Abstract
Previous studies have shown that the anionic alkali mineral complex BARODON has an immunoenhancing effect on pigs as an adjuvant and as a nonspecific immunostimulant. Likewise, the equine immune system has been defined with various monoclonal antibodies specific to equine leukocyte differentiation antigens to determine the possibility of enhancing equine resistance to respiratory diseases and promoting other immunostimulatory effects with the application of BARODON. Compared with the control group, after 3 weeks of treatment, BARODON-treated groups showed higher proportions of cells (P < 0.05) expressing major histocompatibility complex class II and CD2, CD4+, CD4+ CD25+, CD8+, and CD8+ CD25+ T lymphocytes, dendritic cells, and surface immunoglobulin M+ B lymphocytes in peripheral blood, as well as enhanced cell proliferative responses with phytohemagglutinin and increased phagocytic activity against Streptococcus equi and Staphylococcus aureus strains with high antibiotic resistance, the bacteria frequently identified as etiologic agents of equine respiratory diseases at the Seoul Race Park in Seoul, Korea. This study shows that BARODON may act as an immunostimulator and can be an effective alternative to antimicrobial feed additives for nonspecific improvements in equine immune responses, particularly against respiratory diseases.
Equine respiratory disease is believed to be the second most important cause of poor performance, interruption of training, and premature retirement among performance horses (41, 50, 61, 62, 72). In Seoul, Korea, the Seoul Race Park experienced yearly epizootics of infectious upper respiratory diseases (IURD), with an estimated incidence of 29.6%, from 2001 to 2005. IURD affects the nasal passages and throat region and results in chronic coughing, exercise intolerance, weight loss, and nasal discharge (S. H. Ryu, H. C. Koo, Y. K. Park, J. M. Kim, W. K. Jung, Y. H. Park, W. C. Davis, and C. W. Lee, submitted for publication). As it is often debilitating and is recalcitrant to conventional therapy composed of antibiotics, anti-inflammatory drugs, bronchodilators, and expectorants (20), IURD usually requires an extended recovery period, even though it is not usually fatal. Reducing recovery time would be of great benefit to owners and trainers, as well as to the animal itself. Returning to full activity with a reduced hospital stay is an important financial and medical consideration, since an early discharge from a care facility reduces costs for owners and should also reduce the possibility of the horse being reexposed to IURD pathogens and, subsequently, suffering a relapse. To treat IURD, the owners of the Seoul Race Park paid, collectively, an average of $140,000 per year between 2001 and 2005, which is 11.8% of the total veterinary fees for that period (Ryu et al., submitted).
Compared with those from a healthy control group, blood samples collected in the spring and summer from an IURD patient group of horses at the Seoul Race Park had significantly lower proportions of cells expressing major histocompatibility complex class II (MHC-II) and CD2, CD4+, and CD8+ T lymphocytes, as well as B lymphocytes (Ryu et al., submitted). These observations are in accord with the peak of infection, which typically occurs in spring and summer, when horses come and go from the Seoul Race Park frequently and when there is a high isolation rate of Streptococcus equi subsp. equi and Staphylococcus aureus. This strong correlation between immunologic characteristics and the resistance to strangles, the cause of approximately 15% of IURD at the Seoul Race Park (Ryu et al., submitted), suggested that an analysis of equine lymphocyte subpopulations would be a reliable assay for predicting equine resistance to IURD.
There is an increasing demand in the horse racing industry for new, safe, and efficient methods to enhance the immune responses of horses, because clinical cases due to respiratory disease are often refractory to conventional medical treatment with antibiotics. Recently, the anionic alkali mineral complex BARODON (Barodon-S.F., Ansung, Gyeonggi, Korea) was introduced to improve the productivity of food animals in Korea. BARODON’s properties are based on its mineral composition, which includes silica, sodium, silver, and potassium ions in an alkaline solution (pH 13.5). Although BARODON has been patented as an anionic solution in the United States and in Korea, the exact mechanism of its effects is not clear but is assumed to be related to the stimulation of membrane-associated lymphoid tissue by the mineral component. The immunostimulatory effect of BARODON in pigs has already been demonstrated through the proliferation and activation of porcine immune cells, particularly CD4+ CD8+ double-positive T lymphocytes in peripheral blood and in the secondary lymphoid organ (73, 74). Also, it was shown to have an adjuvant effect on hog cholera vaccine efficiency (45).
Based on the immunostimulatory effects of BARODON in animal husbandry and on the potential of equine lymphocyte subpopulation analysis to predict host responses against respiratory diseases, this study was designed to extend our previous study (Ryu et al., submitted) with more detailed immunologic characteristics and to evaluate BARODON as a nonspecific immunostimulating agent in Thoroughbred horses. A set of monoclonal antibodies (MAbs) specifically reactive with equine leukocyte differentiation antigens and a flow cytometric (FC) analysis were used to determine the proportion of leukocyte subpopulations. Lymphoproliferative responses, particularly the in vivo activation of T cells determined by examining the expression of the activation marker, the α chain of the interleukin 2 receptor (IL-2Rα) (CD25) (23, 25, 30, 31, 32, 49, 55, 57, 58), were analyzed before and after stimulation with mitogen. In addition, the phagocytic activities of immune cells from peripheral blood against S. equi subsp. equi, the most important etiologic bacterium in equine respiratory diseases, and S. aureus, which has been frequently identified in horses with IURD at the Seoul Race Park (Ryu et al., submitted), were examined in Thoroughbred horses treated with BARODON.
Increased proportions of CD4+, CD4+ CD25+, CD8+, CD8+ CD25+, and CD2+ T lymphocytes, dendritic cells, and surface immunoglobulin M (sIgM)-positive B lymphocytes in peripheral blood, as well as higher lymphoproliferative responses to mitogens and phagocytic activities against S. equi subsp. equi and S. aureus (P < 0.05), were observed in BARODON-treated horses compared with control group horses. These results imply that BARODON has immunoenhancing effects on the equine immune system, particularly in terms of enhanced resistance against IURD.
MATERIALS AND METHODS
Nonspecific immunostimulator BARODON.
The composition of the anionic mineral complex BARODON is as follows: 600 g of sodium metasilicate (Na2SiO3 commercialized disodium trioxosilicate extracted from plants; Nederland B.V., The Netherlands), 300 g of potassium carbonate (K2CO3), 9 g of sodium carbonate (Na2CO3), 9 g of sodium borate (Na2B4O7), 900 g of sucrose (C12H22O11), 10 mg of silver nitrate (AgNO3), 30 mg of sodium chloride (NaCl), and 120 mg of sodium thiosulfate (Na2S2O3) dissolved in a total of 1,000 ml of water. The product was patented in the United States (patent no. 6,447,810 B1 and 6,673,375 B2) and in Korea (patent no. 0331952), as well as in the Republic of South Africa (patent no. 2003/3337) (10, 11, 12, 13). The specific gravity of the product is 1.43, and the pH is 13.5. Barodon-biogenic feed is 10% diluent of BARODON in water, and Omolene feed is equine fermented feed composed of 0.05% BARODON and 99.5% other ingredients generally used for feed (soybean hulls, wheat hulls, molasses, corn, soybean meal, wheat, gluten feed, salt, calcium carbonate, and pura mix).
Animals and experimental design.
A total of 24 clinically healthy Thoroughbred horses were divided into four groups. Six heads comprised the control group that was fed feed not containing BARODON (Agribrands Purina Korea Inc., Seongnam, Korea). Six heads (Tx-1) were fed 6 to 7 kg of Omolene feed, which had been mixed with 0.05% Barodon F. Gold, and 60 ml of Barodon-biogenic feed, containing 10% Barodon F. Gold, daily. Another six heads (Tx-2) were fed only Omolene feed. The final six heads (Tx-3) were fed only Barodon-biogenic feed. Each group of Thoroughbred horses was fed as described above daily for the 14 weeks of the study, and their blood was collected at time zero (before BARODON treatment) and also at 2, 4, 6, 10, and 14 weeks after the beginning of BARODON treatment. The sample and data collection and their analysis were performed in a blinded manner by animal handlers as well as by investigators.
Clinical evaluation and sample collection.
The horses underwent clinical observations, and collection of samples composed of nasal swabs and blood was performed. In accordance with the farm managers' request that stress in the horses be reduced and to avoid the confounding of results by frequent handling, multiple clinical examinations were not conducted.
Proportion of equine leukocyte subpopulations.
About 100 ml of jugular venous blood was collected from each animal. A set of MAbs specifically reactive with equine leukocyte differentiation antigens and FC were used to examine the proportion of leukocyte subpopulations in the peripheral blood from each group.
