Abstract
In the mammalian kidney the fine control of Na+ reabsorption takes place in collecting duct principal cells where basolateral Na,K-ATPase provides the driving force for vectorial Na+ transport. In the cortical collecting duct (CCD), a rise in intracellular Na+ concentration ([Na+]i) was shown to increase Na,K-ATPase activity and the number of ouabain binding sites, but the mechanism responsible for this event has not yet been elucidated. A rise in [Na+]i caused by incubation with the Na+ ionophore nystatin, increased Na,K-ATPase activity and cell surface expression to the same extent in isolated rat CCD. In cultured mouse mpkCCDcl4 collecting duct cells, increasing [Na+]i either by cell membrane permeabilization with amphotericin B or nystatin, or by incubating cells in a K+-free medium, also increased Na,K-ATPase cell surface expression. The [Na+]i-dependent increase in Na,K-ATPase cell-surface expression was prevented by PKA inhibitors H89 and PKI. Moreover, the effects of [Na+]i and cAMP were not additive. However, [Na+]i-dependent activation of PKA was not associated with an increase in cellular cAMP but was prevented by inhibiting the proteasome. These findings suggest that Na,K-ATPase may be recruited to the cell membrane following an increase in [Na+]i through cAMP-independent PKA activation that is itself dependent on proteasomal activity.
INTRODUCTION
The kidney plays a major role in the homeostasis of body fluid compartments in mammals. Despite the large quantitative variations in dietary intake of solutes and water, the kidneys are able to maintain within a narrow range the composition and volume of extracellular and intracellular fluid compartments. The fine-tuning of Na+ reabsorption, tightly controlled by hormonal and nonhormonal factors, occurs at the level of the renal collecting duct. In this nephron segment, Na+ reabsorption takes place via a transcellular route in collecting duct principal cells. Na+ enters into principal cells via the luminal epithelial Na+ channel (ENaC) and is extruded by the basolateral Na,K-ATPase. The Na,K-ATPase, which provides the driving force for active Na+ and K+ transport, and secondary active transport of other solutes (Skou, 1998), is tightly regulated (Therien and Blostein, 2000; Féraille and Doucet, 2001). Long-term regulation of Na,K-ATPase relies mainly on alteration of the expression of its subunits, whereas short-term control is mediated by changes in enzymatic turnover and/or redistribution between cell surface and intracellular compartments.
In the mammalian cortical collecting duct (CCD), a rise in intracellular Na+ concentration ([Na+]i) rapidly increases the activity of Na,K-ATPase and the number of specific ouabain binding sites (Barlet-Bas et al., 1990b; Blot-Chabaud et al., 1990). It has been shown that [Na+]i-dependent increase of Na,K-ATPase activity does not require transcriptional regulation and/or de novo protein synthesis (Barlet-Bas et al., 1990b). These findings raise the possibility that silent Na-pumps already located at the cell membrane are activated or alternatively, that preexisting intracellular Na,K-ATPase units are shuttled to the cell surface. The latter hypothesis was supported by recent experimental evidence showing that an intracellular pool of Na,K-ATPase units can be rapidly recruited to the cell surface in response to cAMP (Gonin et al., 2001) or to aldosterone (Summa et al., 2001).
The aim of the present study was to investigate the effect of an increase in [Na+]i on Na,K-ATPase cell surface expression in CCD cells and to determine the intracellular signaling pathway mediating this effect. To answer these questions, we used both microdissected intact rat CCDs and confluent cultured mouse principal collecting duct mpkCCDc14 cells (Bens et al., 1999), a cell line characterized by retained-expression of transporters specific for CCD principal cells including ENaC and aquaporin-2 as well as by controlled transepithelial Na+ transport by aldosterone and vasopressin (Bens et al., 1999; Vandewalle et al., 1999; Robert-Nicoud et al., 2001; Hasler et al., 2002),
MATERIALS AND METHODS
Isolated Rat Kidney Tubules
Male Wistar rats (150–200 g body weight; Centre Médical Universitaire, Genève, Switzerland) were anesthetized with intraperitoneal injection of pentobarbital (5 mg/100 g of body weight). After laparotomy, the left kidney was perfused with an incubation solution (120 mM NaCl, 5 mM RbCl, 4 mM NaHCO3, 1 mM CaCl2, 1 mM MgSO4, 0.2 mM NaH2PO4, 0.15 mM Na2HPO4, 5 mM glucose, 10 mM lactate, 1 mM pyruvate, 4 mM essential and nonessential amino acids, 0.03 mM vitamins, 20 mM HEPES, pH 7.45) supplemented with 0.44% (wt/vol) collagenase (CLSII, 0.75–0.87 U/mg). Afterward, the kidney was removed, sliced into small pyramids, and incubated for 20 min at 30°C in an oxygenated (95% O2 and 5% CO2) incubation solution containing 0.08% (wt/vol) collagenase, as described previously (Gonin et al., 2001). Single CCDs were isolated by microdissection in the ice-cold oxygenated incubation solution containing aprotinin (1 μg/ml) and leupeptin (20 mg/ml) to preserve the integrity of proteins. Isolated CCDs were incubated with or without drugs for 2 h at 37°C as described in RESULTS. The length of tubular segments, which served as reference for Na,K-ATPase activities and Western blotting analysis was determined from photographs of microdissected CCDs.
Cell Culture
The mpkCCDc14 cells (passages 20–25) were grown in defined medium (DM: DMEM:Ham's F12′ 1:1 [vol/vol], 60 nM sodium selenate, 5 μg/ml transferrin, 2 mM glutamine, 50 nM dexamethasone, 1 nM triiodothyronine, 10 ng/ml epidermal growth factor, 5 μg/ml insulin, 20 mM d-glucose, 2% vol/vol, fetal calf serum, and 20 mM HEPES, pH 7.4) at 37°C in 5% CO2/95% air atmosphere, and the medium was changed every 2 d. Experiments were performed on confluent cells seeded on semipermeable polycarbonate filters (Transwell, 0.4-μm pore size, 1-cm2 growth area, Corning Costar, Cambridge, MA). Cells were kept for 6–8 d in DM medium and then placed in serum-free, hormone-deprived medium 24 h before the experiments. Except as otherwise noted, the serum-free, hormone-deprived medium was supplemented with 10–6 M aldosterone. For experiments, cells were preincubated for 30 min at 37°C with or without drugs in an incubation solution (5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 0,2 mM NaH2PO4, 0.15 mM Na2HPO4, 5 mM glucose, 4 mM NaHCO3, 12 mM essential amino acids, 2 mM nonessential amino acids, vitamins, 1 mM pyruvic acid, 10 mM lactic acid, 20 mM HEPES, pH 7.4) supplemented with either 120 mM choline chloride (low-Na+condition; 15 mM Na+) or 120 mM NaCl (high-Na+ condition; 135 mM Na+), with or without drugs. Incubation was carried out for 1 h at 37°C after adding 1 μg/ml amphotericin B or 0.1 U/ml nystatin to the basolateral side of the filters. In some cases, experiments were carried out using an incubation solution devoided of K+ (K+-free condition).
