Abstract
We have previously demonstrated that the CrkII and CrkL adapter proteins are required for the spreading of epithelial colonies and the breakdown of adherens junctions in response to hepatocyte growth factor. When overexpressed, CrkII and CrkL promote lamellipodia formation, cell spreading, and the loss of epithelial adherens junctions in the absence of hepatocyte growth factor. The exact mechanism by which Crk proteins elicit these changes is unclear. We show that the overexpression of CrkII or CrkL, but not Src homology 2 or amino-terminal Src homology 3 domain mutant Crk proteins, promotes the relocalization of Paxillin to focal contacts throughout the cell and within lamellipodia in a Rac-dependent manner. In stable cell lines overexpressing CrkII, enhanced lamellipodia formation and cell spreading correlate with an increased association of CrkII with Paxillin, GIT2 (an ARF-GAP) and β-PIX (a Rac1 exchange factor). Mutants of Paxillin that fail to associate with Crk or GIT2, or do not target to focal adhesions inhibit Crk-dependent cell spreading and lamellipodia formation. We conclude from these studies that the association of Crk with Paxillin is important for the spreading of epithelial colonies, by influencing the recruitment of Paxillin to focal complexes and promoting the enhanced assembly of Paxillin/GIT2/β-PIX complexes.
INTRODUCTION
Epithelial-mesenchymal (EM) transitions are characterized by the loss of epithelial cell-cell junctions and cell polarity and the acquisition of a motile mesenchymal phenotype (Boyer et al., 2000). The dispersal of epithelial colonies is a dynamic process initiated by the reorganization of the actin cytoskeleton and the formation of membrane protrusions within cells at the edge of the colony (Lauffenburger and Horwitz, 1996). As cells spread, new focal contacts are formed at the leading edge of the colony, whereas existing ones are remodeled (Webb et al., 2002). On loss of cell-cell junctions, this process is complete and dispersed cells acquire a fibroblastic morphology with enhanced cell motility (Lauffenburger and Horwitz, 1996).
EM transitions and epithelial dispersal are tightly regulated and require the coordinated activation and targeting of structural and signaling complexes that modulate the remodeling of the actin and microtubule network required for cell migration (Sastry and Burridge, 2000; Wittmann and Waterman-Storer, 2001; Webb et al., 2002). Hepatocyte growth factor (HGF) is a potent modulator of EM transitions in vitro (Weidner et al., 1993; Zhu et al., 1994) and in vivo (Birchmeier and Gherardi, 1998). HGF stimulates the breakdown of cell-cell junctions and the dispersal of sheets of epithelial cells, increasing their invasiveness (Stoker et al., 1987; Weidner et al., 1990). In a search for signals downstream from the HGF/Met receptor tyrosine kinase involved in the dispersal of epithelial sheets, we recently demonstrated that Crk adapter proteins are required for HGF-induced lamellipodia formation and cell spreading (Lamorte et al., 2002b). Moreover, overexpression of the CrkII or CrkL adapter protein promotes lamellipodia formation, cell spreading, and loss of adherens junctions independently of HGF (Lamorte et al., 2002b). CrkII and CrkL are composed of a single Src homology 2 (SH2) and two Src homology 3 (SH3) domains (SH2-SH3-SH3) (Reichman et al., 1992; ten Hoeve et al., 1993). Crk proteins function as adapter proteins to assemble signaling complexes. The Crk SH2 domain binds tyrosine phosphorylated proteins involved in cell spreading, actin reorganization, and cell migration, including p130Cas and Paxillin (Feller, 2001), as well as Gab1, a docking protein involved in epithelial morphogenesis (Maroun et al., 1999; Lamorte et al., 2002a). Through its amino terminal SH3 domain Crk interacts constitutively with proline rich motifs present within several protein, including C3G, an exchange factor for Rap1 (Gotoh et al., 1995) and DOCK180, an exchange factor for Rac1 (Kiyokawa et al., 1998a; Nolan et al., 1998) as well as the Abl tyrosine kinase (Feller et al., 1994). Genetic studies in Caenorhabditis elegans have demonstrated a role for CrkII and DOCK180 in phagocytosis and polarized cell migration required for normal pathfinding of the distal tip cells of the developing gonad (Reddien and Horvitz, 2000). In tissue culture, the overexpression of CrkII or CrkL enhances the migration of mammalian cells when assayed as single cells in Boyden chambers (Klemke et al., 1998; Uemura and Griffin, 1999; Cho and Klemke, 2000; Spencer et al., 2000; Hemmeryckx et al., 2001) or on collagen matrices (Petit et al., 2000). However, the mechanism through which Crk proteins promote the spreading and motility of epithelial colonies is not completely understood.
