Abstract
The fission yeast spindle pole body (SPB) is a nucleus-associated organelle that duplicates once each cell cycle during interphase. Duplicated SPBs serve as the poles of an intranuclear mitotic spindle after their insertion into the nuclear envelope in mitosis (Ding et al., Mol. Biol. Cell 8, 1461–1479). Here, we report the identification and characterization of Schizosaccharomyces pombe cdc31p, a member of the conserved calcium-binding centrin/CDC31 family. Immunofluorescence and immunoelectron microscopy show that cdc31p is a SPB component localized at the half-bridge structure of the SPB. cdc31 is an essential gene and Δcdc31 cells and cdc31 conditional mutant cells arrest in mitosis with a monopolar mitotic spindle organized from a single SPB. EM analysis demonstrates that mutant cdc31 cells fail to duplicate the SPB. In addition, cdc31p exhibits genetic interactions with the SPB component sad1p and is required for sad1p localization. Finally, cdc31 mutant can undergo single or multiple rounds of septation before the exit from mitosis, suggesting that cdc31p activity or SPB duplication may be required for the proper coordination between the exit from mitosis and the initiation of septation.
INTRODUCTION
To accurately control the segregation of chromosomes between sister cells, eukaryotes have developed a very sophisticated apparatus, the mitotic spindle. In this apparatus, microtubules are most often focused on two poles organized by a specialized organelle, the centrosome. This organelle, which both nucleates and anchors microtubule (MTs), duplicates only once in a cell cycle. During duplication, new centrosomal structures usually appear in close vicinity to the preexisting ones. In animal cells for instance, new centrioles form perpendicularly to the proximal end of the preexisting pair of centrioles. This feature is also true for budding yeast centrosome, a layered organelle embedded in the nuclear envelope, also called spindle pole body (SPB): SPB duplication proceeds through the formation of a cytoplasmic satellite next to a substructure of the SPB lying on the nuclear envelope called the half-bridge (Byers and Goetsch, 1974; see Adams and Kilmartin, 2000 for a review).
In fission yeast, the SPB is a nuclear-associated organelle composed of a layered cytoplasmic part and an electron dense nuclear part separated by the nuclear envelope. An electron dense particle, lying on the nuclear envelope beside the layered cytoplasmic part of the SPB, has been proposed to be analogous to budding yeast half-bridge. Fission yeast SPB duplication is thought to occur in the cytoplasm in late G2 when the single SPB structure is replaced by two smaller structures connected by an electron dense bridge. No intermediate state in SPB duplication such as the formation of a satellite has been described to date. At the onset of mitosis, the SPBs are inserted into the nuclear envelope and organize the assembly of an intranuclear spindle (Ding et al., 1997).
Molecular mechanisms that underline centrosome duplication process are still poorly understood. However, molecular characterization of centrosomes has revealed that key components controlling centrosome functions have been conserved despite the large structural diversity of centrosomes among eukaryotes. For instance, a gamma-tubulin containing complex controlling microtubule nucleation has been characterized both in yeast and in animal cells (for reviews see Tassin and Bornens, 1999 and Knop et al., 1999; see also Vardy et al., 2002). Molecular mechanisms controlling the centrosome duplication process may have also been conserved because orthologues of genes required for SPB duplication in budding yeast also appear to regulate centrosome duplication in Schizosaccharomyces pombe or in animal cells (Baum et al., 1986; Winey et al., 1993; West et al., 1998; Middendorp et al., 2000).
One of the budding yeast genes implicated in SPB duplication, CDC31, encodes a Ca2+-binding protein of the centrin family (Baum et al., 1986). It is a component of the half-bridge structure of the SPB (Spang et al., 1993). Analysis of thermosensitive mutants has demonstrated that this essential gene is required in an early stage of SPB duplication because formation of the satellite does not occur in these mutants (Byers, 1981; Schild et al., 1981). ScCdc31p association with the half-bridge depends on another half-bridge component Kar1p (Biggins and Rose, 1994; Spang et al., 1995). How ScCdc31p mediates the formation of the satellite at the end of the half-bridge remains completely unknown.
Human centrin 3 (CTN3 gene in human genome database) belongs to the same subfamily of centrins as ScCdc31p and is concentrated at the centrosome in the distal lumen of centrioles (Middendorp et al., 1997). Functional experiments have shown that expression of HsCen3p has a dominant negative effect on centrosome duplication both in yeast and in Xenopus eggs (Middendorp et al., 2000), suggesting the HsCen3p participate in centrosome duplication in human cells and shares a common function with ScCdc31p.
Here, we report the characterization of ScCDC31/HsCen3 orthologue in fission yeast. We demonstrate that S. pombe cdc31p is a component of the half-bridge of the SPB required for SPB duplication. In addition, our data suggest that cdc31p and sad1p, another SPB component required for bipolar spindle formation, may act in the same pathway. Finally, because cdc31 cells blocked in mitosis proceed to septation and display a multiseptation phenotype, we propose that cdc31p activity or SPB duplication may be required for the proper coordination between the exit from mitosis and the initiation of septation.
MATERIALS AND METHODS
Sequence Comparison and Dendrogram Construction
Sequence comparison and dendrogram construction were performed using ClustalW (http://clustalw.genome.ad.jp/) and Box-shade servers (http://ludwig-sun1.unil.ch:8080/software/BOX_form.html).
Yeast Strains and Genetic Methods
Standard S. pombe genetic techniques and cultures were performed as described at http://wwww.bio.uva.nl/pombe/handbook/. All S. pombe strains were isogenic to 972 and are listed in Table 1. Yeast transformations were performed by electroporation (Kelly et al., 1993) or by the lithium acetate-DMSO method for integration of linear DNA (Bahler et al., 1998b).
Table 1.
List of strains used
| Strain | Genotype | Origin |
|---|---|---|
| FC418 | ade6-M210 ura4-D18 leu1-32 h- | Fred Chang |
| FC420 | ade6-M216 ura4-D18 leu1-32 h+ | Fred Chang |
| MFP7 | ade1-D25 ade6-M210 S65T-GFP::cam1 leu1-32 ura4D18 h- | Moser et al. (1997) |
| AP91 | ade6-M210 ura4-D18 leu1-32 h- + pSLF172 (ura4+) | This study |
| AP131 | ade6-M210 ura4-D18 leu1-32 h- + pREP41X (LEU2) | This study |
| AP634 | ade6-M210 ura4-D18 leu1-32 h- + pREP42X (ura4+) | This study |
| AP137 | ade6-M210 ura4-D18 leu1-32 h- + pAP83 (pnmt*-GFPcdc31 ura4+) | This study |
| AP257 | ade6-M210 ura4-D18 leu1-32 h- + pAP100 (pnmt*-HsCen3 ura4+) | This study |
| AP258 | ade6-M210 ura4-D18 leu1-32 h- + pAP101 (pnmt*-cdc31 ura4+) | This study |
| AP260 | ade6-M210 ura4-D18 leu1-32 h- + pAP103 (pnmt*-ScCDC31 ura4+) | This study |
| AP360 | ade6-M210 ura4-D18 leu1-32 h + pAP123 (pnmt-cdc31 ura4+) | This study |
| AP555 | ade6-M210 ura4-D18 leu1-32 h- + pAP158 (pnmt-HA3cdc31 ura4+) | This study |
| FC584 | ade6-M210/ade6-M216 ura4-D18/ura4-D18 leu1-32/leu1-32 h + | Fred Chang |
| AP54 | cde31::kanMX/cde31+ ade6-M210/ade6-M216 ura4-D18/ura4-D18 leu1-32/leu1-32 h+/h- | This study |
| AP274 | cdc31::kanMX ade6-M210 leu1-32 ura4D18 + pAP101 (pnmt*cdc31 ura4+) h- | This study |
| AP390 | cdc31::kanMX ade6-M210 leu1-32 ura4D18 h- + pRH20 (pnmt*-cdc31 leu1+) integrated | This study |
| AP388 | cdc31::kanMX ade6-M210 leu1-32 ura4D18 h- + pRH19 (pnmt*-E147Kcdc31 leu1+) integrated | This study |
| IH274 | sad1-1 leu1-32 ura4-D18 h- | Iain Hagan |
| AP635 | sad1-1 leu1-32 ura4-D18 h- + pREP42X | This study |
| AP633 | sad1-1 leu1-32 ura4-D18 h- + pAP123 (pnmt-cdc31 ura4+) | This study |
| AP681 | sad1-1 leu1-32 ura4-D18 h- + pAP158 (pnmt-HA3cdc31 ura4+) | This study |
| AP600 | sad1-1 leu1-32 ura4-D18 ade6-M216 h+ | This study |
| AP607 | sad1-1 leu1-32 ura4-D18 ade6-M210 h + + pRH19 (pnmt*-E147Kcdc31 leu1+) integrated | This study |
| AP662 | sad1-1 cdc31::kanMX ade6-M210 leu1-32 ura4D18 h + + pRH19 (pnmt*-E147Kcdc31 leu1 +) integrated | This study |
| AP666 | cdc31::kanMX ade6-M210 leu1-32 ura4D18 h- + pRH19 (pnmt* E147Kcdc31 leu1+) integrated | This study |
| AP843 | alp16::kanMX leu1-32 ura4-D18 his7 (ade6) h + + pAP158 | This study |
| AP846 | cut12-1 leu1-32 ura4-D18 his2 h+ + pAP158 | This study |
| AP890 | alp4-1891 ade6-M216 ura4-D18 leu1-32 h- + pAP158 | This study |
| AP891 | alp4-1891 ade6-M216 ura4-D18 leu1 32 h- + pAP123 | This study |
| AP892 | alp4-1891 ade6-M216 ura4-D18 leu1-32 h- + pREP42X | This study |
| AP893 | alp6-719 ade6-M216 leu1-32 ura4-D18 h- + pAP158 | This study |
| AP896 | cam1-E14 ade6-M210 ura4-D18 leu1-32 ade1 D25 h- + pAP158 | This study |
| AP837 | alp4-1891 ade6-M216 ura4-D18 leu1-32 h + | This study |
| AP899 | alp4-1891 leu1-32 ura4-D18 ade6-M216 h- + pRH19 (pnmt*-E147Kcdc31 leu1 +) integrated | This study |
| AP900 | alp4-1891 cdc31::kanMX ade6 leu1-32 ura4-D18 + pRH19 (pnmt*-E147Kcdc31 leu1+) integrated | This study |
| AP901 | cdc31::kanMX ade6 leu1-32 ura4-D18 h- + pRH19 (pnmt*-E147Kcdc31 leu1 +) integrated | This study |
Cloning of cdc31, HsCen3, and ScCDC31
To clone cdc31, a 780-base pair (bp) fragment of DNA containing cdc31 ORF was amplified by PCR from genomic DNA (forward oligo: cacactcgagGTCATGTAAATACTCACACAC; reverse oligo: ttggatccGTAAATTAGCATGTGTTCTCC).