Measurement of Na,K-ATPase Activity
The hydrolytic activity of the Na,K-ATPase was determined under Vmax conditions by the release of Pi from [γ-32P]ATP (Amersham, United Kingdom) on isolated rat CCDs permeabilized by freeze/thawing as described (Deschenes and Doucet, 2000). Measurements were performed in quadruplicate on pools of four to six microdissected rat CCDs. The Na,K-ATPase activity was taken as the difference between the means total and Na,K-independent ATPase activities measured in the presence (100 mM NaCl and 5 mM KCl) and in the absence of Na+ and K+ (150 mM choline chloride, 2 mM ouabain), respectively. The results were expressed as pmol ATP × mm–1 × h–1.
Measurement of Total and Cell Surface Na,K-ATPase
For measurement of total cellular Na,K-ATPase content, confluent mpkCCDcl4 cells grown on filters were lysed in homogenizing buffer (HB: 20 mM Tris-HCl, pH 7.4, 2 mM EDTA, 2 mM EGTA, 20 μg/ml leupeptin, 10 millitrypsin-inhibitor units/ml aprotinin, 30 mM NaF, 30 mM Na pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 1 mM AEBSF, 0.1% [wt/vol] SDS, and 1% [vol/vol] Triton X-100), and protein content was determined using the BCA assay (Pierce, Rockford, IL). Lysed samples were resuspended in Laemmli's buffer (Laemmli, 1970) and processed for SDS-PAGE. After electrophoresis on 7% polyacrylamide gels, proteins were electrotransferred to polyvinylidene difluoride membranes (Immobilion-P, Millipore, Waters, MA). Membranes were incubated with a polyclonal antibody (dilution 1/10,000) raised against the α-subunit of Na,K-ATPase (Carranza et al., 1996a) in Tris-buffered saline (TBS) NP-40 (150 mM NaCl, 50 mM Tris, 0.2% NP-40, pH 7.4) with 5% (wt/vol) nonfat dried milk. After washing, membranes were incubated with an anti-rabbit IgG antibody (dilution 1/10,000) coupled to horseradish peroxidase (BD Transduction Laboratories, San Di-ego, CA). The antigen-antibody complexes were detected by chemiluminescence with the Super Signal Substrate method (Pierce) according to the manufacturer's instructions. The protein bands revealed were quantified using a video densitometer and Image-Quant software (Molecular Dynamics, Sunnyvalle, CA), and the results were expressed as percent of the intensity of the protein band from control samples.
Measurements of cell surface Na,K-ATPase were performed on isolated rat CCDs and cultured mpkCCDcl4 cells as described (Gonin et al., 2001) using EZ-Link sulfosuccinimidobiotin (Sulfo NHS-S-S-Biotin, Pierce) to label cell surface proteins. After lysis in HB buffer, equal amounts of protein were precipitated in the presence of streptavidin-agarose beads (Immunopure immobilized streptavidin, Pierce) diluted in an antiprotease-supplemented buffer solution (TLB: 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 20 μg/ml leupeptin, 1 μg/ml aprotinin). The beads were then washed three times with the TLB solution and once with 10 mM Tris-HCl, pH 7.4. After resuspension in Laemmli's buffer (Laemmli, 1970), samples were processed for SDS-PAGE and Western blotting as described above.
Measurement of Intracellular cAMP Content
After incubation under various experimental conditions, the cAMP content of individual microdissected rat CCDs was measured using a previously described radioimmunoassay (Chabardès et al., 1984). Results were expressed as fmol cAMP × mm–1.
Confluent mpkCCDcl4 cells grown on filters under various experimental conditions were scraped off the filters and homogenized in a buffer containing 50 mM Tris, 4 mM EDTA, pH 7.4. IBM-X (10–5 M) was added during the incubation period and in the homogenization buffer to prevent cAMP degradation by phosphodiesterases. Cellular cAMP levels were then measured using the cyclic AMP (3H) system (Amersham, United Kingdom) according to the manufacturer's instructions. Results were expressed as a pmol cAMP × μg protein–1.
Measurement of PKA Activity
Confluent mpkCCDcl4 cells grown on filters and incubated under various experimental conditions were scraped off and homogenized in an extraction buffer (25 mM Tris-HCl, pH 7.4, 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, 1 μg/ml leupeptin, and 1 μg/ml aprotinin). Protein Kinase A activity was then measured using the SignaTECTR cAMP-Dependent Protein Kinase (PKA) Assay System (Promega, Madison, WI) according to the manufacturer's instructions. Results were expressed as pmol ATP × min–1 × μg protein–1.
Statistics
Results are given as the mean ± SE from (n) independent experiments. Each experiment was performed on microdissected tubules from the same animal or on cultured cells from the same passage. Statistical differences in Na,K-ATPase activity, PKA activity, and cellular cAMP content measured under various conditions were done using the unpaired Student t test or by analysis of variance for comparison of two or more than two groups, respectively. Statistical analysis of Na,K-ATPase α-subunit immunoreactivity was done using the Mann-Whitney U test or the Kruskal-Wallis test for comparison of two or more than two groups, respectively. p < 0.05 was considered significant.
RESULTS
Rising Intracellular Na+ Content Increases Na,K-ATPase Cell Surface Expression in Intact Rat CCDs and in Mouse mpkCCDcl4 Cells
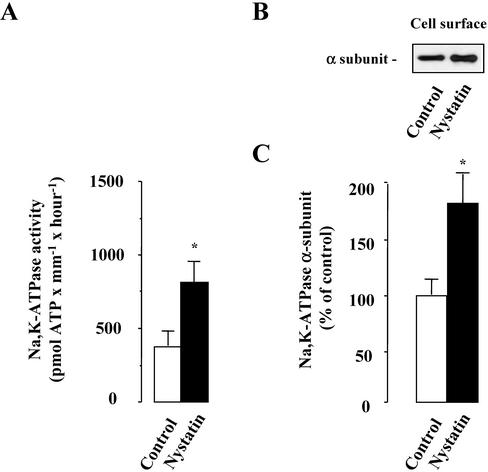
We first assessed the consequences of increased [Na+]i on Na,K-ATPase activity, and cell surface expression. [Na+]i was increased by incubating isolated rat CCDs with the Na+ ionophore nystatin (0.1 U/μl)for 2 h at 37°C. Figure 1 shows that nystatin induced a significant increase in both Na,K-ATPase activity measured at Vmax (as percentage of control ± SE; 191 ± 14%; n = 5; p < 0.05) and Na,K-ATPase cell surface expression (as percentage of control ± SE; 181 ± 40%; n = 10; p < 0.05). These results indicate that the increased levels of [Na+]i increased maximal Na,K-ATPase activity and Na-pump cell surface expression to the same extent in rat CCD.
