Abstract
Laminins are extracellular matrix proteins that participate in neuronal development, survival, and regeneration. During excitotoxin challenge in the mouse hippocampus, neuron interaction with laminin-10 (α5,β1,γ1) protects against neuronal death. To investigate how laminin is involved in neuronal viability, we infused laminin-1 (α1,β1,γ1) into the mouse hippocampus. This infusion specifically disrupted the endogenous laminin layer. This disruption was at least partially due to the interaction of the laminin-1 γ1 chain with endogenous laminin-10, because infusion of anti-laminin γ1 antibody had the same effect. The disruption of the laminin layer by laminin-1 1) did not require the intact protein because infusion of plasmin-digested laminin-1 gave similar results; 2) was posttranscriptional, because there was no effect on laminin mRNA expression; and 3) occurred in both tPA–/– and plasminogen–/– mice, indicating that increased plasmin activity was not responsible. Finally, although tPA–/– mice are normally resistant to excitotoxin-induced neurodegeneration, disruption of the endogenous laminin layer by laminin-1 or anti-laminin γ1 antibody renders the tPA–/– hippocampal neurons sensitive to kainate. These results demonstrate that neuron interactions with the deposited matrix are not necessarily recapitulated by interactions with soluble components and that the laminin matrix is a dynamic structure amenable to modification by exogenous molecules.
INTRODUCTION
Laminins are heterotrimeric molecules that are critical components of the extracellular matrix (ECM). At present, five α chains, three β chains, and three γ chains have been identified, and of the possible 45 potential trimeric molecules that could be generated from these chains, 15 have been observed (Colognato and Yurchenco, 2000; Grimpe et al., 2002). Laminins are known to play an important role in the nervous system (Venstrom and Reichardt, 1993), for example, in the neuromuscular junction (Noakes et al., 1995; Patton et al., 1998; Patton et al., 2001; Sanes and Lichtman, 2001), in brain development (Luckenbill-Edds, 1997; Miner et al., 1998; Colognato and Yurchenco, 2000; Liesi et al., 2001), and in pathology (Murtomaki et al., 1992). Laminins have been found in the adult brain, but the role for these proteins in the adult central nervous system is not well established (Hagg et al., 1989; Jucker et al., 1996; Hagg et al., 1997; Tian et al., 1997).
There are some studies that bear on the role of the ECM in adult central nervous system function. In the mouse hippocampus, injection of glutamate analogs, known as excitotoxins, can cause massive neuronal death (Coyle et al., 1978). It has been shown that mice deficient in the protease tissue plasminogen activator (tPA) or its zymogen substrate plasminogen are resistant to this excitotoxin-induced death (Tsirka et al., 1995; Tsirka et al., 1997), implicating this extracellular proteolytic system in neuronal degeneration. Further experiments showed that these proteases affect neuronal viability by degrading the laminin matrix that is associated with the neurons (Chen and Strickland, 1997; Nagai et al., 1999). Thus, there is evidence that in the mouse brain neurons depend to some extent for survival on their interaction with the ECM, as reported in other systems (Meredith et al., 1993; Boudreau et al., 1995; Coucouvanis and Martin, 1995; Lukashev and Werb, 1998; Frisch and Screaton, 2001).
It is thought that the supramolecular structure of the ECM is maintained by binding interactions between the various components, such as laminins, collagens, etc., both to themselves and to other molecules (Colognato and Yurchenco, 2000). The potential complexity of these interactions makes it difficult to recapitulate ECM structure and function in cell culture. Furthermore, there is evidence that cells interact differently with soluble components of the ECM compared with their interaction with the same molecules in the context of an insoluble matrix (Hayman et al., 1985). Thus, it is important to design ways to evaluate the maintenance and function of ECM structures in vivo.
In studies on laminin expression in the mouse hippocampus, we observed that the prominent, endogenous laminin-10 matrix (Indyk et al., 2003) was disrupted by infusion of soluble mouse laminin-1. In this report, we analyze the mechanism of this observation. Our results indicate that in the hippocampus, laminin is loosely held in the matrix and can be displaced by competition with its soluble counter-part. In contrast to the deposited, insoluble material, the soluble laminin does not protect neurons from excitotoxic death. The disruption of the laminin matrix sensitizes neurons to kainate-induced death, further establishing the critical role for the laminin matrix in neuronal survival.
MATERIALS AND METHODS
Intrahippocampal Infusions
The mice used were adult C57Bl/6 male mice, tPA-deficient male mice (tPA–/–) (Carmeliet et al., 1994), or plasminogen-deficient (plg–/–) (Bugge et al., 1995; Ploplis et al., 1995) male mice (both tPA–/– and plg–/– were) backcrossed to C57Bl/6 for eight generations. The mice were injected intraperitoneally with atropine (0.6 mg/kg body weight) and then were anesthetized deeply with 2.5% avertin (0.02 ml/gm of body weight). They were placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL). A microosmotic pump (Alza, Palo Alto, CA) containing 100 μl of phosphate-buffered saline (PBS) (for control animals), 100 μl of purified mouse laminin-1 (0.5 mg/ml except 0.1 mg/ml for one lower dose experiment; Sigma-Aldrich, St. Louis, MO), 100 μl of anti-laminin γ1 polyclonal antibody (0.2 mg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), 100 μl of plasmin-digested laminin-1 (0.5 mg/ml), or control buffers used for laminin digestion were placed subcutaneously in the back of the animals. A brain infusion cannula connected to the pump was positioned at coordinates bregma, –2.5 mm; medial-lateral, 0.5 mm; and dorsoventral, 1.6 mm to deliver the compound into the hippocampus. The infusion rate was 0.5 μl/h. The pumps were allowed to infuse the designated solution for 7 d, and the animals were anesthetized and perfused through the heart with PBS, followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed, postfixed in the same fixative overnight at 4°C, and then left in 30% sucrose in PBS for 48 h at 4°C. Coronal brain sections (30 μm) were cut on a microtome, collected in PBS, and then processed for cresyl violet, Fluoro Jade B staining, and immunohistochemistry. For the kainate injection experiments, the infusion pumps were allowed to infuse for 5 d and then kainate was injected as described below. Only mice that showed efficient infusion were used.