After purification of the fragment (PCR purification kit, Qiagen, Valencia, CA), fragment ends were digested by XhoI and BamH1 (sites added on oligos) and ligated at same sites in pREP41X (Forsburg, 1993) to generate a LEU2+ pnmt*-cdc31 plasmid (pAP 80). Absence of PCR-induced mutations was checked by sequencing. Then, an XhoI-BamH1 fragment from pAP80 was subcloned at XhoI and BglII sites of pSLF273 and pSLF173 (Forsburg and Sherman, 1997) to generate ura4+ pnmt*-cdc31 and pnmt-cdc31 plasmids (pAP101 and pAP123), respectively.
To generate an in frame N-terminal fusion of GFP with cdc31p a NotI-BamH1 fragment including cdc31 ORF was amplified from genomic DNA using oligonucleotides ggaagaattgcggccgcATGTTTGCTAACGCACGGG and ttggatccGTAAATTAGCATGTGTTCTCC. This fragment was ligated to NotI and BglII sites in pSGP573 (generous gift from S. Forsburg) to obtain pAP81. Finally, nmt promoter was replaced by nmt* promoter from pREP41X plasmid using the PstI and XhoI sites to obtain a ura4+ pnmt*-GFPcdc31 plasmid (pAP83). pnmt-HA3cdc31 plasmid (pAP158) was obtained by subcloning a NotI-SacI fragment from pAP81 into pSLF173.
For expression of HsCen3 and ScCDC31 in fission yeast, an SalI-BamHI fragment from pUF10 (gift from E. Schiebel) containing ScCDC31 complete ORF was subcloned in pSLF273 at XhoI and BglII sites to obtain plasmid pnmt*-ScCDC31 (pAP103). A XhoI-BamHI fragment encoding HsCen3p was amplified by PCR from HsCen3 pBS-KS plasmid (Middendorp et al., 1997) using oligos cacactcgagatgagtttagctctgagaagtg and ttggatccttaaatgtcaccagtcataatagc and ligated in pSLF273 at XhoI and BglII sites to obtain pnmt*-HsCen3 plasmid (pAP100).
All fragments amplified by PCR were checked by sequencing (Genomexpress, Meylan, France).
Deletion of cdc31, Tetrad Analysis, and Germination of Random Spores
Deletion of cdc31 ORF was achieved by homologous recombination according to Bahler et al. (1998b): KanMX cassette was amplified by PCR from kanMX4 plasmid using a forward oligonucleotide corresponding to 80 bp right upstream of cdc31 ATG codon and a reverse oligonucleotide corresponding to 80 bp right downstream of cdc31 stop codon and transformed into the diploid strain FC584. Stable transformants were then selected to obtain a Δcdc31/cdc31+ strain (AP54). Presence of the Δcdc31 allele was confirmed by PCR.
To analyze the progeny of the Δcdc31/cdc31+ strain, sporulation was induced on ME plates (BIO 101, Carlsbad, CA) for 2 d and tetrads dissected using an automated tetrad dissector (MSM, Singer Instruments, Somerset, UK). Spores were allowed to germinate and form colonies at 25°C on YE5S medium. Finally, colonies were replicated on YE5S plates containing 50 mg/l G418. Or, to generate random spores, parental diploid cells were digested by overnight treatment with 5 μl/ml glusulase (NEN, Boston, MA) in H2O. Germination was then allowed by incubation of spores in YE5S liquid medium at 25°C for 15 h.
Production of Conditional cdc31 Mutants
A point mutation leading to an E-to-K substitution at position 147 of cdc31p was first introduced in cdc31 sequence by double PCR: in a first round, a 660-bp 5′ fragment was amplified by PCR from pAP80 using oligos cacactcgagGTCATGTAAATACTCACACAC and GTCGATGTTTTCATTAAGCTC. A 140-bp 3′ fragment containing the mutation and overlapping the 5′ fragment on 20 base pairs was amplified by PCR using oligos GAGCTTAATGAAAACATCGACGATCAGAAATTGGAAGC and ggatccGTAAATTAGCATGTGTTCTCC. The full-length E147K-cdc31 fragment was obtained in second round of PCR using the purified 5′ and 3′ fragments and oligos ctcgagGTCATGTAAATACTCACACAC and ggatccGTAAATTAGCATGTGTTCTCC and cloned at XhoI-BamH1 sites in pREP41X to obtain pRH3. Presence of the mutation was checked by sequencing.
The mutated construct or wild-type cdc31 were then cloned in integrative plasmid JK148 (Keeney and Boeke, 1994) together with nmt* promoter and nmt termination sequence from pREP41X in two steps: A PstI-BamH1 fragment from pAP80 containing nmt*promoter and cdc31 was cloned at similar sites in pJK148. Then a SacI-SacI fragment from pAP80 or pRH3 containing the wild-type or mutated 3′ of cdc31 and nmt* termination sequence was cloned in the resulting plasmid to obtain pRH19 and pRH20.
Plasmids pRH19 and pRH20 were linearized in leu1 sequence using NruI and transformed into AP274 strain (Δcdc31 + pAP101). Stable integrants were selected. Finally, pAP101 plasmid (ura4+) was lost spontaneously after three rounds of growth in patches on plates of minimum medium containing adenine and uracil, resulting in strains AP388 (pnmt*-E147Kcdc31 mutant) and AP390 (pnmt*cdc31 mutant).
Production of sad1-1 pnmt*-E147Kcdc31 and alp4-1491 pnmt*-E147Kcdc31 Double Mutants
Strain IH274 was first crossed to strain FC420 to obtain an h+ sad1-1 strain (strain AP600). Strain AP600 was then crossed to strain AP388 (Δcdc31 + pnmt*-E147Kcdc31 integrated). No double mutant was recovered but strain AP607 (sad1-1 + pnmt*-E147Kcdc31 integrated) was selected and further crossed to strain AP388 transformed by pAP101 (pnmt*-cdc31 ura4+ plasmid) to complement cdc31 function. Finally, strains AP662 and AP666 (sad1-1 Δcdc31 + pnmt*-E147Kcdc31 integrated and Δcdc31 + pnmt*-E147Kcdc31 integrated) were obtained from two strains selected from this cross submitted to two successive incubations on EMM plates containing 1 mg/ml 5FOA, 50 mg/l uracil, and 225 mg/l adenine at 25°C to induce the loss of pAP101 plasmid.
Strain DH1891 (alp4-1891 leu1-32 h-; generous gift from T. Toda) was crossed to strain FC420 to obtain an h+ alp4-1891 strain (AP837). Strain AP837 was then crossed to AP388 transformed by pAP101 to obtain strains AP899 (alp4-1891 + pnmt*-E147Kcdc31 integrated), AP900 (alp4-1891 Δcdc31 + pnmt*-E147Kcdc31 integrated), and AP901 (Δcdc31 + pnmt*-E147Kcdc31 integrated) after incubation on 5FOA as described above.
Analysis of Growth after Serial Dilutions and Growth in Liquid Culture
Growth of mutant strains AP388, AP390, AP607, AP662, AP666, AP899, AP900, and AP901 was evaluated after serial 1/10 dilutions of exponentially growing cultures at OD 595 nm 0.3. Drops of 5 μl were deposited on plates of minimum medium containing uracil, adenine, 0.250 ml/l YE-phloxin B solution (BIO101, Carlsbad, CA) with or without 5 μg/ml thiamine and incubated at 30 or 36°C until the colony formed. Growth of strains overexpressing cdc31p or HA3cdc31p (AP360, AP555, AP634, AP633, AP635, AP681, AP843, AP846, AP890, AP893, and AP 896) was evaluated similarly.
For cytological studies, pnmt*-E147Kcdc31 cells were grown in exponential phase in liquid EMM supplemented with adenine and uracil at 25°C and shifted to 36°C in presence of 0.5 μg/ml thiamine.
Western Blots
Anti-HsCen3p Igs were produced as follows: 6 Histidine-tagged HsCen3p was produced in bacteria, purified on nickel columns, and injected to rabbits. For purification of specific Igs, 6 Histidine-tagged HsCen3p was coupled to sepharose (Aminolink plus coupling gel, Pierce). Igs adsorbed on the column in PBS buffer were eluted in 100 mM glycine, pH 2.7, immediately neutralized by addition of 1 M Tris, pH 8.9, dialyzed, and concentrated to 1 mg/ml in PBS.
