Abstract
The innexins represent a highly conserved protein family, the members of which make up the structural components of gap junctions in invertebrates. We have isolated and characterized a Caenorhabditis elegans gene inx-6 that encodes a new member of the innexin family required for the electrical coupling of pharyngeal muscles. inx-6(rr5) mutants complete embryogenesis without detectable abnormalities at restrictive temperature but fail to initiate postembryonic development after hatching. inx-6 is expressed in the pharynx at all larval stages, and an INX-6::GFP fusion protein showed a punctate expression pattern characteristic of gap junction proteins localized to plasma membrane plaques. Video recording and electropharyngeograms revealed that in inx-6(rr5) mutants the anterior pharyngeal (procorpus) muscles were electrically coupled to a lesser degree than the posterior metacorpus muscles, which caused a premature relaxation in the anterior pharynx and interfered with feeding. Dye-coupling experiments indicate that the gap junctions that link the procorpus to the metacorpus are functionally compromised in inx-6(rr5) mutants. We also show that another C. elegans innexin, EAT-5, can partially substitute for INX-6 function in vivo, underscoring their likely analogous function.
INTRODUCTION
Intercellular communication is essential for coordinating cellular processes such as pattern formation during development, maintenance of metabolic homeostasis, and synchronous muscle contraction (Bruzzone et al., 1996; Phelan and Starich, 2001). This communication is mediated in part via gap junctions, which permit small molecule flow between the cytoplasm of connected cells. Gap junctions between excitable cells, i.e., muscles and neurons, allow these cells to be electrically coupled, facilitating synchronous changes in membrane potential (Bruzzone et al., 1996).
Two protein families can form gap junctions: the vertebrate-specific connexins and the innexins, which are typical to invertebrates. The connexins form integral membrane protein assemblies, called connexons, consisting of six subunits. Connexons in adjacent cells interact in the extracellular space to form the gap junction (Yeager and Nicholson, 1996; Unger et al., 1999).
Although gap junctions were first characterized in invertebrate organisms (Furshpan and Potter, 1957), the genes that encode their structural proteins were only recently characterized, mainly because the innexins have no significant sequence similarity to the connexins (Phelan et al., 1998a).
Nevertheless, innexins are both necessary and sufficient to form gap junctional channels. Innexin mutants lack dye coupling between muscle and neuronal cells (Starich et al., 1996; Todman et al., 1999). Moreover, the innexins Ce-inx-3, Dm-inx-2, and shaking-B can form gap junctional channels when expressed in the paired Xenopus oocyte system. (Phelan et al., 1998b; Landesman et al., 1999; Stebbings et al., 2000). Structure predictions based on primary sequence indicate that, like the connexins, innexins possess four transmembrane domains with the same overall topology (Phelan and Starich, 2001). Thus, homology between connexins and innexins may be evident at the structural level.
Both the connexins and innexins constitute large gene families, presumably reflecting their diverse roles in organisms. About 20 connexins have been identified in vertebrates (White and Paul, 1999), 25 innexins in C. elegans, and 8 in Drosophila (Phelan and Starich, 2001). Because both connexins (Nicholson et al., 1987) and innexins (Stebbings et al., 2000) can form heteromeric channels, the subunit composition of which can have important effects on the properties of the gap junction formed, a large number of distinct gap junctional channels could assemble in vivo.
Several human diseases have been linked to connexin polymorphisms (Bergoffen et al., 1993; Kelsell et al., 1997; Shiels et al., 1998) and directed mutations in mice have revealed diverse roles for connexins in transplacental nutrient transfer (Gabriel et al., 1998), ovulation (Simon et al., 1997), and cardiac development and function (Cx40, Cx43, and Cx45) (Kanter et al., 1994; Reaume et al., 1995; Ewart et al., 1997; Simon et al., 1998).
Mutations in four innexins have been characterized in Drosophila (Curtin et al., 1999; Bauer et al., 2002; Tazuke et al., 2002). The shaking-B (lethal) mutation causes animals to die early, possibly due to feeding defects (Crompton et al., 1995), whereas the shaking-B (neural) mutation disrupts the gap junctions between giant fibers and its postsynaptic motorneuron partners (Thomas and Wyman, 1984; Krishnan et al., 1993; Phelan et al., 1996; Sun and Wyman, 1996). Ogre mutations lead to defects in optic lobe development and abnormal electrical activity in the eye (Lipshitz and Kankel, 1985; Watanabe and Kankel, 1990).
UNC-7 was the first innexin identified in C. elegans, and mutations in unc-7 cause impaired forward locomotion and ivermectin resistance. The uncoordinated phenotype could result from the aberrant formation of an UNC-7-dependent channel or may reflect ectopic electrical junctions between motorneurons and interneurons in unc-7 mutants (Starich et al., 1993; Dent et al., 2000). The mutant phenotype of another innexin gene, unc-9, is very similar to that of unc-7, indicating that UNC-9 subunit may partner with UNC-7 to form the functional gap junction (Barnes and Hekimi, 1997).
Several C. elegans innexins are expressed in the pharynx (Phelan and Starich, 2001), probably because the control of current flow through coupled muscles requires gap junctions with diverse properties in this organ. The pharynx is a neuromuscular pump that has some developmental and functional similarities to the heart (Haun et al., 1998; Maduro et al., 2001) and is required for ingesting food (Figure 4, A and B). The pharyngeal muscles are extensively gap junctioned so that a pump results in a compound action potential and simultaneous contraction of most of the pharyngeal muscles. This compound action potential can be measured by a simple electrophysiological technique, the electropharyngeogram (EPG) (Avery and Thomas, 1997).
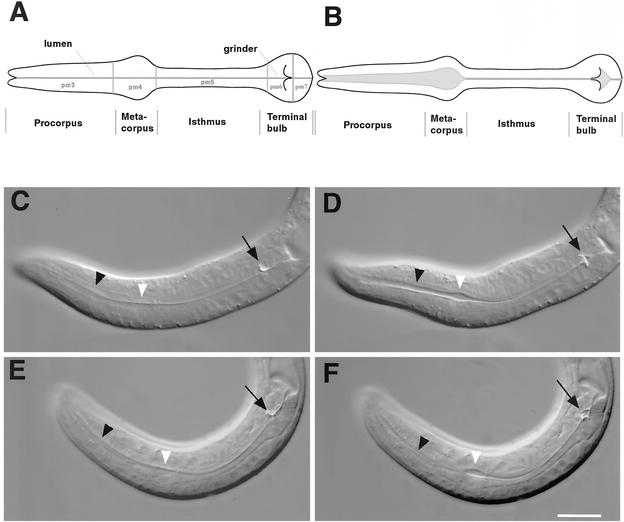
Figure 4.
inx-6(rr5) disrupts appropriate lumenal opening of the procorpus during pumping at restrictive temperature. (A) Lateral view of C. elegans pharyngeal anatomy with anterior to the left. The pharynx is divided into three functional components, which consist of five types of large muscles: the corpus, which can be further subdivided into the procorpus (pm3) and the metacorpus (pm4), the isthmus (pm5), and the terminal bulb (pm6 and pm7). (B) During pumping, the corpus contracts simultaneously with the terminal bulb, thereby opening the lumen of the pharynx to allow the entry of bacteria. This is then followed by simultaneous muscle relaxation (Avery and Thomas, 1997). (C and E) Pharyngeal muscles in a relaxed state between pumps in N2 and inx-6(rr5) L1 larvae. The black arrows indicate the grinder in the terminal bulb. The black arrowheads indicate the procorpus lumen, and the white arrowheads indicate the metacorpus lumen. In wild-type animals (D), the terminal bulb muscle contraction inverts the grinder and the procorpus and metacorpus contract simultaneously, thereby opening the lumen of the whole corpus and allowing the influx of bacteria. In inx-6(rr5) animals (F), the terminal bulb is inverted during pumping (black arrow). However, the procorpus lumen (black arrowhead) does not open. Bar, 10 μm.
One explanation for the large number of innexins in the pharynx is that the control of current flow through coupled muscles requires gap junctions with diverse properties. eat-5, for instance, is expressed only in the muscles of the metacorpus and in the muscles of the isthmus, which connects the metacorpus with the terminal bulb of the pharynx (Avery, 1993). A mutation in eat-5 uncouples the muscles of the terminal bulb from those of the metacorpus but leaves intermuscular junctions within each bulb intact. In eat-5 mutants the metacorpus muscles contract in synchrony and the terminal bulb muscles contract in synchrony but, unlike in wild-type animals, contraction of the anterior and posterior pharynx is asynchronous (Starich et al., 1996).
To further understand the functions of innexins in C. elegans behavior and development, we characterized a temperature-sensitive mutation of the innexin family member inx-6. inx-6(rr5) mutants are unable to initiate development after hatching at restrictive temperature due to defects in pharyngeal pumping. Our characterization of this mutant suggests that inx-6 is required to couple muscle cells of the anterior pharynx.