Intrahippocampal Injections
Adult mice as indicated in each experiment were anesthetized and infused with PBS, control buffer, mouse laminin-1, plasmin-digested laminin-1, or anti-laminin γ1 antibody for 5 d as described above. Then a total volume of 300 nl of 0.83 mM kainate (Tocris Cookson, Ellisville, MO) for a dose of 0.25 nmol was delivered unilaterally using a microinjection apparatus (Stoelting) via a 2.5 μl-Hamilton syringe equipped with a 33-gauge needle, over the course of 60 s. In our previous study (Tsirka et al., 1995), we injected 1.5 nmol of kainate (Sigma-Aldrich). This kainate preparation is discontinued, and the new kainate preparation (Tocris Cookson) is more potent by weight. Injection of 0.25 nmol of kainate induced similar neurodegeneration in wild-type mice to the 1.5 nmol used previously, and therefore this lower dose was used in the experiments reported herein. After retracting the needle 0.1 mm, the needle was kept in place for 2 min to allow diffusion of the kainate, and then was completely removed. The area was then cleaned and the wound sutured. To control for mechanical damage, injections were performed with vehicle alone (PBS) and consistently showed no neuronal death in the hippocampus (our unpublished data). Two days after the injection, the animals were sacrificed and their brains were processed as described above.
Fluoro Jade B Staining
Fluoro Jade B staining was performed as described previously (Schmued and Hopkins, 2000). Briefly, free-floating brain sections were mounted onto slides, dried at 55°C for 2 h, and incubated at room temperature in the following baths: 1% NaOH in 80% ethanol, 5 min; 70% ethanol, 2 min; H2O, 2 min; 0.06% potassium permanganate, 10 min; H2O, 2 min; 0.0004% Fluoro Jade B (Histo-Chem, Jefferson, AR), 20 min; and three H2O washes, 1 min each. Slides were then dried, immersed in Histoclear, and coverslipped with DPX mounting medium (Sigma-Aldrich). Fluorescence was visualized using a fluorescein isothiocyanate filter on an Axioskop2 microscope (Carl Zeiss, Thornwood, NY).
Immunohistochemistry
Mouse brain sections, manipulated as described above, were incubated with 1) affinity-purified rabbit anti-mouse laminin polyclonal antibody (Sigma-Aldrich), at 1:1000 dilution; this antibody has previously been shown to react with both the β1 and γ1 chains of laminin-1 (Chen and Strickland, 1997); 2) affinity-purified rabbit anti-M2 muscarinic acetylcholine receptor polyclonal antibody (Chemicon International, Temecula, CA), at 1:400 dilution; 3) rabbit anti-mouse fibronectin polyclonal antibody (Chemicon International), at 1:500 dilution (our unpublished data; control for Figure 2D). Biotinylated secondary antibodies were used (Vector Laboratories, Burlingame, CA), and the avidin-biotin-peroxidase complex (ABC reaction) was visualized using a Nova Red kit (Vector Laboratories).
Figure 2.
Anti-laminin γ1 antibody or soluble laminin-1 specifically disrupts the endogenous laminin matrix. Adult male mice were unilaterally infused with purified laminin-1 (0.5 mg/ml), fibronectin (0.5 mg/ml), or anti-laminin γ1 antibody (0.2 mg/ml) for 7 d, brain sections were prepared and stained with anti-laminin antibody (A, B, and D), or anti-M2 muscarinic acetylcholine receptor antibody (C). Infusion of soluble laminin-1 or anti-laminin γ1 antibodies disrupted endogenous laminin in the hippocampal neuronal cell layers in the CA1 and DG regions (A and B), but did not affect the distribution of the M2 muscarinic acetylcholine receptor in the same region (C). Infusion of soluble fibronectin did not affect endogenous laminin (D). Ln, laminin-1 immunostaining; M2, M2 muscarinic acetylcholine receptor immunostaining. Bar in D, 500 μm.
Imaging Analysis
The brain sections through the hippocampus after staining were photographed using an AxioVision System. The images were processed using Photoshop 5.5 and figures were prepared using PowerPoint.
Laminin-1 Digestion and SDS-PAGE Analysis
Laminin-1 was purchased from either Sigma-Aldrich or Chemicon International. For plasmin digestion, laminin-1 was incubated in 100 μg/ml human plasminogen, 4.8 μg/ml recombinant tPA, 20 mM Tris-HCl, pH 7.2, 150 mM NaCl, 1 mM CaCl2 at 37°C for 4 h. To assess the ability of tPA to cleave this protein directly, laminin-1 was incubated in the above-mentioned buffer without human plasminogen. After digestion, the solutions were denatured at 100°C for 10 min in 100 mM dithiothreitol (DTT), and then dialyzed against PBS. Residual tPA and plasminogen or plasmin activity was checked by casein zymography, and revealed that this procedure completely destroyed tPA and plasminogen or plasmin activity (our unpublished data). Intact or digested laminin was denatured at 100°C for 10 min in 100 mM DTT and run on a reducing 10–20% gradient SDS-PAGE, stained with Coomassie Blue, and then destained. The gel was then scanned into Photoshop.