Total extracts were prepared as follows: exponentially growing cells were centrifuged and concentrated in 250 μl PBS containing 2 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, and 1 μg/ml pepstatin and leupeptin. Two hundred fifty microliters acid-washed glass beads (Sigma, St. Louis, MO) were added and tubes submitted to 15 min of vortexing at maximum speed on a IKA-vibrax VXA shaker. After addition of 250 μl of 2× sample buffer (125 mM Tris, pH 6.8, containing 6% SDS, 10% 2-mercaptoethanol and 20% glycerol), extracts were boiled for 5 min, centrifuged at 10,000 × g for 15 min, and supernatants were recovered.
Protein concentration was assayed by Coomassie blue staining. Equal amounts of extracts were loaded on 12% SDS-PAGE and blotted on nitrocellulose according to Towbin et al. (1979). Proteins were fixed on nitrocellulose by a 15-min incubation in TBS containing 0.2% glutaraldehyde. cdc31p was revealed using anti-HsCen3p affinity-purified Igs (1:250), peroxidase-coupled anti-rabbit Igs (1: 10,000; Jackson ImmunoResearch, West Grove, PA) and a chemoluminescent revelation kit (Pierce, Rockford, IL).
Immunofluorescence and Microscopy
For immunofluorescence, cells were fixed by plunging cells in –20°C methanol after filtration on a HVLP 0.45-μm filter (Millipore, Bedford, MA). Cells were incubated in methanol for 5 min before rehydratation in PEM buffer (0.1 M NaPipes, pH 6.8, 1 mM EGTA, 1 mM MgCl2). Cells were further processed as described (Snell and Nurse, 1993). Anti-HsCen3p affinity-purified Igs were used (1:100) together with a Cy3-conjugated anti-rabbit secondary antibody (1:1000; Jackson ImmunoResearch). Tubulin was stained using mAb TAT1 (1:10; generous gift of K. Gull) and an Oregon Green 488-coupled anti-mouse antibody (1:200; Molecular Probes, Eugene, OR).
For γ-tubulin, sad1p, MTs, or mid1p stainings, cells were fixed with 4% formaldehyde for 1 h by addition of a 2:1 mix of 16% EM-grade formaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) and PEM buffer and further processed as described (Snell and Nurse, 1993). Sad1 serum was diluted 1:3 and TAT1 1:10. γ-tubulin mAb (Sigma) was diluted 1/100.
Anti-mid1p antibody was obtained by monthly injection of a rabbit (BabCo, Tucson, AZ) with 150 μg GST-mid1 (aa 309–505) fusion protein produced in XL1blue bacteria from pGex-Dmf1 plasmid (Sohrmann et al., 1996; generous gift from V. Simanis) and purified by SDS-PAGE from the Triton-insoluble fraction. Serum was affinity-purified by retroelution on a His3-tagged mid1p fragment (aa 300–506) as described in Paoletti and Chang (2000), except that elution was performed at pH 2.2. Purified Igs were diluted 1:5. Secondary antibodies were respectively Cy3-conjugated anti-rabbit secondary antibody (1:1000, Jackson ImmunoResearch) and Oregon Green 488-coupled anti-mouse antibody (1:200, Molecular Probes).
Cell wall and septum staining was performed as reported for calcofluor staining in Paoletti et al. (2000), using 2 μg/ml fluostain I (Sigma). For nuclei staining, cells were fixed in formaldehyde 4% for 5 min, permeabilized in PEM containing 1% Triton X-100, and stained with DAPI (1 μg/ml). Two microliters of stained cells was mounted between slide and coverslip and readily observed.
Images were acquired with a DMZ Leica microscope and a Hamamatsu CCD camera. For electron microscopy cells were prepared as previously described (Giddings et al., 2001). Briefly, cells were rapidly frozen by high-pressure freezing (BAL-TEC HPM-010, Technotrade International, Manchester, NH), freeze-substituted at –90°C in 2% osmium tetroxide plus 0.1% uranyl acetate in acetone followed by embedding in Epon-Araldite. Serial sections (60 nm) were cut on an Ultracut E microtome (Leica Microsystems, Bannockburn, IL.), poststained with uranyl acetate and lead citrate, and imaged in a Philips CM10 electron microscope operating at 80 kV. For immunolocalization, cells were frozen as above and then freeze substituted in 0.25% glutaraldehyde plus 0.05% uranyl acetate in acetone and then embedded in Lowicryl HM20 resin and UV-polymerized at –35°C. Embedded cells were sectioned as above and immunostained as previously described using anti-HsCen3p Igs (1:100) and secondary antibodies conjugated to 10-nm colloidal gold (BB International, Cardiff, UK). Immunostained sections were poststained and imaged as above.
Synchronization of pnmt*-E147K cdc31 Mutant
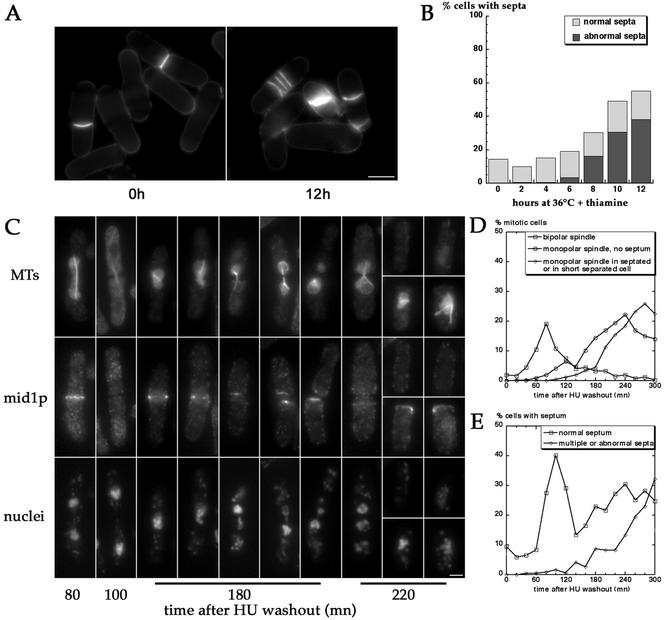
To synchronize pnmt*-E147Kcdc31 mutant, cells were grown exponentially at 30°C in EMM medium complemented with adenine and uracil and shifted at 36°C in presence of 0.5 μg/ml thiamine and 11 mM hydroxyurea (HU; Sigma) for 3 h. Cells were then filtered on 0.2-μm HVLP filters (Millipore) to remove HU, resuspended in fresh medium containing thiamine equilibrated at 36°C (see t = 0 in Figure 10), and further incubated at 36°C. The percentage of cells with a bipolar or monopolar spindle was determined from MT staining on 400 cells at each time point. Percentage of cells with a monopolar spindle and a septum or short separated cell with a monopolar spindle was determined on 200 cells presenting a monopolar spindle according to mid1p staining. Percentage of cells with a normal septum or multiple or abnormal septa was determined on 400 cells stained with fluostain I.
Figure 10.
Septation defects in pnmt*-E147Kcdc31 cells. (A and B) Accumulation of abnormal septa under restrictive conditions in pnmt*-E147Kcdc31 strain (AP388). Septation was monitored 2–12 h after shift to 36°C in presence of thiamine. Misorganized and multiple septa accumulate from 8 h on. Bar in A, 5 μm. (C–E) Synchronized pnmt*-E147Kcdc31 cells proceed to septation before mitosis exit. pnmt*-E47Kcdc31 mutant (AP388) was synchronized by growth for 3 h at 36°C in presence of thiamine and HU. On HU washout, cells were further incubated at 36°C in presence of thiamine, fixed, and stained for MTs, mid1p, and DNA at 20-min intervals (C and D) or stained for septum at same time points (E). In D, the percentage of cells with a bipolar spindle (squares), of nonseptated cells with a monopolar spindle (circles), or of septated cells or short cells after sister cell separation containing a monopolar spindle (diamonds) was determined according to MTs and mid1p staining. The percentage of cells presenting normal (squares) or abnormal and multiple septa (diamonds) is represented in E. Cells undergo a first normal round of mitosis (t = 80 min) and cytokinesis (t = 100 min). In the second round of mitosis, cells assemble monopolar spindles and proceed to septation 20–40 min later, before spindle breakdown (t = 180 and t = 220). Note the strong mid1p staining in the septum region in septated cells or at the new end in short separated cells containing monopolar mitotic spindles (right cells at t = 180 and bottom cells at t = 220). Note also the two supernumerary mid1p rings in the left cell at t = 220 min on each side of the septum. Bar in C, 2 μm.
RESULTS
Identification of an S. pombe Protein Belonging to the Centrin 3/ScCdc31p Subfamily of Centrins
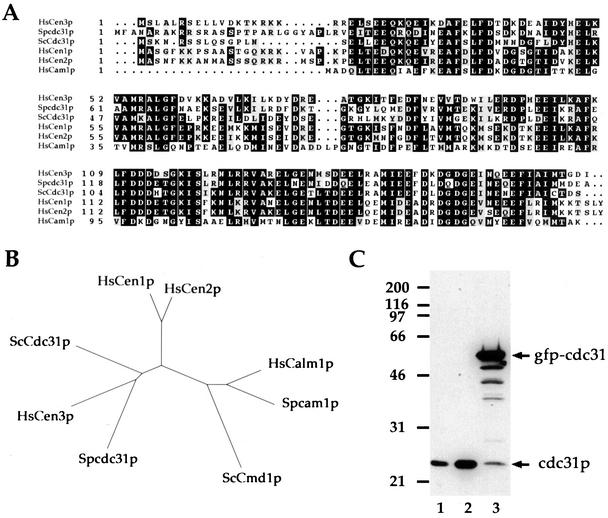
Analysis of centrin in animal cells has revealed the existence of two distinct subfamilies of centrins (Middendorp et al., 1997). In budding yeast however, a single centrin gene, named CDC31 is present. Similarly, in the fission yeast genome database, we found a single ORF encoding a putative member of the centrin family (SPCC1682–04; GenBank accession no. CA20670). Closer sequence comparison between the protein encoded by the ORF and centrins (Figure 1A) and dendrogram construction (Figure 1B) clearly identified this protein as a member of the centrin 3/ScCdc31p subfamily. We have therefore named the S. pombe gene cdc31 (for clarity, cdc31 always refers to S. pombe gene. Spcdc31 is only used when there is possible confusion with budding yeast CDC31 gene noted ScCDC31).