Preparation of peripheral blood leukocytes.
The separation of peripheral blood leukocytes was performed by methods detailed previously (15). Briefly, collected blood was mixed with an equal volume of acid-citrate-dextrose (ACD)-EDTA, and leukocytes were separated by Hypaque Ficoll (density, 1.086; Sigma-Aldrich, St. Louis, Mo.) density gradient centrifugation at 670 × g for 30 min. Finally, live cells were counted by the trypan blue (Invitrogen Life Technologies, Carlsbad, Calif.) exclusion technique, and the final concentration was adjusted to 1 × 107 cells/ml.
MAbs specific to equine leukocyte differentiation antigens.
A panel of MAbs specifically reactive with equine leukocyte differentiation antigens is shown in Table 1. MAbs (VMRD Inc., Pullman, Wash.) specific to MHC-I (E18A), MHC-II (TH81A5), CD2 (HB88A), CD4 (HB61A), CD5 (HB19A), CD8 (HT14A), CD172a (DH59B), and sIgM+ B cells (B29A, H58A) were used to examine the proportions of leukocyte subpopulations.
TABLE 1.
MAbs specifically reactive with the equine leukocyte differentiation antigens used in this study
| MAb | MAb isotype | Moleculea | References |
|---|---|---|---|
| HB19A | IgG2a | EqCD5 | 9, 35 |
| HB88A | IgG1 | EqCD2 | 6, 35 |
| B29A | IgG2a | sIgM | 35, 65 |
| E18A | IgG2a | sIgM | 21, 35 |
| DH59B | IgG1 | CD172a | 15, 28, 36, 46, 54, 56, 65, 68 |
| H58A | IgG2a | MHC-I | 6, 15, 28, 54 |
| TH81A5 | IgG2a | MHC-II | 1, 2, 15, 54 |
| HB61A | IgG1 | EqCD4 | 6, 35, 66 |
| HT14A | IgG1 | EqCD8 | 6, 21, 35, 66 |
Equine (Eq) leukocyte differentiation molecules.
FC analysis.
The proportion of leukocyte subpopulations was determined and analyzed by FC (FACSCalibur) using the CellQuest program (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) and FCS Express software (De Novo; Thornton, Ontario, Calif.), respectively. About 50 μl (15 μg/ml) of MAbs were reacted with 100 μl of cells at 1 × 107 cells/ml in a V-bottomed 96-well microplate. After the first incubation on ice for 30 min, plates were washed three times with the first washing buffer (phosphate-buffered saline [PBS], 450 ml; ACD, 50 ml; 20% NaN3, 5 ml; gamma globulin-free horse serum [Sigma-Aldrich], 10 ml; 250 mM EDTA, 20 ml; 0.5% phenol red, 1 ml) with centrifugation at 670 × g for 5 min. The pellet was disrupted by vortexing, mixed with 50 μl of a 100× dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG2a antibody and a 200× dilution of phycoerythrin (PE)-conjugated goat anti-mouse IgG1 antibody (Caltag Lab, Burlingame, Calif.), and incubated on ice for 30 min in the dark. The cells were then washed three times with a second washing buffer (which was the same as the first washing buffer, excluding horse serum) by centrifugation at 670 × g for 5 min. After the final wash, the cells were mixed with 200 μl of 2% PBS-formaldehyde (38% formalin, 20 ml; PBS, 980 ml) and were kept in the refrigerator for FC analysis.
Mitogen-stimulated lymphoproliferative responses.
A total of 107 peripheral blood mononuclear cells (PBMC) in a volume of 10 ml of medium were placed in a tissue culture flask and were incubated upright in the presence or absence of mitogen (one control flask for culture with only RPMI 1640 medium, the other flask for culture with 1 μg/ml of phytohemagglutinin [PHA] [Sigma-Aldrich]) for 72 h to stimulate equine leukocytes (31). Cells were then stained for IL-2Rα (CD25) expression on CD4+ or CD8+ T lymphocytes with biotinylated recombinant human IL-2 from the FLUOROKINE kit (R&D Systems Inc., Minneapolis, Minn.) and with either MAb HB61A or MAb HT14A (24, 31, 44, 52, 53). After three washes with the first washing buffer and RDF buffer (R&D Systems Inc.), 30 μl of streptavidin-conjugated FITC (FLUOROKINE; R&D Systems Inc.) and 50 μl of a 200× dilution of PE-conjugated goat anti-mouse IgG1 antibody (Caltag Lab) were added for a further 1 h, followed by three washes with the second washing buffer and RDF buffer and final fixation with 2% PBS-formaldehyde.
The proportions of blasting or activated proliferating cells were analyzed by examination of an R2 gate, where large lymphocytes and monocytes were located, upon FC analysis with FCS Express software (De Novo).
Phagocytosis.
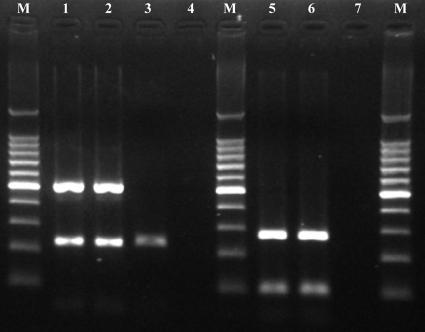
S. equi subsp. equi and S. aureus bacteria isolated from nasal swabs of Thoroughbred horses with IURD during our previous study (Ryu et al., submitted) were confirmed by PCR amplification (Fig. 1) and selected by their high level of resistance to amikacin, penicillin, or trimethoprim-sulfamethoxazole (Table 2), which have been used frequently for the treatment of IURD. They were labeled with propidium iodide (PI) (Becton Dickinson) at a concentration of 100 μg of bacteria/1 ml of working solution of PI (100 μg/ml in 0.1 M carbonate buffer, pH 9.6) by shaking the mixed solution in the dark at 4°C for 24 h. Aliquots of 1 ml (100 μg) of the PI-bacteria solution were centrifuged at 16,600 × g (Fotodyne Microcentrifuge, Hartland, Wis.) for 30 s and were washed twice with PBS.
FIG. 1.
PCR products from Streptococcus equi subsp. equi and Staphylococcus aureus bacteria isolated from nasal swab specimens of horses with IURD, the phagocytic strains used in this study, amplified with corresponding species-specific primer pairs. Lanes: M, 100-bp DNA ladder (Takara Bio Inc., Otsu, Shiga, Japan); 1, S. equi subsp. equi isolate using sodA primer sets (230 bp of PCR product) and seeI primer sets (520 bp of PCR product); 2, S. equi subsp. equi ATCC 33398 using sodA primer sets and seeI primer sets; 3, S. equi subsp. zooepidemicus ATCC 43079 using sodA primer sets and seeI primer sets (notice the negative reactions of S. equi subsp. zooepidemicus with seeI primer sets, which are specifically reactive to S. equi subsp. equi); 4 and 7, negative control (distilled water); 5, S. aureus isolate using nuc primer sets and Sa-442 primer sets; 6, S. aureus ATCC 25923 using nuc primer sets and Sa-442 primer sets.
TABLE 2.
Antimicrobial susceptibilities of the Streptococcus equi subsp. equi and Staphylococcus aureus strains used for in vitro phagocytosis in this study
| Bacterium | Susceptibilitya
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillins
|
Cephalosporins
|
Aminoglycosides
|
Tetracyclines (TE) | Macrolides (E) | Sulfonamides
|
Polypeptides
|
Quinolones (ENR) | |||||||||||
| AM | AMC | OX | P | CF | CEF | AN | GM | K | N | STR | SXT | TMP | B | PB | ||||
| S. equi subsp. equi | S | R | S | R | R | I | R | I | S | R | S | S | ||||||
| S. aureus | R | S | S | R | S | S | S | S | S | S | S | S | R | S | S | S | ||
Abbreviations for antimicrobials (Becton Dickinson and Company, Sparks, Md.): AM, ampicillin; AMC, amoxicillin; OX, oxacillin; P, penicillin; CF, cephalothin; CEF, ceftiofur; AN, amikacin; GM, gentamicin; K, kanamycin; N, neomycin; STR, streptomycin; TE, tetracycline; E, erythromycin; SXT, trimethoprim-sulfamethoxazole; TMP, trimethoprim; B, bacitracin; PB, polymyxin B; ENR, enrofloxacin. Abbreviations for susceptibilities: S, susceptible; R, resistant; I, intermediate.