In Situ Hybridization
mRNA in situ hybridization was performed according to Chen et al. (1995). DNA clones for mouse laminin γ1 were obtained from Drs. J.H. Miner and J.R. Sanes (Washington University, St. Louis, MO), and the plasmid DNA was sequenced to verify the correct insert sequence (Indyk et al., 2003). Digoxigenin (DIG)-labeled sense or antisense RNA probes (Roche Applied Science, Indianapolis, IN) were synthesized from laminin γ1 chain-specific clones by using T3- or T7-polymerase transcription. Probes were purified by LiCl/EtOH precipitation, and correct probe size was verified by formaldehyde gel electrophoresis. In situ hybridizations were performed on mouse brain sections. Briefly, the animals were anesthetized and perfused through the heart with diethyl pyrocarbonate (DEPC)-treated PBS, followed by DEPC-treated 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed, postfixed in the same fixative overnight at 4°C, and then left in DEPC-treated 30% sucrose in PBS for 48 h at 4°C. Coronal brain sections (30 μm) were cut on a microtome, collected in DEPC-treated PBS, and kept in 4°C. For prehybridization treatment, the brain sections were washed in DEPC-treated PBS for 4 times for 5 min each, followed by two 5-min incubations in DEPC-treated PBS containing 100 mM glycine. The brain sections were then treated with DEPC-treated PBS containing 0.3% Triton X-100 for 15 min and washed in DEPC-treated PBS two times for 5 min each. The sections were permeabilized in 100 mM Tris-HCl, 50 mM EDTA, pH 8.0, 15 μg/ml RNase-free proteinase K for 30 min and postfixed for 5 min in DEPC-treated PBS containing 4% paraformaldehyde. The sections were then washed in DEPC-treated PBS four times for 5 min each and incubated in 0.1 M triethanolamine buffer, pH 8.0, containing 0.25% acetic anhydride two times for 5 min each. The sections were then prehybridized in 4× SSC containing 50% deionized formamide for 30 min and then incubated overnight at 55°C in hybridization solution containing RNA probe (sense or antisense, 1 μg/ml hybridization solution), 10% dextran sulfate, 20 mM Tris HCl, pH 8.0, 300 mM NaCl, 0.2% sarcosyl, 1× Denhardt's solution, 1 mg/ml salmon sperm DNA, 1 mg/ml yeast tRNA, 40% formamide, 10 mM DTT. Posthybridization washes at 65°C consisted of 4× SSC and 50% formamide in 2× SSC, followed by 37°C washes in 10 mM Tris HCl, 1 mM EDTA, 500 mM NaCl. Sections were then treated with 20 μg/ml RNase A in RNase buffer (10 mM Tris HCl, 1 mM EDTA, 500 mM NaCl) for 30 min at 37°C, followed by washes in RNase buffer, 50% formamide in 2× SSC at 65°C, and buffer 1 (0.1 M Tris HCl, pH 7.5, 150 mM NaCl). Sections were then blocked with 1.5% blocking reagent (Roche Diagnostics) in buffer 1 and incubated overnight with anti-DIG antibody (1:500 anti-DIG Fab fragment-AP conjugated; Roche Applied Science) and 1% blocking reagent in buffer 1. After several washes in 0.1 M Tris HCl, pH 7.5, 150 mM NaCl, sections were washed in buffer 3 (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 50 mM MgCl2) and color reaction performed in the dark by using 75 mg/ml nitro blue tetrazolium and 50 mg/ml 5-bromo-4-chloro-3-indolyl phosphate (Roche Applied Science) in buffer 3. Reaction was stopped by washes in buffer 3 and TE, and sections coverslipped with 10 mM Tris, 1 mM disodium EDTA buffer/glycerol and photographed. Sense probes were used as negative controls in all experiments.
Quantification of hippocampal neuronal laminin layers and neuronal loss C57Bl/6 mice were infused into the hippocampus with intact soluble laminin (n = 5) or plasmin digested laminin (n = 5) for 7 d, and their brains were processed for laminin immunostaining. tPA-deficient mice were infused with control buffer (n = 6), or intact soluble laminin-1 (n = 5), or plasmin-digested laminin (n = 5) for 5 d, and kainate was injected. Two days after the kainate injection their brains were processed for cresyl violet staining. We used camera lucida tracings to quantitate the lengths of neuronal laminin layers or the numbers of neuronal cell loss (Tsirka et al., 1995; Tsirka et al., 1997; Chen et al., 1999). Four sections from the hippocampus of each mouse in each group were matched, and the linear lengths of disrupted neuronal laminin layers or dead pyramidal cell layers were determined on each section. The values from each category were averaged across the subjects in a group using the Sigma Plot program (Jandel Scientific, Corte Madera, CA).
RESULTS
Soluble Laminin-1 Disrupts the Laminin Matrix In Vivo
In the mouse hippocampus, a laminin matrix is present along the neuronal cell layers (Chen and Strickland, 1997; Nakagami et al., 2000). This laminin matrix is composed primarily of laminin-10, containing the α5, β1, and γ1 chains (Indyk et al., 2003). During excitotoxic challenge in the mouse hippocampus, tPA secretion and expression is induced (Gualandris et al., 1996; Parmer et al., 1997; Baranes et al., 1998), and due to the presence of plasminogen (Sappino et al., 1993; Tsirka et al., 1997; Matsuoka et al., 1998), increased plasmin activity is generated (Chen and Strickland, 1997; Tsirka et al., 1997). This increased plasmin activity leads to the degradation of the laminin-10 matrix (Chen and Strickland, 1997; Indyk et al., 2003) and is a critical component of neuronal death promoted by excitotoxins.
In investigating the role of laminin in neuronal cell death, we infused purified soluble mouse laminin-1 into the C57Bl/6 mouse hippocampus. Surprisingly, infusion of soluble mouse laminin-1 disrupted the normal structure of the endogenous laminin matrix in the hippocampal CA1 and dentate gyrus (DG) neuronal cell layers (compare Figure 1A, infused with buffer, with B, infused with soluble laminin), whereas the laminin in CA3 was largely unaffected. The reason for the lack of response in CA3 is unknown, but previous work has noted that neurons in the CA3 region have stronger laminin staining and are more resistant to neuronal death than those in CA1 (Chen and Strickland, 1997).
Figure 1.
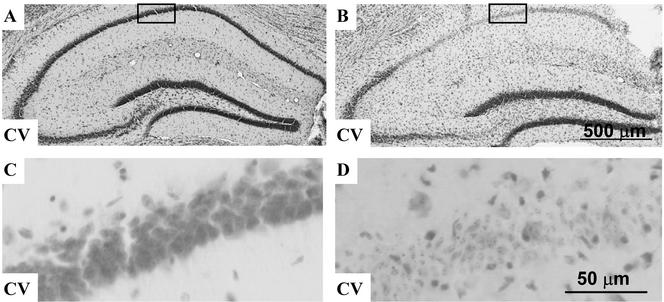
Infusion of soluble laminin-1 disrupts endogenous laminin in the wild-type mouse hippocampus but is not neurotoxic. Wild-type mice were unilaterally infused with buffer or purified mouse laminin-1 (0.5 mg/ml) for 7 d, brain sections were prepared and stained with anti-laminin-1 antibody. Laminin in the hippocampal neuronal call layers in buffer infused animals is intact (A), but in the soluble laminin-infused animals the endogenous laminin in the CA 1 and DG neuronal cell layers of the infused side is disrupted (B). Cresyl violet (C) and Fluoro Jade B (E) staining show that infusion of soluble laminin is not neurotoxic. Higher magnification of the boxed area in C is shown in D. (F) A positive control for Fluoro Jade B staining, which stains degenerating neurons after kainate injection. CA1, CA3, and DG denote regions of the hippocampus. Ln, Laminin-1 immunostaining; CV, cresyl violet staining; FJB, Fluoro Jade B staining. Bar in C for A–C, 500 μm; bar in D for D–F, 25 μm.