Figure 1.
cdc31 encodes a 23-kDa protein of the centrin 3/ScCdc31p subfamily of centrins. (A) Alignment of cdc31p with human centrins, ScCdc31p, and human calmodulin. Identical residues are boxed in black. Similar residues are shaded in gray. cdc31p presents 58.6% and 58.3% identity with HsCen3p and ScCdc31p, respectively, 50.0% and 51.1% identity with HsCen1p and HsCen2p, and 38.2% identity with human calmodulin. (B) Dendrogram of centrins and calmodulins. cdc31p clusters with the HsCen3p/ScCdc31p subfamily of centrins. (C) Western blot analysis of cdc31p using affinity-purified anti-HsCen3p Igs. 1, Wild-type cells; 2, wild-type cells overexpressing cdc31p from pnmt*-cdc31 plasmid; 3, wild-type cells expressing a GFPcdc31 fusion protein. Molecular weights (kDa) are shown on the left.
An immunoblot of total S. pombe protein extract with an anti-HsCen3p affinity-purified antibody revealed a protein of 23 kDa, suggesting that the antibody was able to cross-react with the product of S. pombe cdc31 gene (Figure 1C). To test whether this cross-reacting protein was cdc31p, we placed the cdc31 gene alone, or in frame with the GFP coding sequence, under the control of the nmt* thiamine-sensitive promoter (pnmt*-cdc31 and pnmt*-GFPcdc31 plasmids; see MATERIAL AND METHODS). Plasmids were transformed into wild-type cells and expression of cdc31p or GFPcdc31 fusion protein was induced by removal of thiamine from the medium for 16 h. Anti-HsCen3p Igs revealed an increased 23-kDa band in extracts from cells transformed with the pnmt*-cdc31 plasmid. In the case of the pnmt*-GFPcdc31 plasmid, a 50-kDa band, corresponding to the expected molecular weight of the GFPcdc31 fusion protein was observed as well as a degradation pattern underneath (Figure 1C).
These results indicate that cdc31 gene encodes a 23-kDa protein that is recognized by the anti-HsCen3p Igs.
cdc31p Is a Component of the Half-bridge of the Spindle Pole Body
To determine the subcellular localization of cdc31p, we performed immunofluorescence experiments on wild-type cells fixed in –20°C methanol using anti-HsCen3p Igs and antitubulin mAb TAT1 (Figure 2A). Cdc31p was present in one or two bright juxta-nuclear spots. Interphase cells exhibited a single cdc31p spot associated with one bundle of microtubules, whereas mitotic cells exhibited two bright spots at the poles of the mitotic spindle. In postmitotic cells presenting a postanaphase array of MTs, a single spot was associated with each nucleus. In addition, an irregular background was consistently observed all over the cell, suggesting that the protein may also be present in other cellular compartments as reported for centrins in animal cells (Paoletti et al., 1996). No specific staining was found at the equatorial MTOCs during septation nor at interphase MTOCs on the nuclear envelope.
Figure 2.
cdc31p localizes to the SPB at all phases of the cell cycle. (A) Top: Triple staining of wild-type cells for cdc31p (red), MTs (green), and DNA (blue). Bar, 5 μm. Bottom: Series of similarly stained cells at different cell cycle stages. From left to right: early G2, early mitosis, early and late anaphase, and postmitotic cell. (B) Staining of a GFP-cam1 strain for cdc31p. cdc31p staining (left) and GFP fluorescence (right) after formaldehyde fixation and processing for immunofluorescence are shown. Arrows point at SPBs. Bar, 5 μm.
This data suggested that the protein was concentrated at the SPB. To further test the colocalization of cdc31p with the SPB, we stained a strain expressing a cam1-GFP fusion protein that localizes to the SPB (Moser et al., 1997) with anti-HsCen3p Igs. cdc31p and cam1-GFP clearly colocalized at the SPB (Figure 2B).
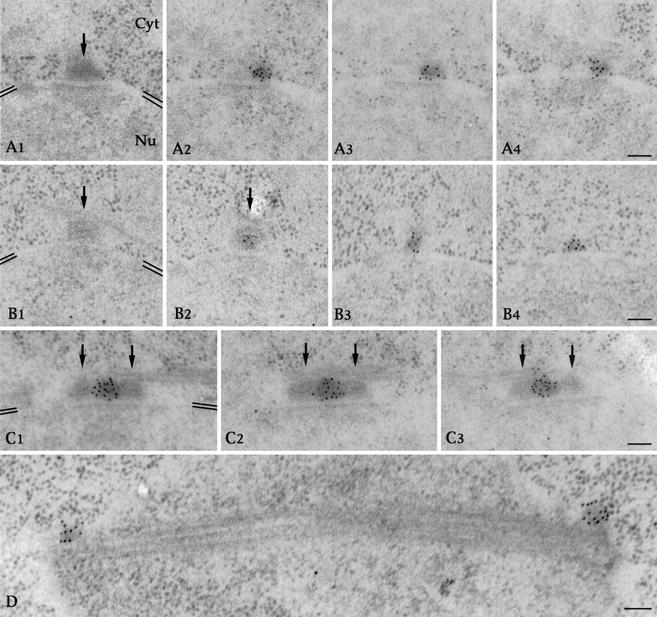
Finally, to determine the ultrastructural localization of the protein, we performed immunogold labeling of cdc31p on wild-type cells. Clusters of gold particles were found on adjacent serial sections in association with an electron-dense appendage corresponding to the half-bridge of the SPB (Figure 3, A2–A4, and B3 and B4). No gold particle was detected on the SPB-layered structure (Figure 3, arrows in A–C). cdc31p was also detected in the bridge connecting the two duplicated SPBs (Figure 3C) and on the side of spindle poles during mitosis (Figure 3D). Interestingly, gold beads were distributed throughout the half-bridge appendage rather than on the nuclear facing surface. Therefore, cdc31p association with the half-bridge is unlikely to depend on a membrane spanning protein as reported for ScCDC31 in budding yeast (Spang et al., 1993, Biggins and Rose, 1994; see DISCUSSION).
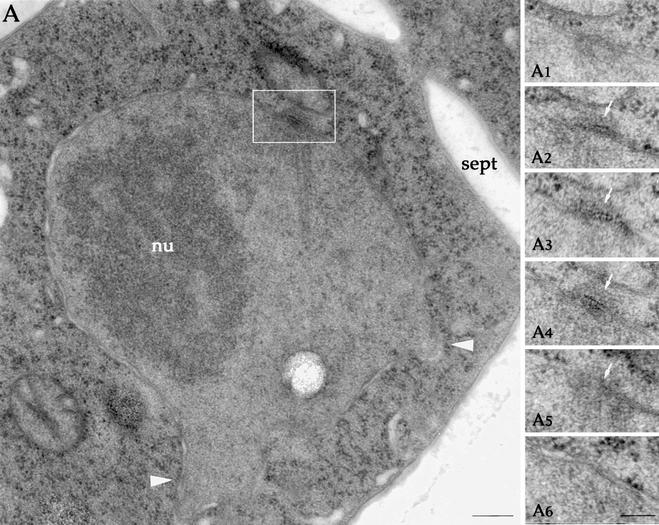
Figure 3.
cdc31p is a component of the half-bridge. Postembedding immunogold localization of cdc31p in wild-type cells. (A–C) Serial sections through unduplicated (A1–A4 and B1–B4) or duplicated (C1–C3) SPBs. Gold particles are clustered on the half-bridge on sections adjacent to the SPB-layered structure pointed by black arrows or on the bridge between the duplicated SPBs. Position of the nuclear envelope is highlighted by double black lines in A1, B1, and C1. Orientation of cytoplasm (Cyt) and nucleus (Nu) in always as in A1. Bars, 100 nm. (D) Section through a mitotic spindle. Gold particles are located on the side of the spindle poles. Bar, 100 nm. Note that the gold particles are distributed throughout the half-bridge electron-dense structure rather than on the nuclear facing surface of it.
cdc31 Is an Essential Gene Required for Bipolar Spindle Formation
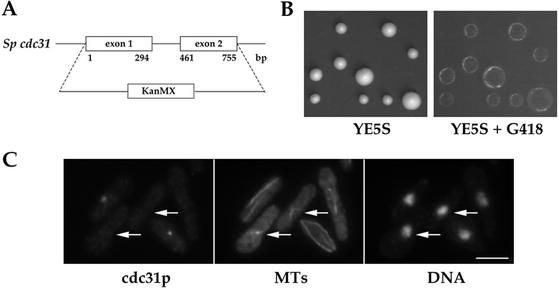
To determine the function of cdc31 in fission yeast, we deleted in a diploid strain one copy of the whole gene and replaced it by homologous recombination by the kanMX cassette conferring resistance to G418 (strain AP54; Figure 4A and Table 1). After sporulation, tetrads were dissected and spores allowed to germinate and form colonies on YE5S plates at 25°C. Only two spores per tetrad gave rise to colonies. These colonies were not resistant to G418, indicating that none of the Δcdc31 segregants were viable (Figure 4B). We thus conclude that cdc31 is an essential gene.
Figure 4.