MATERIALS AND METHODS
C. elegans Strains and Culture
C. elegans strains were cultured using standard techniques described by Brenner (1974). All experiments were performed at 20°C unless otherwise noted.
The Bristol strain N2 was used as the wild-type throughout. The following strains were also used: Bergerac strain RW7000, DA465 [eat-2(ad465) II], DA491 [dpy-20(e1282) unc-30(e191) IV], DR107 [unc-26(e205) dpy-4(e1166) IV], DR282 [dpy-13(e184) unc-31(e169) IV], JD118 [inx-6(rr5) IV; avr-15(ad1051) V], JD125 [inx-6(rr5) IV; exp-2(sa26ad1426) avr-15(ad1051) V], MT2115 (nDf27/nT1 IV; +/nT1 V), PD4792 (mIs11 IV), and VT765 [unc-36(e251); maIs103 [rnr::GFP unc-36(+)] X].
A Genetic Screen for Temperature-sensitive Mutants Defective in the Initiation of Postembryonic Development
The strain VT765 [unc-36(e251); maIs103 [rnr::GFP unc-36(+)]] (Hong et al., 1998) was mutagenized with 47 mM ethylmethanesulfonate as described previously (Brenner, 1974). To select mutants efficiently, we used a potent inhibitor of DNA replication, hydroxyurea (HU), as a tool to select against animals that initiate postembryonic development at 25°C. HU-affected adult animals are uncoordinated and sterile due to effects on the development of the neuroblast lineage and germline. However, HU has no effect if worms do not initiate the cell divisions typical of early postembryonic development. Arrested L1s were transferred to individual NGM plates without HU and allowed to recover at 15°C for further analysis.
Temperature-Shift Experiments
Mutant embryos were isolated with alkaline/hypochlorite and hatched in M9 buffer (20 mM KH2PO4, 40 mM Na2HPO4, 85 mM NaCl 85, 1 mM MgSO4). The hatched L1 larvae were cultured at 15°C and after every 8 h, 50 worms were transferred to plates that were preequilibrated at 25°C. Animals that grew to reproductive maturity were scored as a percentage of the total population present on the plate after 48 h.
Plasmid Constructs
pMR341 contains a 8.2-kb SalI fragment from the cosmid T23F6, which was subcloned into vector p-Bluescript (Stratagene, La Jolla, CA). pMR342 contains a 6.2-kb SacII/SmalI fragment from the cosmid T23F6 that was subcloned into p-Bluescript (Stratagene). pMR346 (inx-6::GFP) is a transcriptional green fluorescent protein (GFP) fusion containing 3 kb of sequence 5′ to the XbaI site upstream of the inx-6 translation start site. pMR347 (inx-6::INX-6::GFP) is a translational GFP fusion containing the same upstream sequence described above, driving an inx-6 cDNA amplified from a cDNA library (a gift from Dr. A. La Volpe, International Institute of Genetics and Biophysics, Napoli, Italy) by using the primers 5′catgtctagaatggcgtcgcaagttggag3′ (upstream) and 5′atgggatccagtatgcttaatcgatttgacaaatg3′ (downstream) and inserted in frame into the Fire Lab vector pPD95.77. In pMR348, the inx-6 promoter of pMR342 was removed by digestion with SacII/XbaI and replaced with a SacII/XbaI fragment of the myo-2 promoter amplified from the Fire Lab vector pPD30.69 by using primers 5′catgcatctagaacctttgggtcctttggc3′ (upstream) and 5′atatccgcggaggatccccagcttgcat3′ (downstream). In pMR350 (inx-6::EAT-5), the coding sequence of inx-6 in pMR342 was replaced with a XbaI/PstI polymerase chain reaction fragment containing eat-5 coding sequence.
Germline Transformation
Germline transformation was performed as described previously (Mello et al., 1991). Cosmids and plasmids for rescue experiments were injected at a concentration of 20 μg/ml, whereas the cotransformation marker pRF4 (rol-6 D) was injected at a concentration of 100 μg/ml. For rescue experiments, mutant animals were injected and maintained at 15°C. Adult F2 animals exhibiting a Rol phenotype were transferred to 25°C, and rescue of L1 arrest of their progeny (F3) was scored.
RNA Interference
inx-6 double-stranded RNA (dsRNA) was produced and injected according to Fire et al. (1998). The gel-purified template (1 μg) was used for in vitro transcription reactions and the RNA was then phenol/chloroform extracted, ethanol precipitated, and annealed. inx-6 dsRNA was injected into N2 or MR127 animals at a concentration of 1 mg/ml. The injected animals were transferred daily to new plates, and development of F1 progeny was monitored.
Video Recording and Electropharyngeogram
inx-6(rr5) mutant worms grown at 25°C were incubated in 1 mM levamisole (to induce paralysis) and 10 mM serotonin (to induce pumping) in M9 buffer for 30 min (both drugs from Sigma-Aldrich, St. Louis, MO). Worms were mounted in M9 buffer on 35 × 60-mm coverslips. A recording chamber was created by applying a ring of gasket sealant to the surface of the coverslip and allowing it to cure overnight. Worms were viewed on an Olympus IX-70 inverted microscope with Nomarski optics and a 40× lens. EPGs were recorded using 1.2 OD borosilicate suction pipettes pulled on a Sutter P-97 pipette puller (Sutter Instrument, Novato, CA). Recording of EPGs was essentially as described in Raizen et al. (1994) by using a Warner Instrument (Hamden, CT), patch-clamp PC-501A with a 1-GΩ headstage but without filtering in the amplifier. EPGs were digitized using a Digitdata 1322A and recorded using Clampex 8.1 software (Axon Instruments, Union City, CA). Recordings were formatted and digitally filtered using a 1-kHz Gaussian filter with Clampfit 8.1 software (Axon Instruments).
Video images were recorded using a Hitachi KP-M1U charge-coupled device camera and frames were captured using a Matrox Meteor-II frame grabber (Matrox Electronic Systems, Dorval, QC, Canada). The EPG signal was used to trigger the frame grabber to collect 15 frames. To do this, the EPG signal was sent to the a Digitimer D.130 spike processor (Medical Systems, Great Neck, NY) which, upon encountering an E-spike, simultaneously sent a signal to the Digidata 1322A and to the frame grabber. The signal to the Digidata initiated the recording of the EPG and the signal to the frame grabber initiated image acquisition (acquisition software, written by J.A. Dent with MIL-lite, available on request). Grabbed images were deinterlaced and digitally condensed in the x-axis to restore the aspect ratio yielding an image every ∼17 ms. By simultaneously recording the EPG in one channel and the camera synchronization signal in a second channel, it was possible to correlate in time both the images and the EPG.
Dye Diffusion Experiment
Newly hatched L1 larvae were washed with M9 buffer and soaked in saturated carboxyfluorescein (Sigma-Aldrich) solution (∼20 mM) for at least 3 h. Due to the pharyngeal abnormalities in inx-6(rr5) animals, inx-6(rr5) larvae were initially soaked in the carboxyfluorescein solution at permissive temperature (15°C). Animals were then transferred to 25°C for 5 h to allow worms to eliminate the wild-type inx-6 gene product before laser treatment. After thoroughly washing with M9 buffer, animals were mounted on a 2% agarose pad as described with 1 mM levamisole and 10 mM serotonin. Dye was introduced into the pharyngeal muscles by focusing a low-intensity laser pulse at the posterior edge of the grinder. Images of the diffusion of the fluorescent dye were captured at 10-s intervals by using identical exposure time. The dye diffusion pattern was verified 10 min after the laser treatment to confirm that the dye distribution remained unchanged.
Microscopy and Image Processing
Light microscopy was performed using a Leica DMR compound microscope with Nomarski optics. Images were captured with a Hamamatsu C4742–95 digital camera. Image processing, analysis and computational deconvolution were performed using Openlab 3.07 software (Improvision, Lexington, MA) and Adobe Photoshop.
RESULTS
rr5 Is a Temperature-sensitive Mutation that Blocks the Initiation of Postembryonic Development
In a screen to isolate temperature-sensitive mutants that are unable to initiate postembryonic development, we isolated a mutant (rr5) that could not initiate postembryonic development at restrictive temperature (25°C). Growth at restrictive temperature does not affect embryogenesis in rr5 animals because they show no embryonic lethality and their morphology at hatching seems identical to wild-type animals after culture at 25°C. However, newly hatched rr5 larvae are unable to initiate postembryonic development at restrictive temperature, and worms seem starved and remain at the L1 stage for several days before dying. After 5–6 d at 25°C, developmental events typical of L1 stage development, including cell divisions or P-neuroblast nuclear migrations, were undetectable in rr5-arrested animals. After transfer from restrictive temperature to permissive temperature (15°C), animals recover from their arrested state and grow into normal adults without obvious developmental or reproductive consequence.