To determine whether this laminin change was due to neuronal damage or neuronal cell death, we performed cresyl violet staining, which assesses cell viability. The infusion of soluble laminin did not change the neuronal morphology or cause any cell death (Figure 1, C and D). The fact that neurons did not show any dispersal upon disruption of the laminin layer (Figure 1C) indicates that they are fixed in place by intercellular and other matrix interactions and are not dependent on laminin alone for structural integrity.
To further confirm that the change in the laminin matrix was not due to neuronal cell death, we performed Fluoro Jade B staining, which sensitively identifies degenerating neurons. As a positive control for the method, we stained a kainate-injected hippocampus, which showed substantial neuronal death as evidenced by Fluoro-Jade B staining (Figure 1F). In contrast, there was no Fluoro-Jade B staining in the soluble laminin-infused hippocampus (Figure 1E), indicating no cell death. These results indicate that soluble laminin disrupted the endogenous laminin matrix in the hippocampus and that this disruption was not due to and did not lead to neuronal death.
One concern about this observation was the possibility that the large amount of soluble laminin in the infused sample might be exhausting the anti-laminin antibody used for the immunostaining. We considered this possibility unlikely, because laminin in the CA3 layer, which is resistant to disruption, was still stained by the antibody. Nevertheless, to assess the status of our antibody, we first stained laminin-infused sections identical to the method used for Figure 1B, recovered this primary antibody, and used it to stain untreated brain sections. The antibody showed normal staining of the control section (our unpublished data), indicating an abundance of anti-laminin antibodies, and ruling out antibody depletion as contributing to our observations. These results show that infusion of soluble laminin-1 can disrupt the laminin matrix in vivo.
Laminin-1 consists of α1, β1, and γ1 chains, whereas the principal laminin in the hippocampus is laminin-10 (Indyk et al., 2003), with α5, β1, and γ1 chains. It was therefore not clear how laminin-1 was affecting the laminin-10 matrix. One possibility was that the effects were mediated by either the β1 or γ1 chains, which are common subunits of both laminin-1 and laminin-10. To test this possibility, antibody against the γ1 chain was infused into the hippocampus. This antibody was capable of disrupting the endogenous laminin matrix (Figure 2B, compare with A), indicating that γ1 interactions are important in maintaining this matrix.
To determine whether soluble laminin infusion also affected other proteins expressed in the mouse hippocampus, we examined the muscarinic acetylcholine receptor m2, which is highly expressed in the hippocampus (Levey et al., 1991). After laminin-1 infusion, sections stained with anti-muscarinic acetylcholine receptor m2 antibody showed normal expression of this protein in the CA1 region (Figure 2C).
To determine whether infusion of another ECM protein would affect endogenous laminin, we infused purified fibronectin into the mouse hippocampus. The infusion was efficient as revealed by anti-fibronectin antibody staining (our unpublished data), but had no effect on the laminin matrix (Figure 2D). These results together indicate that the effects observed are specific with respect to the infused laminin and the targeted laminin matrix, and are due at least in part to interactions with the γ1 chain.
Plasmin-digested Laminin Disrupts the Laminin Matrix
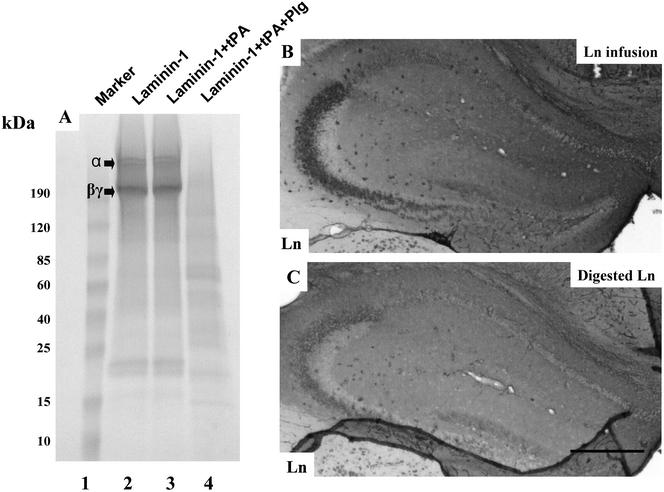
To investigate the structural requirements for the effect of infused laminin, we examined whether digestion of laminin-1 with plasmin would affect its ability to disrupt endogenous laminin when infused into the mouse hippocampus. We first digested purified laminin-1 with plasmin. Our previous studies have shown that after plasmin digestion, there was a lack of intermediate bands except for two bands at around 80- and 20-kDa when visualized by anti-laminin antibody staining (Chen and Strickland, 1997). It was possible that the antibody could not recognize the digested fragments, and therefore other intermediate bands could not be visualized. To address this question, we electrophoresed both intact and plasmin-digested laminin on SDS-PAGE, and stained the gel with Coomassie Blue. Laminin-1 occurred as an α1 band at ∼400 kDa, and a combined β1γ1 band at ∼220 kDa (Figure 3A, lane 2). After digestion with tPA alone, laminin-1 remained intact, demonstrating that tPA was not able to cleave laminin-1 (Figure 3A, lane 3). However, in the presence of both tPA and plasminogen, laminin-1 was digested to multiple intermediates. The α1 chain was completely cleaved (compare lane 4 with lane 2). For the β1γ1 band, there was some undigested material, which could be due to incomplete digestion or resistance of one of the chains to plasmin cleavage. However, when laminin-1 was digested for longer times, the β1γ1 bands were completely degraded, indicating that both chains are susceptible to plasmin. These results clearly show that plasmin can cleave laminin-1 at multiple sites.
Figure 3.
Plasmin digested laminin-1 can effectively disrupt the endogenous laminin matrix. (A) Purified mouse laminin-1 was digested with tPA alone or tPA plus human plasminogen at 37°C for 4 h, electrophoresed on a 10–20% reducing gradient SDS-PAGE, and stained with Coomassie Blue. The migration positions of the α chain and the βγ chains are indicated. tPA alone does not cleave laminin-1 (lane 3), but plasmin (tPA + plasminogen)-digested laminin-1 to multiple fragments (lane 4). (B and C) Wild-type mice were infused with either purified soluble laminin-1 (B) or plasmin digested laminin-1 (C), brain sections were prepared, and stained with anti-laminin-1 antibody. Bar in C, 500 μm.