Deletion of cdc31 is lethal and induces monopolar spindle formation. (A) cdc31 ORF was replaced in a diploid strain by the kanMX cassette conferring resistance to G418. A scheme of cdc31 exons and intron is represented. Position of their ends relative to cdc31 ATG codon is indicated in base pairs. (B) Analysis of tetrads from a Δcdc31/cdc31+ strain (AP54). Four spores from five tetrads were deposited vertically. Only two spores per tetrad form colonies on YE5S (left), which cannot grow in presence G418 (replica on the right side). (C) Random spores from the Δcdc31/cdc31+ strain were grown in YE5S, fixed in methanol, and stained for cdc31p, MTs, and DNA. Arrows point at spores depleted in cdc31p presenting monopolar spindles. Bar, 5 μm.
Random spores were then produced from the diploid strain and allowed to grow in liquid YE5S medium for 15 h, fixed in –20°C methanol, and processed for immunofluorescence with anti-HsCen3p Igs and antitubulin mAb TAT1. Two populations of cells were observed: cdc31+ cells with a cdc31p staining on the SPB and Δcdc31 cells with no cdc31p SPB staining. The Δcdc31 cells exhibited abnormal aster or V-shaped microtubule structures likely representing monopolar spindles and hypercondensed chromosomes (Figure 4C). This phenotype suggested that cdc31 is necessary for bipolar spindle formation.
HsCen3p Expression in Fission Yeast Is Toxic
We next investigated the ability of genes of the centrin 3/Cdc31p subfamily of centrins to complement cdc31 deletion in S. pombe. We found that neither ScCdc31p, nor HsCen3p expression, could complement cdc31 deletion in a plasmid loss assay (our unpublished results).
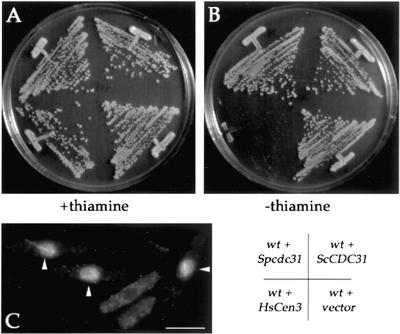
Then, we examined the effect of overexpressing Spcdc31p or expressing HsCen3p or ScCdc31p in fission yeast from the medium strength nmt* promoter. cdc31p overexpression from nmt* promoter did not affect growth (Figure 5, A and B), nor from the full-strength nmt promoter (see Figure 9C). ScCdc31p expression had no effect either. In contrast, HsCen3p production inhibited colony formation. Analysis of cells expressing HsCen3p by immunofluorescence revealed an accumulation of cells with monopolar spindles (Figure 5C). This suggests that HsCen3p has a dominant negative effect on SPB duplication in fission yeast as reported in budding yeast (Figure 5A; Middendorp et al., 2000). These results also indicate that ScCdc31p and HsCen3p cannot carry out S. pombe cdc31p function.
Figure 5.
HsCen3p is toxic in S. pombe. (A and B) Wild-type cells carrying pnmt*-cdc31, pnmt*-HsCen3, or pnmt*-ScCDC31 plasmids or an empty vector (pSLF172) were grown on EMM plates with (A) or without thiamine (B). HsCen3p prevents colony formation when the medium strength nmt* promoter is induced. (C) Wild-type cells carrying pnmt*-HsCen3 plasmids grown for 24 h at 30°C in absence of thiamine and stained for tubulin. Arrowheads point at cells with monopolar spindles. Bar, 5 μm.
Figure 9.
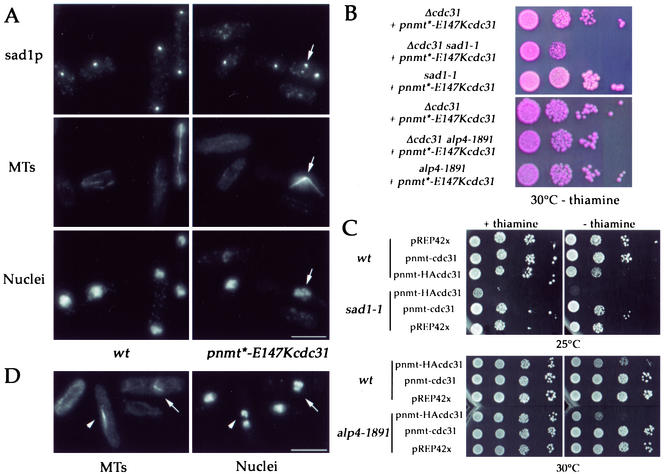
sad1p staining redistributes into several spots in pnmt*-E147cdc31 mutant and sad1 interacts genetically with cdc31. (A) Triple staining of wild-type cells (left; strain AP131) and pnmt*-E147Kcdc31 mutant (strain AP388) grown for 18 h at 30°C in presence of thiamine for sad1p (top), MTs (middle), and DNA (bottom). In restrictive conditions, a splitting of sad1p staining is observed in cells presenting monopolar spindles (arrows). Maximum projections of z series throughout the cells are shown. Bars, 5 μm. (B) Growth of sad1-1 pnmt*-E147Kcdc31 double mutant (AP662, sad1-1 Δcdc31 + pnmt*-E147Kcdc31 plasmid integrated) compared with simple mutants (AP666, Δcdc31 + pnmt*-E147Kcdc31 plasmid integrated and AP607, sad1-1 + pnmt*-E147Kcdc31 plasmid integrated) and of alp4-1891 pnmt*-E147Kcdc31 double mutant (AP900) compared with simple mutants (AP899 and AP901) at 30°C in absence of thiamine (permissive conditions). sad1-1 pnmt*-E147Kcdc31 double mutant is pinker in presence of phloxin and shows reduced growth compared with the two simple mutants. (C) Hypertoxicity of HA3cdc31p in sad1-1 strain. sad1-1, alp4-1891, and wild-type strains transformed with pnmt-cdc31 and pnmt-HA3cdc31 plasmids (full-strength nmt promoter), and pREP42X as a control were grown at 25 or 30°C in presence or absence of thiamine. High levels of expression of cdc31p do no affect growth. In wild-type and alp4-1891 cells, HA3cdc31p has a dominant negative effect when expressed at high levels only. In sad1-1 strain, low levels of expression of HA3cdc31p are sufficient to strongly reduce growth, and high levels of expression are lethal. (D) Double staining of sad1-1 cells expressing HA3cdc31p for tubulin and DNA. HA3cdc31p expression was induced for 24 h at 25°C. Arrow: monopolar spindle; arrowhead: bipolar spindle. Bar, 5 μm.
Construction of cdc31 Conditional Mutants
To further characterize cdc31 function in fission yeast, we generated conditional cdc31 mutants. These mutants were obtained by stable integration in a Δcdc31 strain of plasmids in which wild-type cdc31 or mutated E147Kcdc31 were placed under the control of the repressible nmt* promoter (E147K mutation is analogous to the mutation present in cdc31–2 thermosensitive mutant in Saccharomyces cerevisiae; Biggins and Rose, 1994).
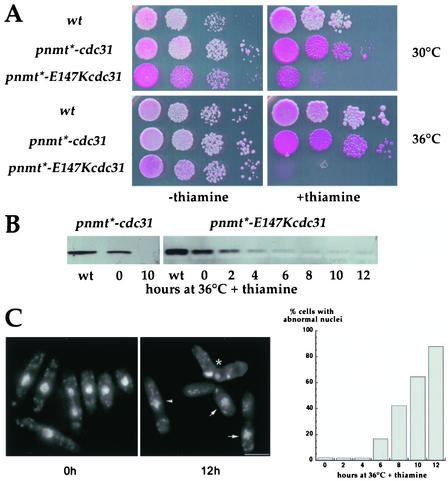
Growth of these mutant strains (AP390 and AP388 hereafter named pnmt*-cdc31 and pnmt*-E147Kcdc31 mutants) was analyzed on plates after serial dilutions of exponentially growing liquid cultures (Figure 6A). In thiamine-free medium, both strains grew similarly to wild-type cells at 30 or 36°C. But both strains were thiamine sensitive: pnmt*-cdc31 mutant turned pink on minimum medium containing phloxin and thiamine, and pnmt*-E147Kcdc31 mutant was not able to form colonies. For this strain, any residual growth was abolished when cells were grown at 36°C in the presence of thiamine.
Figure 6.
Analysis of Spcdc31 conditional strains. (A) Growth of pnmt*-cdc31 (Δcdc31 + pnmt*-cdc31 plasmid integrated, strain AP390) and pnmt*-E147Kcdc31 (Δcdc31 + pnmt*-E147Kcdc31 plasmid integrated, strain AP388) mutants at 30 or 36°C, in permissive (–thiamine) or restrictive (+thiamine) conditions. Wild-type strain: AP131. Serial 1:10 dilutions were spotted on EMM plates containing adenine, uracil, and phloxin B. (B) Western blot analysis of cdc31p or E147Kcdc31p expression levels in mutant strains. Total extracts from wild-type cells (wt) are compared with total extracts from pnmt*-cdc31 or pnmt*-E147Kcdc31 cells grown at 30°C in absence of thiamine (t = 0) or for 2–12 h at 36°C in presence of thiamine. (C and D) Accumulation of abnormal nuclei under restrictive conditions in pnmt*-E147Kcdc31 strain: nuclei morphology was monitored 2–12 h after shift to 36°C in presence of thiamine. Cells with hypercondensed chromosomes (arrowhead), cut phenotype (*), missegregated nuclei (arrows), or disrupted nuclei start accumulating 6 h after shift to 36°C in presence of thiamine. Bars, 5 μm.