Therefore, rr5 seems to be absolutely required to initiate postembryonic development. Because this allele is temperature sensitive, we sought to determine when rr5 activity was required during development. By down-shifting to permissive or up-shifting to restrictive temperature, respectively, an approximate temporal window can be obtained to describe when a gene product is required for wild-type function. Normally this would be performed by executing an up-shift and a down-shift time course; however, due to the highly penetrant developmental arrest phenotype of rr5 animals at 25°C, only up-shift experiments could be performed (Table 1). By shifting rr5 animals to restrictive temperature at various stages during development, we found that rr5 disrupts a gene that is necessary during the L1 and L2 stages for correct larval development. All post-L2 stage up-shifted animals grew to become fertile at restrictive temperature and produce viable progeny that arrested as hatchlings.
Table 1.
rr5 disrupts a gene required during early larval development
| Time (h) | Stage | Adult animals after up-shift (%) |
|---|---|---|
| 8 | L1 | 0.0 ± 0.0 |
| 16 | L1 | 0.6 ± 1.1 |
| 24 | L1 | 2.1 ± 1.0 |
| 32 | L2 | 8.7 ± 1.3 |
| 40 | L2 | 40.1 ± 0.5 |
| 48 | L3 | 47.1 ± 5.8 |
| 56 | L3 | 75.4 ± 3.1 |
| 64 | L3 | 96.6 ± 0.5 |
| 72 | L4 | 100 ± 0.0 |
rr5 mutant embryos maintained at 15°C were hatched in M9 buffer and then cultured at 15°C. At 8-h intervals, 50 worms were upshifted to restrictive temperature (25°C), and animals that reached the adult stage after 48 h in culture were scored. Results are expressed as percentage of the total worm population present on the plate. All progeny derived from upshifted rr5 animals that grew to adulthood at 25°C successfully terminated embryogenesis before recapitulating the L1 arrest phenotype typical of rr5 at 25°C.
rr5 Is a Temperature-sensitive Allele of an unc-7-like Protein inx-6
To understand how rr5 functions at the molecular level, we mapped rr5 initially using a sequence-tagged site mapping approach (Williams et al., 1992). Our results indicated that the mutation is on LG IV and to the left of dpy-20. Further three-point recombination mapping showed that rr5 is between dpy-20 and unc-31 and close to unc-31 (∼0.03 map units) (Figure 1A).
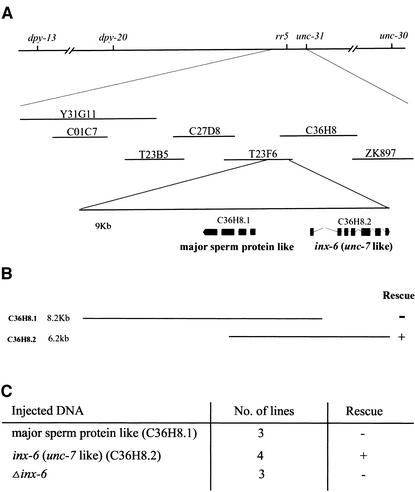
Figure 1.
Mutation in the C. elegans inx-6 gene causes the temperature-sensitive L1 arrest phenotype of rr5. (A) Genetic and physical map of the rr5 region. rr5 maps between dpy-20 and unc-31, 0.03 map units left of unc-31. (B) Two cosmids, T23F6 and C36H8, which share 9 kb of overlapping sequence, were found to completely rescue the larval arrest phenotype at 25°C. (C) Subclones of the overlapping region were used to test for transformation rescue. The C36H8.2 subclone, which contains a predicted gene inx-6, could fully rescue the mutant phenotype, whereas a second predicted gene on this cosmid C36H8.1 had no effect. The inx-6 coding region was mutated by inserting a fragment into one of the exons, which theoretically caused a frame-shift in the predicted inx-6 coding region. The resulting Δinx-6 could no longer rescue the mutant phenotype. All constructs were injected as described in MATERIALS AND METHODS with the dominant coinjection marker pRF4 (rol-6 D).
Six cosmids and one YAC that span this interval were chosen by consulting the C. elegans physical map (Figure 1A). Two cosmids, T23F6 and C36H8, were found to completely rescue the larval arrest phenotype of rr5 at 25°C, suggesting that a common region to both cosmids carried the wild-type rr5 gene product. The 9-kb overlapping region contains two predicted genes that encode a major sperm protein-like product and the innexin gene inx-6, an unc-7 homolog (Figure 1B). These two predicted genes were individually subcloned and injected to test for rescue. Transformation rescue experiments indicated that the inx-6–containing construct could fully rescue the mutant phenotype, whereas the second candidate had no effect on the rr5 phenotype (Figure 1C).
To confirm that inx-6 is indeed the mutated gene in rr5, the inx-6 coding region was frame shifted, and the mutant variant was assessed for its ability to rescue. The Δinx-6 construct could no longer complement the rr5 mutation, suggesting that rr5 is an allele of inx-6, which we will refer to as inx-6(rr5).
To address whether inx-6(rr5) is a weak or strong hypomorphic allele, and/or whether maternal products could rescue embryonic functions in this mutant background we compared the phenotype of inx-6(rr5) to that of an inx-6(RNAi) animal. Double-stranded RNA to any gene of interest can elicit a potent response in C. elegans referred to as RNA-mediated interference that is mediated by degrading targeted transcripts posttranscriptionally (Fire et al., 1998). In the F1 progeny of wild-type animals injected with double-stranded RNA corresponding to the inx-6 coding region, 5–10% animals displayed a postembryonic arrest phenotype very similar to that observed with inx-6(rr5) at 25°C, albeit at lower penetrance. To test whether residual wild-type gene activity may be present in the inx-6(rr5) mutant, we performed a similar RNAi experiment as described above in an inx-6(rr5) background. The F1 progeny of injected parents show the identical phenotype, in severity and penetrance, to the F1 progeny from uninjected inx-6(rr5) mutant parents kept at 25°C. These data suggest that the inx-6(rr5) mutant phenotype at 25°C likely represents a strong hypomorph or the inx-6 null phenotype. This was further confirmed by placing the inx-6(rr5) mutant chromosome in trans to a deficiency that uncovers this region (our unpublished data).
inx-6 Is a Member of a Highly Conserved Protein Family
inx-6 encodes a protein that belongs to the innexin family (invertebrate connexin analogs), which encode components of invertebrate gap junctions. The closest homolog to INX-6 is UNC-7 with 33% identity at the amino acid level. The strongest homology is seen among the conserved cysteine residues in the extracellular loops and in the transmembrane domains that play roles in normal channel regulation (Figure 2).
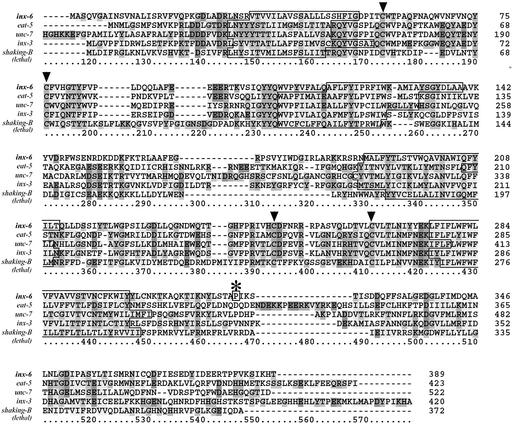
Figure 2.
INX-6 shares substantial homology with other C. elegans and Drosophila innexin family members. The pileup alignment of INX-6, Drosophila melanogaster shaking-B (lethal) and the other close homologs in C. elegans was done using Clustal method. Four predicted transmembrane regions typical of INNEXINS are outlined, and the conserved cysteine residues presumably involved in maintaining the connections between hemichannels in the extracellular loops are indicated by the arrowheads. * indicates the point mutation identified in rr5.
The inx-6 genomic sequence from rr5 was sequenced to identify a lesion within this gene that would cause the inx-6(rr5) arrest phenotype. A G/C to A/T transition, typical of EMS-induced lesions was identified, and caused a P353L change in the INX-6 protein near the C termini. That a point mutation in the C termini could disrupt the protein function at the restrictive temperature is consistent with a previous study in which the C terminus was shown to be very important for the normal channel function of connexins (Yeager and Nicholson, 1996; Morley et al., 1996; Wang and Peracchia, 1998).
inx-6 Is Expressed Exclusively in Pharyngeal Tissues
To understand more about how inx-6 affects postembryonic development, we examined its expression pattern by using GFP reporters. Two reporter constructs were generated: a transcriptional fusion that consisted of the inx-6 promoter driving GFP and a translational fusion that fused the inx-6 promoter to the inx-6 cDNA, which in turn was fused in frame to GFP. Results from both GFP-expressing transgenes indicated that inx-6 is first detected during embryogenesis at the comma stage, in anterior cells that are likely to be the pharyngeal precursors. This pharynx-specific expression expands during the development of the pharynx to the end of embryogenesis, whereas inx-6 continues to be expressed in the corpus muscles and isthmus marginal cells of the pharynx throughout the larval and adult stages (Figure 3).
