Abstract
We evaluated a one-step sandwich-format microplate enzyme immunoassay for detecting dengue virus NS1 antigen (Ag) in human serum by use of Platelia Dengue NS1 Ag kits (Bio-Rad Laboratories, Marnes La Coquette, France). We collected 299 serum samples from patients with dengue disease and 50 serum samples from patients not infected with dengue virus. For the 239 serum samples from patients with acute infections testing positive by reverse transcription-PCR and/or virus isolation for one of the four dengue virus serotypes, the sensitivity of the Platelia Dengue NS1 Ag kit was 88.7% (95% confidence interval, 84.0% to 92.4%). None of the serum samples from patients not infected with dengue virus tested positive with the Platelia Dengue NS1 Ag kit. A diagnostic strategy combining the Platelia Dengue NS1 Ag test for acute-phase sera and immunoglobulin M capture enzyme-linked immunosorbent assay for early-convalescent-phase sera increased sensitivity only from 88.7% to 91.9%. Thus, NS1 antigen detection with the Platelia Dengue NS1 Ag kit could be used for first-line testing for acute dengue virus infection in clinical diagnostic laboratories.
Dengue fever is a viral disease transmitted by mosquitoes and caused by four serotypes of dengue virus (DEN): DEN-1, DEN-2, DEN-3, and DEN-4. Dengue fever is the most important arthropod-borne viral disease affecting humans, and according to World Health Organization (WHO) estimates, its incidence has increased by a factor of 30 over the last 50 years (21). It occurs in tropical areas and affects up to 100 million people each year, including 500,000 cases of dengue hemorrhagic fever and around 30,000 deaths, mostly among children. It is now endemic in more than 100 countries (the Americas, the eastern Mediterranean, Southeast Asia, and the western Pacific) and poses a threat to more than 2.5 billion people (5). The African continent seems less affected by the dengue virus.
Dengue viruses are transmitted to humans by Stegomyia aegypti (formerly Aedes aegypti) mosquitoes and cause a wide range of symptoms, from unapparent or mild disease (dengue fever) to a severe hemorrhagic form (dengue hemorrhagic fever) (4). In a small percentage of cases, they may also cause hepatitis (9) or encephalitis (8, 15).
Dengue viruses belong to the Flavivirus genus of the Flaviviridae family. Flaviviruses are enveloped, single-strand RNA viruses. The genomic RNA is approximately 11 kb in size and encodes three structural proteins: C (core protein), M (membrane protein), and E (envelope protein). The genomic RNA is translated to generate a large polyprotein precursor, which is cotranslationally processed by host cell- and virus-encoded proteases to yield the individual viral proteins. The nonstructural (NS) proteins are NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5 (3). NS1 is a highly conserved glycoprotein that seems to be essential for virus viability but has no established biological activity. Unusually for a viral glycoprotein, NS1 is produced in both membrane-associated and secreted forms. Enzyme-linked immunosorbent assays (ELISA) directed against the NS1 antigen have demonstrated that this antigen is present at high concentrations in the sera of dengue virus-infected patients during the early clinical phase of the disease (1, 24).
Dengue virus infection is currently detected by means of several biological tests: virus isolation on mosquito cells (7), viral RNA detection by reverse transcription-PCR (RT-PCR) (6, 12), or serological tests, such as immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay (MAC-ELISA) (2, 11, 20). RT-PCR remains expensive, and its routine use in clinical diagnostic laboratories is difficult. Commercial MAC-ELISA kits are available, but they cannot be used for early diagnosis, because IgM does not become detectable until 5 to 10 days after the onset of illness in cases of primary dengue virus infection and until 4 to 5 days after the onset of illness in secondary infections (22). In this study, we evaluated a new diagnostic tool for acute dengue virus infection, based on an enzyme immunoassay for detecting dengue virus NS1 antigen (Ag) in human serum, using the Platelia Dengue NS1 Ag kit (Bio-Rad Laboratories, Marnes La Coquette, France).
