± SD)
|
95% CI of LOA and biasa | ||
|---|---|---|---|---|---|
| Recommended | User-modified | Mean difference | |||
| Cayman EIA | 5 | 58.6 ± 3.1 | 67.0 ± 5.7 | −8.4 ± 6.2 | Biasb (−16.1, −0.7) |
| LOAc (−20.5, 3.7) | |||||
| Lower LOAd (−33.8, −7.2) | |||||
| Upper LOAd (−9.6, 17.0) | |||||
| Assay Designs EIA | 5 | 183.2 ± 51.2 | 28.4 ± 5.8 | −154.9 ± 51.5 | Bias (−218.8, −91.0) |
| LOA (−255.8, −54.0) | |||||
| Lower LOA (−366.6, −145.1) | |||||
| Upper LOA (−164.7, 56.8) | |||||
95% CI bias = mean difference ± t critical value on n − 1 degrees of freedom × standard error of mean difference (SE).
95% CI LOA = mean difference ± 1.96 × SD.
95% CI lower LOA = (mean difference − 1.96 × SD) ± t critical value on n − 1 degrees of freedom × 1.732 × SE; 95% CI upper LOA = (mean difference + 1.96 × SD) ± t critical value on n − 1 degrees of freedom × 1.732 × SE.
DISCUSSION
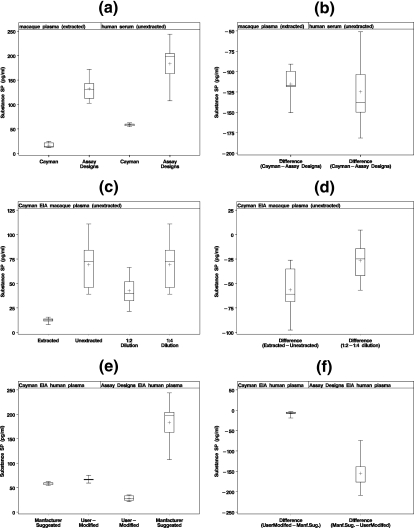
In view of the significant variability in the reported levels of SP in similar control materials, namely, human plasma and serum, the present study was initiated to identify potential variables (biological, methodological, or analytical) that might contribute to the observed differences. In addition to comparison of the performances of two similar EIA for the measurement of SP in human and rhesus macaque plasmas, a comparison of the influence of sample preparation was also evaluated. These studies found that procedural differences between two very similar assays can contribute to significant differences in SP values in the parallel evaluation of plasma and serum. Figure 1a and b illustrates the results obtained in the parallel measurement of SP in extracted macaque plasma and unextracted human plasma using two similar antigen competition EIA. The two assays yielded significant mean SP difference values. The mean SP value obtained from unextracted normal human plasma using the Assay Designs EIA (Fig. 1a) is similar to that reported by Bondy et al. (4) (216.87 ± 81.9 pg/ml), who used the same Assay Designs EIA in the evaluation of human sera. Studies using similar extraction methods and the Cayman EIA for the measurement of SP have consistently yielded significantly lower mean SP values than studies using the Assay Designs EIA (Table 1).
FIG. 1.
(a and b) Parallel evaluation of five extracted rhesus macaque plasma and unextracted human plasma samples for SP using two different SP antigen competition EIA kits. (c and d) Comparison of SP levels in 18 paired extracted and unextracted plasma samples (1:4 dilution prior to analysis) and the influence of sample dilution prior to analysis in rhesus macaque plasma using the Cayman SP EIA. (e and f) Influence of modification of the standard curve on SP values using the Cayman EIA and the Assay Designs EIA in the evaluation of unextracted human plasma at a 1:4 dilution. The upper and lower whiskers of the box-and-whisker plots are the maximum and minimum SP values. The first and third quartiles (25th and 75th percentiles) are represented by the lower and upper edges of the box, respectively. The mean is represented by the plus sign, and the median (50th percentile) is the line inside the box.