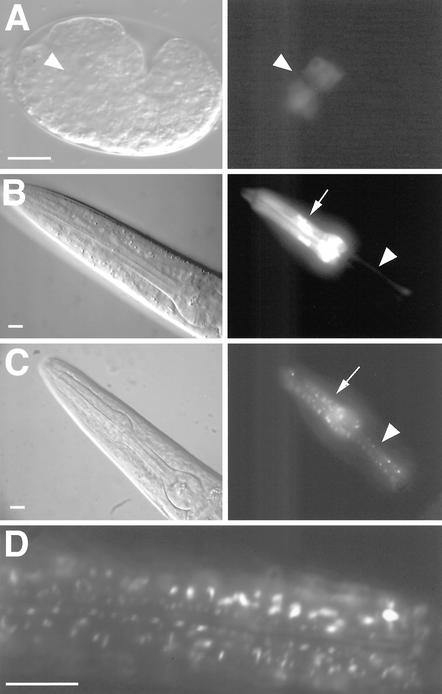
Figure 3.
inx-6 expression pattern during development. Differential interference contrast (left) and GFP fluorescence images (right) of transcriptional (inx-6::GFP) and translational (inx-6::INX-6::GFP) reporters. (A) Expression of the inx-6::GFP in embryo (anterior to the left) begins at the comma stage in anterior cells within the presumptive pharyngeal mesoderm (indicated by arrowhead). (B) inx-6::GFP is expressed in the corpus muscles (arrow) and isthmus marginal cells (arrowhead) throughout postembryonic development. (C) Expression of the inx-6::INX-6::GFP translational fusion reporter in a young adult hermaphrodite reveals a characteristic punctate expression pattern in corpus muscles (arrow) and isthmus marginal cells (arrowhead). The translational fusion construct fully rescued the mutant phenotype at 25°C, suggesting that the punctate GFP expression pattern may faithfully depict the expression of INX-6 in vivo. (D) A magnified image shows the punctate expression pattern in the corpus. Bars, 10 μm.
The translational GFP fusion protein is expressed in a punctate expression pattern (Figure 3, C and D), which is very different from the transcriptional fusion (Figure 3B). Gap junctional channels often aggregate in plasma membranes to form plaques, an observation that was confirmed using immunocytochemistry in vertebrate cells (Kumar and Gilula, 1996). In invertebrates, this particular pattern has also been shown by the innexin protein wEST01007 (INX-3) in C. elegans (Starich et al., 1996). The characteristic expression pattern of INX-6 likely faithfully represents the normal expression of the INX-6 protein because it can fully rescue the mutant phenotype, suggesting that the punctate pattern that we observed for the full-length INX-6::GFP fusion may reflect the actual subcellular localization of INX-6. We were unable to determine what membrane surface the plaques associate with. The presence of plaques along the length of the muscle could indicate that gap junctions are being formed between the muscle and the intercalated marginal cells (Albertson and Thomson, 1976). However, we cannot rule out that some of the plaques are aggregated proteins moving between intracellular compartments.
inx-6(rr5) Mutant Pharyngeal Muscles Contract and Respond to Neuronal Stimuli
If inx-6 is expressed in the pharynx because it is necessary for efficient pharyngeal pumping, then the inx-6(rr5) mutant might arrest at the restrictive temperature because it cannot feed. To address this possibility, we scored the rate of pharyngeal pumping in recently hatched L1 stage larvae at both the permissive and restrictive temperatures. Pumping was scored by looking at the rhythmic motion of the grinder in the terminal bulb, the most obvious indicator of muscle contraction. In addition, we scored the worms both in the presence and absence of serotonin. In adult worms, serotonin increases pumping from a basal rate of ∼40 pumps/min to a maximal rate of ∼250 pumps/min via activation of a pacemaker motor neuron MC. At the permissive temperature, inx-6(rr5) showed the same pumping rate as wild-type animals, both in the presence and absence of serotonin, whereas at the restrictive temperature, its pumping rate was much slower, consistent with an effect of inx-6(rr5) on pharyngeal function (Table 2).
Table 2.
inx-6(rr5) reduces pharyngeal contraction frequency but does not affect the capacity to respond to serotonin (5HT)
|
Pumping rate (contractions/min)
|
|||
|---|---|---|---|
| Genotype | -5HT | +5HT | |
| 15°C | N2 | 134.4 ± 11.2 | 149.3 ± 16.6 |
| inx-6(rr5) | 131.9 ± 12.1 | 141.2 ± 15.1 | |
| eat-2(ad465) | 17.9 ± 4.6 | 16.9 ± 4.6 | |
| 25°C | N2 | 165.8 ± 15.3 | 182.6 ± 15.1 |
| inx-6(rr5) | 76.8 ± 12.3 | 103.6 ± 16.2 | |
| eat-2(ad465) | 16.6 ± 5.1 | 17.4 ± 5.8 | |
Wild-type N2 animals were used as positive control. All animals were examined at the L1 stage by mounting on 2% agarose pads with M9 buffer with (+5HT) or without (-5HT) 5 mM serotonin. The pumping rate was monitored using a compound microscope by counting the rhythmic motion of the grinder in the terminal bulb of individual animals over the course of 1 min beginning immediately after location of the specimens and adjustment of focus. Values are expressed as contractions/min ± SD observed from 20 different animals (n = 20).
To see whether the lower pumping frequency of inx-6(rr5)at the restrictive temperature was sufficient to explain the developmental arrest, we compared it with an eat-2 mutant. eat-2 encodes a pharyngeal muscle nicotinic acetylcholine receptor that is necessary for neurotransmission by the MC pacemaker motor neuron (Raizen et al., 1995). Because neurotransmission from the MC neuron is absent in the eat-2 mutant, the worms pump at a slow rate and are severely starved. Nevertheless, eat-2 mutants complete development and are fertile. Even at the restrictive temperature, inx-6(rr5) mutants pump faster than the eat-2 mutants, indicating that pumping rate alone is not sufficient to explain the developmental arrest of rr5. Unlike eat-2, the inx-6(rr5) mutant still responds to serotonin with an increased pumping rate, indicating that the MC motor neuron is still functional, and the pharyngeal muscle is still regulated by the nervous system (Table 2).
Pharyngeal Muscle Contraction is Unsynchronized in inx-6(rr5) Mutants
Because differences in pumping rate alone are not sufficient to explain the developmental arrest of inx-6(rr5), we wanted to know whether there was a defect in muscle coordination. On closer examination of L1 arrested inx-6(rr5) larvae, we found that >90% (136/150) of the worms lacked procorpus contraction when the terminal bulb was contracted compared with <2% (2/150) of wild-type starvation-arrested L1 worms (Figure 4). Without procorpus contraction, no food can enter the pharynx and worms therefore arrest development as a result of starvation.
The lack of muscle contraction could result from defects in muscle contraction or from defects in electrical excitability. If there were a defect in electrical activity, we should be able to see this in the EPG. Specifically, we hypothesized that inx-6(rr5) affected the flow of excitation from the metacorpus to the procorpus. To test this, we simultaneously recorded video and the EPG of mutant L3-L4 stage larvae that escaped arrest at the restrictive temperature (currents generated by L1 larvae are too small to measure by EPG).
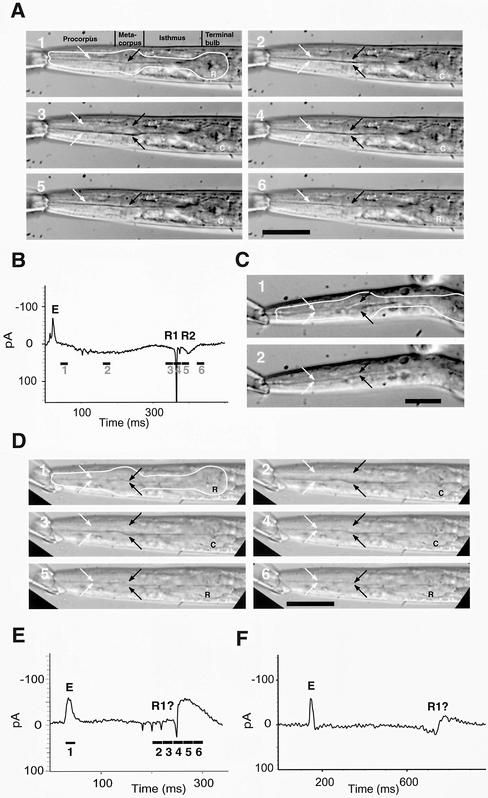
We first examined wild-type L3 stage larvae grown at 25°C, which showed the muscle motions typical of wild-type adults (Figure 5, A and B). In wild-type worms, the radially oriented muscles of the corpus contract slowly over a period of ∼200 ms (Avery and Thomas, 1997). The contraction pulls the walls of the pharyngeal lumen open and the opening of the lumen is the most obvious visual manifestation of muscle contraction. This contraction is terminated by a very rapid (<20 ms) relaxation of the muscles and closing of the lumen. The terminal bulb muscles are fully contracted for most of this time, and their relaxation is delayed slightly relative to corpus relaxation.