Half of the PI-bacteria solution was opsonized with 40% horse serum diluted in PBS in the dark at 37°C for 30 min. Next, it was centrifuged, washed twice with cold PBS, and resuspended with PBS (10 μg PI-bacteria/100 μl PBS). A separate aliquot was not treated with the serum for nonopsonized phagocytosis (19, 38, 39).
For in vitro phagocytosis, isolated peripheral blood phagocytes (2 × 106 cells/ml PBS) were incubated with 10 μg of opsonized or nonopsonized PI-bacteria solution at 37°C for 30 min. The solutions were centrifuged, washed with cold PBS, and transferred into a 96-well V-bottomed plate. Those cells were then secondarily labeled with DH59B (VMRD Inc.), which is specific for granulocytes and monocytes, and subsequently with FITC-labeled anti-mouse IgG, to confirm that the R3-gated cells in the FC analysis were granulocytes and monocytes.
FC of FITC (FL1) and PI (FL2) mean fluorescence was measured before and after the addition of 50 μl of a 0.4% solution of trypan blue (Invitrogen Life Technologies) to each tube in order to quench the fluorescent signals imparted by nonphagocytosed bacteria or by extracellular bacteria that adhere nonspecifically to the surface of the phagocytes (22).
Statistical analysis.
The proportions of leukocytes expressing the various cell surface markers in peripheral blood and their phagocytic activities were compared between the BARODON-treated groups (Tx-1 to Tx-3) and the control group by the Kruskal-Wallis one-way analysis of variance by ranks. Analyses were performed with the Analyze-it program (Analyze-it Software Ltd., Leeds, United Kingdom).
RESULTS
Proportions of equine leukocyte subpopulations.
The changes in the proportions of equine leukocyte subpopulations were investigated by MAbs and FC.
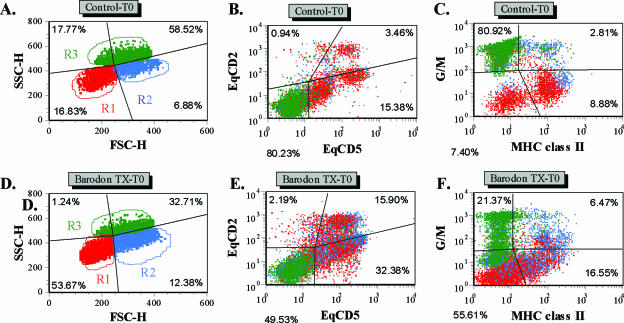
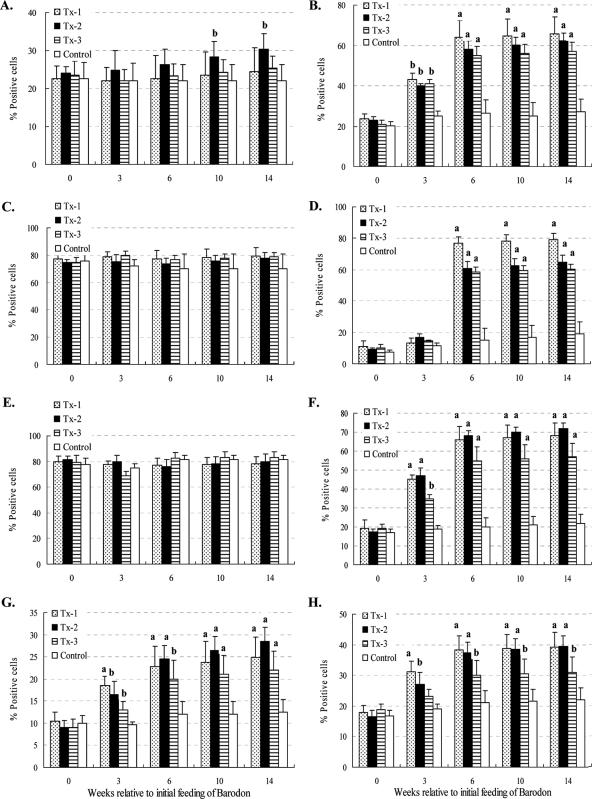
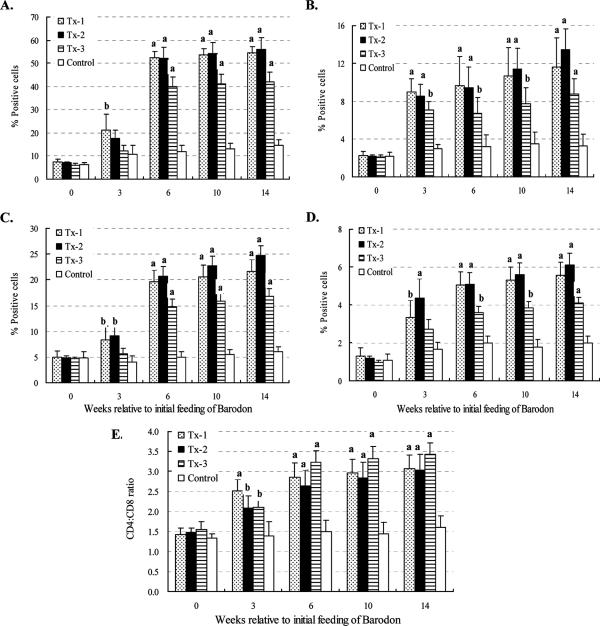
Based on side and forward light scatter, the R1 and R3 gates were used to identify small lymphocytes and granulocytes, respectively. Additionally, the R2 gate was used to define monocytes and large lymphocytes at the beginning of the culture, as well as large lymphocytes undergoing blastogenesis and proliferation at 3 days of culture (Fig. 2). There was no significant difference between the BARODON-treated groups (Tx-1 to Tx-3) and the control group in the proportions of granulocytes (R3) or MHC-I-expressing cells (P > 0.05) (Fig. 3C and E). However, compared with control groups, the proportions of lymphocytes (R1 and R2) and blasting cells (R2) or CD2+ cells (i.e., all thymocytes, T lymphocytes, and natural killer [NK] cells) in BARODON-treated groups were significantly higher (P < 0.05) at only 10 and 14 weeks and from 3 or 6 weeks to 14 weeks of treatment, respectively, which was the day of the last blood sampling (Fig. 2 and 3A, B, and D).
FIG. 2.
Representative dot plot profiles of PBMC from a control horse (A to C) and a BARODON-treated horse (D to F) labeled with two MAbs (Table 1) for equine CD2 (EqCD2) and EqCD5 (B and E) or for granulocytes and monocytes (G/M) and MHC-II (C and F) before (time zero, T0) 3 days of culture in RPMI alone or with PHA. In profiles A and D, the quadrants show the division between small lymphocytes in gate 1 (R1), large lymphocytes and monocytes in gate 2 (R2), and granulocytes in gate 3 (R3), as well as the relative frequencies of cells in each gate at T0. Profiles B and E, with gates placed only on 1, 2, and 3, show the frequency of EqCD2− EqCD5− cells (lower left quadrant), EqCD2+ EqCD5− cells (upper left quadrant), EqCD2+ EqCD5+ cells (upper right quadrant), and EqCD2− EqCD5+ cells (lower right quadrant). Profiles C and F, with gates on 1, 2, and 3, show the frequency of G/M− MHC-II− cells (lower left quadrant), G/M+ MHC-II− cells (upper left quadrant), G/M+ MHC-II+ cells (upper right quadrant), and G/M− MHC class II+ cells (lower right quadrant).
FIG. 3.
Summary of FC analysis of R1 plus R2 (lymphocytes) (A), R2 (blasting and proliferating cells) (B), R3 (granulocytes) (C), CD2+ cells (all thymocytes, T lymphocytes, and NK cells) (D), MHC-I+ cells (E), MHC-II+ cells (F), dendritic cells (G), and sIgM+ B lymphocytes (H) in peripheral blood with gates placed only on 1, 2, and 3 at time zero. Horses were fed daily both with 6 to 7 kg of Omolene feed, which had already been mixed with 0.05% Barodon F. Gold, and with an additional 60 ml of Barodon-biogenic feed containing 10% Barodon F. Gold (Tx-1; n = 6). Another group was fed only Omolene feed (Tx-2; n = 6), while a third group was fed only Barodon-biogenic feed (Tx-3; n = 6). The fourth group, an untreated control group, was fed general feed with no BARODON (Control; n = 6). Significant differences between control animals and animals treated with BARODON are as indicated in the figure (a, P < 0.01; b, 0.01 < P < 0.05). See Materials and Methods for details on the analysis of subsets by selective gating used to show the frequency of each cell population with gates placed on 1, 2, and 3.