Expression levels of the mutant protein E147Kcdc31 were then analyzed by Western blotting. At 30°C in absence of thiamine E147Kcdc31p was slightly less expressed than cdc31p in wild-type cells. Depletion of E147Kcdc31p could readily be observed 2–4 h after addition of thiamine at 36°C. Almost no protein was detected from 6 h on. Comparable levels of expression were observed in pnmt*-cdc31 mutant.
In conclusion, E147K point mutation, does not confer a thermosensitive phenotype when expressed at endogenous levels, and depletion of cdc31p by addition of thiamine in pnmt*-cdc31 mutant is not sufficient to stop growth. However, combining E147K mutation and depletion effectively abolishes cdc31 function.
Two other strains carrying point mutations in cdc31 (A62T and P108S; corresponding to cdc31-1, cdc31–5 mutants in S. cerevisiae; Biggins and Rose, 1994) behaved similarly to pnmt*-E147Kcdc31 mutant (unpublished data) and displayed similar phenotypes (see below).
Chromosome Segregation Defects and Monopolar Spindle Formation in Conditional cdc31 Mutant
Because the growth of pnmt*-E147Kcdc31 mutant was completely abolished in thiamine at 36°C, we choose these experimental conditions to further analyze the function of cdc31 gene. Cells were grown in exponential phase in absence of thiamine and shifted to 36°C in presence of thiamine for 2–12 h, fixed in formaldehyde, and stained with DAPI. Various abnormal nuclear structures were observed after 6 h. These include hypercondensed chromosomes, nuclei cut by a septum, missegregated nuclei, and cells with disrupted nuclei (Figure 6C). These events started to accumulate 6 h after the shift at 36°C in presence of thiamine and affected the whole population (>90%) after 12 h.
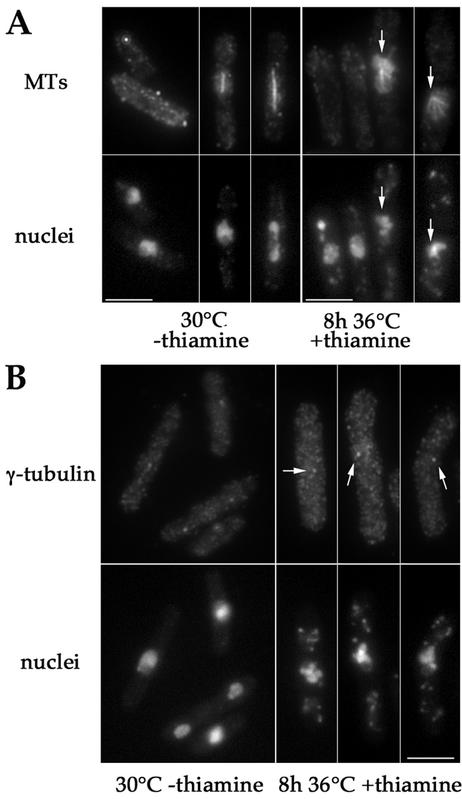
Thus, pnmt*E147-cdc31 mutants exhibit strong defects in chromosome segregation. We then looked at SPBs and spindle formation in pnmt*-E147cdc31 mutant. For that purpose, cells were fixed after 8 h at 36°C in presence of thiamine and double-stained for MTs and nuclei or γ-tubulin and nuclei (Figure 7). We could observe that 30% of cells presented monopolar spindles and hypercondensed chromosomes, indicating that they were blocked in mitosis (Figure 7A). In these cells, a single dot of gamma-tubulin was observed (Figure 7B).
Figure 7.
Depletion of E147Kcdc31p induces monopolar spindle formation. pnmt*-E147Kcdc31 cells (strain AP388) were double-stained for MTs and DNA (A) or γ-tubulin and DNA (B) after growth at 30°C in absence of thiamine (left) or after 8 h of growth at 36°C in presence of thiamine (right). In repressive conditions cells with hypercondensed chromosomes and monopolar spindles are observed (arrows). Cells with hypercondensed chromosomes present a single spot of γ-tubulin (arrows), suggestive of a failure in SPB duplication. Bars, 5 μm.
These data show that cdc31 mutant cells arrest in mitosis with a monopolar spindle, consistent with Δcdc31 spore germination experiments, and further suggest that a single SPB structure is present at the spindle pole.
Absence of SPB Duplication in pnmt*-E147Kcdc31 Mutants
To test whether this spindle assembly defect may be a consequence of a failure in SPB duplication, ultrastructural analysis of the mutant cells was performed after 8 h of growth at 36°C in presence of thiamine. Cells presenting a monopolar spindle were analyzed by serial sectioning (n = 9). All exhibited a single SPB structure (Figure 8, A1–A6). Some cells exhibited MTs that emanated from the SPB, reached the opposite surface of the nucleus, and induced deformations in the nuclear envelope. In addition, some cells exhibited an incomplete septum (n = 4, Figure 8A), which could cut the nucleus (n = 2). One cell exhibited three incomplete septa. This suggested that some cells had proceeded to septum formation while blocked in mitosis.
Figure 8.
Monopolar spindles are organized by an unduplicated SPB. (A) Electron microscopy of pnmt*-E147Kcdc31 mutant (strain AP388) grown for 8 h at 36°C in presence of thiamine. Note that nuclear MTs emanating from the single SPB induce deformations in the nuclear envelope on the opposite side of the nucleus (arrowheads). Note also the presence of a incomplete septum (sept). nu, nucleolus. Bar, 200 nm. (A1–A6) Serial sections through the SPB. A4 corresponds to the white inset in A. White arrows point at the single SPB. Bar, 100 nm.
In conclusion, this analysis clearly indicates that cdc31p is required in an early stage of SPB duplication and further suggests that the control of septation initiation is perturbed in the mutant.
Genetic Interactions between sad1 and cdc31
We also looked at the distribution of sad1p, another SPB component required for bipolar spindle formation (Hagan and Yanagida, 1995) using anti-sad1p antibody. pnmt*-E147Kcdc31 or wild-type cells were grown at 30°C for 18 h in presence of thiamine and triple-stained for sad1p, MTs, and DNA. Surprisingly, sad1p was found in multiple spots in pnmt*-E147Kcdc31 cells, instead of being concentrated in a single spot at the SPB like in wild-type cells (Figure 9A). These data suggest that cdc31p is required for the localization or concentration of sad1 protein at the SPB.
Next, we compared the growth rates of single or double sad1-1 and pnmt*-E147Kcdc31 mutants in permissive conditions (30°C without thiamine). We found that the double mutant grew more slowly and exhibited more cell death compared with the single mutants, indicating a synthetic effect between the mutations (Figure 9B).
Finally, we compared the effect of overexpressing a nonfunctional dominant negative HA3cdc31 fusion protein in wild-type cells and in sad1-1 mutant grown at permissive temperature. We found that HA3cdc31p expression at low levels in presence of thiamine (nmt full-strength promoter) severely affected colony number and size in sad1-1 mutant, whereas it had no effect on wild-type cells. Moreover, high expression in absence of thiamine was lethal in sad1-1 strain and only mildly perturbed growth in a wild-type background, indicating that HA3cdc31p is hyper-toxic in sad1-1 strain (Figure 9C). Immunofluorescence analysis of sad1-1 cells expressing HA3cdc31p revealed the presence of cells blocked in mitosis with monopolar spindles (Figure 9D).
To check whether this effect was specific to sad1-1 strain or could be observed in any SPB mutant, HA3cdc31p was also expressed in alp4-1891, alp6-719 (Vardy and Toda, 2000), alp16Δ (Fujita et al., 2002), cam1-E14 (Moser et al., 1997), and cut12–1 (Bridge et al., 1998) strains. None of the strains grown at permissive temperature was affected by low levels of expression. At high levels of expression, HA3-cdc31 was slightly more toxic than in wild-type cells but not lethal like in sad1-1 strain (Table 2). In addition, double alp4-1491 pnmt*-E147Kcdc31 (Figure 9C) and double alp6-719 pnmt*-E147Kcdc31 mutants (our unpublished results) grew as well as single mutants.
Table 2.
Growth of SPB mutant strains expressing HA3cdc31p
| Strain | +Th | -Th |
|---|---|---|
| wild type | +++ | ++ |
| sad1-1 | +/- | - |
| cut12-1 | ++ | +/- |
| alp4-1891 | +++ | + |
| alp6-719 | +++ | + |
| alp16Δ | +++ | + |
| cam1-E14 | +++ | + |
Serial dilutions of wild type, sad1-1, cut12-1, alp16Δ, alp4-1891, alp6-719 and cam1-E14 strains expressing HA3cdc31p under the control of pnmt promoter were spotted on plates of minimum medium with or without thiamine and incubated at 30°C until colony formed. Th, thiamine.
These results indicate that cdc31p may interact directly or indirectly with sad1p and regulate its localization and function.
Mis-timing of Septum Formation before Mitosis Exit in pnmt*-E147Kcdc31 Mutants
Because ultrastructural analysis suggested that septum formation may occur untimely in pnmt*-E147Kcdc31 mutant (see above), we monitored septum formation.
First, cells were grown in exponential phase in absence of thiamine and shifted to 36°C in presence of thiamine for 2–12 h and stained for septa. Abnormal septa including multiple or misshaped septa started accumulating 8 h after shift and reached ∼40% of the population after 12 h (Figure 10, A and B), indicating that abnormal septation events occur after the formation of monopolar spindles.