To determine whether the effect of soluble laminin-1 was dependent on its heteromeric structure, plasmin-digested laminin-1 was infused into the hippocampus. The plasmin-digested material was equally capable of disrupting the laminin matrix as the intact soluble molecule (Figure 3, compare B and C). Quantification of these results showed the following percentages of neuronal disruption: intact laminin, 59.4 ± 5.5; plasmin-digested laminin, 61.3 ± 4.8. Thus, the effect of laminin-1 does not require the intact molecule.
Soluble Laminin Does Not Affect Laminin mRNA
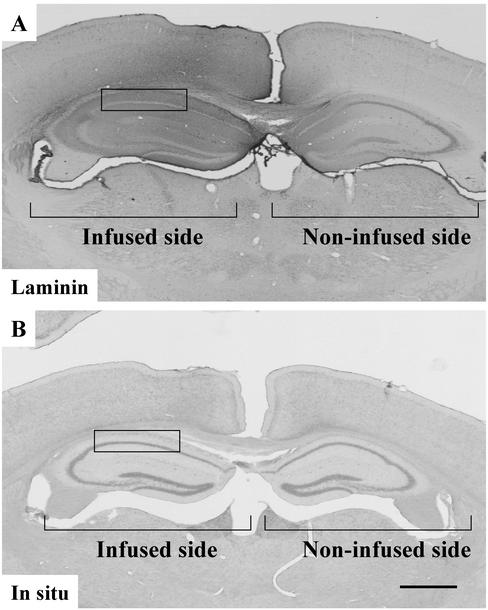
The above-mentioned results showed that infusion of soluble laminin-1 specifically disrupts endogenous laminin in the mouse hippocampus. The maintenance of the laminin matrix is likely to be a steady-state balance between deposition and degradation. The infused laminin could act by reducing laminin mRNA expression, thereby reducing laminin protein synthesis, which might lead to the eventual disappearance of the protein in the matrix. Therefore, we examined whether soluble laminin could affect endogenous laminin mRNA synthesis. To this end, we performed laminin γ1 chain in situ hybridization to determine whether the expression of this mRNA is affected. After laminin infusion, even though the endogenous laminin was clearly disrupted in the CA1 region of the hippocampus (Figure 4A), the adjacent sections hybridized to an antisense laminin γ1 probe showed no significant changes compared with the CA1 region of the noninfused side (Figure 4B). Consistent with our previous results, the laminin γ1 mRNA showed a neuronal pattern. Hybridization to a sense probe showed no significant staining (our unpublished data). The above-mentioned results showed that infusion of soluble laminin-1 did not affect the transcription of the endogenous laminin.
Figure 4.
Infusion of soluble laminin-1 does not affect endogenous laminin mRNA expression. Wild-type mice were infused with purified soluble laminin-1, brain sections were prepared and stained with anti-laminin-1 antibody (A) or processed for laminin γ1 chain in situ hybridization (B). Infusion of soluble laminin-1 disrupts endogenous laminin protein in the neuronal cell layers in the hippocampus (for example, in the boxed area) but does not significantly affect endogenous laminin mRNA expression in the same area (compare infused and noninfused sides). Bar in B, 500 μm.
Soluble Laminin-1 Disrupts the Laminin Matrix in Plasminogen–/– Mice
Previous results showed that during excitotoxic neurodegeneration, the tPA/plasmin proteolytic cascade is up-regulated and degrades laminin. Therefore, another explanation for the effects of soluble laminin could be that it induced tPA or plasmin generation, thereby leading to disappearance of the laminin matrix. To test this possibility, soluble laminin-1 was infused into plasminogen–/– mice. In these mice, the endogenous laminin matrix was disrupted without cell death by infusion of soluble laminin equivalent to that observed in wild-type mice (Figure 5). These results exclude the possibility that the soluble laminin is acting via an induction of plasmin activity, since there can be no plasmin generated in the plasminogen–/– mice.
Figure 5.
Infusion of soluble laminin-1 disrupts endogenous laminin in plasminogen-deficient mice. Plasminogen-deficient mice were infused with purified mouse laminin-1, and brain sections were prepared and processed for antilaminin-1 immunostaining (A), cresyl violet staining (B), or Fluoro Jade B staining (C). Infusion of soluble laminin-1 disrupted the endogenous laminin in the hippocampal neuronal cell layers (A) as in wild-type mice (compare Figure 1B) but did not cause significant neuronal cell death (B and C). Bar in C, 200 μm.
Soluble Laminin-1 and Anti-γ1 Chain Antibody Promotes Neurodegeneration
Previous results have shown that degradation of the laminin matrix via plasmin can sensitize neurons to cell death. Because soluble laminin can also disrupt the laminin matrix, we examined whether this material could also facilitate neuronal degeneration after kainate challenge. For these experiments, we used tPA–/– mice, in which hippocampal neurons are normally resistant to kainite-induced degeneration. We speculated that tPA–/– mice, which cannot degrade laminin during excitotoxic injury and are resistant to neurodegeneration, would become sensitive to death if their neuronal laminin were disrupted by soluble laminin infusion. To test this hypothesis, we infused soluble laminin-1 into the hippocampus of tPA–/– mice for 5 d and then injected kainate. In parallel controls, the mice were infused with buffer for 5 d and then kainate was injected. As expected, tPA–/– mice infused with buffer were resistant to neurodegeneration (Figure 6, A–C). However, mice infused with soluble laminin-1 mice were now sensitive to neurodegeneration, mostly in the CA1 region where the endogenous laminin disruption is most dramatic (Figure 6, D–F). As shown above, plasmin-digested laminin-1 can also disrupt endogenous laminin, and we therefore also tested whether plasmin-digested laminin-1 could promote neurodegeneration. Plasmin-digested laminin-1 also promoted neurodegeneration in the tPA–/– mice (Figure 6, G–I), and the effect of intact laminin and plasmin-digested-laminin were similar (percentage of hippocampal neuronal death: buffer, 4.2 ± 1.3; intact laminin, 65.6 ± 5.2; and plasmin-digested laminin, 67.4 ± 6.1). These results demonstrate that when endogenous laminin is disrupted either by intact soluble laminin or plasmin-digested-laminin, the neurons are sensitized to neuronal injury.