The proportions of MHC-II+ cells, dendritic cells, and sIgM+ B lymphocytes in BARODON-treated groups increased significantly more (P < 0.05) than those in the control group from 3 to 14 weeks of treatment. Among BARODON-treated groups, the proportions of MHC-II+ cells, dendritic cells, and sIgM+ B lymphocytes were significantly higher in the Tx-1 and Tx-2 groups than in the Tx-3 group (P < 0.05), with no significant difference between the Tx-1 and Tx-2 groups (P > 0.05) (Fig. 2 and 3F to H).
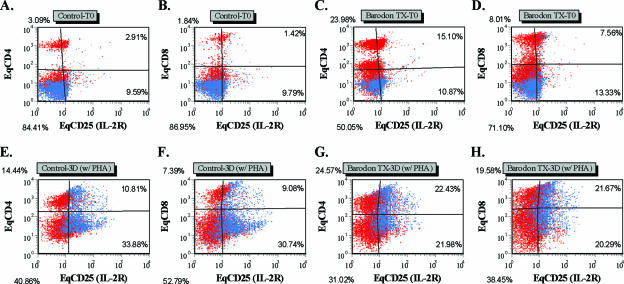
Before 3 days of culture with mitogen, significantly higher proportions of CD4+, CD4+ CD25+, CD8+, and CD8+ CD25+ T lymphocytes, and higher CD4/CD8 ratios in PBMC from BARODON-treated groups compared with the control group, were seen after 3 weeks of treatment (P < 0.05), particularly in the Tx-1 and Tx-2 groups compared with the Tx-3 group (P < 0.05). However, no significant difference was observed between the Tx-1 and Tx-2 groups (P > 0.05) (Fig. 4A to D and Fig. 5).
FIG. 4.
Representative dot plot profiles of PBMC at time zero with gates placed only on 1 and 2 from a control horse (A, B, E, and F) and a BARODON-treated horse (C, D, G, and H) labeled with two MAbs (Table 1) for equine CD4 (EqCD4) or EqCD8 and EqCD25 (IL-2Rα) at time zero (A to D) and after 3 days (3D) culture with PHA (E to H). The profiles A, C, E, and G show the frequency of EqCD4− EqCD25− cells (lower left quadrant), EqCD4+ EqCD25− cells (upper left quadrant), EqCD4+ EqCD25+ cells (upper right quadrant), and EqCD4− EqCD25+ cells (lower right quadrant). In profiles B, D, F, and H, the quadrants show the frequency of EqCD8− EqCD25− cells (lower left quadrant), EqCD8+ EqCD25− cells (upper left quadrant), EqCD8+ EqCD25+ cells (upper right quadrant), and EqCD8− EqCD25+ cells (lower right quadrant).
FIG. 5.
Summary of FC analysis of CD4+ (A), CD4+ CD25+ (B), CD8+ (C), and CD8+ CD25+ (D) T lymphocytes and the CD4/CD8 ratio (E) from the proportions of CD4+ and CD8+ T lymphocytes in peripheral blood with gates placed only on 1 and 2 at time zero. Horses in one group were fed Omolene feed as well as Barodon-biogenic feed (Tx-1; n = 6), group two was fed only Omolene feed (Tx-2; n = 6), group three was fed only Barodon-biogenic feed (Tx-3; n = 6), and the untreated control group was fed general feed with no BARODON (Control; n = 6). Significant differences between control animals and animals treated with BARODON are as indicated in the figure (a, P < 0.01; b, 0.01 < P < 0.05). Detailed information on how much BARODON was included in each feed and how subsets were analyzed by selective gating, which was used to show the frequency of each cell population, is provided in Materials and Methods.
Mitogen-stimulated lymphoproliferative activities of cells from peripheral blood.
To examine the mitogen-stimulated lymphoproliferative responses, live PBMC were harvested from all groups following stimulation with PHA for 3 days and were analyzed by FC dual-color staining.
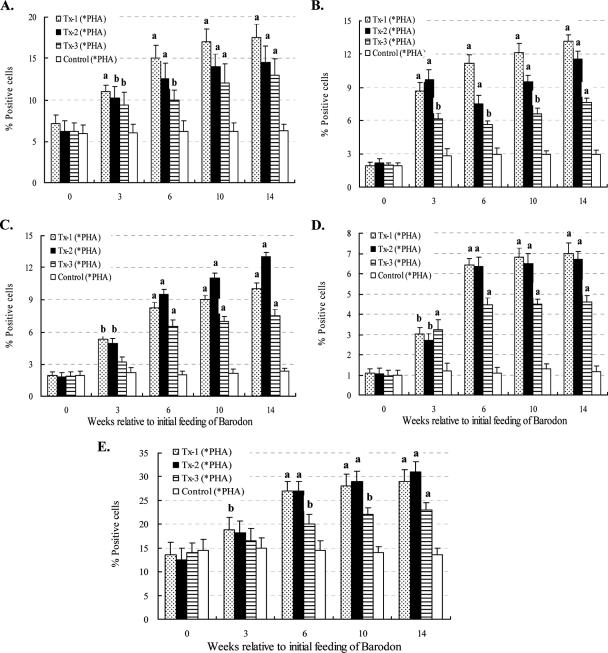
The proportions of large proliferating cells (R2)—CD4+, CD4+ CD25+, CD8+, and CD8+ CD25+ T lymphocytes—in BARODON-treated groups were significantly higher (P < 0.05) than those in the control group after 3 weeks of treatment (P < 0.05). Those significant differences were also found between the Tx-1 or Tx-2 and Tx-3 groups but not between the Tx-1 and Tx-2 groups (Fig. 4E to H and Fig. 6).
FIG. 6.
Summary of FC analysis of CD4+ (A), CD4+ CD25+ (B), CD8+ (C), and CD8+ CD25+ (D) T lymphocytes in peripheral blood with gates placed only on 1 and 2 and blast, proliferating lymphocytes (E) in peripheral blood with a gate placed only on 2 after 3 days of culture in RPMI alone or with PHA. Horses in one group were fed both Omolene feed and Barodon-biogenic feed (Tx-1; n = 6), while a second group was fed only Omolene feed (Tx-2; n = 6) and a third group was fed only Barodon-biogenic feed (Tx-3; n = 6). The untreated control group was fed general feed with no BARODON (Control; n = 6). Significant differences in the proportions of CD4+ (A), CD4+ CD25+ (B), CD8+ (C), and CD8+ CD25+ (D) T lymphocytes and cells in gate R2 (E) after culture in RPMI alone and with PHA, between control horses and BARODON-treated horses, are as indicated in the figure (*PHA = 3 days of PHA − 3 days of RPMI; a, P < 0.01; b, 0.01 < P < 0.05). See Materials and Methods for detailed information on the analysis of subsets by selective gating, used to show the frequency of each cell population.
Phagocytosis.
To examine in vitro phagocytic activity, PBMC were obtained from all groups and were compared following an experimental inducement of phagocytosis with opsonized or nonopsonized S. equi subsp. equi and S. aureus bacteria isolated from the Korea Racing Association's respiratory patient group and having high-level resistance to antibiotics (amikacin, penicillin, or trimethoprim-sulfamethoxazole) for respiratory disease (Ryu et al., submitted).
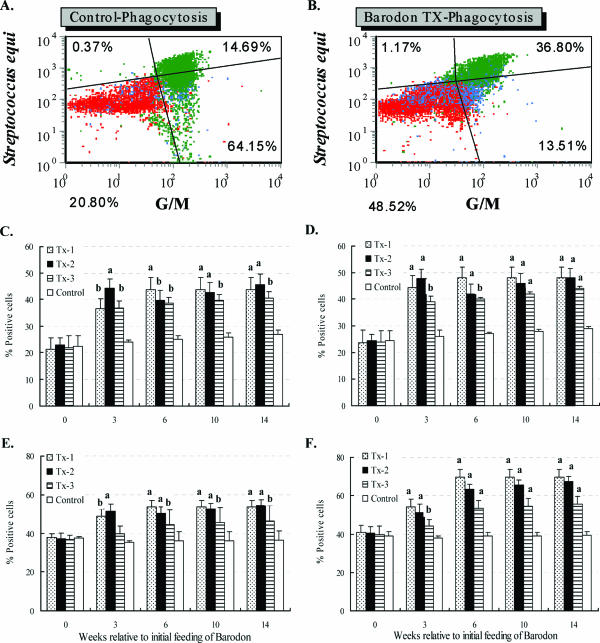
After trypan blue treatment, the PI (FL2) mean fluorescence for phagocytosis was reduced due to the quenching of PI signals by nonphagocytosed extracellular bacteria. However, there was no positive or negative effect from the addition of trypan blue on the FITC (FL1) signal for granulocytes and monocytes. The phagocytic activity in BARODON-treated groups was significantly higher (P < 0.05) than that in the control group after 3 weeks of treatment (P < 0.05). Among BARODON-treated groups, the phagocytic activity was significantly greater in the Tx-1 and Tx-2 groups than in Tx-3, and there was no significant difference between the Tx-1 and Tx-2 groups (P < 0.05). The phagocytic activities against the opsonized S. equi subsp. equi and S. aureus were significantly greater than those against the nonopsonized S. equi subsp. equi and S. aureus (P < 0.05) (Fig. 7).