Second, to test if septation did occur in cells blocked in mitosis and evaluate the timing of septation events, cells were synchronized in S phase by incubation for3hinHUat36°Cin presence of thiamine. After HU washout, cells were further incubated at 36°C in presence of thiamine, fixed every 20 min, and stained for MTs, DNA, and mid1p, a protein that colocalizes with the contractile ring in mitosis until it starts contracting in late anaphase. Synchronized cells performed a normal first round of mitosis and septation (Figure 10C, t = 80 and 100 min, and d and E). Cells assembled monopolar spindles in the second round of mitosis and proceeded to septation 20–40 min later (Figure 10C, t = 180 and 220 min, and d and E) despite a sustained mitotic block as judged by the presence of long septated cells with monopolar spindles and of short cells with monopolar spindles after sister cell separation. In these cells, hypercondensed chromosomes were missegregated or “cut” by the septum. In addition, these cells maintained a strong mid1p staining in the septum region or at the new end, a situation never observed in the first round of normal mitosis and septation (see t = 100 in Figure 10C), nor in wild-type cells (Sorhmann et al., 1996; Paoletti et al., 2000). Additional rings or strands of mid1p were also sometimes observed next to the septum (see left cell t = 220), suggesting that multiseptation events that accumulate after the second round of mitosis (see Figure 10C) may arise from continuous induction of septation in cells blocked in mitosis that form supernumerary cytokinetic rings.
Our results indicate that cdc31p is required for the proper coordination between mitotic exit and septation.
DISCUSSION
cdc31 Encodes a Half-bridge Component of the SPB Required for SPB Duplication
Centrins are a conserved family of centrosomal proteins that have been implicated in centrosome duplication (Baum et al., 1986; Middendorp et al., 2000; Salisbury et al., 2002). Here, we show that fission yeast has one centrin-like gene that encodes a component of the SPB and is essential for SPB duplication: cdc31 mutant arrests in mitosis with a single unduplicated SPB and a monopolar mitotic spindle. cdc31p is the first identified S. pombe protein required for SPB duplication.
cdc31p resides in a cytoplasmic electron dense appendage on the side of the SPB called the half-bridge by functional analogy with S. cerevisiae half-bridge or bridge, which connects the two duplicated SPBs (Byers and Goetsch, 1974; Ding et al., 1997). The comparison of fission yeast cdc31p and ScCdc31p localizations highlights the structural differences between the half-bridges in the two yeasts: whereas the ScCdc31p resides on a thin layer tightly associated with the nuclear envelope, fission yeast cdc31p is distributed throughout a thick electrondense appendage, which transiently dissociate from the nuclear envelope in early mitosis when duplicated SPBs are inserted into the nuclear envelope (Ding et al., 1997).
It has been shown that ScCdc31p association with the half-bridge depends on another half-bridge component, Kar1p, which is directly anchored to the nuclear envelope via its C-terminal domain (Biggins and Rose, 1994; Spang et al., 1995). Because cdc31p distribution is not restricted to the nuclear side of the half-bridge, it seems unlikely that cdc31p anchoring to the half-bridge is mediated by a membrane-associated protein like Kar1p in S. pombe. Accordingly, no gene homologous to KAR1 has been described in S. pombe genome. It will be of great interest in the future to determine how cdc31p association with the half-bridge is mediated in S. pombe.
A number of other mutants in which bipolar spindle formation is compromised because of perturbations of SPB function have been described previously in fission yeast. These include mutants in components of the fission yeast gamma-tubulin complex alp4p and alp6p (Vardy et al., 2002), calmodulin (Moser et al., 1997), cut11p (West et al., 1998), cut12p (Bridge et al., 1998), and sad1p (Hagan and Yanagida, 1995). pnmt*-E147Kcdc31 is the first mutant in which spindle defects result from a complete lack of SPB duplication. Because we observed that in cdc31 mutant, the single SPB was properly inserted into the nuclear envelope, we can also infer from our data that SPB duplication is not a prerequisite for SPB insertion into the nuclear envelope in fission yeast.
Conservation of a Network of Genes Controlling Centrosome Duplication
Our data on cdc31p indicate that the key factors controlling the initiation of SPB duplication have been conserved between the two distantly related yeasts and further illustrate the important function of the centrin 3/Cdc31p family in the control of centrosome reproduction. However, our attempts to complement cdc31 deletion by the expression of HsCen3p or ScCdc31p failed. Moreover, HsCen3p expression was toxic, as reported previously for HsCen3 expression in budding yeast (Middendorp et al., 2000). This suggests that partners of the three proteins may have diverged more than centrins during evolution.
How members of the centrin 3/Cdc31p family may trigger centrosome duplication remains poorly understood. In S. cerevisiae, a network of SPB duplication genes including CDC31, KAR1, and two ubiquitin like genes, DSK2 and RAD23 acting upstream of CDC31 (Biggins et al., 1996) has been described. Recent data also indicate that this network may be regulated by the PKC1 pathway (Khalfan et al., 2000). As mentioned above, no gene homologous to KAR1 has been identified in S. pombe genome, but S. pombe genes homologous to DSK2 and RAD23 have been characterized (dph1 and rhp23, respectively; He et al., 1998; Elder et al., 2002). These genes are not essential and direct evidence for their implication in SPB duplication is lacking. However, the fact that overexpression of dph1 induces monopolar spindle formation like DSK2 (He et al., 1998) suggests that dph1 and DSK2 may share a conserved function in SPB duplication.
Our studies suggest that sad1p is a potential downstream target of cdc31p. sad1p is similar to C. elegans Unc-84 (Malone et al., 1999). A sad1-1 mutant exhibits a monopolar spindle phenotype like cdc31 mutant (Hagan and Yanagida, 1995). Ultrastructural localization of sad1p is not known, although sad1p is likely anchored to the nuclear envelope because it contains a putative transmembrane domain and overexpression leads to an accumulation on the nuclear envelope (Hagan and Yanagida, 1995). We observed that sad1p localization at the SPB is compromised in pnmt*-E47Kcdc31 mutant, whereas cdc31p is still concentrated at the SPB in the sad1-1 mutant (unpublished data). This suggests that cdc31p may control the accumulation of sad1p at the SPB. We also found that the two genes display extensive genetic interactions. In budding yeast, there is no sad1 homologue, and no gene acting directly down-stream of CDC31 has been identified, although CDC31 interacts genetically with SPB satellite components such as Spc29p (Adams and Kilmartin, 1999; Elliott et al., 1999).
An Additional Function for cdc31 in Regulating the Septation Initiation Network?
In budding yeast, it has been shown that ScCDC31p has additional functions independent of SPB duplication (Sullivan et al., 1998; Ivanovska and Rose, 2001). In particular, ScCDC31p is required for KIC1 kinase activity, which plays a role in the maintenance of cellular integrity. In pnmt*-E147Kcdc31 mutant, corresponding to cdc31–2 allele in S. cerevisiae, we did not observe any cell lysis phenotype nor in two other mutants corresponding, respectively, to cdc31-1 and cdc31–5 (pnmt*-A42Tcdc31 and pnmt*-P108Scdc31; unpublished data).
In pnmt*-E147Kcdc31 mutant, we observed cells displaying multiple or aberrant septation events as well as cells containing both mitotic spindles and septa. In synchronous cultures, cells blocked in mitosis with a monopolar spindle proceeded to septation in less than an hour. Cells even maintained a mitotic state after full separation of sister cells.
This suggests that spindle checkpoints controlling mitotic exit are functioning normally in pnmt*-E147Kcdc31 strain, but that septation is allowed to occur before mitosis completion. Accordingly, the strain is not hypersensitive to thiabendazole (our unpublished results) as reported for strains defective in spindle checkpoints (He et al., 1997, 1998). This situation also differs from what has been described in APC mutants cut9–665 and lid1–6 in which delayed septation events are preceded by cdc2p inactivation (Chang et al., 2001).
Induction of septation depends on a signaling cascade called the SIN (septation initiation network; see Le Goff et al., 1999; Balasubramanian et al., 2000; Sawin, 2000; and McCollum and Gould, 2001 for reviews). Activation of the cascade occurs in mitosis when the small GTP-binding protein spg1p switches from the GDP to the GTP-bound form (Schmidt et al., 1997). However, activation of the last steps in the SIN requires the inactivation of cdc2p at the exit of mitosis, ensuring that septation does not take place before chromosome segregation is achieved (Guertin et al., 2000; Chang et al., 2001). This requirement seems to be abolished in the pnmt*-E147Kcdc31 mutant.
Because several components of the SIN network including the small GTP-binding protein spg1 (Schmidt et al., 1997), the GAPs controlling its activity (Cerutti and Simanis, 1999; Li et al., 2000) and downstream kinases of the SIN (Sohrmann et al., 1998; Sparks et al., 1999; Guertin et al., 2000) are permanently or transiently located at the SPB, one intriguing possibility is that the localization of SIN components is defective in cdc31 mutant and allows the last steps of SIN to occur before cdc2 inactivation.
Untimely activation of septation before mitosis exit as well as a multiseptation phenotype have recently been reported for alp4-1891 mutant (Vardy et al., 2002), and a novel role for the γ-tubulin complex in inhibiting the SIN activation during mitosis has been proposed. However, because γ-tubulin is still associated with the spindle pole in pnmt*-E147Kcdc31 mutant, it seems unlikely that the septation defects are caused solely through the γ-tubulin complex.
It will be important to determine whether the septation defects result from an independent function of cdc31 in regulating the initiation of septation or are a consequence of the absence of SPB duplication. Characterization of the behavior of various components of the SIN in pnmt*-E147Kcdc31 strain and of additional alleles of cdc31 will help to distinguish these possibilities.
Acknowledgments
We thank Trisha Davis, Keith Gull, Iain Hagan, Elmar Schiebel, Viesturs Simanis, and Takashi Toda for strains, antibodies and plasmids; Valérie Doye, Anne-Marie Tassin, and Phong Tran for critical reading of the manuscript; and Richard McIntosh and Paul Nurse for helpful discussion.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–10–0661. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-10-0661.
Abbreviations used: HU, hydroxyurea; MTs, microtubules; MTOC, microtubule organizing center; SPB, spindle pole body.