MATERIALS AND METHODS
Clinical samples.
Serum samples from the collection of the Centre National de Reference des Arbovirus et virus influenza (the French National Reference Center for Arboviruses and Influenza Viruses), Région Antilles-Guyane (the CNR), based at the Institut Pasteur de la Guyane, were used for this study. The database provided information about the age and sex of the patient, the date of serum collection, and the date on which symptoms occurred (onset of fever was taken as day 0, i.e., first 24 h).
We tested 369 sera for NS1 antigen with the Platelia Dengue NS1 Ag kit. The sera tested included (i) 42 cases of DEN type 1 infection, 43 of DEN type 2 infection, and 109 of DEN type 3 infection occurring during outbreaks in French Guiana between 1997 and 2005, (ii) 49 cases of DEN type 4 infection from the 2005 outbreak in the French West Indies, and (iii) 56 nonserotyped serum samples containing dengue virus IgM antibody taken from patients (5 acute-phase serum samples collected on days 3 and 4 of infection and 51 early-convalescent-phase serum samples collected between 5 and 10 days after the onset of fever). Two additional groups of serum samples were also tested: (i) 50 acute-phase serum samples (days 1 to 4) from patients presenting dengue-like syndrome (temperature of ≥38.5°C, arthralgia, headache, and/or myalgia) for whom recent dengue virus infection was ruled out, as confirmed with a second blood sample, and (ii) 20 serum samples from patients with yellow fever, provided by the WHO Collaborating Center for Arbovirus Reference and Research at Instituto Evandro Chagas, Belém, Brazil (four sera collected before day 5, seven sera collected between days 5 and 10, two sera collected after day 10, and seven sera collected at unknown times). The diagnosis of yellow fever was based on the IgM detection system routinely used in this laboratory (10).
The mean age of the study population was 33 years (range, 17 to 49 years), and the sex ratio (male/female) was 1.3. Yellow fever vaccination status was known for 208 of the 303 individuals living in French Guiana, where yellow fever vaccination is mandatory: 203 (97.5%) had been vaccinated against yellow fever. Serum samples from patients infected with dengue virus were divided into two groups: serum samples collected during the acute phase (day 0 to day 4) and sera collected during early convalescence (day 5 to day 10). All samples tested were collected from patients with clinical symptoms of dengue fever (fever, headache, myalgia, and/or arthralgia) with or without a rash and minor hemorrhagic manifestations. No patient presented severe clinical symptoms of dengue hemorrhagic fever/dengue shock syndrome.
Dengue virus was isolated from an Aedes pseudoscutellaris cell culture (AP61) and/or detected by RT-PCR, as previously described, in acute-phase serum samples (12, 16). Acute-phase and early-convalescent-phase serum samples were tested for IgM antibodies against dengue virus by use of MAC-ELISA, as previously described (19).
The Platelia Dengue NS1 Ag test is a one-step sandwich-format microplate enzyme immunoassay for detecting dengue virus NS1 antigen in human serum or plasma. It makes use of murine monoclonal antibodies for capture and detection. Tests were carried out on samples from human patients and controls according to the manufacturer's recommendations. Briefly, 50 μl of the sample or control serum was incubated directly and simultaneously with 50 μl of diluent and 100 μl of diluted conjugate for 90 min at 37°C in microplate wells sensitized with anti-NS1 monoclonal antibodies. The plate was washed, and immune complexes were detected using a color development reaction. After 30 min of incubation at room temperature, the enzymatic reaction was stopped by adding sulfuric acid. Optical density (OD) was determined at 450/620 nm and was proportional to the amount of NS1 antigen present in the sample. The decision as to whether NS1 antigen was considered to be present or absent in an individual sample was based on comparisons of the OD for the sample with that of the cutoff control serum.