FIG. 7.
Representative dot plot profiles of PBMC with gates placed only on 1, 2, and 3 at time zero from a control horse (A) and a BARODON-treated horse (B) labeled with one MAb (Table 1) for granulocytes and monocytes (G/M) following phagocytosis with opsonized Streptococcus equi subsp. equi, which had been previously stained overnight with PI, and a summary of FC analysis of PBMC following phagocytosis with nonopsonized (C) or opsonized (D) S. equi subsp. equi and with nonopsonized (E) or opsonized (F) Staphylococcus aureus. Profiles A and B show the frequency of S. equi-negative G/M− cells (lower left quadrant), S. equi-negative G/M+ cells (upper left quadrant), S. equi-positive G/M+ cells (upper right quadrant), and S. equi-positive G/M− cells (lower right quadrant). Horses in one group were fed both Omolene feed and Barodon-biogenic feed (Tx-1; n = 6), while horses in the second group were fed only Omolene feed (Tx-2; n = 6) and horses in the third group were fed only Barodon-biogenic feed (Tx-3; n = 6). The untreated control group was fed general feed with no BARODON (Control; n = 6). Significant differences between the phagocytic capabilities of control animals and animals treated with BARODON are as indicated in the figure (a, P < 0.01; b, 0.01 < P < 0.05). Detailed information on the concentration of BARODON in each feed and instructions on how to perform phagocytosis and opsonization, stain S. equi subsp. equi and S. aureus with PI, and analyze subsets by selective gating to determine the frequency of each cell population are provided in Materials and Methods.
DISCUSSION
Nonspecific immunostimulants are substances that induce an enhancement of the body's native or acquired defense mechanisms regardless of the mode of antigen specificity (26, 51). Immunomodulators, also termed biologic response modifiers, may either enhance or suppress innate immune responses in a non-antigen-specific way. The proposed action mechanism of nonspecific immunomodulatory preparations is macrophage activation and the subsequent release of cytokines that might enhance the immune response. Macrophages may recognize nonspecific immunomodulator particulate matter and become activated, resulting in the production of interferon (IFN), IL-1, tumor necrosis factor, or IL-6 (48, 59). These cytokines may affect humoral and cellular immune functions, including phagocytic activity, antibody production, and lymphocyte cytotoxicity. Mild fever, anorexia, and lethargy may be observed after the administration of immunostimulant preparations. This reaction likely reflects the increased circulating IL-1 and is not considered an adverse side effect. On the contrary, it may indicate recognition of the immunomodulator and activation of the immune system.
Regardless of the host species, immunostimulant preparations are used most often for treatment of chronic viral or bacterial infections with evidence of secondary immunosuppression (51). In equine medicine, nonspecific immunostimulant products such as Baypamun P, Baypamun N, Lobelin, natural human IFN-α, and inactivated Propionibacterium acnes have been used for treatment of sarcoid skin tumors, equine respiratory disease complex, chronic respiratory disease, and inflammatory airway disease and as a respiratory stimulant (27, 37, 42, 43, 47, 60, 64, 67, 75).
Recently, it has become possible to define the host immune system more specifically with MAbs against leukocyte differentiation antigens of various animals, including horses (5, 7, 69, 70). The efficacy of vaccines and new drugs can be evaluated in vivo by comparing the host response before and after application of those reagents (14, 17, 29). BARODON′s immunoenhancing effects on pigs as an adjuvant and as a nonspecific immunostimulant have been approved as follows (45, 73, 74): (i) increases in antibody titers and immune cell proportions in hog cholera- and Actinobacillus pleuropneumoniae-vaccinated pigs after BARODON treatment; (ii) improvements in average daily weight gain rates and feed conversion rates; (iii) increased proportions of CD4+ and CD8+ T lymphocytes, MHC-II+ lymphocytes, non-T/non-B (N) cells, and, particularly, CD4+ CD8+ double-positive T lymphocytes from peripheral blood and the mesenteric lymph nodes; and (iv) a higher stimulatory activity to mitogen (PHA, concanavalin A, and pokeweed mitogen). Likewise, the equine immune system was defined by using various MAbs specific to equine leukocyte differentiation antigens after application of the anionic alkali mineral complex BARODON to determine the horse's ability to resist respiratory diseases as well as the immunostimulatory effects of BARODON and its potential as an immunostimulant and alternative to antimicrobial feed additives for improving host immune responses in Thoroughbred horses.
The increased proportions of cells expressing MHC-II, which play a major role in bacterial defense mechanisms, phagocytosis, and antigen presentation, as well as of CD4+, CD4+ CD25+, CD8+, CD8+ CD25+, and CD2+ T lymphocytes, dendritic cells, and B lymphocytes in peripheral blood, from BARODON-treated horses indicate that BARODON has immunoenhancing effects on equine immune systems. The comparatively higher proportions of activated immune cells in the healthy control group than in the IURD patient group were also found to be associated with resistance to IURD, as noted in our previous study (Ryu et al., submitted).
The phagocytic activity against S. equi subsp. equi and S. aureus in the BARODON-treated group was significantly higher than that of the control group after 3 weeks of treatment. After mitogen (PHA) stimulation of PBMC for 3 days, the proportions of CD4+, CD4+ CD25+, CD8+, and CD8+ CD25+ T lymphocytes in the BARODON-treated group increased significantly compared with the control group. Significant differences were observed after 3 weeks of treatment. The cells expressing CD2+ (all thymocytes, T lymphocytes, and NK cells) and MHC-I antigen were not significantly different within treatment groups (Tx-1, Tx-2, and Tx-3). However, when the immunological characteristics within the BARODON treatment groups were analyzed, the increased proportions of cells expressing MHC-II antigen, large blasting cells (R2), CD4+, CD4+ CD25+, CD8+, and CD8+ CD25+ T lymphocytes, dendritic cells, and B lymphocytes in peripheral blood, as well as the enhanced cell proliferative responses against PHA and phagocytic activity against S. equi subsp. equi and S. aureus, were all significantly greater in the Tx-1 and Tx-2 groups than in Tx-3. No significant difference was observed between the Tx-1 and Tx-2 groups. The increased proportions of these immune cells should influence the activated lymphoproliferative responses by mitogen stimulus. Further studies using MAbs against other activation or regulatory molecules on equine immunomodulating cells and an analysis of their cytokine gene expression and protein secretion, including IL-10 or transforming growth factor beta, can more specifically elucidate the activity of BARODON.
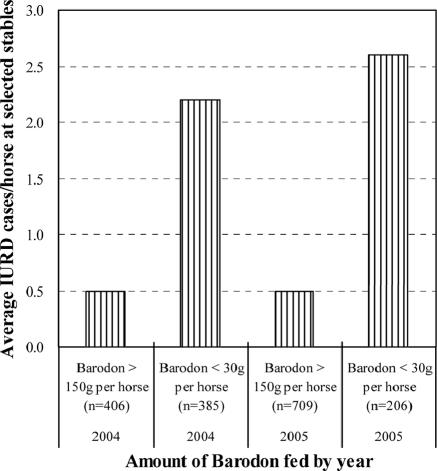
Under stress, such as with strenuous exercise and long-distance transportation, alveolar macrophage activities and CD4+ T lymphocytes can be suppressed (71). According to clinical experience and observations at the Seoul Race Park, the advance administration of BARODON in either an anionic feed additive form or a drinking water form reduced many clinical complications, including stress-induced respiratory disease, suggesting activation of immune cell populations, which is a result similar to that obtained after the treatment of horses with inactivated P. acnes (18, 43). Therefore, BARODON′s immunoenhancing effect in equine herds can improve the immune responses of horses to equine respiratory bacterial infection, as shown by the increased phagocytosis and, moreover, possibly treatment against S. equi subsp. equi and S. aureus strains with high-level resistance to common antibiotics for IURD. Considering that (i) the doping control system has prohibited the preventive use of antimicrobial feed additives and (ii) veterinarians have been very much concerned about this when selecting antibiotics in a clinical situation, BARODON’s immunoenhancing effect, with its mineral composition, in equine herds can be useful when aimed at equine respiratory bacterial infections under racetrack conditions. Clinical experience with Omolene feed containing 0.05% BARODON at selected stables in the Seoul Race Park showed that the feeding of more than 150 g of BARODON per year reduced significantly the prevalence of IURD compared with groups fed less than 30 μg of BARODON or general feed not containing BARODON from 2004 to 2005 (Fig. 8). Further controlled field studies will elucidate the potential effects of BARODON against equine respiratory disease.