The role of the Rho family of small GTPases in regulating actin cytoskeletal dynamics is well established (Hall, 1998). The activation of Rac1 is required for lamellipodia formation, Cdc42 for filopodial extensions, and RhoA for the bundling of actin stress fibers and the formation of mature focal adhesions. More recently, members of the ADP-ribosylation factor (ARF) family of GTPases have been implicated in the remodeling of the actin cytoskeleton. ARF proteins have been characterized primarily based on their role in the regulation of membrane traffic (Chavrier and Goud, 1999). Moreover, ARF6 activity regulates the targeting of Rac1 to the membrane and is required for Rac1-induced lamellipodia formation (Radhakrishna et al., 1999). In addition, ARF6 activity is involved in the breakdown of epithelial cell-cell junctions through the internalization of E-cadherin/β-catenin complexes in response to HGF (Palacios et al., 2001). In further support of the regulation of actin reorganization and cell migration by ARF GTPases, ARF guanine nucleotide exchange factors and ARF-GTPase activating proteins (ARF-GAP) regulate these processes as well (Franco et al., 1999; Turner et al., 1999; Di Cesare et al., 2000; Jackson et al., 2000; Kondo et al., 2000; Randazzo et al., 2000; Mazaki et al., 2001; Santy and Casanova, 2001; Uchida et al., 2001; West et al., 2001; Brown et al., 2002; Liu et al., 2002a; Manabe Ri et al., 2002). For example, the overexpression of various ARF-GAP proteins modulates the formation and/or turnover of focal adhesions (Di Cesare et al., 2000; Jackson et al., 2000; Kondo et al., 2000; Randazzo et al., 2000; Mazaki et al., 2001; Liu et al., 2002a) and the overexpression of an ARF guanine nucleotide exchange factor, ARNO, enhances the spreading and dispersal of epithelial cells (Santy and Casanova, 2001). In addition to their role as GAPs, ARF-GAP proteins may also influence signaling pathways through additional protein–protein interactions. GIT2/PKL is a Paxillin binding protein with an ARF-GAP domain (Turner et al., 1999; Premont et al., 2000) that localizes to focal adhesions (Brown et al., 2002) and links Paxillin to an exchange factor for Rac1, β-PIX/Cool (Bagrodia et al., 1998; Manser et al., 1998).
Focal adhesions are multiprotein complexes, containing integrins, focal adhesion kinase (FAK), Paxillin, and other molecules that serve to anchor the actin cytoskeleton to the plasma membrane and provide attachments with the extracellular matrix (Geiger et al., 2001). Fibroblasts isolated from Paxillin null mice display defects in focal adhesion signaling, together with reduced cell migration and impaired cell spreading on fibronectin (Hagel et al., 2002). Paxillin is one of the earliest proteins recruited into adhesions at the leading edge of ruffling cells (Laukaitis et al., 2001) and becomes tyrosine phosphorylated after integrin ligation (Burridge et al., 1992). Tyrosine phosphorylation of Paxillin is necessary for focal adhesion formation and the reorganization of the actin cytoskeleton in motile cells (Nakamura et al., 2000). As a scaffold protein, Paxillin recruits several structural and signaling proteins into focal adhesions (reviewed in Turner, 2000).