References
- Adams, I.R., and Kilmartin, J.V. (1999). Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae. J. Cell Biol. 145, 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, I.R., and Kilmartin, J.V. (2000). Spindle pole body duplication: a model for centrosome duplication? Trends Cell Biol. 10, 329–335. [DOI] [PubMed] [Google Scholar]
- Bahler, J., Wu, J.Q., Longtine, M.S., Shah, N.G., McKenzie, A. r., Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998b). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M.K., McCollum, D., and Surana, U. (2000). Tying the knot: linking cytokinesis to the nuclear cycle. J. Cell Sci. 113, 1503–1513. [DOI] [PubMed] [Google Scholar]
- Baum, P., Furlong, C., and Byers, B. (1986). Yeast gene required for spindle pole body duplication: homology of its product with Ca2+- binding proteins. Proc. Natl. Acad. Sci. USA 83, 5512–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins, S., and Rose, M.D. (1994). Direct interaction between yeast spindle pole body components: Kar1p is required for Cdc31p localization to the spindle pole body. J. Cell Biol. 125, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins, S., Ivanovska, I., and Rose, M.D. (1996). Yeast ubiquitin-like genes are involved in duplication of the microtubule organizing center. J. Cell Biol. 133, 1331–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge, A.J., Morphew, M., Bartlett, R., and Hagan, I.M. (1998). The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev. 12, 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers, B., and Goetsch, L. (1974). Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harbor Symp. Quant. Biol. 38, 123–131. [DOI] [PubMed] [Google Scholar]
- Byers, B. (1981). Cytology of the yeast life cycle. In: The Molecular Biology of the Yeast Saccharomyces: Life cycle and Inheritance, eds. J.N. Strathern, E.W. Jones, and J.R. Broach, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 59–96.
- Cerutti, L., and Simanis, V. (1999). Asymmetry of the spindle pole bodies and spg1p GAP segregation during mitosis in fission yeast. J. Cell Sci. 112, 2313–2321. [DOI] [PubMed] [Google Scholar]
- Chang, L., Morrell, J.L., Feoktistova, A., and Gould, K.L. (2001). Study of cyclin proteolysis in anaphase-promoting complex (APC) mutant cells reveals the requirement for APC function in the final steps of the fission yeast septation initiation network. Mol. Cell. Biol. 21, 6681–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, R., West, R.R., Morphew, D.M., Oakley, B.R., and McIntosh, J.R. (1997). The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol. Biol. Cell 8, 1461–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder, R.T., Song, X.Q., Chen, M., Hopkins, K.M., Lieberman, H.B., and Zhao, Y. (2002). Involvement of rhp23, a Schizosaccharomyces pombe homolog of the human HHR23A and Saccharomyces cerevisiae RAD23 nucleotide excision repair genes, in cell cycle control and protein ubiquitination. Nucleic Acids Res. 30, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, S., Knop, M., Schlenstedt, G., and Schiebel, E. (1999). Spc29p is a component of the Spc110p subcomplex and is essential for spindle pole body duplication. Proc Natl Acad Sci USA, 96, 6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg, S.L. (1993). Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 21, 2955–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg, S.L., and Sherman, D.A. (1997). General purpose tagging vectors for fission yeast. Gene 191, 191–195. [DOI] [PubMed] [Google Scholar]
- Fujita, A., Vardy, L., Garcia, M.A., and Toda, T. (2002). A fourth component of the fission yeast gamma-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when gamma-tubulin function is compromised. Mol. Biol. Cell 13, 2360–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings, T.H., Jr., O'Toole, E.T., Morphew, M., Mastronarde, D.N., McIntosh, J.R., and Winey, M. (2001). Using rapid freeze and freeze-substitution for the preparation of yeast cells for electron microscopy and three-dimensional analysis. Methods Cell Biol. 67, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin, D.A., Chang, L., Irshad, F., Gould, K.L., and McCollum, D. (2000). The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J. 19, 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, I., and Yanagida, M. (1995). The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol. 129, 1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X., Patterson, T.E., and Sazer, S. (1997). The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA 94, 7965–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X., Jones, M.H., Winey, M., and Sazer, S. (1998). Mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J. Cell Sci. 111, 1635–1647. [DOI] [PubMed] [Google Scholar]
- Ivanovska, I., and Rose, M.D. (2001). Fine structure analysis of the yeast centrin, Cdc31p, identifies residues specific for cell morphology and spindle pole body duplication. Genetics 157, 503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, J.B., and Boeke, J.D. (1994). Efficient targeted integration at leu1-32 and ura4–294 in Schizosaccharomyces pombe. Genetics 136, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, T.J., Martin, G.S., Forsburg, S.L., Stephen, R.J., Russo, A., and Nurse, P. (1993). The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell 74, 371–382. [DOI] [PubMed] [Google Scholar]
- Khalfan, W., Ivanovska, I., and Rose, M.D. (2000). Functional interaction between the PKC1 pathway and CDC31 network of SPB duplication genes. Genetics 155, 1543–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., Pereira, G., and Schiebel, E. (1999). Microtubule organization by the budding yeast spindle pole body. Biol. Cell 91, 291–304. [PubMed] [Google Scholar]
- Le Goff, X., Utzig, S., and Simanis, V. (1999). Controlling septation in fission yeast: finding the middle, and timing it right. Curr. Genet. 35, 571–584. [DOI] [PubMed] [Google Scholar]
- Li, C., Furge, K.A., Cheng, Q.C., and Albright, C.F. (2000). Byr4 localizes to spindle-pole bodies in a cell cycle-regulated manner to control Cdc7 localization and septation in fission yeast. J. Biol. Chem. 275, 14381–14387. [DOI] [PubMed] [Google Scholar]
- Malone, C.J., Fixsen, W.D., Horvitz, H.R., and Han, M. (1999). UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development 126, 3171–3181. [DOI] [PubMed] [Google Scholar]
- McCollum, D., and Gould, K.L. (2001). Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 11, 89–95. [DOI] [PubMed] [Google Scholar]
- Middendorp, S., Paoletti, A., Schiebel, E., and Bornens, M. (1997). Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiae CDC31 gene. Proc. Natl. Acad. Sci. USA 94, 9141–9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorp, S., Kuntziger, T., Abraham, Y., Holmes, S., Bordes, N., Paintrand, M., Paoletti, A., and Bornens, M. (2000). A role for centrin 3 in centrosome reproduction. J. Cell Biol. 148, 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, M.J., Flory, M.R., and Davis, T.N. (1997). Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J. Cell Sci. 110, 1805–1812. [DOI] [PubMed] [Google Scholar]
- Paoletti, A., Moudjou, M., Paintrand, M., Salisbury, J.L., and Bornens, M. (1996). Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 109, 3089–3102. [DOI] [PubMed] [Google Scholar]
- Paoletti, A., and Chang, F. (2000). Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell 11, 2757–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury, J., Suino, K., Busby, R., and Springett, M. (2002). Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 12, 1287. [DOI] [PubMed] [Google Scholar]
- Sawin, K.E. (2000). Cytokinesis: Sid signals septation. Curr. Biol. 10, R547–550. [DOI] [PubMed] [Google Scholar]
- Schild, D., Ananthaswany, H.N., and Mortimer, R.K. (1981). An endomitotic effect of a cell cycle mutation of Saccharomyces cerevisiae. Genetics 97, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, S., Sohrmann, M., Hofmann, K., Woollard, A., and Simanis, V. (1997). The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev. 11, 1519–1534. [DOI] [PubMed] [Google Scholar]
- Snell, V., and Nurse, P. (1994). Genetic analysis of cell morphogenesis in fission yeast—a role for casein kinase II in the establishment of polarized growth. EMBO J. 13, 2066–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrmann, M., Fankhauser, C., Brodbeck., C., and Simanis, V. (1996). The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 10, 2707–2719. [DOI] [PubMed] [Google Scholar]
- Spang, A., Courtney, I., Fackler, U., Matzner, M., and Schiebel, E. (1993). The calcium-binding protein cell division cycle 31 of Saccharomyces cerevisiae is a component of the half bridge of the spindle pole body. J. Cell Biol. 123, 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang, A., Courtney, I., Grein, K., Matzner, M., and Schiebel, E. (1995). The Cdc31p-binding protein Kar1p is a component of the half bridge of the yeast spindle pole body. J. Cell Biol. 128, 863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks, C.A., Morphew, M., and McCollum, D. (1990). Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J Cell Biol. 146, 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, D.S., Biggins, S., and Rose, M.D. (1998). The yeast centrin, cdc31p, and the interacting protein kinase, Kic1p, are required for cell integrity. J. Cell Biol. 143, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin, A.M., and Bornens, M. (1999). Centrosome structure and microtubule nucleation in animal cells. Biol. Cell 91, 343–354. [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy, L., and Toda, T. (2000). The fission yeast gamma-tubulin complex is required in G(1) phase and is a component of the spindle assembly checkpoint. EMBO J. 19, 6098–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy, L., Fujita, A., and Toda, T. (2002). The gamma-tubulin complex protein Alp4 provides a link between the metaphase checkpoint and cytokinesis in fission yeast. Genes Cells 7, 365–373. [DOI] [PubMed] [Google Scholar]
- West, R.R., Vaisberg, E.V., Ding, R., Nurse, P., and McIntosh, J.R. (1998). cut11(+): A gene required for cell cycle-dependent spindle pole body anchoring in the nuclear envelope and bipolar spindle formation in Schizosaccharomyces pombe. Mol. Biol. Cell 9, 2839–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey, M., Hoyt, M.A., Chan, C., Goetsch, L., Botstein, D., and Byers, B. (1993). NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J. Cell Biol. 122, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]