Figure 6.
Infusion of soluble laminin-1 promotes neurodegeneration in tPA-deficient mice. TPA-deficient mice were infused with mouse laminin-1, plasmin-digested laminin-1, or buffer for 5 d, and then kainate was injected. Two days after kainate injection, the mice were killed and brain sections were prepared. The brain sections were processed for anti-laminin-1 immunostaining (A, D, and G) or cresyl violet staining (B, C, E, F, H, and I). Infusion of soluble laminin-1 or plasmin-digested laminin-1 promoted neurodegeneration in tPA–/– mice (E, F, H, and I). Higher magnification of the boxed areas in B, E, and H is shown in C, F, and I, respectively. Ln, laminin immunostaining; CV, cresyl violet staining. Bar in H for A, B, D, E, G, and H, 500 μm; bar in I for C, F, and I, 25 μm.
When tPA–/– mice were infused with a reduced dose of laminin-1, the laminin matrix was disrupted in a very limited region of CA1, and as expected did not cause any cell death. Kainate injection into these mice caused neurodegeneration in precisely the region of CA1 where the matrix was disrupted, whereas neurons survived in adjacent areas in which the matrix was not perturbed (our unpublished data). This result strengthens the conclusion that disruption of the laminin matrix was responsible for sensitizing neurons to cell death.
To further investigate the mechanism of laminin-1 on neuronal death, we infused antibody against the laminin γ1 chain into tPA–/– mice and then injected kainate into some of these mice. The infused antibody was not toxic alone (Figure 7, A and C), but promoted neuronal death when the tPA–/– mice were injected with kainate (Figure 7, B and D). This result, coupled with the observation that this antibody disrupts the laminin matrix (Figure 2), indicated that the γ1 chain is a critical component for maintaining the laminin layer and protecting neurons against excitotoxic death.
Figure 7.
Infusion of anti-laminin γ1 antibody promotes neurodegeneration in tPA-deficient mice. TPA-deficient mice were infused with anti-laminin γ1 antibody (0.2 mg/ml) for 5 d (A and B) and then kainate was injected (B). Two days after kainate injection, the mice were killed and brain sections were prepared. The brain sections were processed for cresyl violet staining (A and B). Infusion of anti-laminin γ1 antibody was not neurotoxic by itself (A and C), but when combined with kainate injection promoted neurodegeneration in tPA-deficient mice (B and D). Higher magnification of the boxed areas in A and B are shown in C and D, respectively. CV, cresyl violet staining.
DISCUSSION
Our experiments demonstrate that infusion of soluble laminin-1 or anti-laminin γ1 chain antibody into the mouse hippocampus can disrupt the laminin matrix that normally lies along the neuronal cell layers. This disruption is specific Because other ECM proteins such as fibronectin had no effect on the laminin matrix, and the soluble laminin did not affect the hippocampal expression of other proteins. The effect did not require the intact heterotrimeric molecule, because laminin-1 extensively digested with the protease plasmin was still capable of disrupting the in vivo matrix. There is substantial precedence for small regions of matrix proteins being able to function as effective cellular ligands (Ruoslahti, 1996).
One function of mouse hippocampal laminin is to provide survival support for neurons. When excitotoxins are injected into the hippocampus, tPA activity is dramatically increased leading to plasmin generation, and to degradation of the laminin matrix (Chen and Strickland, 1997; Nagai et al., 1999). This loss of matrix renders the neurons sensitive to neuronal death. If laminin degradation is prevented by lack or inhibition of the proteases responsible, neurons are protected. These results indicate that the neuron-matrix interaction is an important aspect of cell viability, as has been observed in many other systems (Ernsberger et al., 1989; Meredith et al., 1993; Boudreau et al., 1995; Coucouvanis and Martin, 1995; Frisch and Screaton, 2001). In support of this concept, disruption of the laminin matrix with soluble laminin also facilitates neuronal death after kainate injection, even in animals that lack tPA. Thus, two different experimental paradigms, one involving proteolytic degradation and one competitive disruption, demonstrate that the integrity of the laminin matrix in the hippocampus is critical for protecting neurons against degeneration.
There are numerous mechanisms by which a soluble matrix protein could affect the amount of that protein in the matrix in vivo. It is possible that transcription of the gene encoding the protein could be affected, leading to less mRNA and eventually less protein in the matrix. However, it is clear from in situ hybridization that the soluble laminin did not affect laminin mRNA levels in the hippocampus. Another possibility is that the soluble protein could induce the tPA/plasminogen system, leading to degradation of the matrix. However, laminin-1 infusion led to disappearance of the matrix laminin in plasminogen–/– mice, indicating that at least this proteolytic system is not responsible.
The most likely mechanism for the observed effect is that the high concentration of soluble laminin competes with the matrix laminin for binding sites with other components of the ECM. Once dissociated from the matrix, these previously tethered laminin molecules might be free to diffuse out of the hippocampus or be degraded by proteases. The infused molecule presumably would not have the same affinity for interaction with other matrix components, so it would not be effectively bound to the ECM, and would not remain with the neuronal cell layers.
With respect to how laminin might protect neurons against excitotoxic death, it is likely that neuronal laminin receptors mediate this effect. There have been multiple studies indicating that integrins α3β1, α6β1, and α6β4 are the major receptors for laminin-10 (Ferletta and Ekblom, 1999; Gu et al., 1999; Tani et al., 1999; Kikkawa et al., 2000; Pouliot et al., 2000; Nielsen and Yamada, 2001; Doi et al., 2002). However, it is not known which, if any, integrin might be playing a primary role in the hippocampus. The infusion of antibodies against potential receptor molecules, as with the anti-laminin γ1 experiments shown in Figures 2 and 7, might help to identify which receptors are most important for interaction of laminin with neurons.
These results suggest a dynamic and plastic nature for the hippocampal ECM. If the matrix proteins were extremely fixed, one would not expect them to be dislodged by the presence of a competing soluble molecule. On the other hand, if a dynamic equilibrium exists in which association of the components is favored, but dissociation is also significant, then a high concentration of a soluble but imperfect molecule could foster the dissociation without being held firmly by the association forces. In this sense, the soluble molecule would function as an antagonist of ECM maintenance. It has been shown in other contexts that a soluble ECM protein can have different effects that the same protein embedded in a matrix (Hayman et al., 1985).