Figure 5.
Repolarization and relaxation of corpus muscles is desynchronized in the inx-6(rr5) mutant. (A and B) Video and EPG, respectively, of wild-type L3 larva. In all frames, the white arrows indicate the position of the lumenal walls in the procorpus and the black arrows indicate the position of the lumenal walls in the metacorpus. In the first frame, the outline of the pharynx is traced in white. In the terminal bulb (TB) a C indicates that the TB muscles are contracted and an R indicates they are relaxed. The period of time over which each video image was captured is indicated by a black bar under the EPG trace with the video frame number corresponding to that time point indicated below the bar. E marks the excitation spike that signals the beginning of the action potential and R1 and R2 mark the repolarization of the corpus and terminal bulb muscle, respectively. In frame 1, depolarization has occurred but there is almost no contraction of the corpus or terminal bulb. In frame 2, the terminal bulb is contracted but the corpus contraction is modest. In frame 3, the corpus reaches maximal contraction just before repolarization. In frame 4, the corpus muscle repolarizes and relaxes almost simultaneously although relaxation is not quite complete. In frame 5, the terminal bulb has repolarized but is still contracted. In frame 6, all muscles are relaxed. Bar, 50 μm. (C) L3 inx-6(rr5) that escaped the restrictive temperature. In frame 1, the metacorpus has reached the point of maximum contraction during the pump but the procorpus is still relaxed. In comparison, frame 2 shows complete relaxation of the pharyngeal muscle during an interpump interval. Bar, 25 μm. (D and E) Video and EPG of a 3-d-old L4 inx-6(rr5); avr-15(ad1051) mutant worm that escaped the restrictive temperature. The E spike(s) is broadened and there are four downward spikes in spite of the absence of M3 neurotransmission as a result of the avr-25(ad1051) mutation. Frame 1, the pharynx just before contraction begins. All muscles are fully relaxed. Frame 2, all muscles are at their point of maximum contraction indicated by the wide-open lumen. Frame 3, the procorpus muscle, and to a lesser extent, the metacorpus muscles, have begun to relax and the lumen is more constricted. Frame 4, the procorpus muscles have relaxed but the metacorpus is still mostly contracted. Frame 5, the terminal bulb has now relaxed but the metacorpus is still slightly open. Frame 6, all of the muscles are completely relaxed. Bar, 50 μm. (F) EPG trace of inx-6(rr5); exp-2(ad1426) avr-15(ad1051) that escaped restrictive temperature. The exp-2(ad1426) mutation eliminates the potassium channel that mediates fast muscle repolarization and hence the R spikes. With exp-2(ad1426) in the background, there are no R spikes, only a slow wave indicating the repolarization.
The timing of muscle relaxation is precisely coordinated with the electrical activity of the pharynx as revealed by the EPG. The EPG is an extracellular recording technique whose trace reflects the time derivative of the muscle membrane potential. In the EPG trace, one or two E spikes correspond to the depolarization of the pharyngeal muscles and one or two R spikes represent the repolarization and return to resting potential. Inhibitory postsynaptic potentials (IPSPs) of the motor neuron M3 usually occur as downward spikes during the plateau phase, which is the period of muscle depolarization delimited by the E and R spikes. Corpus and terminal bulb contraction begin immediately after the E spikes. The corpus muscles are maximally contracted just before the first R spike (R1) and relax immediately after. There is often a much smaller R spike (R2) that follows R1 and precedes terminal bulb relaxation. The only unusual feature of the EPGs we recorded from wild-type L3 stage larvae was the absence of M3 IPSPs during the plateau phase. This is an effect of levamisole, which we used to paralyze the worms for video recording.
Video recording of inx-6(rr5) L3 stage worms confirmed that the coordination of pharyngeal muscle contraction is abnormal. Contraction of the procorpus muscles is weak and their relaxation is premature. As shown in Figure 5C, often the muscles of the procorpus do not contract at all, whereas the muscles of the metacorpus and terminal bulb (not visible) contract almost normally and in synchrony. The EPG trace of the stunted inx-6(rr5) L3 stage worms was faint and difficult to interpret (our unpublished data).
To better understand the effects of inx-6 on the electrical activity of the pharynx, we examined the somewhat larger L4 and adult inx-6(rr5) escapers. In spite of the presence of levamisole, we found negative spikes characteristic of M3 IPSPs in the EPG trace of the inx-6(rr5) mutants. If these were M3 IPSPs, they would be absent in an avr-15(ad1051) mutant background because avr-15 encodes a glutamategated chloride channel subunit that is necessary to form the M3 postsynaptic receptor on the pharyngeal muscle (Dent et al., 1997). However, even in the inx-6(rr5); avr-15(ad1051) double mutant background, these negative spikes persist (Figure 5, D and E).
The only other electrical activity known to produce negative spikes is the spontaneous repolarization of the pharyngeal muscles, mediated in part by the exp-2 voltage-gated potassium channel (Davis et al., 1999). Usually, there are at most two of these spikes: R1 and R2 (described above). Because a loss-of-function allele (ad1426) of the exp-2 gene results in the reduction or absence of negative spikes resulting from muscle repolarization, we made the inx-6; exp-2 avr-15 triple mutant. The negative spikes were absent in this triple mutant, indicating that the spikes in inx-6(rr5) are the result of EXP-2 mediated muscle repolarization (Figure 5F). The fact that there are a series of downward spikes in the inx-6(rr5) single mutant indicates that muscle repolarization is uncoordinated. It is also interesting to note that whereas in wild-type animals there are one or two distinct E spikes, initiation of the action potential in inx-6(rr5) is characterized by a broad upward deflection rather than a spike, which is consistent with a lack of coordination in muscle depolarization as well.
The effect of the inx-6(rr5) mutation on the motion of the pharyngeal muscle of L4 stage worms is also consistent with premature and uncoordinated muscle repolarization and relaxation. Instead of a rapid relaxation that occurs immediately after the first R spike, relaxation occurs slowly over a period of 30–60 ms beginning shortly after the second negative spike and well before the last spike (Figure 5E). The premature relaxation is evident first in the procorpus, indicating that these muscles are less electrically coupled than the metacorpus.
Cell-Cell Coupling of the Corpus Is Compromised in inx-6(rr5) Mutants
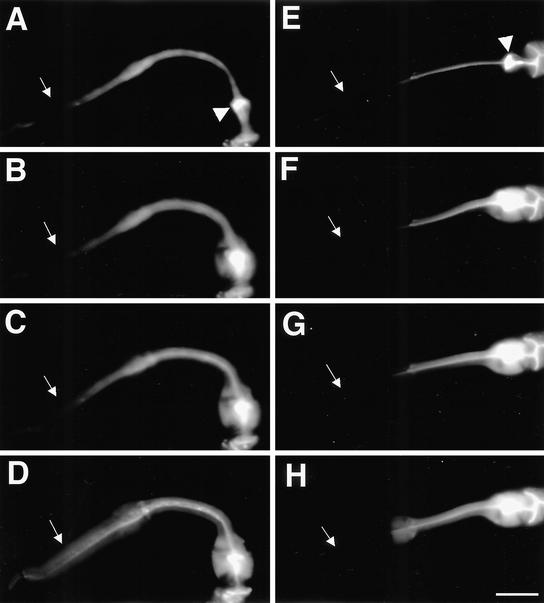
Because our electrophysiological data indicate that the procorpus of the inx-6(rr5) mutant has abnormalities in electrical coupling, we investigated whether inx-6(rr5) may cause gap junction defects. One effective way to test whether the procorpus of inx-6(rr5) mutants is appropriately coupled by gap junctions would be to monitor the diffusion of a fluorescent dye (carboxyfluorescein) among pharyngeal muscles. When animals are soaked in saturated carboxyfluorescein solution, the dye diffuses into all the tissues with the exception of the pharyngeal muscles. Instead it accumulates in the pharyngeal lumen, including the grinder in the terminal bulb. A single weak laser pulse directed at the posterior edge of the grinder is enough to perforate the pharyngeal lumen to allow the dye in the grinder to diffuse into terminal bulb muscles. The dye then diffuses via endogenous functional gap junctions to progressively more anterior muscles. Because the laser pulse can sometimes permanently damage the pharynx, we only considered animals that continued pumping and responded to serotonin appropriately after laser pulse.