The observed differences in SP levels in the parallel evaluation of plasmas from both healthy human subjects and rhesus macaques using two commercially available EIA could be attributable to qualitative differences in the reagents used in the assays, procedural differences of the two assays, or differences in the analytical treatment of data. Since both methods are antigen competition-based assays that use similar capture and probe antibodies, as well as tracers, and both recommend four-parameter logistic fit analyses of data, it was felt that the most likely source for the observed differences was in the formulation of the standard curves used to interpolate unknown values. This assumption was based on the extraordinary differences in the measurable-concentration ranges for the two assays, namely, 3.9 to 500 pg/ml and 9.76 to 10,000 pg/ml for the Cayman assay and Assay Designs assay, respectively. To test this hypothesis using reagents supplied with each kit, identical standard curves were formulated for each assay system to incorporate several data points between 15.6 pg/ml and 250 pg/ml that reflect the range of SP values most often reported in the literature for control human serum and plasma specimens. Unextracted control human plasmas evaluated with both assay systems according to the manufacturer's recommendations (Fig. 1a and b) were reevaluated using identical standard curves formulated with reagents from each assay system. Similar but statistically different results (mean difference, −8.4 ± 6.2) were obtained with the Cayman EIA compared to values obtained using the manufacturer's recommended standard curve in the evaluation of control human plasma (Fig. 1e and f). In contrast, the Assay Design EIA gave very different results (mean difference, −154.9 ± 51.5) (Fig. 1e and f). These findings raise questions about the accuracy of such a broad standard curve in an antigen competition assay when most clinical-specimen values are interpolated from the extreme lower end of the curve. To address the possibility of significant qualitative and/or quantitative differences in each assay's SP standard, known concentrations (based on the manufacturer's labeling) of each standard were evaluated in the other EIA. As was found with unknown samples, the observed values obtained with the Cayman SP standard when evaluated with the Assay Designs EIA were considerably higher than the expected values (Fig. 2), while the observed values obtained with the Assay designs SP standard when evaluated in the Cayman EIA were considerably lower than the expected values. Although these data suggest possible qualitative and/or quantitative differences in the two SP standards, possible differences in the analytical interpolation (based on four-parameter logistic fit) of values from standard curves that differ significantly in their measurable ranges may also contribute to the observed discrepancies.
In addition to methodological factors contributing to significant differences in observed SP levels, differences in sample preparation also played a role in contributing to discrepant results. Extracted samples reproducibly yielded lower SP values than did unextracted samples (Fig. 1c and d). These data support the observations of Corbally et al. (10), which found SP to exist in serum and plasma in free and bound states, with a majority of SP bound in a reversible manner to high- and intermediate-molecular-weight serum and plasma proteins. Using radiolabeled SP and glutaraldehyde cross-linking techniques, Corbally et al. (10) found that SP bound to both high-molecular-weight proteins and human serum albumin. This interaction between SP and serum and plasma proteins was weak, as the bound SP was easily dissociated by repeated gel filtration. This finding suggests the possibility of SP binding reversibly and nonspecifically to plasma or serum proteins via weak hydrogen bonding. Further support of these observations lies in the ability to recover additional SP from serum or plasma by diluting samples prior to evaluation by EIA (Fig. 1c and d). Serial dilution of the sample prior to introduction of a constant amount of probe antibody effectively increases the ratio of probe antibody to serum or plasma proteins competing for available SP, thus enhancing the possibility of antibody-mediated capture of SP, an interaction with much higher avidity than simple nonspecific binding, as previously described by Corbally et al. (10).
Our study demonstrates that a number of factors can have significant impacts on the measurement of SP in both serum and plasma. These findings should be considered in the selection of the conditions needed to optimally and accurately evaluate SP levels in both serum and plasma. Commercially available EIA kits do not give comparable results when used to assay SP in plasma using the recommended protocols. These differences can be reduced by using identical standard curves that more closely encompass the SP levels found in both sera and plasma. These findings indicate that one must carefully consider the experimental design when interpreting quantitative SP data reported in the literature. The results of our study indicate that there is no benefit to the extraction of serum or plasma for the recovery of SP. Further, dilution of unextracted serum or plasma (collected in EDTA) prior to analysis enhances the recovery of SP due to its reversible and nonspecific interaction with serum and plasma proteins. Antigen competition assays for the measurement of SP should make use of standard curves for the analytical interpolation of unknown values that have measurable ranges that closely encompass the anticipated SP levels of the specimens to be evaluated and should not exceed 2 log units of concentration range.