Such a dynamic view of the ECM has obvious implications for tissue architecture, especially in the brain. It has been shown that tPA can affect new synapse formation (Baranes et al., 1998; Müller and Griesinger, 1998), long-term potentiation (Huang et al., 1996), and learning and memory (Madani et al., 1999). The plasticity demonstrated by these studies could be partially dependent on proteolytic remodeling of the matrix that surrounds the neurons, and laminin degradation can also affect LTP (Nakagami et al., 2000). Thus, to achieve a structure that can be modified with experience, it would be important to have a supporting matrix that is stable but not too stable. Our results provide clear evidence from in vivo experiments that this situation exists in the mouse hippocampus.
In addition, our experimental paradigm provides a strategy for unraveling some of the complex intermolecular interactions in the matrix in vivo. Other ECM proteins can be tested for their effect on the matrix, and for those that are active, small regions or even peptides that recapitulate the effect could be determined. Putting these data together should improve our understanding of how the ECM is maintained and provide reagents for disassembling the matrix to further study its structure and function.
Acknowledgments
This work was supported by National Institutes of Health grants NS-35704 and NS-38472 (to S.S.). We are grateful to all the members of the Strickland laboratory for input and support.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–12–0832. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0832.
References
- Baranes, D., Lederfein, D., Huan, Y., Chen, M., Bailey, C.H., and Kandel, E.R. (1998). Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21, 813–825. [DOI] [PubMed] [Google Scholar]
- Boudreau, N., Sympson, C.J., Werb, Z., and Bissell, M.J. (1995). Suppression of ICE and apoptosois in mammary epithelial cells by extracellular matrix. Science 267, 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge, T.H., Flick, M.J., Daugherty, C.C., and Degen, J.L. (1995). Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 9, 794–807. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., Schoonjans, L., Kieckens, L., Ream, B., Degen, J.L., Bronson, R., De Vos, J.J., van den Oord, D., Collen, D., and Mulligan, R.C. (1994). Physiological consequences of loss of plasminogen activator gene function in mice. Nature 368, 419–424. [DOI] [PubMed] [Google Scholar]
- Chen, Z.-L., Indyk, J.A., Bugge, T.H., Kombrinck, K.W., Degen, J.L., and Strickland, S. (1999). Neuronal death and blood-brain barrier breakdown after excitotoxic injury are independent processes. J. Neurosci. 19, 9813–9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z.-L., and Strickland, S. (1997). Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 91, 917–925. [DOI] [PubMed] [Google Scholar]
- Chen, Z.L., et al. (1995). Expression and activity-dependent changes of a novel limbic-serine protease gene in the hippocampus. J. Neurosci. 15, 5088–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H., and Yurchenco, P.D. (2000). Form and function: the laminin family of heterotrimers. Dev. Dynamics 218, 213–234. [DOI] [PubMed] [Google Scholar]
- Coucouvanis, E., and Martin, G.R. (1995). Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell 83, 279–287. [DOI] [PubMed] [Google Scholar]
- Coyle, J.T., Molliver, M.E., and Kuhar, M.J. (1978). In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J. Comp. Neurol. 180, 301–323. [DOI] [PubMed] [Google Scholar]
- Doi, M., Thyboll, J., Kortesmaa, J., Jansson, K., Iivanainen, A., Parvardeh, M., Timpl, R., Hedin, U., Swedenborg, J., and Tryggvason, K. (2002). Recombinant human laminin-10 (α5β1γ1). Production, purification, and migration-promoting activity on vascular endothelial cells. J. Biol. Chem. 277, 12741–12748. [DOI] [PubMed] [Google Scholar]
- Ernsberger, U., Edgar, D., and Rohrer, H. (1989). The survival of early chick sympathetic neurons in vitro is dependent on a suitable substrate but independent of NGF. Dev. Biol. 135, 250–262. [DOI] [PubMed] [Google Scholar]
- Ferletta, M., and Ekblom, P. (1999). Identification of laminin-10/11 as a strong cell adhesive complex for a normal and a malignant human epithelial cell line. J. Cell Sci. 112, 1–10. [DOI] [PubMed] [Google Scholar]
- Frisch, S.M., and Screaton, R.A. (2001). Anoikis mechanisms. Curr. Opin. Cell Biol. 13, 555–562. [DOI] [PubMed] [Google Scholar]
- Grimpe, B., Dong, S., Doller, C., Temple, K., Malouf, A.T., and Silver, J. (2002). The critical role of basement membrane-independent laminin gamma1 chain during axon regeneration in the CNS. J. Neurosci. 22, 3144–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Y., Sorokin, L., Durbeej, M., Hjalt, T., Jonsson, J.I., and Ekblom, M. (1999). Characterization of bone marrow laminins and identification of alpha5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells. Blood 93, 2533–2542. [PubMed] [Google Scholar]
- Gualandris, A., Jones, T.E., Strickland, S., and Tsirka, S.E. (1996). Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 16, 2220–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg, T., Muir, D., Engvall, E., Varon, S., and Manthorpe, M. (1989). Laminin-like antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment. Neuron 3, 721–732. [DOI] [PubMed] [Google Scholar]
- Hagg, T., Portera-Cailliau, C., Jucker, M., and Engvall, E. (1997). Laminins of the adult mammalian CNS; laminin-alpha2 (merosin M-) chain immunoreactivity is associated with neuronal processes. Brain Res. 764, 17–27. [DOI] [PubMed] [Google Scholar]
- Hayman, E.G., Pierschbacher, M.D., and Ruoslahti, E. (1985). Detachment of cells from culture substrate by soluble fibronectin peptides. J. Cell Biol. 100, 1948–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y.-Y., et al. (1996). Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc. Natl. Acad. Sci. USA 93, 8699–8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indyk, J.A., Chen, Z.-L., and Strickland, S. (2003). Laminin chain expression suggests that laminin-10 is a major isoform in the mouse hippocampus and is degraded by the tPA/plasmin system during excitotoxic injury. Neuroscience 116, 359–371. [DOI] [PubMed] [Google Scholar]