Figure 1.
Effect of high [Na+]i on Na,K-ATPase activity and cell surface expression in isolated rat CCDs. The activity (A) and cell surface expression (B and C) of Na,K-ATPase were analyzed on microdissected CCDs incubated without (control) or with 0.1 U/μl nystatin for 1 h at 37°C. (A) The activity of Na,K-ATPase was determined under Vmax conditions on freeze/thawing permeabilized CCDs. Results are expressed as pmol ATP × mm–1 × h–1 and are means ± SE from five animals. *p < 0.05. (B and C) The Na,K-ATPase α-subunit was detected by Western blotting performed after biotinylation and streptavidin precipitation of cell surface proteins. (B) Representative immunoblot showing Na,K-ATPase cell surface expression. (C) Bars represent densitometric values expressed as the percentage of the optical density value obtained from untreated samples (control). Results are means ± SE from eight animals. *p < 0.05 vs. control values.
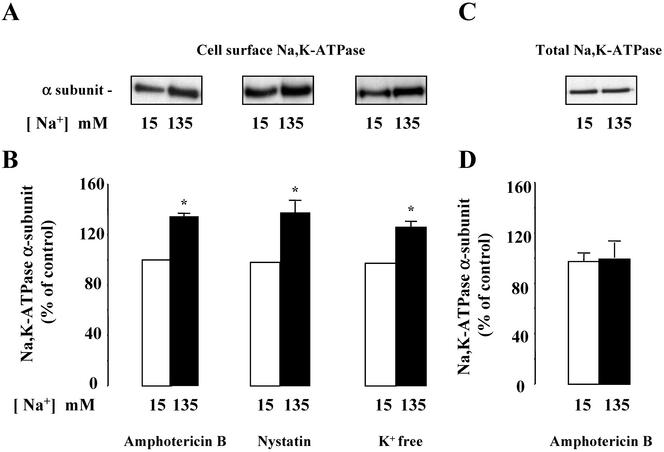
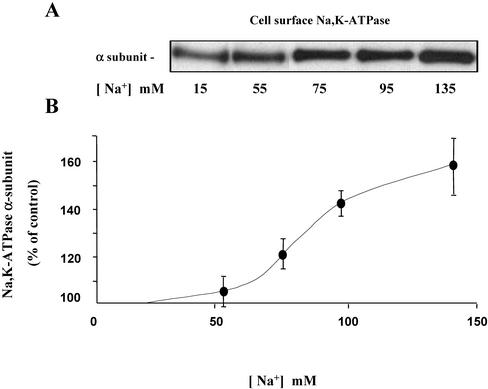
Similar experiments were performed in confluent mpkCCDcl4 cells grown on filters and incubated with high (135 mM) or low (15 mM) Na+ solutions. In this case, cultured cells were incubated with two different Na+ ionophores, nystatin (0.1 U/μl) or amphotericin B (1 μg/ml), in order to enhance plasma membrane permeability to Na+ and to decrease the concentration gradient between intracellular and extracellular Na+. Experiments were also performed using a K+ free solution, which reversibly inhibits Na,K-ATPase and therefore disrupts the physiological Na+ concentration gradient across the plasma membrane (Blot-Chabaud et al., 1990). Under these conditions, mpkCCDc14 cells incubated in a solution containing 135 mM Na+ in order to increase [Na+]i, exhibited a greater cell surface expression of Na,K-ATPase as compared with the same set of cells incubated in a solution containing 15 mM Na+ (Figure 2, A and B). The increase in Na,K-ATPase cell surface expression occurred after a lag time of 30 min and was maximal after 1-h incubation at 37°C (our unpublished results). In contrast, a rise in [Na+]i did not alter Na,K-ATPase total cellular content (Figure 2, C and D). A graded increase in [Na+]e from 15–135 mM produced a Na+ concentration-dependent increase in Na,K-ATPase cell surface expression in amphotericin B-permeabilized mpkCCDcl4 cells (Figure 3). Control experiments were performed to assess the effect of amphotericin B permeabilization on mpkCCDc14 cell viability. Addition of 10–9 M vasopressin during the last 10 min of the 1-h incubation in the presence of amphotericin B produced a close to sixfold increase in cellular cAMP content (see Figure 7) indicating that cellular ATP content was not depleted by ionophore treatment. In addition, although transepithelial resistance and potential fell to zero in amphotericin B permeabilized confluent cells, a full recovery was observed 24 h after ionophore washout. Moreover, addition of vasopressin (10–9 M) for 24 h after ionophore washout decreased transepithelial resistance from 4928 ± 142 to 3500 ± 214 Omhs/cm2 (n = 4), increased transepithelial potential from 41 ± 2 to 53 ± 2 mV and induced aquaporin-2 water channel expression (our unpublished results), as previously described in nonpermeabilized cells (Vandewalle etal., 1999; Hasler et al., 2002). Therefore, short-term (1 h) amphotericin B permeabilization did not significantly alter the viability and vasopressin responsiveness of mpkCCDcl4 cells. These results indicate that cultured mpkCCDcl4 cells represent a suitable ex vivo cell system for extensive analysis of mechanism(s) underlying the Na,K-ATPase recruitment to the cell surface in response to high [Na+]i.
Figure 2.
Effect of high [Na+]i on Na,K-ATPase cell surface and total expression in mpkCCDc14 cells. Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of 15 or 135 mM Na+ for 30 min at 37°C and then permeabilized by 1 μg/ml amphotericin B or 0.1 U/μl nystatin, or incubated in K+-free medium for 1 h at 37°C. The Na,K-ATPase α-subunit was detected by Western blotting performed after biotinylation and streptavidin precipitation of cell surface proteins (Cell surface Na,K-ATPase; A and B) or on cell lysates (Total Na,K-ATPase; C and D). (A and C) Representative immunoblots showing cell surface (A) and total (C) expression of Na,K-ATPase. (B and D) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from five independent experiments. *p <0.05 vs. 15 mM Na+ values.
Figure 3.
[Na+]i-dependency of Na,K-ATPase cell surface expression in mpkCCDc14 cells. Amphotericin B–permeabilized mpkCCDcl4 cells were incubated for 1 h at 37°C in a medium containing increasing concentrations of Na+. Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (A) Representative immunoblots showing Na,K-ATPase cell surface expression. (B) Densitometric values were expressed as the percentage of the optical density value measured in the presence of 15 mM Na+ and plotted against Na+ concentration in the medium. Results are means ± SE from four independent experiments. *p < 0.05 vs. 15 mM Na+ values.
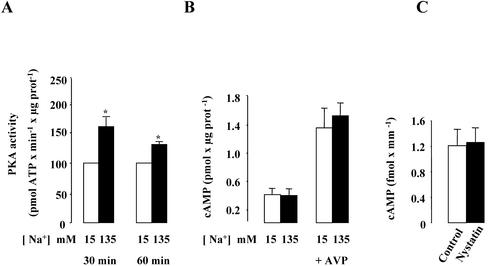
Figure 7.