The cutoff value (CO) corresponded to the mean OD values for the cutoff control duplicates provided in the kit. Sample results were expressed as a ratio, using the following formula: sample ratio = S/CO, where S is the OD obtained for the sample. All sera were tested in a single well. The technicians carrying out the NS1 Dengue test were blind to the results of RT-PCR and/or viral isolation and MAC-ELISA. In cases of discordance with dengue diagnosis results (virological and/or serological results), the sample was retested.
According to the manufacturer's recommendations, samples were considered (i) nonreactive for dengue virus NS1 antigen if this ratio was less than 0.5, (ii) equivocal for dengue virus NS1 antigen if this ratio was in the range of 0.5 to 1.0, and (iii) reactive for dengue virus NS1 antigen if a ratio of 1.0 or more was obtained.
Statistical analysis was performed with STATA, version 8.0 (STATA Corporation, College Station, TX), and figures were drawn with Sigmaplot software (version 8.0 for Windows; SPSS Science, Chicago, IL).
RESULTS
Of the 243 samples testing positive for dengue virus by RT-PCR and/or virus isolation, 212 (87.2%) tested positive with the Platelia Dengue NS1 Ag test. However, four sera (1.7%) were considered equivocal: one of the DEN-2 serotype (ratio of 0.549), two of the DEN-3 serotype (ratios of 0.560 and 0.752), and one of the DEN-4 serotype (ratio of 0.742). These serum samples were collected 0, 1 (n = 2), and 2 days after the onset of fever. All four samples identified as equivocal by Platelia Dengue NS1 Ag testing were IgM negative by MAC-ELISA and were excluded from further analysis. Table 1 shows the proportions of samples testing positive by the Platelia Dengue NS1 Ag test according to serotype. The total sensitivity was 88.7% (212/239; 95% confidence interval [95% CI], 84.0% to 92.4%). No significant difference was observed between the four dengue virus serotypes (χ2 = 0.51, P = 0.91). The sensitivity of dengue virus NS1 antigen detection with respect to RT-PCR was 85.0% (113/133; 95% CI, 77.7% to 90.6%), and that with respect to viral culture was 94.1% (143/152; 95% CI, 89.1% to 97.3%). Table 2 shows the sensitivity of the Platelia Dengue NS1 Ag test as a function of the timing of the test (days after onset of fever) and the sensitivity of the dengue MAC-ELISA. For the NS1 antigen assay, sensitivity was optimal, at 87.6% (95% CI, 82.3% to 91.7%), between days 0 and 4, whereas the sensitivity of the MAC-ELISA during this period was only 8.6% (95% CI, 5.2% to 13.3%). Data concerning the timing of the test were missing for 29 samples testing positive by the Platelia Dengue NS1 Ag test.
TABLE 1.
Platelia Dengue NS1 Ag test sensitivity according to dengue virus serotype detected by RT-PCR and/or virus isolation (n = 239)
| DEN serotype | No. of sera tested | No. of positive NS1 Ag tests | Sensitivity (%) | 95% CI |
|---|---|---|---|---|
| DEN-1 | 42 | 38 | 90.5 | 77.4-97.3 |
| DEN-2 | 42 | 37 | 88.1 | 74.4-96.0 |
| DEN-3 | 107 | 94 | 87.9 | 80.1-93.4 |
| DEN-4 | 48 | 43 | 89.6 | 77.3-96.5 |
| All serotypes | 239 | 212 | 88.7 | 84.0-92.4 |
TABLE 2.