We have addressed the mechanism through which Crk adapter proteins promote the spreading of colonies of epithelial cells. We report herein that the microinjection of CrkII or CrkL into colonies of epithelial cells promotes the formation of lamellipodia together with relocalization of Paxillin into focal complexes. The association of Crk with Paxillin is important for epithelial cell spreading and correlates with enhanced CrkII/Paxillin/GIT2/β-PIX complex formation in Madin-Darby canine kidney (MDCK) cells overexpressing CrkII. Paxillin mutants that fail to associate with Crk or GIT2, or fail to target to focal adhesions, inhibit Crk-dependent lamellipodia formation and cell spreading. We suggest that the coupling of Crk with Paxillin and their relocalization to focal contacts is important for the remodeling of the actin cytoskeleton and cell spreading, events critical for cell migration and invasion.
MATERIALS AND METHODS
Materials and Antibodies
A polyclonal p130Cas antibody was obtained from Dr. Michel Tremblay (McGill University, Montreal, QC, Canada). Antibodies to p1306 CrkII and Paxillin were purchased from Transduction Laboratories (Lexington, KY). CrkL and Cbl antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HA.11 and c-Myc (9E10) antibodies were obtained from Babco (Richmond, CA). FLAG-M2 antibodies were purchased from Sigma (Oakville, ON, Canada). An antibody raised against PKL, the chicken homolog of GIT2, was described previously (West et al., 2001). Alexa Fluor 488-phalloidin, Texas Red-X-phalloidin, and secondary antibodies conjugated to Alexa Fluor 488 were purchased from Molecular Probes (Eugene, OR). Secondary antibodies conjugated to CY3 were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Human HGF was generously provided by Dr. George Vande Woude (Van Andel Research Institute, Grand Rapids, MI) and human epidermal growth factor (EGF) was purchased from Roche Diagnostics (Laval, QC, Canada). Y27632 was purchased from Calbiochem (La Jolla, CA).
Plasmids
Expression plasmids for CrkI/II and CrkL were obtained from Dr. Bruce Mayer (University of Connecticut Health Center, Farmington, CT) and Dr. John Groffen (Childrens Hospital of Los Angeles Research Institute, Los Angeles, CA), respectively. pcDNA3-p130Cas, pRK5-mycN17Rac1, and pcdef3-β-PIX plasmids were obtained from Dr. Michel Tremblay (McGill University), Dr. Alan Hall (University College London, London, United Kingdom), and Dr. Arthur Weiss (University of California, San Francisco, CA), respectively. pcDNA3-Paxillin, pcDNA3-Paxillin Y31/118F, pcDNA3-Paxillin Δ263–282 (ΔLD4), pcDNA3-Paxillin Δ444–494 (ΔLIM3), and GFP-PKL expression plasmids were reported previously (Brown et al., 1996; Turner et al., 1999; Petit et al., 2000).
Microinjection
MDCK cells were maintained in DMEM containing 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin (Invitrogen Canada, Burlington, ON, Canada). MDCK cells (7 × 103) were plated on glass coverslips (Bellco Glass, Vineland, NJ) 3 days before microinjection. DNA plasmids were diluted in phosphate-buffered saline (PBS) as indicated in the figure legends. Occasionally, rabbit immunoglobulin G (Pierce Chemical, Rockford, IL) was included at a concentration of 0.6 μg/μl to detect injected cells. Small colonies of 10–50 cells were injected using an Eppendorf micromanipulator (Eppendorf Scientific, Westbury, NY). Microinjected cells were incubated for 5 h and fixed as described below.