FIG. 8.
Relationship between the amount of BARODON (in grams) fed per horse and the prevalence of IURD from 2004 to 2005 at selected stables in the Seoul Race Park.
The major ingredient in BARODON is a mineral that may affect vital biological processes, including immune responses. Silica, among several candidate substances in BARODON, may be responsible for these immunoenhancing effects. Subchronic and short-term exposure to silica was shown to enhance respiratory defense mechanisms by increasing the proportions of neutrophils, T lymphocytes, and NK cells and by activating phagocytes to release more reactive oxygen species, which have been known to help in the pulmonary clearance of infectious agents in lungs (3, 4, 33). More specifically, in the thoracic lymph nodes of rats with subchronic silicosis, a significantly higher percentage of CD8+ T cells and, to a lesser degree, CD4+ T cells was proliferated and activated, with the expression of IL-2R and intercellular adhesion molecule 1 and enhanced expression of IFN-γ mRNA, which may be an important priming cytokine for a systemic preactivation not only of alveolar macrophages but also of peritoneal macrophages that have experienced no direct contact with silica particles (8, 23, 34, 40).
The immunostimulant's function was found to be mediated predominantly by macrophage activation, with stimulus-induced gene expression, and finally by increased functional competence (63). Therefore, multiple doses are expected to give pulses of immune stimulation, such as cytokine release, due to the increased persistence of the product within macrophages. In this study, 6 to 7 kg of daily feed intake of Omolene feed included 3 to 3.5 g of Barodon F. Gold. However, a daily intake of 60 ml of Barodon-biogenic feed can provide horses with 8.58 g of Barodon F. Gold, given its concentration and specific gravity. Nevertheless, BARODON’s effect on the enhancement and activation of immune responses without clinical side effects in horses was higher in the anionic feed additive form of BARODON, Omolene feed, than in the drinking water form of BARODON, Barodon-biogenic feed. This difference cannot be fully explained in this study.
Further studies are needed to determine the optimal amounts for daily intake and the best method of ingestion of BARODON for the efficient stimulation of equine mucosal immunity without possible adverse side effects. In a study with the nonspecific immunostimulant OM-85 BV in human patients, stimulatory effects on T-lymphocyte subpopulations persisted during treatment but decreased to baseline values within 3 months after discontinuation of immunotherapy (16). However, the duration of immunomodulatory effects after discontinuation of BARODON was not investigated in this study. Although more studies are needed to elucidate the exact mechanism of action of BARODON and its enhancing effect on the equine immune system, this study suggests that BARODON is a potential immunostimulant and an alternative to antimicrobial feed additives for improving equine immune responses and that its use results in the improved capability of horses to endure an attack of infectious respiratory diseases.
Acknowledgments
We thank Sook Shin and Yang Hee Kim for outstanding technical assistance and the many veterinarians of the Korea Racing Association who helped to collect equine blood and check clinical observations during this study.
This study was supported in part by funding from Agribrands Purina Korea Inc. and by the Brain Korea 21 program for Veterinary Science and the Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University. Further support was also provided by the Korea Research Foundation (grant KRF-2006-005-J0293).
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Ababou, A., W. C. Davis, and D. Levy. 1993. The DA6-147 monoclonal antibody raised against HLA-DR alpha chain identifies a cryptic epitope on the BoLA-DR alpha chain. Ann. Rech. Vet. 24:402-407. [PubMed] [Google Scholar]
- 2.Ababou, A., J. Goyeneche, W. C. Davis, and D. Levy. 1994. Evidence for the expression of three different BoLA-class II molecules on the bovine BL-3 cell line: determination of a non-DR non-DQ gene product. J. Leukoc. Biol. 56:182-186. [DOI] [PubMed] [Google Scholar]
- 3.Antonini, J. M., J. R. Roberts, H. M. Yang, M. W. Barger, D. Ramsey, V. Castranova, and J. Y. Ma. 2000. Effect of silica inhalation on the pulmonary clearance of a bacterial pathogen in Fischer 344 rats. Lung 178:341-350. [DOI] [PubMed] [Google Scholar]
- 4.Antonini, J. M., H. M. Yang, J. Y. Ma, J. R. Roberts, M. W. Barger, L. Butterworth, T. G. Charron, and V. Castranova. 2000. Subchronic silica exposure enhances respiratory defense mechanisms and the pulmonary clearance of Listeria monocytogenes in rats. Inhal. Toxicol. 12:1017-1036. [DOI] [PubMed] [Google Scholar]
- 5.Biddison, W. E., and S. Shaw. 1989. CD4 expression and function in HLA class II-specific T cells. Immunol. Rev. 109:5-15. [DOI] [PubMed] [Google Scholar]
- 6.Bilzer, T., O. Planz, W. I. Lipkin, and L. Stitz. 1995. Presence of CD4+ and CD8+ T cells and expression of MHC class I and MHC class II antigen in horses with borna disease virus-induced encephalitis. Brain Pathol. 5:223-230. [DOI] [PubMed] [Google Scholar]
- 7.Bolin, S. R., A. W. McClurkin, and M. F. Colia. 1985. Effects of bovine viral diarrhea virus on the percentages and absolute numbers of circulating B and T lymphocytes in cattle. Am. J. Vet. Res. 46:884-886. [PubMed] [Google Scholar]
- 8.Borm, P. J., N. Palmen, J. J. Engelen, and W. A. Buurman. 1988. Spontaneous and stimulated release of tumor necrosis factor-alpha (TNF) from blood monocytes of miners with coal workers' pneumoconiosis. Am. Rev. Respir. Dis. 138:1589-1594. [DOI] [PubMed] [Google Scholar]
- 9.Brodersen, R., F. Bijlsma, K. Gori, K. T. Jensen, W. Chen, J. Dominguez, K. Haverson, P. F. Moore, A. Saalmuller, D. Sachs, W. J. Slierendrecht, C. Stokes, O. Vainio, F. Zuckermann, and B. Aasted. 1998. Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet. Immunol. Immunopathol. 64:1-13. [DOI] [PubMed] [Google Scholar]
- 10.Choi, S. I., H. S. Choi, K. S. Jeon, B. W. Yoo, and Y. H. Park. 10. September 2002. Composition of multipurpose high functional alkaline solution composition, preparation thereof, and for the use of nonspecific immunostimulators. U.S. patent 6,447,810 B1.
- 11.Choi, S. I., H. S. Choi, K. S. Jeon, B. W. Yoo, and Y. H. Park. 26. March 2002. Composition of multipurpose high functional alkaline solution composition, preparation thereof, and for the use of nonspecific immunostimulators. Patent no. 0331952, Korea (application no. 2000-0070054).
- 12.Choi, S. I., H. S. Choi, K. S. Jeon, B. W. Yoo, and Y. H. Park. 6. January 2004. Composition of multipurpose high functional alkaline solution composition, preparation thereof, and for the use of nonspecific immunostimulators. U.S. patent 6,673,375 B2.
- 13.Choi, S. I., H. S. Choi, K. S. Jeon, B. W. Yoo, and Y. H. Park. 28. January 2004. Composition of multipurpose high functional alkaline solution composition, preparation thereof, and for the use of nonspecific immunostimulators. Patent no. 2003/3337, Republic of South Africa.