Sensitivities of the Platelia Dengue NS1 Ag test and of the MAC-ELISA related to number of days after onset of fever (n = 266)
| Days after onset of fevera | No. of sera tested | Platelia Dengue NS1 Ag test
|
MAC-ELISA
|
||||
|---|---|---|---|---|---|---|---|
| No. of positive tests | Sensitivity (%) | 95% CI | No. of positive ELISA | Sensitivity (%) | 95% CI | ||
| 0 | 15 | 15 | 100.0 | 78.2-100.0b | 0 | 0.0 | 0.0-23.2b |
| 1 | 70 | 61 | 87.1 | 77.0-93.9 | 1 | 1.4 | 0.0-7.6 |
| 2 | 59 | 54 | 91.5 | 81.3-97.2 | 3 | 5.1 | 1.1-14.2 |
| 3 | 40 | 34 | 85.0 | 70.2-94.3 | 6 | 15.0 | 5.7-29.8 |
| 4 | 25 | 19 | 76.0 | 54.9-90.6 | 8 | 32.0 | 15.0-53.5 |
| 5 | 19 | 8 | 42.1 | 20.3-66.5 | 16 | 84.2 | 60.4-96.6 |
| 6 | 18 | 17 | 94.4 | 75.7-99.8 | 18 | 100.0 | 81.0-100.0b |
| ≥7 | 20 | 9 | 45.0 | 23.1-68.5 | 20 | 100.0 | 83.1-100.0b |
| Total | 266c | 217 | 81.6 | 76.4-86.1 | 72 | 26.7 | 21.8-32.8 |
Onset of fever is defined as day 0 if blood samples were collected within the first 24 h following the onset of fever.
One-sided, 97.5% confidence interval.
Patients with a known date of fever onset.
The 50 serum samples from patients not infected with dengue virus and the 20 serum samples from patients with recent yellow fever infection all tested negative with the Platelia Dengue NS1 Ag test. The specificity of the Platelia Dengue NS1 Ag test was 100% (97.5% CI, 84.9% to 100%, one-sided).
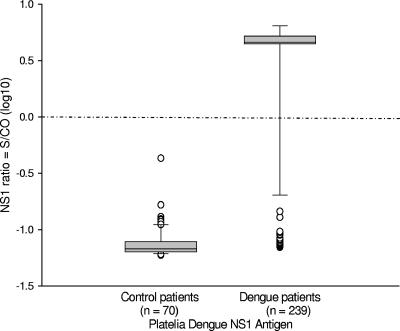
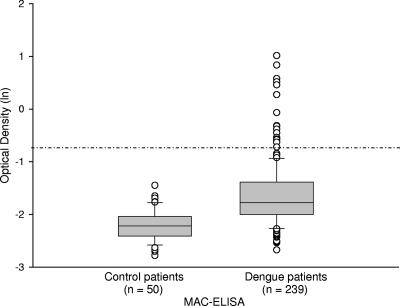
An analysis of the Platelia Dengue NS1 Ag test ratio distribution between control and dengue fever patients indicates strong, clear discrimination between positive and negative serum samples (Fig. 1). A similar analysis for MAC-ELISA distribution demonstrated that IgM serological testing was less useful (Fig. 2).
FIG. 1.
Platelia Dengue NS1 Ag test results for control patients and for dengue-positive patients (confirmed by RT-PCR and/or viral culture) are shown. The y axis indicates ratio = S/CO, where S is the obtained OD and CO is the mean cutoff value of the OD for the cutoff control duplicates provided in the kit. In this figure, the ratio values are expressed as log10 values and the dashed-dotted line indicates the cutoff value, set to 1 (giving 0 following the logarithmic transformation used to plot the figure). The box indicates statistical values. The boundary of the box closest to the x axis indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from the x axis indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles. The outlying points (○) have also been plotted on the figures.
FIG. 2.
MAC-ELISA results for control patients and for dengue patients (confirmed by RT-PCR and/or viral culture) are shown. The y axis indicates the OD values, expressed as natural logarithms (ln), and the dashed-dotted line indicates the cutoff value of the assay, set to 0.5 (giving −0.69 after natural log transformation). The ODs for dengue MAC-ELISA were not available for the serum samples from the 20 patients with recent yellow fever. Box plot details are described in the legend for Fig. 1.