Effect of high [Na+]i on PKA activity in mpkCCDc14 cells and cellular cAMP content in mpkCCDc14 cells and isolated rat CCDs. (A and B) Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of 15 or 135 mM Na+ for 30 min at 37°C. (A) After permeabilization by 1 μg/ml amphotericin B, cells were incubated for 30–60 min at 37°C. The PKA activity was measured on homogenized cells using the SignaTECT cAMP-dependent Protein Kinase Assay System as described in MATERIALS AND METHODS. Results expressed as pmol ATP × min–1 × μg protein–1 are means ± SE from four independent experiments. *p <0.05 vs. 15 mM Na+ values. (B) After permeabilization by 1 μg/ml amphotericin B, cells were incubated for 60 min at 37°C and 10–9 M vasopressin (AVP) was added or not to the cell medium during the last 10 min of incubation. The cellular cAMP content was measured on homogenized cells using the cyclic AMP (3H) assay system as described in MATERIALS AND METHODS. Results expressed as pmol cAMP × μg protein–1 are means ± SE from four independent experiments. (C) Microdissected CCDs were incubated in the absence (control) or presence of 0.1 U/μl nystatin for 1 h at 37°C. Cellular cAMP content was determined using a radioimmunoassay as described in MATERIAL AND METHODS. Results expressed as fmol cAMP × mm–1 are means ± SE from six animals.
The Increase in Na,K-ATPase Cell Surface Expression Induced by a Rise in [Na+]i Is Enhanced by Corticosteroids and Independent of Ongoing Transcription and De Novo Translation
The recruitment of active Na,K-ATPase units induced by a rise in [Na+]i has been shown to require the presence of aldosterone in isolated mammalian CCDs (Barlet Bas et al., 1990; Blot-Chabaud et al., 1990). Experiments were performed on confluent mpkCCDc14 cells grown in serum-free, hormone-deprived medium and in the absence or presence of 10–6 M aldosterone for 72 h to assess the aldosterone-dependency of [Na+]i-induced increase in cell surface expression of Na,K-ATPase. The [Na+]i-dependent increase in Na,K-ATPase cell surface expression measured on amphotericin B-permeabilized cells was significantly reduced in mpkCCDc14 cells grown in hormone-free medium (as percentage of control ± SE; 111 ± 1.6%) compared with aldosterone-treated cells (as percentage of control ± SE; 126 ± 0.3; n = 6; p <0.05; our unpublished results). Therefore, these results indicate that corticosteroid hormones potentiate Na,K-ATPase cell membrane recruitment caused by a rise in [Na+]i in mpkCCDcl4 cells.
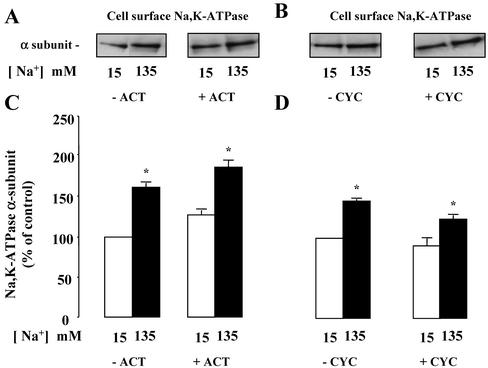
Because the time course of [Na+]i-induced recruitment of Na,K-ATPase units to the cell surface (30–60 min) is compatible with de novo protein synthesis, we assessed the effects of actinomycin D and cycloheximide, two classical inhibitors of transcription and protein synthesis, respectively. In amphotericin B–permeabilized cells, neither 5 μM actinomycin D nor 20 μM cycloheximide prevented the effect of [Na+]i on Na,K-ATPase cell surface expression (Figure 4). These results indicate that recruitment of Na,K-ATPase by a rise in [Na+]i does not rely on transcriptional activity or on de novo protein synthesis.
Figure 4.
Effect of transcription and translation inhibition on [Na+]i-dependent Na,K-ATPase cell surface expression in mpkCCDcl4 cells. Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of 15 mM or 135 mM Na+ without (–) or with (+)5 μM actinomycin D (Act; A and C) or 20 μM cycloheximide (Cyc; B and D) for 30 min at 37°C. After permeabilization by 1 μg/ml amphotericin B cells were incubated for 1 h at 37°C. Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (A and B) Representative immunoblots showing Na,K-ATPase cell surface expression. (C and D) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from six independent experiments. *p < 0.05 vs. 15 mM Na+ values.
The [Na+]i-dependent Increase of Na,K-ATPase Cell Surface Expression Relies on PKA Activation
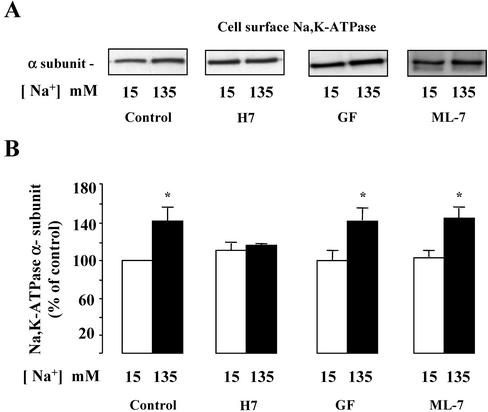
We next examined the involvement of serine/threonine kinase in [Na+]i-dependent Na,K-ATPase cell surface expression. In amphotericin B-permeabilized mpkCCDcl4 cells incubated in low- or high-Na+ media, the broad-range serine/threonine kinases inhibitor H-7 (10–5 M) abolished the [Na+]i-dependent increase of Na,K-ATPase cell surface expression (Figure 5).
Figure 5.
Effect of serine/threonine kinase inhibitors on the [Na+]i-dependent Na,K-ATPase cell surface expression in mpkCCDcl4 cells. Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of 15 or 135 mM Na+ without or with 10–5 M H-7, 10–6 M GF109203X (GF), or 10–5 M ML-7 for 30 min at 37°C. After permeabilization by 1 μg/ml amphotericin B cells were incubated for 1 h at 37°C. Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (A) Representative immunoblot showing Na,K-ATPase cell surface expression. (B) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from six independent experiments. *p < 0.05 vs. 15 mM Na+ values.