In order to improve upon those conditions found in the present study to more accurately quantify SP in unextracted plasma, additional studies are planned to investigate the influence of mild dissociation of hydrogen bonds on SP recovery in unextracted plasma. Such conditions should not only improve recovery, but also improve sensitivity by not requiring dilution of specimens prior to analysis.
Acknowledgments
This study was supported by NIH grants MH49981 and MH76388 to S.D.D. and by Public Health Service Grant RR00164 to A.A.L.
We thank Stephen Jasionowski for editorial assistance.
Footnotes
Published ahead of print on 13 September 2006.
REFERENCES
- 1.Anichini, M., S. Cesaretti, M. Lepori, S. Maddali Bongi, M. Maresca, and M. Zoppi. 1997. Substance P in the serum of patients with rheumatoid arthritis. Rev. Rhum. Engl. Ed. 64:18-21. [PubMed] [Google Scholar]
- 2.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 3.Bland, J. M., and D. G. Altman. 2003. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet. Gynecol. 22:85-93. [DOI] [PubMed] [Google Scholar]
- 4.Bondy, B., T. C. Baghai, C. Minov, C. Schule, J. M. Schwarz, P. Zwanzger, R. Rupprecht, and J.-H. Moller. 2003. Substance P serum levels are increased in major depression: preliminary results. Biol. Psychiatry 53:538-542. [DOI] [PubMed] [Google Scholar]
- 5.Bost, K. L. 2004. Tachykinin-mediated modulation of the immune response. Front. Biosci. 9:3331-3332. [DOI] [PubMed] [Google Scholar]
- 6.Bozic, C. R., B. Lu, U. E. Hopken, C. Gerard, and N. P. Gerard. 1996. Neurogenic amplification of immune complex inflammation. Science 273:1722-1725. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, W. B., O. B. Holland, C. E. Gomez-Sanchez, R. M. Graham, W. A. Pettinger, and A. C. White. 1981. Plasma substance P levels in normotensive and hypertensive subjects. Clin. Exp. Hypertens. 3:183-193. [DOI] [PubMed] [Google Scholar]
- 8.Chang, M. M., S. E. Leeman, and H. D. Niall. 1971. Amino acid sequence of substance P. Nat. New Biol. 232:86-87. [DOI] [PubMed] [Google Scholar]
- 9.Clark, J. W., P. D. Senanayake, G. D. Solomon, and C. Gallagher. 1994. Substance P: correlation of CSF and plasma levels. Headache 34:261-264. [DOI] [PubMed] [Google Scholar]
- 10.Corbally, N., D. Powell, and K. F. Tipton. 1990. The binding of endogenous and exogenous substance-P in human plasma. Biochem. Pharmacol. 39:1161-1166. [DOI] [PubMed] [Google Scholar]
- 11.Dionne, R. A., S. M. Max, S. M. Gordon, S. Parada, C. Sang, R. H. Gracly, N. F. Sethna, and D. B. MacLean. 1998. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin. Pharmacol. Ther. 64:562-568. [DOI] [PubMed] [Google Scholar]
- 12.Douglas, S. D., W.-Z. Ho, D. R. Gettes, A. Cnaan, H. Zhao, J. Leserman, J. M. Petitto, R. N. Golden, and D. L. Evans. 2001. Elevated substance P levels in HIV-infected men. AIDS 15:2043-2045. [DOI] [PubMed] [Google Scholar]
- 13.Faulhaber, H. D., R. Rathsack, G. Rostock, V. Homuth, D. Pfeiffer, E. Naumann, W. Hartrodt, R. C. Gorne, and P. Oehme. 1983. Evidence of decreased plasma substance P levels in human essential hypertension and influence of prazosin treatment. Biomed. Biochim. Acta 42:1019-1025. [PubMed] [Google Scholar]