A diagnostic strategy combining Platelia Dengue NS1 Ag testing of serum samples collected within 5 days of the onset of fever and MAC-ELISA for serum samples collected in the early convalescent phase would potentially make it possible to diagnose 91.9% (271/295) of dengue virus infections (95% CI, 88.1% to 94.7%). In our study, the efficiency of this diagnostic strategy is estimated at 93.4% [(271 + 70)/(295 + 70)], with a 95% CI of 90.4% to 95.7%.
DISCUSSION
This study confirms that the detection of dengue virus NS1 antigen in Platelia Dengue NS1 Ag tests is useful for the rapid, early biological diagnosis of dengue disease. Serological MAC-ELISA are currently routinely used for dengue fever diagnosis (2, 22); however, as shown in Table 2, the sensitivity of this test does not become acceptable until 5 days after the onset of fever. Virus isolation is carried out only by reference laboratories and is a time-consuming and expensive technique. The use of dengue RT-PCR is now well documented (6, 12, 14, 18). However, its use in most laboratories is currently difficult, due largely to the stringent requirements concerning storage temperature, transportation, time between collection and extraction, and laboratory workflow. These two techniques are therefore largely restricted to surveillance systems and research.
Alcon et al. reported that the NS1 antigen was found circulating from the first day after the onset of fever up to day 9: NS1 levels ranged from 0.04 to 2 μg/ml in acute-phase serum samples (from day 0 to 7), and the level for a convalescent-phase serum (day 8 and later) was 0.04 μg/ml. In secondary infection, NS1 levels ranged from 0.01 to 2 μg/ml and were not detectable for convalescent-phase sera (1). Shu et al. reported data from acute-phase sera with either primary or secondary infection that were in agreement with those of Alcon et al. Moreover, their data suggested that the NS1 antigen was detectable during days 1 to 8 of illness (17). A third recent study showed that the NS1 antigen, limited to dengue virus serotype 1, could be detected until day 18 after the onset of symptoms. NS1 antigen and IgM antibodies were detected concomitantly during the acute phase, but from day 1 to day 3, NS1 antigen showed a more sensitive detection (23). According to these studies and to our results, the presence of NS1 in human sera can be confirmed between days 0 and 9 (with day 0 corresponding to the onset of fever), whereas IgM antibodies do not begin to appear until the early convalescent phase (Table 2).
The positive detection of NS1 at the onset of fever, together with other clinical symptoms, reflects the known onset of clinical symptoms about 5 days after dengue virus infection (4). The low sensitivity obtained on day 5 in our study may be linked to poor reporting for this variable (date of testing) in the database. Although not demonstrated, we cannot rule out the possibility that immune complexes begin to form from day 5 onwards. In this context, the use of NS1 detection as a first-line test for the diagnosis of acute dengue virus infection, using serum samples collected during the onset of clinical symptoms (day 0 to day 4), could help to accelerate the diagnosis of dengue fever in clinical diagnostic laboratories. The use of a combination of MAC-ELISA and NS1 antigen detection for early-convalescent-phase serum samples (days 5 to 10) could increase the overall clinical sensitivity of both assays. It should be noted that a positive result for the MAC-ELISA was used as an inclusion criterion for serum samples from dengue patients; this accounts for the MAC-ELISA sensitivity for these samples reaching 100%, due to the “serological” bias in favor of serological sensitivity.
The Platelia Dengue NS1 Ag assay is a one-step microplate enzyme immunoassay. It is simple, rapid, subject to quality assurance, and robust, based on strong discrimination between positive and negative samples. It would be easy to implement this test in any clinical diagnostic laboratory equipped with an incubator and an ELISA reader. Automation should facilitate the testing of large numbers of samples over periods of time useful for physicians.