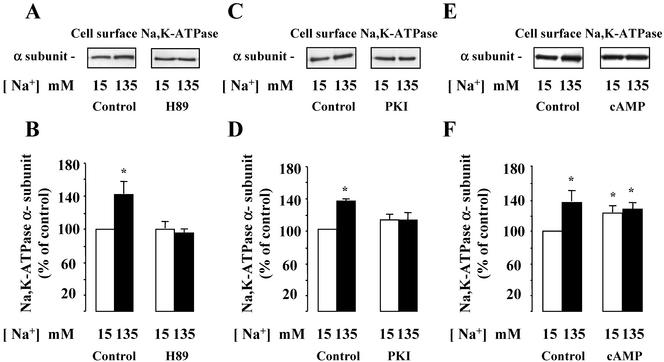
We next refined our analysis of protein kinases involved in the [Na+]i-induced upregulation of cell surface-expressed Na,K-ATPase by investigating the effects of various narrow-range serine/threonine kinase inhibitors. The results obtained indicate that neither GF109203X (10–6 M), a protein kinase C inhibitor, nor ML-7 (10–5 M), a myosin light chain kinase inhibitor, altered the stimulatory effect of increased [Na+]i on cell surface expression of Na,K-ATPase (Figure 5). In contrast, H89 (5 × 10–5 M), a preferential but not fully specific protein kinase A (PKA) inhibitor (Davies et al., 2000), prevented the [Na+]i-dependent recruitment of Na,K-ATPase to the cell surface of mpkCCDcl4 cells (Figure 6, A and B). Figure 6, C and D, shows that 50 μM myristoylated PKI, a cell-permeable peptide inhibitor of PKA, also prevented the effect of increased [Na+]i on cell surface expression of Na,K-ATPase. In addition, H89 also abolished the increase of Na,K-ATPase activity induced by nystatin in isolated rat CCDs (as pmol × mm–1 × h–1 ± SE; Control: 501 ± 83; Nystatin: 844 ± 64*; H89: 570 ± 40; H89 + Nystatin: 569 ± 21; n = 4*; p <0.05 vs. control). Because we have previously shown that cAMP, a classical PKA activator, induced the translocation of an intracellular pool of Na,K-ATPase to the plasma membrane (Gonin et al., 2001), we studied the additivity of Na,K-ATPase cell surface expression induced by both [Na+]i and cAMP. In amphotericin B–permeabilized mpkCCDcl4 cells incubated in a low Na+ (15 mM) solution, addition of db-cAMP (10–3 M), a cell-permeant cAMP analog, for 10 min at 37°C increased Na,K-ATPase cell surface expression by 25 ± 5%. In contrast, db-cAMP did not further increase Na,K-ATPase cell surface expression in amphotericin B–permeabilized cells incubated in a high-Na+ (135 mM) solution (Figure 6, E and F). These results strongly suggest that PKA activity is stimulated by a rise in [Na+]i.
Figure 6.
Effect of PKA inhibition and activation on [Na+]i-dependent Na,K-ATPase cell surface expression of in mpkCCDcl4 cells and Na,K-ATPase activity in isolated rat CCDs. (A and B) Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of either 15 or 135 mM Na+ without or with 5 × 10–5 M H-89 for 30 min at 37°C. After permeabilization by 1 μg/ml amphotericin B, cells were incubated for 1 h at 37°C. Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (A) Representative immunoblot showing Na,K-ATPase cell surface expression. (B) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from four independent experiments. *p <0.05 vs. 15 mM Na+ values. (C and D) Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of either 15 or 135 mM Na+ without or with 50 μM myristoylated PKI for 30 min at 37°C. After permeabilization by 1 μg/ml amphotericin B, cells were incubated for 1 h at 37°C. Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (A) Representative immunoblot showing Na,K-ATPase cell surface expression. (B) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from four independent experiments. *p < 0.05 vs. 15 mM Na+ values. (E and F) Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of 15 or 135 mM Na+ for 30 min at 37°C. After permeabilization by 1 μg/ml amphotericin B, cells were incubated for 1 h at 37°C and 10–3 M db-cAMP was added or not for the last 10 min of incubation. Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (D) Representative immunoblot showing Na,K-ATPase cell surface expression. (E) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from four independent experiments. *p < 0.05 vs. 15 mM Na+ values.
To further confirm that the stimulatory effect of high [Na+]i on Na,K-ATPase cell surface expression was dependent on PKA activation, PKA activity was measured in amphotericin B–permeabilized mpkCCDcl4 cells. As shown in Figure 7A, incubation of permeabilized cells for 30–60 min at 37°C in the presence of 135 mM Na+ induced a sustained increase in PKA activity. Because PKA activation is classically related to an increase in cellular cAMP content, cellular cAMP content was measured in amphotericin B–permeabilized mpkCCDc14 cells. Unexpectedly, the amount of cellular cAMP content did not change in cells incubated in mediums containing either 15 mM or 135 mM Na+ (Figure 7B). Similarly, increasing [Na+]ii did not alter the cAMP content of cells preincubated with the phosphodiesterase inhibitor IBMX (10–4 M; our unpublished results). In contrast, 10–9 M vasopressin, a classical adenylate cyclase activator, added during the last 10 min of incubation, induced a large increase in cellular cAMP content in amphoterincin B–permeabilized cells (Figure 7B). This AVP-induced increase in cellular cAMP content was not influenced by increased [Na+]i. Therefore, amphotericin B permeabilization did not induce a large leak of cAMP out of mpkCCDcl4 cells and increasing [Na+]i did not alter the adenylate cyclase responsiveness to AVP. Furthermore, cellular cAMP content was not altered in nystatin-permeabilized isolated rat CCDs (Figure 7C). Altogether, these results indicate that a rise in [Na+]i induces PKA activation independently of any detectable increase in cellular cAMP content.