In 100% of the wild-type animals (n >30) that were successfully operated, the dye diffused evenly throughout all pharyngeal muscles within 60 s after the laser pulse (Figure 6, A–D). In successfully operated inx-6(rr5) animals, dye spread into the terminal bulb, the isthmus, and the metacorpus at a similar rate as wild type, but failed to diffuse into the procorpus in all cases observed (n >50) (Figure 6, E–H). These data are consistent with the inx-6::GFP expression pattern and strongly indicate that the gap junctions required for coupling the procorpus with the metacorpus are functionally compromised in inx-6(rr5) mutants.
Figure 6.
Cell-cell coupling is abnormal in the pharyngeal muscle of inx-6(rr5) mutants. Wild-type N2 (A) and inx-6(rr5) mutant animals (E) were allowed to ingest a saturated carboxyfluorescein solution. Dye was accumulated in the pharyngeal lumen and was selectively excluded from the pharyngeal muscle in wild-type and inx-6(rr5) animals but could enter the muscle and diffuse anteriorly after a single laser pulse focused at the posterior of the grinder. (A–D) Dye-coupling process in the wild-type L1 stage animal, showing diffusion of the fluorescent dye toward the procorpus after the laser pulse. (E–H) Same process in the inx-6(rr5) L1 mutant preincubated at restrictive temperature. Arrowheads indicate the grinder in the terminal bulb where the laser pulse was applied. White arrows indicate the procorpus. In wild-type animals, dye spreads anteriorly through the isthmus and throughout the whole corpus evenly within 60 s after laser treatment, whereas in inx-6(rr5) animals, dye quickly spreads into the metacorpus but could not cross into the procorpus. Images were captured within 60 s after laser treatment by using the same exposure time for each acquisition. The final dye-coupling pattern reached equilibrium and did not change as verified 10 min after the initial laser application. Bar, 10 μm.
EAT-5 Can Partially Substitute for INX-6 Function In Vivo
Previous studies showed that the eat-5 mutants also demonstrated a pharyngeal pumping defect similar to that observed in inx-6(rr5) mutants. EAT-5 is another C. elegans innexin family member and is a close homolog to INX-6, sharing >30% sequence identity over the length of the entire protein.
The inx-6 and eat-5 expression patterns overlap in the pharyngeal muscles of the metacorpus, and they are expressed in adjacent cells (marginal cells and muscles, respectively) of the isthmus. Based on the above-mentioned information, it is possible that inx-6 and eat-5 may function together or have analogous roles in regulating pharyngeal pumping during larval development.
To test whether EAT-5 and INX-6 are functionally interchangeable, we performed a gene substitution experiment in which the inx-6 promoter was used to drive the eat-5 coding sequence to induce EAT-5 expression in the region where inx-6 would normally be expressed. The construct was injected into inx-6(rr5) mutants, and stable transgenic animals were monitored at restrictive temperature to determine whether the inx-6::EAT-5 transgene could rescue the inx-6(rr5) mutant phenotype. Strikingly, we found that the newly hatched transgenic animals could grow to adulthood at the restrictive temperature (Table 3). These transgenic animals are healthy and fertile, although they grow somewhat slower and need ∼88 h to reach the adult stage compared with ∼40 h for wild-type animals at 25°C. The fact that EAT-5 can rescue a mutation in inx-6(rr5) is strong evidence that inx-6 and eat-5 serve similar but not identical functions in vivo.
Table 3.
eat-5 can partially rescue the inx-6(rr5) mutation
|
Distribution of worms at each postembryonic stage after 40 h at 25°C (%)
|
|||||
|---|---|---|---|---|---|
| Genotype | L1 | L2 | L3 | L4 | Adult |
| inx-6; Ex[inx-6::GFP]a | 99 | 1 | 0 | 0 | 0 |
| inx-6; Ex[inx-6::INX-6; inx-6::GFP]b | 0 | 0 | 0 | 0 | 100 |
| inx-6; Ex[inx-6::EAT-5; inx-6::GFP] | 46 | 31 | 16 | 5 | 2 |
Expression of eat-5 under the control of the inx-6 promoter can rescue the inx-6(rr5) mutation although the rescued animals are compromised in growth rate. To identify transgenic animals at the L1 stage, the nonrescuing inx-6 transcriptional fusion with GFP was used as the cotransformation marker: inx-6(rr5) mutants that possess a wild-type inx-6 transgene (inx-6::INX-6) were used as a positive control. inx-6(rr5) mutants that carried the nonrescuing inx-6::GFP transcriptional fusion reporter were used as a negative control. L1 stage animals (100) from each strain were picked and maintained on food at 25°C for analysis. The distribution of animals at each stage was scored after 40 h, when all the animals in the positive control had reached the adult stage. More than 59% of the mutant strain inx-6; Ex[inx-6::EAT-5; inx-6::GFP] reached the adult stage only after 88 h (our unpublished data).
inx-6; Ex[inx-6::GFP] animals behave identically to inx-6(rr5) mutants. Most animals (>98%) did not progress beyond the L1 stage even after 88 h when maintained at 25°C.
inx-6; Ex[inx-6::INX-6; inx-6::GFP] is a fully rescued inx-6(rr5) mutant strain and it grows and develops as wild-type animals.
Ectopic Expression of inx-6 Caused Abnormalities
Because EAT-5 could substitute for INX-6, we were curious whether ectopically expressed INX-6 would contribute in a benign way to endogenous gap junctions or whether it might interfere with the proper regulation of cell-cell coupling. The inx-6 expression pattern showed that inx-6 is only expressed in the corpus and isthmus tissues, suggesting that there must be other kinds of gap junction channels in the terminal bulb muscles to ensure the intact pharyngeal muscle contraction. If the expression of inx-6 were expanded into the terminal bulb, it could potentially interact with endogenous innexins and interfere with their functions, or it could form inappropriate gap junctions between normally isolated cells. To test this, we used the myo-2 promoter to drive inx-6 coding sequence in the corpus, isthmus, and the terminal bulb muscles.
The myo-2::INX-6 construct was injected into inx-6(rr5) mutants and wild-type animals. In the F1 generation, most transgenic animals developed relatively normally, including inx-6(rr5) mutants at restrictive temperature, which grew slightly more slowly than wild-type animals. However, in the F2 generation, all the animals expressing the myo-2::INX-6 transgene died as L1 larvae. We found that the pharyngeal lumen of most transgenic animals was wide open due to the hypercontracted pharyngeal muscles (our unpublished data), which presumably rendered the transgenic animals unable to feed. This phenotype is consistent with electrical coupling of cells that are not normally coupled, abnormally increased coupling between cells that are normally coupled, or the formation of open hemichannels on the surface of muscle cells.
DISCUSSION
Structure of inx-6 Gap Junctions
Temperature-sensitive missense mutations such as inx-6(rr5) are generally thought to interfere with protein stability. Structure predictions based on sequence indicate that both innexins and connexins are membrane proteins with four transmembrane domains with both the amino and carboxyl termini on the cytoplasmic side. The homology between INX-6 and the other innexins is high in the putative transmembrane domains, so it, too, should have this topology. Based on this prediction, the rr5 mutation is in the cytoplasmic C terminus, which has important roles in gap junction gating sensitivity in connexins (Morley et al., 1996; Wang and Peracchia, 1998). However, the P353 residue affected by rr5 is not well conserved among innexins and is in a nonconserved domain. Thus, an alternative hypothesis is that P353 could lie in a protein–protein interaction domain, and association with an INX-6–specific cytoplasmic protein is compromised at high temperatures by the P353L mutation.
Like connexins, innexins are probably multimeric, although whether inx-6 forms a homomeric or heteromeric channel remains to be determined. The precise subunit composition is unknown for any gap junction formed by innexins, but there are indications that innexins do form heteromeric channels. UNC-7 and UNC-9 share considerable sequence similarity and also have similar mutant phenotypes (Starich et al., 1993; Barnes and Hekimi, 1997). These innexins may associate to form a channel expressed in the nervous system. Similarly, direct electrophysiological and genetic evidence indicate that the Drosophila innexins Dm-INX-2 and Dm-INX-3 form obligate heteromeric channels (Stebbings et al., 2000).