- 14.Creemers, P. C. 1987. Determination of co-expression of activation antigens on proliferating CD4+, CD4+CD8+ and CD8+ lymphocyte subsets by dual parameter flow cytometry. J. Immunol. Methods 97:165-171. [DOI] [PubMed] [Google Scholar]
- 15.Davis, W. C., S. Marusic, H. A. Lewin, G. A. Splitter, L. E. Perryman, T. C. McGuire, and J. R. Gorham. 1987. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet. Immunol. Immunopathol. 15:337-376. [DOI] [PubMed] [Google Scholar]
- 16.Emmerich, R., K. Pachmann, and D. Milatovic. 1992. Influence of OM-85 BV on different humoral and cellular immune defense mechanisms of the respiratory tract. Respiration 59:19-23. [DOI] [PubMed] [Google Scholar]
- 17.Erf, G. F., W. G. Bottje, and T. K. Bersi. 1998. CD4, CD8 and TCR defined T-cell subsets in thymus and spleen of 2- and 7-week old commercial broiler chickens. Vet. Immunol. Immunopathol. 62:339-348. [DOI] [PubMed] [Google Scholar]
- 18.Flaminio, M. J., B. R. Rush, and W. Shuman. 1998. Immunologic function in horses after non-specific immunostimulant administration. Vet. Immunol. Immunopathol. 63:303-315. [DOI] [PubMed] [Google Scholar]
- 19.Flaminio, M. J., B. R. Rush, E. G. Davis, K. Hennessy, W. Shuman, and M. J. Wilkerson. 2002. Simultaneous flow cytometric analysis of phagocytosis and oxidative burst activity in equine leukocytes. Vet. Res. Commun. 26:85-92. [DOI] [PubMed] [Google Scholar]
- 20.Freeman, K. P., J. F. Roszel, J. M. McClure, R. Mannsman, P. E. Patton, and S. Naile. 1993. A review of cytological specimens from horses with and without clinical signs of respiratory disease. Equine Vet. J. 25:523-526. [DOI] [PubMed] [Google Scholar]
- 21.Furr, M. O., M. V. Crisman, J. Robertson, O. Barta, and W. S. Swecker, Jr. 1992. Immunodeficiency associated with lymphosarcoma in a horse. J. Am. Vet. Med. Assoc. 201:307-309. [PubMed] [Google Scholar]
- 22.Gabrilivich, D., and L. Serebrovskaya. 1991. Assessment of phagocytic activity in whole blood using laser flow cytometry. J. Immunol. Methods 140:289-290. [DOI] [PubMed] [Google Scholar]
- 23.Garn, H., A. Friedetzky, G. S. Davis, D. R. Hemenway, and D. Gemsa. 1997. T-lymphocyte activation in the enlarged thoracic lymph nodes of rats with silicosis. Am. J. Respir. Cell. Mol. Biol. 16:309-316. [DOI] [PubMed] [Google Scholar]
- 24.Grabstein, K., J. Eisenman, K. Shanebeck, C. Rauch, S. Srinivasan, V. Fung, C. Beers, J. Richardson, M. A. Schoenborn, M. Ahdieh, L. Johnson, M. R. Alderson, J. D. Watson, D. M. Anderson, and J. G. Giri. 1994. Cloning of T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264:965. [DOI] [PubMed] [Google Scholar]
- 25.Greene, W. C., J. M. Depper, M. Kronke, and W. J. Leonard. 1986. The human interleukin-2 receptor: analysis of structure and function. Immunol. Rev. 92:29-48. [DOI] [PubMed] [Google Scholar]
- 26.Hadden, J. W. 1993. Immunostimulants. Immunol. Today 14:275. [DOI] [PubMed] [Google Scholar]
- 27.Herholz, C., R. Straub, and A. Busato. 2001. The variability and repeatability of indices derived from the single-breath diagram for CO2 in horses with chronic obstructive pulmonary disease and the effect of lobelin hydrochloride on these indices. Vet. Res. Commun. 25:401-412. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann, L. M., W. P. Cheevers, W. C. Davis, D. P. Knowles, and K. I. O'Rourke. 2003. CD21-positive follicular dendritic cells: a possible source of PrPSc in lymph node macrophages of scrapie-infected sheep. Am. J. Pathol. 162:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins, D. A. 1981. Markers for T and B lymphocytes and their application to animals. Vet. Bull. 51:925-963. [Google Scholar]
- 30.Horohov, D. W., A. Dimock, P. Guirnalda, R. W. Folsom, K. H. McKever, and K. Malinowski. 1999. Effect of exercise on the immune response of young and old horses. Am. J. Vet. Res. 60:643-647. [PubMed] [Google Scholar]
- 31.Horohov, D. W., J. H. Kydd, and D. Hannant. 2002. The effect of aging on T cell responses in the horse. Dev. Comp. Immunol. 26:121-128. [DOI] [PubMed] [Google Scholar]
- 32.Hviid, L., A. Felsing, and T. G. Theander. 1993. Kinetics of human T-cell expression of LFA-1, IL-2 receptor, and ICAM-1 following antigenic stimulation in vitro. J. Clin. Lab. Immunol. 40:163-171. [PubMed] [Google Scholar]
- 33.Kumar, R. K. 1989. Quantitative immunohistologic assessment of lymphocyte populations in the pulmonary inflammatory response to intratracheal silica. Am. J. Pathol. 135:605-614. [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar, R. K., W. Li, and R. O'Grady. 1990. Activation of lymphocytes in the pulmonary inflammatory response to silica. Immunol. Investig. 19:363-372. [DOI] [PubMed] [Google Scholar]
- 35.Kydd, J., D. F. Antczak, W. R. Allen, D. Barbis, G. Butcher, W. Davis, W. P. Duffus, N. Edington, G. Grünig, M. A. Holmes, D. P. Lunn, J. McCulloch, A. O'Brien, L. E. Perryman, A. Tavernor, S. Williamson, and C. Zhang. 1994. Report of the First International Workshop on Equine Leucocyte Antigens, Cambridge, United Kingdom, July 1991. Vet. Immunol. Immunopathol. 42:3-60. [DOI] [PubMed] [Google Scholar]
- 36.Lunney, J. K., K. Walker, T. Goldman, B. Aasted, A. Bianchi, R. Binns, S. Licence, R. Bischof, M. Brandon, F. Blecha, T. L. Kielian, D. S. McVey, R. M. Chu, M. Carr, C. Howard, P. Sopp, W. Davis, P. Dvorak, J. Dominguez, A. Canals, J. M. Sanchez Vizcaino, Y. B. Kim, H. Laude, C. R. Mackay, U. Magnusson, K. McCullough, M. Misfeldt, M. Murtaugh, T. Molitor, C. Choi, R. Pabst, R. M. Parkhouse, S. Denham, H. Yang, M. Pescovitz, R. Pospisil, H. Tlaskalova, A. Saalmueller, E. Weiland, H. Salmon, D. Sachs, S. Arn, M. Shimizu, C. Stokes, K. Stevens, L. Valpotic, F. Zuckermann, and R. Husmann. 1994. Overview of the first international workshop to define swine leukocyte cluster of differentiation (CD) antigens. Vet. Immunol. Immunopathol. 43:193-206. [DOI] [PubMed] [Google Scholar]
- 37.Marlin, D. J., C. A. Roberts, R. C. Schroter, and P. Lekeux. 2000. Respiratory responses of mature horses to intravenous lobeline bolus. Equine Vet. J. 32:200-207. [DOI] [PubMed] [Google Scholar]
- 38.Marr, K. A., M. Koudadoust, M. Black, and S. A. Balajee. 2001. Early events in macrophage killing of Aspergillus fumigatus conidia: new flow cytometric viability assay. Clin. Diagn. Lab. Immunol. 8:1240-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massoco, C., and J. Palermo-Neto. 2003. Effects of midazolam on equine innate immune response: a flow cytometric study. Vet. Immunol. Immunopathol. 95:11-19. [DOI] [PubMed] [Google Scholar]
- 40.Mohr, C., D. Gemsa, C. Graebner, D. R. Hemenway, K. O. Leslie, P. M. Absher, and G. S. Davis. 1991. Systemic macrophage stimulation in rats with silicosis: enhanced release of tumor necrosis factor-alpha from alveolar and peritoneal macrophages. Am. J. Respir. Cell. Mol. Biol. 5:395-402. [DOI] [PubMed] [Google Scholar]
- 41.Moore, B. R. 1996. Lower respiratory tract disease. Vet. Clin. N. Am. Equine Pract. 12:457-472. [DOI] [PubMed] [Google Scholar]
- 42.Moore, B. R., S. Krakowka, J. M. Cummins, and J. T. Robertson. 1996. Changes in airway inflammatory cell populations in standard bred racehorses after interferon-alpha administration. Vet. Immunol. Immunopathol. 49:347-358. [DOI] [PubMed] [Google Scholar]
- 43.Moore, B. R., S. Krakowska, and J. T. Robertson. 1996. Evaluation of an immunostimulant in preventing shipping related respiratory disease. J. Equine Vet. Sci. 16:78. [Google Scholar]
- 44.Noguchi, M., Y. Nakamura, S. M. Russell, S. F. Ziegler, M. Tsang, X. Cao, and W. J. Leonard. 1993. Interleukin-2 receptor (gamma) chain: a functional component of the interleukin-7 receptor. Science 262:1877. [DOI] [PubMed] [Google Scholar]
- 45.Park, B. K., Y. H. Park, and K. S. Seo. 1999. Lymphocyte subpopulations of peripheral blood in pigs treated with an ionized alkali mineral complex. Seoul Univ. J. Vet. Sci. 24:67-74. [PubMed] [Google Scholar]
- 46.Pighetti, G. M., and L. D. Sordillo. 1996. Specific immune response of dairy cattle after primary inoculation with recombinant bovine interferon-gamma as an adjuvant when vaccinating against mastitis. Am. J. Vet. Res. 57:819-824. [PubMed] [Google Scholar]
- 47.Ragle, C., R. A. Jack, and C. Klimczak. 1992. Round-table discussion: EqStim immunostimulant. J. Equine Vet. Sci. 12:209. [Google Scholar]
- 48.Rees, R. C., and H. Parry. 1991. Macrophages in tumor immunity, p. 3-423. In C. E. Lewis and J. O. McGee (ed.), Macrophage. IRL Press, New York, N.Y.