Inhibitors of the Proteasomal Degradation Pathway Prevent [Na+]i-induced PKA Activation and Increase in Na,K-ATPase Cell Surface Expression
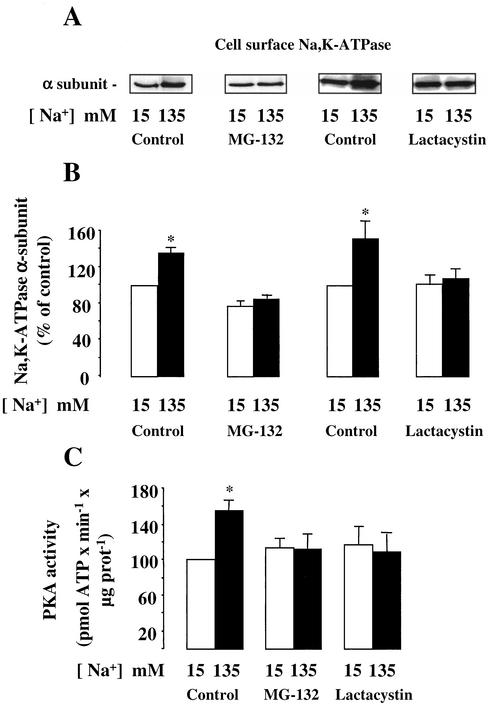
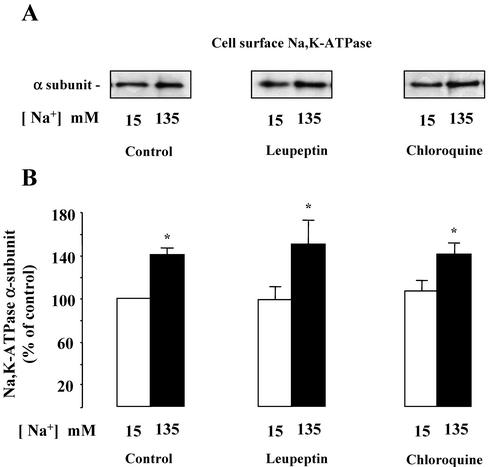
It has been reported that cytokines may trigger cAMP-independent activation of PKA (Zhong et al., 1997) through a pathway that requires proteasomal activity (Dulin et al., 2001). Experiments were performed to investigate the possible roles of the proteasomal and lysosomal protein degradation pathways in Na,K-ATPase recruitment to the mpkCCDcl4 cell surface induced by a rise [Na+]i. Proteasomal activity was inhibited by addition of 10–6 M MG132 or lactacystin, two specific and structurally unrelated proteasome inhibitors. To analyze the contribution of the lysosome, we used either 2 × 10–6 M leupeptin, an inhibitor of cysteine proteases, or 10–7 M chloroquine, a weak base that increases lysosomal pH and thereby inhibits the proteolytic activity of lysosomal enzymes. We first checked that these drugs did not modify the high transepithelial electrical resistance of confluent mpkCCDC14 cells (our unpublished results). Figure 8 shows that the presence of proteasomal inhibitors completely abolished the [Na+]i-induced increase of Na,K-ATPase expressed at the cell surface and blocked the stimulation of PKA activity in amphotericin B–permeabilized mpkCCDcl4 cells. In contrast, none of the lysosomal inhibitors used altered the increase in Na,K-ATPase cell surface expression caused by a rise in [Na+]i (Figure 9). These results indicate that the proteasomal but not the lysosomal degradation pathway is involved in [Na+]i-dependent recruitment of Na,K-ATPase to the cell surface of mpkCCDcl4 cells.
Figure 8.
Effect of proteasomal inhibitors on [Na+]i-dependent Na,K-ATPase cell surface expression and PKA activation in mpkCCDcl4 cells. Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of either 15 or 135 mM Na+ without or with 10–6 M lactacystin or 10–6 M MG-132 for 30 min at 37°C. After permeabilization by 1 μg/ml amphotericin B, cells were incubated for 1 h at 37°C. (A and B) Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (A) Representative immunoblot showing Na,K-ATPase cell surface expression. (B) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from six independent experiments. *p < 0.05 vs. 15 mM Na+ values. (C) PKA activity was measured on homogenized cells using the SignaTECT cAMP-dependent Protein Kinase Assay System as described in MATERIALS AND METHODS. Results expressed as pmol ATP × min–1 × μg protein–1 are means ± SE from four independent experiments. *p < 0.05 vs. 15 mM Na+ values.
Figure 9.
Effect of lysosomal inhibitors on [Na+]i-dependent Na,K-ATPase cell surface expression in mpkCCDcl4 cells. Confluent mpkCCDcl4 cells grown on polycarbonate filters were first preincubated in the presence of either 15 or 135 mM Na+ without or with 10–6 M leupeptin or with 10–7 M choloroquine for 30 min at 37°C. After permeabilization by 1 μg/ml amphotericin B, cells were incubated for 1 h at 37°C. (A and B) Cell surface Na,K-ATPase was detected as described in the legend of Figure 2. (A) Representative immunoblot showing Na,K-ATPase cell surface expression. (B) Bars represent densitometric values expressed as the percentage of the optical density value measured in the presence of 15 mM Na+. Results are means ± SE from six independent experiments. *p < 0.05 vs. 15 mM Na+ values.
DISCUSSION
This study provides several lines of evidence that 1) translocation of an intracellular reservoir of Na-pumps to the plasma membrane is responsible for the stimulation of Na,K-ATPase activity observed in response to an increase in [Na+]i in mammalian CCD principal cells and 2) this recruitment of Na,K-ATPase units is at least in part mediated by cAMP-independent PKA activation and requires the proteasomal degradation of a yet unidentified regulatory factor that maintains PKAc in an inactive state.
[Na+]i, aldosterone, and vasopressin (Gonin et al., 2001; Summa et al., 2001) all control Na,K-ATPase cell surface expression in collecting duct principal cells. [Na+]i is the major limiting factor for Na,K-ATPase activity in intact cells (Skou, 1998). In resting renal epithelial cells, [Na+]i is maintained below levels required for half-maximal Na,K-ATPase activation (K0.5), which explains why this enzyme works at only 20–30% of its maximal rate (Cheval et al., 1990; Féraille et al., 1995). It has therefore been assumed that the only rate limiting step for Na+ reabsorption in collecting duct principal cells was the Na+ influx occurring through the apical epithelial Na+ channel (ENaC) and that the kinetic control of Na,K-ATPase by [Na+]i was sufficient to maintain the balance between the apical entry and basolateral exit of Na+. However, several studies have shown that aldosterone and vasopressin enhance both apical and basolateral steps of vectorial Na+ transport in collecting duct principal cells through the coordinated stimulation of ENaC and Na,K-ATPase (Palmer et al., 1982; Schafer and Troutman, 1990; Bens et al., 1999; Gonin et al., 2001; Summa et al., 2001). Such coordinated control seems to be of special importance in the renal collecting duct. Under normal conditions, luminal Na+ concentrations in CCD are quite low and any increase in [Na+]i secondary to increased Na+ influx should theoretically reduce the electrochemical driving force for apical Na+ entry (Silver et al., 1993). However, because the apparent affinity of Na,K-ATPase for Na+ is twice as high in the collecting duct than in the proximal tubule and thick ascending limb (Barlet-Bas et al., 1990a; Féraille et al., 1994, 1995), the kinetic reserve for a [Na+]i activation of Na,K-ATPase is much lower in the collecting duct than in the more proximal nephron segments. In view of ENaC downregulation by high [Na+]i (Kellenberger et al., 1998; Awayda, 1999), the observed increase of Na,K-ATPase cell surface expression that allows a more efficient decrease in [Na+]i suggests that the restoration of a normal [Na+]i is a priority with respect to Na+ reabsorption in collecting duct principal cells. Therefore, acute fluctuations of [Na+]i may exert a rapid kinetic effect on Na,K-ATPase activity whereas sustained changes in [Na+]i would also control the number of active Na pumps present at the cell surface of CCD cells.