Physicians using this proposed acute and early convalescence diagnostic strategy on inpatients and outpatients should be able to obtain rapid, specific dengue diagnosis within a few hours for acute-phase samples, but such diagnoses should also be possible for patients presenting at a late stage of the disease. With the expansion of the geographic range of dengue fever and the increasing number and severity of reported cases, this strategy could allow clinical diagnostic laboratories to identify dengue virus infections early enough to adjust patient management, reducing the time between detection of the first cases, based on NS1 antigen detection, and the notification of public health authorities, including vector control teams. However, reference laboratories will continue to use virus isolation and/or RT-PCR to confirm the outbreak, to identify the circulating dengue virus serotypes, and to test serum samples from suspected cases of dengue fever for which negative results were obtained for NS1 antigen detection.
One limitation of this study is that we did not used paired sera, and this lack of serological data made it impossible to distinguish between primary and secondary dengue virus infections. We were also unable to analyze the NS1 antigen as a potential early marker of severe dengue disease, because we did not include patients with severe dengue disease (dengue hemorrhagic fever/dengue shock syndrome) and because the current Platelia Dengue NS1 Ag test is not a quantitative test. However, a previous study has demonstrated that the level of NS1 of dengue-2 virus in plasma was correlated with viremia levels and was significantly higher in patients with dengue hemorrhagic fever than in patients with dengue fever within 72 h of illness onset (13). The development of a quantitative test of NS1 could probably be of interest as a prognosis factor in the future.
Yellow fever still occurs in Latin America, despite the tremendous efforts invested in the immunization program, and cross-reactive antibody responses may be developed, resulting in a risk of high levels of false-positive serological results with current assays. However, the NS1 antigen was not detected in any of the 20 patients with recent yellow fever tested in this study. These findings should be confirmed with other acute-phase sera from patients with yellow fever and validated using sera from patients with Japanese encephalitis infections in Asia.
In conclusion, this evaluation of the new dengue diagnostic tool Platelia Dengue NS1 Ag test indicates that this test is highly appropriate to field testing conditions, particularly for early-acute-phase samples, as its sensitivity and specificity far exceed those of the MAC-ELISA for such samples. NS1 antigen detection has great potential value for use in epidemic situations, as it could facilitate the early screening of patients and limit disease expansion. Prospective studies should be carried out to confirm the clinical value of this new test. The development of a new generation of NS1 antigen type-specific assays should be considered in the near future.
Acknowledgments
Platelia Dengue NS1 Ag kits were provided by Bio-Rad Laboratories, Marnes La Coquette, France. We thank Marc Tabouret, Patrice Sarfati, and Christophe Salanon (Bio-Rad Laboratories) for helpful discussions. We also thank Jacques Rosine, Pascal Chaud, Thierry Cardoso, Alain Blateau, and Philippe Quénel from the CIRE (Cellule Inter Régional d'Epidémiologie—Antilles Guyane) and the CVS (Cellule de Veille Sanitaire) from Martinique, Guadeloupe, and French Guiana for the assistance given to the CNR.
Footnotes
Published ahead of print on 20 September 2006.