- Jucker, M., Tian, M., and Ingram, D.K. (1996). Laminins in the adult and aged brain. Mol. Chem. Neuropathol. 28, 209–218. [DOI] [PubMed] [Google Scholar]
- Kikkawa, Y., Sanzen, N., Fujiwara, H., Sonnenberg, A., and Sekiguchi, K. (2000). Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by α3β1, α6β1 and α6β4 integrins. J. Cell Sci. 113, 869–876. [DOI] [PubMed] [Google Scholar]
- Levey, A.I., Kitt, C.A., Simonds, W.F., Price, D.L., and Brann, M.R. (1991). Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 11, 3218–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi, P., Fried, G., and Stewart, R.R. (2001). Neurons and glial cells of the embryonic human brain and spinal cord express multiple and distinct isoforms of laminin. J. Neurosci. Res. 64, 144–167. [DOI] [PubMed] [Google Scholar]
- Luckenbill-Edds, L. (1997). Laminin and the mechanism of neuronal outgrowth. Brain Res. Brain Res. Rev. 23, 1–27. [DOI] [PubMed] [Google Scholar]
- Lukashev, M.E., and Werb, Z. (1998). ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 8, 437–441. [DOI] [PubMed] [Google Scholar]
- Madani, R., Hulo, S., Toni, N., Madani, H., Steimer, T., Muller, D., and Vassalli, J.-D. (1999). Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 18, 3007–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, Y., Kitamura, Y., and Taniguchi, T. (1998). Induction of plasminogen in rat hippocampal pyramidal neurons by kainic acid. Neurosci. Lett. 252, 119–122. [DOI] [PubMed] [Google Scholar]
- Meredith, J., Fazeli, J.B., and Schwartz, M.A. (1993). The extracellular matrix as a cell survival factor. Mol. Biol. Cell 4, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J.H., Cunningham, J., and Sanes, J.R. (1998). Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J. Cell Biol. 143, 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, C.M., and Griesinger, C.B. (1998). Tissue plasminogen activator mediates reverse occlusion plasticity in visual cortex. Nat. Neurosci. 1, 47–53. [DOI] [PubMed] [Google Scholar]
- Murtomaki, S., Risteli, J., Risteli, L., Koivisto, U.M., Johansson, S., and Liesi, P. (1992). Laminin and its neurite outgrowth-promoting domain in the brain in Alzheimer's disease and Down's syndrome patients. J. Neurosci. Res. 32, 261–273. [DOI] [PubMed] [Google Scholar]
- Nagai, N., Urano, T., Endo, A., Takahashi, H., Takada, Y., and Takada, A. (1999). Neuronal degeneration and a decrease in laminin-like immunoreactivity is associated with elevated tissue-type plasminogen activator in the rat hippocampus after kainic acid injection. Neurosci. Res. 33, 147–154. [DOI] [PubMed] [Google Scholar]
- Nakagami, Y., Abe, K., Nishiyama, N., and Matsuki, N. (2000). Laminin degradation by plasmin regulates long-term potentiation. J. Neurosci. 20, 2003–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, P.K., and Yamada, Y. (2001). Identification of cell-binding sites on the laminin alpha 5 N-terminal domain by site-directed mutagenesis. J. Biol. Chem. 276, 10906–10912. [DOI] [PubMed] [Google Scholar]
- Noakes, P.G., Gautam, M., Mudd, J., Sanes, J.R., and Merlie, J.P. (1995). Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature 374, 258–262. [DOI] [PubMed] [Google Scholar]
- Parmer, R.J., Mahata, M., Mahata, S., Sebald, M.T., O'Connor, D.T., and Miles, L.A. (1997). Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway. Catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J. Biol. Chem. 272, 1976–1982. [DOI] [PubMed] [Google Scholar]
- Patton, B.L., Chiu, A.Y., and Sanes, J.R. (1998). Synaptic laminin prevents glial entry into the synaptic cleft. Nature 393, 698–701. [DOI] [PubMed] [Google Scholar]
- Patton, B.L., Cunningham, J.M., Thyboll, J., Kortesmaa, J., Westerblad, H., Edstrom, L., Tryggvason, K., and Sanes, J.R. (2001). Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat. Neurosci. 4, 597–604. [DOI] [PubMed] [Google Scholar]
- Ploplis, V.A., Carmeliet, P., Vazirzadeh, S., Van Vlaenderen, I., Moons, L., Plow, E.F., and Collen, D. (1995). Effects of disruption of the plasminogen gene on thrombosis, growth and health in mice. Circulation 92, 2585–2593. [DOI] [PubMed] [Google Scholar]
- Pouliot, N., Connolly, L.M., Moritz, R.L., Simpson, R.J., and Burgess, A.W. (2000). Colon cancer cells adhesion and spreading on autocrine laminin-10 is mediated by multiple integrin receptors and modulated by EGF receptor stimulation. Exp. Cell Res. 261, 360–371. [DOI] [PubMed] [Google Scholar]
- Ruoslahti, E. (1996). RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715. [DOI] [PubMed] [Google Scholar]
- Sanes, J.R., and Lichtman, J.W. (2001). Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805. [DOI] [PubMed] [Google Scholar]
- Sappino, A.-P., Madani, R., Huarte, J., Belin, D., Kiss, J.Z., Wohlwend, A., and Vassalli, J.-D. (1993). Extracellular proteolysis in the adult murine brain. J. Clin. Investig. 92, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued, L.C., and Hopkins, K.J. (2000). Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 874, 123–130. [DOI] [PubMed] [Google Scholar]
- Tani, T., Lehto, V.P., and Virtanen, I. (1999). Expression of laminins 1 and 10 in carcinoma cells and comparison of their roles in cell adhesion. Exp. Cell Res. 248, 115–121. [DOI] [PubMed] [Google Scholar]
- Tian, M., Hagg, T., Denisova, N., Knusel, B., Engvall, E., and Jucker, M. (1997). Laminin-alpha2 chain-like antigens in CNS dendritic spines. Brain Res. 764, 28–38. [DOI] [PubMed] [Google Scholar]
- Tsirka, S.E., Gualandris, A., Amaral, D.G., and Strickland, S. (1995). Excitotoxin-induced neuronal degerneration and seizure are mediated by tissue plasminogen activator. Nature 377, 340–344. [DOI] [PubMed] [Google Scholar]
- Tsirka, S.E., Rogove, A.D., Bugge, T.H., Degen, J.L., and Strickland, S. (1997). An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci. 17, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venstrom, K.A., and Reichardt, L.F. (1993). Extracellular matrix. 2: role of extracellular matrix molecules and their receptors in the nervous system. FASEB J. 7, 996–1003. [DOI] [PubMed] [Google Scholar]