Our results also show that high [Na+]i induces a proportional increase in maximal hydrolytic activity and Na,K-ATPase cell surface expression in mammalian CCDs. Therefore, the increase in the number of specific ouabain binding sites, i.e., the number of active Na-pump units at the cell surface, previously observed in response to high [Na+]i (Barlet-Bas et al., 1990b; Blot-Chabaud et al., 1990) most likely relies on an increase in Na-pump cell surface expression. This increase is observed in the absence of a variation of the total cellular pool of Na,K-ATPase and is independent of transcriptional regulation and de novo protein synthesis (Barlet Bas et al., 1990b), strongly suggesting that translocation of intracellular Na-pumps to the plasma membrane occurs in response to increased [Na+]i. This interpretation is further supported by the PKA dependency of this process because we have recently shown the existence of an intracellular pool of Na,K-ATPase, which can be recruited rapidly to the plasma membrane in response to cAMP in collecting duct principal cells (Gonin et al., 2001). Similarly, short-term aldosterone induces the redistribution of intracellular Na-pumps to the cell surface (Summa et al., 2001). The aldosterone-dependency of both high [Na+]i- and cAMP-induced increase in cell surface expression of Na,K-ATPase (Barlet-Bas et al., 1990, Blot-Chabaud et al., 1990, Gonin et al., 2001) may suggest the requirement of (an) aldosterone-induced regulatory protein(s) exerting a permissive effect.
The results of this study also strongly support that PKA activation is required for the [Na+]i-induced recruitment of Na-pumps to the cell surface in collecting duct principal cells: first, pharmacological inhibition of PKA prevented the [Na+]i-induced increase in Na,K-ATPase cell surface expression and activity; second, high [Na+]i concomitantly stimulated PKA activity and increased Na,K-ATPase cell surface expression; third, the effects of [Na+]i and cAMP on Na,K-ATPase cell surface expression are not additive; and finally proteasome inhibitors prevented both [Na+]i-induced PKA activation and Na,K-ATPase increased cell surface expression. It remains to be determined whether PKA directly induces Na,K-ATPase redistribution of, e.g., though phosphorylation of the Na,K-ATPase α-subunit (Beguin et al., 1994; Feschenko and Sweadner, 1994; Fisone et al., 1994; Carranza et al., 1996b, 1998), and/or whether other signaling intermediate(s) is(are) involved.
The PKA holoenzyme is a heterotetramer consisting of two catalytic (PKAc) subunits associated with two regulatory subunits (Scott, 1991; Taylor et al., 1990; Francis and Corbin, 1994). Dissociation of the holoenzyme is induced by binding of cAMP to the regulatory subunits, which consequently alleviates autoinhibitory contacts and releases active PKAc. Our results clearly indicate that the [Na+]i-induced activation of PKA is independent of a rise in cellular cAMP content, because even in the presence of phosphodiesterase inhibitor or AVP, intracellular cAMP concentration is not altered by high [Na+]i in CCD cells. The large increase in cellular cAMP content observed in Na+-loaded amphotericin B–permeabilized cells treated with AVP indicate that cAMP did not leak out the cells and that high [Na]i did not interfere with adenylate cyclase activity. On the other hand, Na+-sensitive adenylyl cyclase stimulation (Cooper et al., 1998) does not seem to be involved in [Na+]i-induced PKA activation. An alternate cAMP-independent mechanism of PKA activation has been recently described. In response to cytokines, free active PKAc is released upon dissociation of a multiprotein complex containing PKA, IκBα, and NF-κB p65 (Zhong et al., 1997; Zieger et al., 2001). Dissociation of the PKAc/IκBα/NF-κB p65 complex is triggered by phosphorylation (Karin, 1999) and subsequent proteasomal degradation of IκBα (Dulin et al., 2001). In addition, the association of a discrete pool of PKAc with the tonicity-responsive enhancer binding protein (TonEBP) and cAMP-independent activation of PKA in response to extracellular hypertonicity have been recently described (Ferraris et al., 2002). Incubation of cells under hypertonic conditions rapidly stimulates the transcriptional activity of TonEBP (Miyakawa et al., 1999) in a PKA-dependent manner (Ferraris et al., 2002). Our results show that PKA activation by [Na+]i is independent of cAMP and requires proteasomal activity, suggesting that [Na+]i controls the degradation rate of a regulatory protein that maintains a discrete cellular pool of PKAc in an inactive state.
Acknowledgments
We thank Michelangelo Foti for helpful discussion. This work was supported in part by grants from the Swiss National Foundation 31–50830.99 and the Carlos et Elsie deReuter Foundation to E.F.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–11–0720. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-11-0720.
Abbreviations used: PKA, protein kinase A; CCD, cortical collecting duct; AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride.
References
- Awayda, M.S. (1999). Regulation of epithelial Na+ channel by intracellular Na+. Am. J. Physiol. 277, C216–C224. [DOI] [PubMed] [Google Scholar]
- Barlet-Bas, C., Cheval, L., Khadouri, C., Marsy, S., and Doucet, A. (1990a). Difference in the Na+ affinity of Na+,K+-ATPase along the rabbit nephron: modulation by K+. Am. J. Physiol. 259, F246–F250. [DOI] [PubMed] [Google Scholar]
- Barlet-Bas, C., Khaodouri, C., Marsy, S., and Doucet, A. (1990b). Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na,K-ATPase whose size is modulated by corticosteroids. J. Biol. Chem. 265, 7799–7803. [PubMed] [Google Scholar]
- Beguin, P., Beggah, A.T., Chibalin, A.V., Burgener-Kairuz, P., Jaisser, F., Mathews, P.M., Rossier, B.C., Cotecchia, S., and Geering, K. (1994). Phosphorylation of the Na,K-ATPase α-subunit by protein kinase A and C in vitro and intact cells. Identification of a novel motif for PKC-mediated phosphorylation. J. Biol. Chem. 269, 24437–24445. [PubMed] [Google Scholar]
- Bens, M., Vallet, V., Cluzeaud, F., Pascual-Letallec, L., Kahn, A., Rafestin-Oblin, M.E., Rossier, B.C., and Vandewalle, A. (1999). Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J. Am. Soc. Nephrol. 10, 923–934. [DOI] [PubMed] [Google Scholar]
- Blot-Chabaud, M., Wanstok, F., Bonvalet, J-P., and Farman, N. (1990). Cell-sodium-induced recruitment of Na+-K+-ATPase pumps in rabbit collecting tubules is aldosterone-dependent. J. Biol. Chem. 265, 11676–11681. [PubMed] [Google Scholar]
- Carranza, M.L., Féraille, E., and Favre, H. (1996a). Protein kinase C-dependent phosphorylation of Na+-K+-ATPase alpha-subunit in rat kidney cortical tubules. Am. J. Physiol. 271, C136–C143. [DOI] [PubMed] [Google Scholar]