REFERENCES
- 1.Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blacksell, S. D., P. N. Newton, D. Bell, J. Kelley, M. P. Mammen, Jr., D. W. Vaughn, V. Wuthiekanun, A. Sungkakum, A. Nisalak, and N. P. Day. 2006. The comparative accuracy of 8 commercial rapid immunochromatographic assays for the diagnosis of acute dengue virus infection. Clin. Infect. Dis. 42:1127-1134. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression and replication. Annu. Rev. Microbiol. 44:649-688. [DOI] [PubMed] [Google Scholar]
- 4.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzmán, M. G., and G. Kouri. 2002. Dengue: an update. Lancet Infect. Dis. 2:33-42. [DOI] [PubMed] [Google Scholar]
- 6.Harris, E., T. G. Roberts, L. Smith, J. Selle, L. D. Kramer, S. Valle, E. Sandoval, and A. Balmaseda. 1998. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J. Clin. Microbiol. 36:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henchal, E. A., J. M. McCown, M. C. Seguin, M. K. Gentry, and W. E. Brandt. 1983. Rapid identification of dengue virus isolates by using monoclonal antibodies in an indirect immunofluorescence assay. Am. J. Trop. Med. Hyg. 32:164-169. [DOI] [PubMed] [Google Scholar]
- 8.Hommel, D., A. Talarmin, V. Deubel, J. M. Reynes, M. T. Drouet, J. L. Sarthou, and A. Hulin. 1998. Dengue encephalitis in French Guiana. Res. Virol. 149:235-238. [DOI] [PubMed] [Google Scholar]
- 9.Kalayanarooj, S., D. W. Vaughn, S. Nimmannitya, S. Green, S. Suntayakorn, N. Kunentrasai, W. Viramitrachai, S. Ratanachu-eke, S. Kiatpolpoj, B. L. Innis, A. L. Rothman, A. Nisalak, and F. A. Ennis. 1997. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 176:313-321. [DOI] [PubMed] [Google Scholar]
- 10.Kuno, G., I. Gomez, and D. J. Gubler. 1987. Detecting artificial anti-dengue IgM immune complexes using an enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 36:153-159. [DOI] [PubMed] [Google Scholar]
- 11.Kuno, G., I. Gomez, and D. J. Gubler. 1991. An ELISA procedure for the diagnosis of dengue infections. J. Virol. Methods 33:101-113. [DOI] [PubMed] [Google Scholar]
- 12.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libraty, D. H., P. R. Young, D. Pickering, T. P. Endy, S. Kalayanarooj, S. Green, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165-1168. [DOI] [PubMed] [Google Scholar]
- 14.Lindegren, G., S. Vene, A. Lundkvist, and K. I. Falk. 2005. Optimized diagnosis of acute dengue fever in Swedish travelers by a combination of reverse transcription-PCR and immunoglobulin M detection. J. Clin. Microbiol. 43:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murgue, B., X. Deparis, E. Chungue, O. Cassar, and C. Roche. 1999. Dengue: an evaluation of dengue severity in French Polynesia based on an analysis of 403 laboratory-confirmed cases. Trop. Med. Int. Health 4:765-773. [DOI] [PubMed] [Google Scholar]
- 16.Reynes, J. M., A. Laurent, V. Deubel, E. Telliam, and J. P. Moreau. 1994. The first epidemic of dengue hemorrhagic fever in French Guiana. Am. J. Trop. Med. Hyg. 51:545-553. [PubMed] [Google Scholar]
- 17.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, H. H. Yang, T. H. Lin, and J. H. Huang. 2002. Potential application of nonstructural protein NS1 serotype-specific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: correlation of results with those of the plaque reduction neutralization test. J. Clin. Microbiol. 40:1840-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu, P. Y., and J. H. Huang. 2004. Current advances in dengue diagnosis. Clin. Diagn. Lab. Immunol. 11:642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talarmin, A., B. Labeau, J. Lelarge, and J. L. Sarthou. 1998. Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever. J. Clin. Microbiol. 36:1189-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn, D. W., A. Nisalak, T. Solomon, S. Kalayanarooj, M. D. Nguyen, R. Kneen, A. Cuzzubbo, and P. I. Devine. 1999. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 60:693-698. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2005. Epidemic and pandemic alert and response: impact of dengue. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/csr/disease/dengue/impact/en/index.html.
- 22.World Health Organization. 1997. Dengue haemorrhagic fever: diagnosis, treatment and control. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/inbex.html.
- 23.Xu, H., B. Di, Y. X. Pan, L. W. Qiu, Y. D. Wang, W. Hao, L. J. He, K. Y. Yuen, and X. Y. Che. 2006. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: implications for early diagnosis and serotyping of dengue virus infections. J. Clin. Microbiol. 44:2872-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young, P. R., P. A. Hilditch, C. Bletchly, and W. Halloran. 2000. An antigen capture enzyme-linked immunosorbent assay reveals high levels of dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 38:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]