- 14.Fehder, W. P., W.-Z. Ho, D. E. Campbell, W. W. Tourtellotte, L. Michaels, J. R. Cutilli, M. Uvaydova, and S. D. Douglas. 1998. Development and evaluation of a chromatographic procedure for partial purification of substance P with quantitation by an enzyme immunoassay. Clin. Diagn. Lab. Immunol. 5:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehder, W. P., J. Sachs, M. Uvaydova, and S. D. Douglas. 1997. Substance P as an immune modulator of anxiety. Neuroimmunomodulation 4:42-48. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Rodriguez, C. M., J. Prieto, J. Quiroga, J. M. Zozoya, A. Andrade, M. Nunez, B. Sangro, and J. Penas. 1995. Plasma levels of substance P in liver cirrhosis: relationship to the activation of vasopressor systems and urinary sodium excretion. Hepatology 21:35-40. [DOI] [PubMed] [Google Scholar]
- 17.Ho, W.-Z, and S. D. Douglas. 2004. Substance P and neurokinin-1 receptor modulation of HIV. Neuroimmunology 157:48-55. [DOI] [PubMed] [Google Scholar]
- 18.Hortnagl, H., E. A. Singer, K. Lenz, G. Klienberger, and H. Lochs. 1984. Substance P is markedly increased in plasma of patients with hepatic coma. Lancet i:480-483. [DOI] [PubMed] [Google Scholar]
- 19.Kramer, H. J., R. Dusing, H. Stelkens, R. Heinrich, J. Kipnowski, and K. Glanzer. 1980. Immunoreactive substance P in human plasma: response to changes in posture and sodium balance. Clin. Sci. 59:75-77. [DOI] [PubMed] [Google Scholar]
- 20.Kramer, M. S., N. Cutler, J. Feighner, R. Shrivastava, J. Carman, J. J. Sramek, S. A. Reines, G. Liu, D. Snavely, E. Wyatt-Knowles, J. J. Hale, S. G. Mills, M. MacCoss, C. J. Swain, T. Harrison, R. G. Hill, F. Hefti, E. M. Scolnick, M. A. Cascieri, G. G. Chicchi, S. Sadowski, A. R. Williams, L. Hewson, D. Smith, E. J. Carlson, R. J. Hargreaves, and N. M. J. Rupniak. 1998. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281:1640-1645. [DOI] [PubMed] [Google Scholar]
- 21.Kukuvitis, A., A. Kourtis, N. Papaiconomou, V. Zournatzi, G. Makedos, and D. Panidis. 2003. Differential effects of unopposed versus opposed hormone therapy, tibolone, and raloxifene on substance P levels. Fertil. Steril. 80:96-98. [DOI] [PubMed] [Google Scholar]
- 22.Kunt, T., T. Forst, S. Schmidt, A. Pfutzner, S. Schneider, O. Harzer, M. Lobig, M. Engelbach, K. Goitom, T. Pohlmann, and J. Beyer. 2000. Serum levels of substance P are decreased in patients with type 1 diabetes. Exp. Clin. Endocrinol. Diabetes 108:164-167. [DOI] [PubMed] [Google Scholar]
- 23.Lee, F. Y., H. C. Lin, Y. T. Tsai, F. Y. Chang, R. H. Lu, M. C. Hou, C. P. Li, C. J. Chu, S. S. Wang, and S. D. Lee. 1997. Plasma substance P levels in patients with liver cirrhosis: relationship to systemic and portal hemodynamics. Am. J. Gastroenterol. 92:2080-2084. [PubMed] [Google Scholar]
- 24.Lotti, T., G. Hautmann, and E. Panconesi. 1995. Neuropeptides in skin. J. Am. Acad. Dermatol. 33:482-496. [DOI] [PubMed] [Google Scholar]