- Carranza, M.L., Féraille, E., Kiroytcheva, M., Rousselot, M., and Favre, H. (1996b). Stimulation of ouabain-sensitive 86Rb+ uptake and Na+, K+-ATPase α-subunit phosphorylation by a cAMP-dependent signaling pathway in intact cells from rat kidney cortex. FEBS Lett. 396, 309–314. [DOI] [PubMed] [Google Scholar]
- Carranza, M.L., Rousselot, M., Chibalin, A.V., Bertorello, A.M., Favre, H., and Féraille, E. (1998). Protein kinase A induces recruitment of active Na+,K+-ATPase units to the plasma membrane of rat proximal convoluted tubule cells. J. Physiol. 511, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabardès, D., Montégut, M., Imbert-Teboul, M., and Morel, F. (1984). Inhibition of α2-adrenergic agonists on AVP-induced cAMP accumulation in isolated collecting tubule of the rat kidney. Mol. Cell. Endocrinol. 37, 263–275. [DOI] [PubMed] [Google Scholar]
- Cooper, D.M., Schell, M.J., Thorn, P., and Irvine, R.T. (1998). Regulation of adenylyl cyclase by membrane potential. J. Biol. Chem. 273, 27703–27707. [DOI] [PubMed] [Google Scholar]
- Davies, S.P., Reddy, H., Caivano, M., and Cohen, P. (2000). Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes, G., and Doucet, A. (2000). Collecting duct Na+, K+-ATPase activity is correlated with urinary sodium excretion in rat nephrotic syndromes. J. Am. Soc. Nephrol. 11, 604–615. [DOI] [PubMed] [Google Scholar]
- Dulin, N.O., Niu, J., Browning, D.D., Ye, R.D., and Voyno-Yasenetskaya, T. (2001). Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J. Biol. Chem. 276, 20827–20830. [DOI] [PubMed] [Google Scholar]
- Féraille, E., Carranza, M.L., Rousselot, M., and Favre, H. (1994). Insulin enhances sodium sensitivity of Na,K-ATPase in isolated rat proximal convoluted tubule. Am. J. Physiol. 267, F55–F62. [DOI] [PubMed] [Google Scholar]
- Féraille, E., Rousselot, M., Rajerison, R., and Favre, H. (1995). Effect of insulin on Na+, K+-ATPase in rat collecting duct. J. Physiol. 488, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féraille, E., and Doucet, A. (2001). Sodium-potassium-adenosinetriphosphate-dependent sodium transport in the kidney: hormonal control. Physiol. Rev. 81, 345–418. [DOI] [PubMed] [Google Scholar]
- Feschenko, M.S., and Sweadner, K.J. (1994). Conformation-dependent phosphorylation of Na,K-ATPase by protein kinase A and protein kinase C. J. Biol. Chem. 269, 30436–30444. [PubMed] [Google Scholar]
- Fisone, G. et al. (1994). Identification of the phosphorylation site for cAMP-dependent protein kinase on Na+, K+-ATPase and effects of site-directed mutagenesis. J. Biol. Chem. 269, 9368–9373. [PubMed] [Google Scholar]
- Francis, S.H., and Corbin, J.D. (1994). Structure and function of cyclic nucleotide-dependent protein kinases. Annu. Rev. Physiol 56, 237–272. [DOI] [PubMed] [Google Scholar]
- Gonin, S., Deschenes, G., Roger, F., Bens, M., Martin, P.Y., Carpentier, J.L., Vandewalle, A., Doucet, A., and Feraille, E. (2001). Cyclic AMP increases cell surface expression of functional Na,K-ATPase units in mammalian cortical collecting duct principal cells. Mol. Biol. Cell 13, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, U., Mordasini, D., Bens, M., Bianchi, M., Cluzeaud, F., Rousselot, M., Vandewalle, A., Feraille, E., and Martin, P.Y. (2002). Long term regulation of aquaporin-2 expression in vasopressin-gresponsive renal collecting duct principal cells. J. Biol. Chem. 277, 10379–10386. [DOI] [PubMed] [Google Scholar]
- Ferraris, J.D., Persaud, P., Williams, C.K., Chen, Y., and Burg, M. (2002). cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/osmotic response element binding protein. Proc. Natl. Acad. Sci. USA 99, 16800–16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin, M. (1999). How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18, 6867–6874. [DOI] [PubMed] [Google Scholar]
- Kellenberger, S., Gautschi, I., Rossier, B.C., and Schild, L. (1998). Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J. Clin. Invest. 12, 2741–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Miyakawa, H., Woo, S.K., Dahl, S.C., Handler, J.S., and Kwon, H.M. (1999). Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. USA 96, 2538–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, L.G., Li, J.H.Y., Lindeman, B., and Edelman, I.S. (1982). Aldosterone control of the density of sodium channels in the toad urinary bladder. J. Membr. Biol. 64, 91–102. [DOI] [PubMed] [Google Scholar]
- Robert-Nicoud, M. et al. (2001). Transcriptome of a mouse kidney cortical collecting duct cell line: Effects of aldosterone and vasopressin. Proc. Natl. Acad. Sci. USA 98, 2712–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, J.A., and Troutman, S.L. (1990). cAMP mediates the increase in apical membrane Na+ conductance produced in rat CCD by vasopressin. Am. J. Physiol. 259, F823–F831. [DOI] [PubMed] [Google Scholar]
- Scott, J.D. (1991). Cyclic nucleotide-dependent protein kinases. Pharmacol. Ther. 50, 123–145. [DOI] [PubMed] [Google Scholar]
- Silver, R.B., Frindt, G., Windhager, E.E., and Palmer, L.G. (1993). Feedback regulation of Na channels in rat CCT. I. Effects of inhibition of Na pump. Am. J. Physiol. 264, F557–F564. [DOI] [PubMed] [Google Scholar]
- Skou, J.C. (1998). Nobel lecture. The identification of the sodiumpotassium pump. Biosci. Rep. 18, 155–169. [DOI] [PubMed] [Google Scholar]
- Summa, V., Mordasini, D., Roger, F., Bens, M., Martin, P.Y., Vandewalle, A., Verrey, F., and Feraille, E. (2001). Short term effect of aldosterone on Na,K-ATPase cell surface expression in kidney collecting duct cells. J. Biol. Chem. 276, 47087–47093. [DOI] [PubMed] [Google Scholar]
- Taylor, S.S., Buechler, J.A., and Yonemoto, W. (1990). cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 59, 971–1005. [DOI] [PubMed] [Google Scholar]
- Therien, A.G., and Blostein, R. (2000). Mechanisms of sodium pump regulation. Am. J. Physiol. 279, C541–C566. [DOI] [PubMed] [Google Scholar]
- Vandewalle, A., Bens, M., and Duong Van Huyen, J.-P. (1999). Immortalized kidney epithelial cells as tools for hormonally regulated ion transport studies. Curr. Opin. Nephrol. Hypertens. 8, 581–587. [DOI] [PubMed] [Google Scholar]
- Zhong, H., SuYang, H., Erdjument-Bromaage, H., Tempst, P., and Ghosh, Z. (1997). The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89, 413–424. [DOI] [PubMed] [Google Scholar]
- Zieger, M., Tausch, S., Henklein, P., Nowak, G., and Kaufmann, R. (2001). A novel PAR-1-Type thrombin receptor signaling pathway: cyclic AMP-independent activation of PKA in SNB-19 glioblastoma cells. Biochem. Biophys. Res. Com. 282, 952–957. [DOI] [PubMed] [Google Scholar]