- 49.Robb, R. J., W. C. Greene, and C. M. Rusk. 1984. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J. Exp. Med. 160:1126-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossdale, P. D., R. Hopes, N. J. Digby, and K. Offord. 1985. Epidemiological study of wastage among racehorses 1982 and 1983. Vet. Rec. 116:66-69. [DOI] [PubMed] [Google Scholar]
- 51.Rush, B. R., and M. J. Flaminio. 2000. Immunomodulation in horses. Vet. Clin. N. Am. Equine Pract. 16:183-197. [DOI] [PubMed] [Google Scholar]
- 52.Russell, S. M., A. D. Keegan, N. Harada, Y. Nakamura, M. Noguchi, P. Leland, M. C. Friedmann, A. Miyajima, R. K. Puri, W. E. Paul, and W. J. Leonard. 1993. Interleukin-2 receptor (gamma) chain: a functional component of the interleukin-4 receptor. Science 262:1880. [DOI] [PubMed] [Google Scholar]
- 53.Russell, S. M., J. A. Johnston, M. Noguchi, M. Kawamura, C. M. Bacon, M. C. Friedmann, M. Berg, D. W. McVicar, B. A. Witthuhn, O. Silvennoinen, A. S. Goldman, F. C. Schmalstieg, J. N. Ihle, J. J. O'Shea, and W. J. Leonard. 1994. Interaction of IL-2R (beta) and (gamma)/sub c/ chains with Ja. Science 266:1042. [DOI] [PubMed] [Google Scholar]
- 54.Sager, H., W. C. Davis, D. A. Dobbelaere, and T. W. Jungi. 1997. Macrophage-parasite relationship in theileriosis. Reversible phenotypic and functional dedifferentiation of macrophages infected with Theileria annulata. J. Leukoc. Biol. 61:459-468. [DOI] [PubMed] [Google Scholar]
- 55.Schwab, R., L. M. Pfeffer, P. Szabo, D. Gamble, C. M. Schnurr, and M. E. Weksler. 1990. Defective expression of high affinity IL-2 receptors on activated T cells from aged humans. Int. Immunol. 2:239-246. [DOI] [PubMed] [Google Scholar]
- 56.Shafer-Weaver, K. A., and L. M. Sordillo. 1997. Bovine CD8+ suppressor lymphocytes alter immune responsiveness during the postpartum period. Vet. Immunol. Immunopathol. 56:53-64. [DOI] [PubMed] [Google Scholar]
- 57.Smith, K. A. 1998. Interleukin-2: inception, impact, and implications. Science 240:1169-1176. [DOI] [PubMed] [Google Scholar]
- 58.Song, L., Y. H. Kim, R. K. Chopra, J. J. Proust, J. E. Nagel, A. A. Nordin, and W. H. Adler. 1993. Age-related effects in T cell activation and proliferation. Exp. Gerontol. 28:313-321. [DOI] [PubMed] [Google Scholar]
- 59.Speert, D. P. 1991. Macrophages in bacterial infection, p. 3-423. In C. E. Lewis and J. O. McGee (ed.), Macrophage. IRL Press, New York, N.Y.
- 60.Studer, U., E. Marti, D. Stornetta, S. Lazary, and H. Gerber. 1997. The therapy of equine sarcoid with a non-specific immunostimulator—the epidemiology and spontaneous regression of sarcoids. Schweiz. Arch. Tierheilkd. 139:385-391. [PubMed] [Google Scholar]
- 61.Sweeney, C. R., R. H. Whitlock, D. A. Meirs, S. C. Whitehead, and S. O. Barningham. 1987. Complications associated with Streptococcus equi infection on a horse farm. J. Am. Vet. Med. Assoc. 191:1446-1448. [PubMed] [Google Scholar]
- 62.Sweeney, C. R., C. E. Benson, R. H. Whitlock, D. A. Meirs, S. O. Barningham, S. C. Whitehead, and D. Cohen. 1989. Description of an epizootic and persistence of Streptococcus equi infections in horses. J. Am. Vet. Med. Assoc. 194:1281-1286. [PubMed] [Google Scholar]
- 63.Tizard, I. 1992. Veterinary immunology, 3rd ed., p. 72-84. W. B. Saunders, Philadelphia, Pa.
- 64.Tizard, I. 1993. Treatment of respiratory disease by means of immunomodulators. Presented at the 12th Annual Meeting of the Veterinary Comparative Respiratory Society, Kennett Square, Pa.
- 65.Tumas, D. B., A. L. Brassfield, A. S. Travenor, M. T. Hines, W. C. Davis, and T. C. McGuire. 1994. Monoclonal antibodies to the equine CD2 T lymphocyte marker, a pan-granulocyte/monocyte marker and a unique pan-B lymphocyte marker. Immunobiology 192:48-64. [DOI] [PubMed] [Google Scholar]
- 66.Tunon, A. M., H. Rodriguez-Martinez, A. Nummijarvi, and U. Magnusson. 1999. Influence of age and parity on the distribution of cells expressing major histocompatibility complex class II, CD4, or CD8 molecules in the endometrium of mares during estrus. Am. J. Vet. Res. 60:1531-1535. [PubMed] [Google Scholar]
- 67.Vail, C. D., A. J. Nestved, and J. B. Martins. 1990. Adjunct treatment of equine respiratory disease complex (ERDC) with the Propionibacterium acnes immunostimulant, EqStim. J. Equine Vet. Sci. 10:399. [Google Scholar]
- 68.Weingartl, H. M., M. Sabara, J. Pasick, E. van Moorlehem, and L. Babiuk. 2002. Continuous porcine cell lines developed from alveolar macrophages: partial characterization and virus susceptibility. J. Virol. Methods 104:203-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westermann, J., and R. Pabst. 1990. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol. Today 11:406-410. [DOI] [PubMed] [Google Scholar]
- 70.Woldehiwet, Z. 1991. Lymphocyte subpopulations in peripheral blood of sheep experimentally infected with tick-borne fever. Res. Vet. Sci. 51:40-43. [DOI] [PubMed] [Google Scholar]
- 71.Wong, C. W., H. L. Thomson, and Y. H. Thong. 1990. Effect of strenuous exercise on chemiluminescence response of equine alveolar macrophages. Equine Vet. J. 22:33-35. [DOI] [PubMed] [Google Scholar]
- 72.Yelle, M. T. 1987. Clinical aspects of Streptococcus equi infection. Equine Vet. J. 19:158-162. [DOI] [PubMed] [Google Scholar]
- 73.Yoo, B. W., S. I. Choi, S. H. Kim, S. J. Yang, H. C. Koo, N. H. Kown, S. H. Seo, B. K. Park, H. S. Yoo, and Y. H. Park. 2001. Immunostimulatory effects of anionic alkali mineral complex solution Barodon in porcine lymphocytes. J. Vet. Sci. 2:15-24. [PubMed] [Google Scholar]
- 74.Yoo, B. W., S. I. Choi, S. H. Kim, S. J. Yang, H. C. Koo, N. H. Kown, S. H. Seo, B. K. Park, H. S. Yoo, and Y. H. Park. 2002. Immunostimulatory effects of anionic alkali mineral complex solution Barodon in porcine lymphocytes. J. Swine Health Prod. 10:265-270. [PubMed] [Google Scholar]
- 75.Ziebell, K. L., H. Steinmann, D. Kretzdorn, T. Schlapp, K. Failing, and N. Schmeer. 1997. The use of Baypamun N in crowding associated infectious respiratory disease: efficacy of Baypamun N (freeze dried product) in 4-10 month old horses. Zentbl. Veterinarmed. B 44:529-536. [DOI] [PubMed] [Google Scholar]