There are several C. elegans innexins expressed in the pharynx with which inx-6 might associate. For example, inx-6 expression overlaps that of eat-5 (Starich et al., 1996). It is interesting, therefore, that eat-5 can substitute for inx-6, especially because eat-5 does not form homomeric junctions in Xenopus oocytes (Landesman et al., 1999). It may be that eat-5 can rescue inx-6(rr5) because it can associate with and stabilize the mutant INX-6 encoded by rr5. On the other hand, ectopic expression of INX-6 under the myo-2 promoter indicates that it may also form a homomeric channel. Work in Drosophila showed that expressing one subunit of an obligate homomer (Dm-inx-2 or Dm-inx-3) has little effect, but when the two subunits are ectopically coexpressed, they cause severe developmental defects (Stebbings et al., 2000). By analogy, the fact that ectopically expressed INX-6 has severe effects on the pharynx suggests that either INX-6 is capable of forming a homomeric channel, or it can associate promiscuously with endogenous innexin subunits.
Innexin Redundancy in the Pharynx
The temperature-sensitive period (TSP) of inx-6(rr5) suggests that other innexins may be functionally redundant. Although inx-6 is expressed from mid-to-late embryogenesis until the adult stage, our TSP experiments indicated that the inx-6 gene product is only required for the L1 and L2 larval stages. In spite of its embryonic expression, there seem to be no abnormalities in the inx-6(rr5) embryos raised at the restrictive temperature. Therefore, other innexins expressed in the pharyngeal muscles may act redundantly with inx-6 to ensure pharyngeal differentiation and morphogenesis. The fact that cell-cell coupling was normal within the metacorpus in inx-6(rr5) animals, where the inx-6::GFP is strongly expressed in wild-type may reflect such redundancy. Other innexin members such as eat-5 in the metacorpus may form functional gap junctions independent of inx-6, and thereby permit diffusion of dye and synchronization of action potentials among metacorpus muscles. That inx-6(rr5) animals can survive at the restrictive temperature as an adult may also reflect redundancy. However, at present we cannot exclude that high temperature prevents assembly or transport of the mutant INX-6 protein, and conversely wild-type protein normally remains stable and persists.
If innexins are largely redundant, then ectopic expression of an innexin in a wild-type background should have no ill effects. In contrast, we found that ectopic expression of inx-6 in the terminal bulb, not replacing but rather supplementing the innexins that are normally expressed there, causes the pharynx to hypercontract. Although we cannot rule out the possibility that overexpression from the multicopy transgene causes this phenotype, it is unlikely because inx-6::INX-6–containing transgenic arrays show no such effect. It is also unlikely that inx-6 is associating with endogenous channels of the terminal bulb and acting as a dominant negative allele because reducing gap junctions should reduce the excitability of muscles, not cause hypercontraction. inx-6, acting alone or in association with endogenous innexins, might increase the degree of coupling. Variations on this model are that ectopically expressed inx-6 is coupling cells that are normally isolated or that it forms permeable hemichannels in the cell membrane. Either way, the severe phenotype of inx-6 expressed in the terminal bulb argues against a simple model of innexin redundancy wherein ectopic expression of an innexin in cells that already have innexins has no effect.
Innexin Specialization and the Role of Gap Junctions in Pharyngeal Muscle Contraction
The 20 muscle cells of the pharynx must contract with precise timing to ensure appropriate function of this organ. Thus, electrical coupling of pharyngeal muscles is of the utmost importance. The need for so many different innexins in a simple organ such as the pharynx is less clear. Our video, electrophysiological, GFP reporter expression, and dye-coupling data all suggest that inx-6 is involved specifically in coupling the metacorpus to the procorpus. Whence the need for an innexin dedicated to this task?
One explanation is that the innexin subunits are functionally equivalent and the specialization is in their pattern of expression. By driving eat-5 with the inx-6 promoter, we showed that this transgene could rescue the larval arrest of the inx-6(rr5) mutants but, unlike an inx-6::INX-6 transgene, could not entirely restore wild-type growth rate. Thus, the functional properties of eat-5 and inx-6 must be similar but not identical. Although eat-5 and inx-6 may be redundant in the metacorpus, inx-6 seems to be better suited to couple the pro- and metacorpus.
If the innexin proteins are not perfectly interchangeable, what might be the properties that make them uniquely suited to couple specific cells? Here, we can only speculate. Subunit specific properties might include ability to form heterotypic interactions, voltage sensitivity, or regulation by second messengers. However, an understanding of the unique requirements of the pro- to metacorpus gap junctions may inform our speculation. Neuronal control is necessary for efficient pharyngeal function and the motor neurons seem to interact primarily with the metacorpus muscles. The motor neurons that regulate pharyngeal contraction and relaxation, MC and M3, are both corpus neurons and M3 synapses onto the metacorpus (Albertson and Thomson, 1976; Raizen and Avery, 1994; Raizen et al., 1995). Presumably, the effects of these neurons on metacorpus muscle membrane potential must be transmitted to the rest of the pharyngeal muscles to maintain synchrony. Thus, it seems that eat-5 ensures the posterior propagation, and inx-6 the anterior propagation, of changes in membrane potential originating in the metacorpus.
Our results indicate that although the metacorpus drives depolarization of both the procorpus and terminal bulb, there are different requirements for the control of current flow in each case. The multiple spikes in the inx-6(rr5) EPG indicate that when coupling to the metacorpus is compromised, the procorpus muscles repolarize prematurely. Although we were not able to correlate these spikes with relaxation of specific muscles, it is unlikely that these are R2 spikes, because, even in wild-type worms, the R2 spike that corresponds to terminal bulb relaxation is usually smaller that the R1 spikes seen in the inx-6(rr5) mutant (compare R2 in Figure 5B to the R spikes in Figure 5E). Rather, it seems that in the L4 stage inx-6(rr5) worms the corpus muscles contract normally (Figure 5D, frame 2) but that individual procorpus muscles begin relaxing prematurely. Thus, these spikes likely represent premature procorpus repolarizations. The implication is that the procorpus needs the metacorpus not only to initiate depolarization but also to maintain it. In contrast, the repolarization and relaxation of the terminal bulb muscles lags those of corpus. Moreover, in the eat-5 mutant, uncoupling the corpus from the terminal bulb reveals that the terminal bulb seems to have its own pacemaker (Starich et al., 1996),
It would make sense then that gap junctions formed by INX-6 would differ from those formed by EAT-5. Assuming that the metacorpus drives both depolarization and repolarization of the procorpus, the INX-6 gap junctions would be relatively passive. However, because (in this model) the metacorpus must drive terminal bulb depolarization but then be immune to the continued depolarization of the terminal bulb after the metacorpus repolarizes and relaxes, one might predict that the gap junction formed by EAT-5 would rectify.
CONCLUSIONS
The identification of a temperature-sensitive allele of the innexin inx-6 offers an important opportunity to study the various cellular roles of gap junctions. Future work will focus on how these important proteins function together with other innexin family members to coordinate the precise electrical coupling essential for pharyngeal function and larval development. Because of the ease of generating mutants and the availability of promoters for ectopic expression, the pharynx could be very useful for elucidating the role of gap junction diversity in controlling current flow through coupled muscles.
Acknowledgments
We thank Todd Starich and Jocelyn Shaw for sharing reagents, information, and discussion; Andy Fire for the GFP vector kit; the C. elegans Genetics Centre for strains; the Sanger Centre for cosmids; and Ron Chase for the spike counter. J.A.D. was supported by the Natural Sciences and Engineering Research Council of Canada. This work was supported by the National Cancer Institute of Canada with funds from the Terry Fox Run.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–11–0716. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-11-0716.