- 25.Mantyh, C. R., T. S. Gates, R. P. Zimmerman, M. L. Welton, E. P. Passaro, Jr., S. R. Vigna, J. E. Maggio, L. Kruger, and L. W. Mantyh. 1988. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc. Natl. Acad. Sci. USA 85:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall, K. W., B. Chiu, and R. D. Inman. 1990. Substance P and arthritis: analysis of plasma and synovial fluid. Arthritis Rheum. 33:87-90. [DOI] [PubMed] [Google Scholar]
- 27.McGillis, J. P., M. Mitsuhashi, and D. G. Payan. 1990. Immunomodulation by tachykinin neuropeptides. Ann. N. Y. Acad. Sci. 594:85-94. [DOI] [PubMed] [Google Scholar]
- 28.McLean, S. 2005. Do substance P and the NK1 receptor have a role in depression and anxiety. Curr. Pharm. Des. 11:1529-1547. [DOI] [PubMed] [Google Scholar]
- 29.Michaels, L. A., K. Ohene-Frempong, H. Zhao, and S. D. Douglas. 1998. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood 92:3148-3151. [PubMed] [Google Scholar]
- 30.Navari, R. M., R. R. Reinhardt, R. J. Gralla, M. G. Kris, P. J. Hesketh, A. Khojasteh, H. Kindler, T. H. Grote, K. Pendergrass, S. M. Grunberg, A. D. Carides, B. J. Gertz, et al. 1999. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. N. Engl. J. Med. 340:190-195. [DOI] [PubMed] [Google Scholar]
- 31.Nicoll, R. A., C. Schenker, and S. E. Leeman. 1980. Substance P as a neurotransmitter candidate. Annu. Rev. Neurosci. 3:227-268. [DOI] [PubMed] [Google Scholar]
- 32.Otsuka, M., and T. Takahashi. 1977. Putative peptide neurotransmitters. Annu. Rev. Pharmacol. Toxicol. 17:425-439. [DOI] [PubMed] [Google Scholar]
- 33.Otsuka, M., and K. Yoshioka. 1993. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 73:229-308. [DOI] [PubMed] [Google Scholar]
- 34.Palma, C., and C. A. Maggi. 2000. The role of tachykinins via NKg1 receptors in progression of human gliomas. Life Sci. 67:985-1001. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds, W. J., B. Chiu, and R. D. Inman. 1988. Plasma substance P levels in fibrositis. J. Rheumatol. 15:1802-1803. [PubMed] [Google Scholar]
- 36.Rissler, K. 1995. Sample preparation, high-performance liquid chromatographic separation and determination of substance P-related peptides. J. Chromatogr. B 665:233-270. [DOI] [PubMed] [Google Scholar]
- 37.Rupniak, N. M. J. 2002. New insights into the antidepressant actions of substance P (NK-1 receptor) antagonists. Can. J. Physiol. Pharmacol. 80:489-494. [DOI] [PubMed] [Google Scholar]
- 38.Singh, D., D. D. Joshi, M. Hameed, J. Qian, P. Gascon, B. P. Maloof, A. Mosenthal, and P. Rameshwar. 2000. Increased expression of preprotachykinin-1 and neurokinin receptors in human breast cancer cells: implications for bone marrow metastasis. Proc. Natl. Acad. Sci. USA 97:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripp, R. A., A. Barskey, L. Goss, and L. J. Anderson. 2002. Substance P receptor expression on lymphocytes is associated with the immune response to respiratory syncytial virus infection. J. Neuroimmunol. 129:141-153. [DOI] [PubMed] [Google Scholar]
- 40.Weinstock, J. V., A. Blum, A. Metwali, D. Elliott, and R. Arsenescu. 2003. IL-18 and IL-12 signal through the NF-kappa B pathway to induce NK-1R expression on T cells. J. Immunol. 170:5003-5007. [DOI] [PubMed] [Google Scholar]