References
- Albertson, D.G., and Thomson, J.N. (1976). The pharynx of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 275, 299–325. [DOI] [PubMed] [Google Scholar]
- Avery, L. (1993). The genetics of feeding in Caenorhabditis elegans. Genetics 133, 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, L., and Thomas, J.H. (1997). Feeding and defecation. In: The Nematode C. elegans II. ed. D. Riddle, Cold Spring Harbor, NY: Cold Spring Harbor Press, 679–716. [PubMed]
- Barnes, T.M., and Hekimi, S. (1997). The Caenorhabditis elegans avermectin resistance and anesthetic response gene unc-9 encodes a member of a protein family implicated in electrical coupling of excitable cells. J. Neurochem. 69, 2251–2260. [DOI] [PubMed] [Google Scholar]
- Bauer, R., Lehmann, C., Fuss, B., Eckardt, F., and Hoch, M. (2002). The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J. Cell Sci. 115, 1859–1867. [DOI] [PubMed] [Google Scholar]
- Bergoffen, J., Scherer, S.S., Wang, S., Scott, M.O., Bone, L.J., Paul, D.L., Chen, K., Lensch, M.W., Chance, P.F., and Fischbeck, K.H. (1993). Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262, 2039–2042. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone, R., White, T.W., and Paul, D.L. (1996). Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 238, 1–27. [DOI] [PubMed] [Google Scholar]
- Crompton, D., Todman, M., Wilkin, M., Ji, S., and Davies, J. (1995). Essential and neural transcripts from the Drosophila shaking-B locus are differentially expressed in the embryonic mesoderm and pupal nervous system. Dev. Biol. 170, 142–158. [DOI] [PubMed] [Google Scholar]
- Curtin, K.D., Zhang, Z., and Wyman, R.J. (1999). Drosophila has several genes for gap junction proteins. Gene 31, 191–201. [DOI] [PubMed] [Google Scholar]
- Davis, M.W., Fleischhauer, R., Dent, J.A., Joho, R.H., and Avery, L. (1999). A mutation in the C. elegans EXP-2 potassium channel that alters feeding behavior. Science 286, 2501–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent, J.A., Davis, M.W., and Avery, L.A. (1997). avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 16, 5867–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent, J.A., Smith, M.M., Vassilatis, D.K., and Avery, L. (2000). The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 197, 2674–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart, J.L., et al. (1997). Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development 124, 1281–1292. [DOI] [PubMed] [Google Scholar]
- Fire, A., Xu, S., Montgomery, M.K., Kostas, S.A., Driver, S.E., and Mello, C.C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811. [DOI] [PubMed] [Google Scholar]
- Furshpan, E.J., and Potter, D.D. (1957). Mechanism of nerve-impulse transmission at a crayfish synapse. Nature 180, 342–343. [DOI] [PubMed] [Google Scholar]
- Haun, C., Alexander, J., Stainier, D.Y., and Okkema, P.G. (1998). Rescue of Caenorhabditis elegans pharyngeal development by a vertebrate heart specification gene. Proc. Natl. Acad. Sci. USA 95, 5072–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Y., Roy, R., and Ambros, V. (1998). Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125, 3585–3597. [DOI] [PubMed] [Google Scholar]
- Gabriel, H.D., Jung, D., Butzler, C., Temme, A., Traub, O., Winterhager, E., and Willecke, K. (1998). Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J. Cell Biol. 140, 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter, H.L., Saffitz, J.E., and Beyer, E.C. (1994). Molecular cloning of two human cardiac gap junction proteins, connexin40 and connexin45. J. Mol. Cell. Cardiol. 26, 861–868. [DOI] [PubMed] [Google Scholar]
- Kelsell, D.P., Dunlop, J., Stevens, H.P., Lench, N.J., Liang, J.N., Parry, G., Mueller, R.F., and Leigh, I.M. (1997). Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83. [DOI] [PubMed] [Google Scholar]
- Krishnan, S.N., Frei, E., Swain, G.P., and Wyman, R.J. (1993). Passover: a gene required for synaptic connectivity in the giant fiber system of Drosophila. Cell 73, 967–977. [DOI] [PubMed] [Google Scholar]
- Kumar, N.M., and Gilula, N.B. (1996). The gap junction communication channel. Cell 84, 381–388. [DOI] [PubMed] [Google Scholar]
- Landesman, Y., White, T.W., Starich, T.A., Shaw, J.E., Goodenough, D.A., and Paul, D.L. (1999). Innexin-3 forms connesin-like intercellular channels. J. Cell Sci. 112, 2391–2396. [DOI] [PubMed] [Google Scholar]
- Lipshitz, H.D., and Kankel, D.R. (1985). Specificity of gene action during central nervous system development in Drosophila melanogaster: analysis of the lethal (1) optic ganglion reduced locus. Dev. Biol. 108, 56–77. [DOI] [PubMed] [Google Scholar]
- Maduro, M.F., Meneghini, M.D., Bowerman, B., Broitman-Maduro, G., and Rothman, J.H. (2001). Restriction of mesendoderm to a single blastomere by the combined action of SKN-1 and a GSK-3β homolog is mediated by MED-1 and -2 in C. elegans. Mol. Cell 7, 475–485. [DOI] [PubMed] [Google Scholar]
- Mello, C.C., Kramer, J.M., Stinchcomb, D., and Ambrose, V. (1991). Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley, G.E., Taffet, S.M., and Delmar, M. (1996). Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys. J. 70, 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, B., Dermietzel, R., Teplow, D., Traub, O., Willecke, K., and Revel, J.P. (1987). Two homologous protein components of hepatic gap junctions. Nature 329, 732–734. [DOI] [PubMed] [Google Scholar]
- Phelan, P., Nakagawa, M., Wilkin, M.B., Moffat, K.G., Okane, C.J., Davies, J.A., and Bacon, J.P. (1996). Mutations in Shaking-B prevent electrical synapse formation in the Drosophila giant fiber system. J. Neurosci. 16, 1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan, P., et al. (1998a). Innexins: a family of invertebrate gap-junction proteins. Trends Genet. 14, 348–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan, P., Stebbings, L.A., Baines, R.A., Bacon, J.P., Davies, J.A., and Ford, C. (1998b). Drosophila shaking-B protein forms gap junctions in paired Xenopus oocytes. Nature 391, 181–184. [DOI] [PubMed] [Google Scholar]
- Phelan, P., and Starich, T.A. (2001). Innexins get into the gap. Bioessays 23, 388–396. [DOI] [PubMed] [Google Scholar]
- Raizen, D.M., and Avery, L. (1994). Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 12, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen, D.M., Lee, R.Y., and Avery, L. (1995). Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 141, 1365–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume, A.G., de Sousa, P.A., Kulkarni, S., Langille, B.L., Zhu, D., Davies, T.C., Juneja, S.C., Kidder, G.M., and Rossant, J. (1995). Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831–1834. [DOI] [PubMed] [Google Scholar]
- Shiels, A., Mackay, D., Ionides, A., Berry, V., Moore, A., and Bhattacharya, S. (1998). A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am. J. Hum. Genet. 62, 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, A.M., Goodenough, D.A., Li, E., and Paul, D.L. (1997). Female infertility in mice lacking connexin 37. Nature 385, 525–529. [DOI] [PubMed] [Google Scholar]
- Simon, A.M., Goodenough, D.A., and Paul, D.L. (1998). Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 8, 295–298. [DOI] [PubMed] [Google Scholar]
- Starich, T.A., Herman, R.K., and Shaw, J.E. (1993). Molecular and genetic analysis of unc-7, a Caenorhabditis elegans gene required for coordinated locomotion. Genetics 133, 527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich, T.A., Lee, R.Y.N., Panzarella, C., Avery, L., and Shaw, J.E. (1996). eat-5 and unc-7 represent a multigene family in Caenorhabditis elegans involved in cell-cell coupling. J. Cell Biol. 134: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings, L.A., Todman, M.G., Phelan, P., Bacon, J.P., and Davies, J.A. (2000). Two Drosophila innexins are expressed in overlapping domains and cooperate to form gap-junction channels. Mol. Biol. Cell 11, 2459–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y.A., and Wyman, R.J. (1996). Passover eliminates gap junctional communication between the neurons of the Giant Fiber System in Drosophila. J. Neurobiol. 30, 340–348. [DOI] [PubMed] [Google Scholar]
- Tazuke, S.I., Schulz, C., Gilboa, L., Fogarty, M., Mahowald, A.P., Guichet, A., Ephrussi, A., Wood, C.G., Lehmann, R., and Fuller, M.T. (2002). A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 129, 2529–2539. [DOI] [PubMed] [Google Scholar]
- Thomas, J.B., and Wyman, R.J. (1984). Mutations altering synaptic connectivity between identified neurons in Drosophila. J. Neurosci. 14, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todman, M.G., Baines, R.A., Stebbings, L.A., Davies, J.A., and Bacon, J.P. (1999). Gap-junctional communication between developing Drosophila muscles is essential for their normal development. Dev. Genet. 24, 57–68. [DOI] [PubMed] [Google Scholar]
- Unger, V.M., Kumar, N.M., Gilula, N.B., and Yeager, M. (1999). Three-dimensional structure of a recombinant gap junction membrane channel. Science 283, 1176–1180. [DOI] [PubMed] [Google Scholar]
- Wang, X.G., and Peracchia, C. (1998). Molecular dissection of a basic COOH-terminal domain of Cx32 that inhibits gap junction gating sensitivity. Am. J. Physiol. 275, 1384–1390. [DOI] [PubMed] [Google Scholar]
- Watanabe, T., and Kankel, D.R. (1990). Molecular cloning and analysis of l(1)ogre, a locus of Drosophila melanogaster with prominent effects on the postembryonic development of the central nervous system. Genetics 126, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, T.W., and Paul, D.L. (1999). Genetic diseases and gene knockouts reveal diverse connexin functions. Annu. Rev. Physiol. 61, 283–310. [DOI] [PubMed] [Google Scholar]
- Williams, B.D., Schrank, B., Huynh, C., Shownkeen, R., and Waterston, R.H. (1992). A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 131, 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager, M., and Nicholson, B.J. (1996). Structure of gap junction intercellular channels. Curr. Opin. J. Struct. Biol. 6, 183–192. [DOI] [PubMed] [Google Scholar]