Indirect Immunofluorescence
Cells were fixed for 15 min in 3.7% formaldehyde and permeabilized with 0.2% Triton X-100. Cell permeabilization with CSK was performed as described previously (Lamorte et al., 2002b). Nonspecific binding sites on the cells were blocked with 1% bovine serum albumin for 30 min. Primary and secondary antibodies were added successively, each for 30 min, with extensive washing between each incubation. 9E10 antibodies were diluted 1:800, CrkL antibodies were diluted 1:200, and Paxillin and FLAG-M2 antibodies were diluted 1:1000. All secondary antibodies were diluted 1:1000. Alexa Fluor 488-phalloidin and Texas Red-X-phalloidin were used at a 1:1000 dilution. All reagents were diluted in PBS supplemented with 1 mM MgCl2 and 1 mM CaCl2, with the exception of phalloidin, which was diluted in PBS supplemented with 0.2% Triton X-100. Donkey αrabbit antibodies conjugated to Alexa Fluor 488 were used to detect cells injected with rabbit immunoglobulin G. For experiments where monoclonal antibodies were used for costaining, CrkL was used instead of CrkII because the CrkL antibody is polyclonal. This was justified as both CrkII and CrkL promote a similar phenotype when microinjected into MDCK colonies (Lamorte et al., 2002b; Figure 2). Coverslips were mounted onto glass slides using Immunofluore mounting medium (ICN, St. Laurent, PQ, Canada). Images were acquired using a Retiga 1300 digital camera (QIMAGING, Burnaby, BC, Canada) and an AxioVert 135 microscope (Carl Zeiss Canada, Toronto, ON, Canada). Image analysis was carried out using Northern Eclipse version 6.0 (Empix Imaging, Mississauga, ON, Canada).
Figure 2.
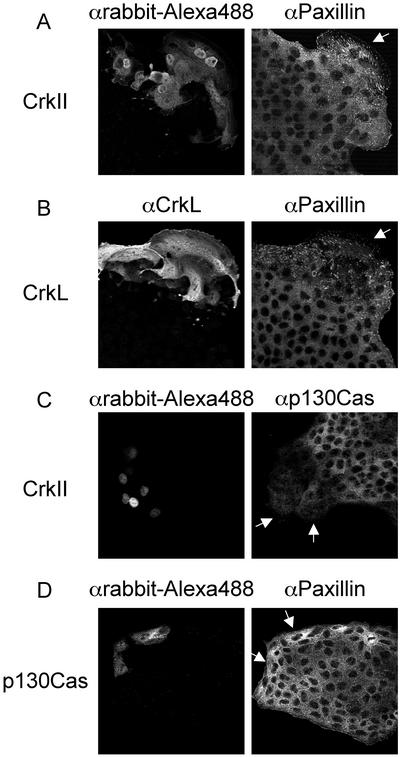
Paxillin but not p130Cas is redistributed in cells microinjected with CrkII or CrkL. (A and B) CrkII expression plasmids (50 ng/μl) and rabbit immunoglobulin G (0.6 μg/μl) (A) or CrkL expression plasmids (50 ng/μl) (B) were microinjected into the nuclei of MDCK cells. Cells were fixed after a 5-h incubation and double stained with αrabbit-Alexa Fluor 488 (A) or αCrkL/αrabbit-Alexa 488 (B) and αPaxillin/αmouse-CY3. (C) The nuclei of MDCK cells were microinjected with CrkII expression plasmids (50 ng/μl) and rabbit immunoglobulin G (0.6 μg/μl) and incubated for 5 h. After fixation, cells were stained with αrabbit-Alexa488 and αp130Cas/αmouse-CY3. (D) The nuclei of MDCK cells were microinjected with p130Cas expression plasmids (100 ng/μl) and rabbit immunoglobulin G (0.6 μg/μl). After a 5-h incubation, cells were fixed and stained with αrabbit-Alexa Fluor 488 and αPaxillin/αmouse-CY3. Arrows indicate microinjected cells.
Immunoprecipitation and Western Blotting
For coimmunoprecipitations, MDCK and MDCK cells overexpressing CrkII were grown to ∼90% confluence and serum starved for 6 h in DMEM containing 0.02% FBS. Cells were lysed with 1.0% Triton X-100 lysis buffer containing 50 mM HEPES, pH 7.5, 150 mM NaCl, 2 mM EGTA, 1.5 mM MgCl2, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 50 mM NaF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Immunoprecipitations and Western blotting were performed as described previously (Fixman et al., 1996).
RESULTS
Paxillin Relocalizes to Focal Contacts in Response to HGF Stimulation or Crk Overexpression
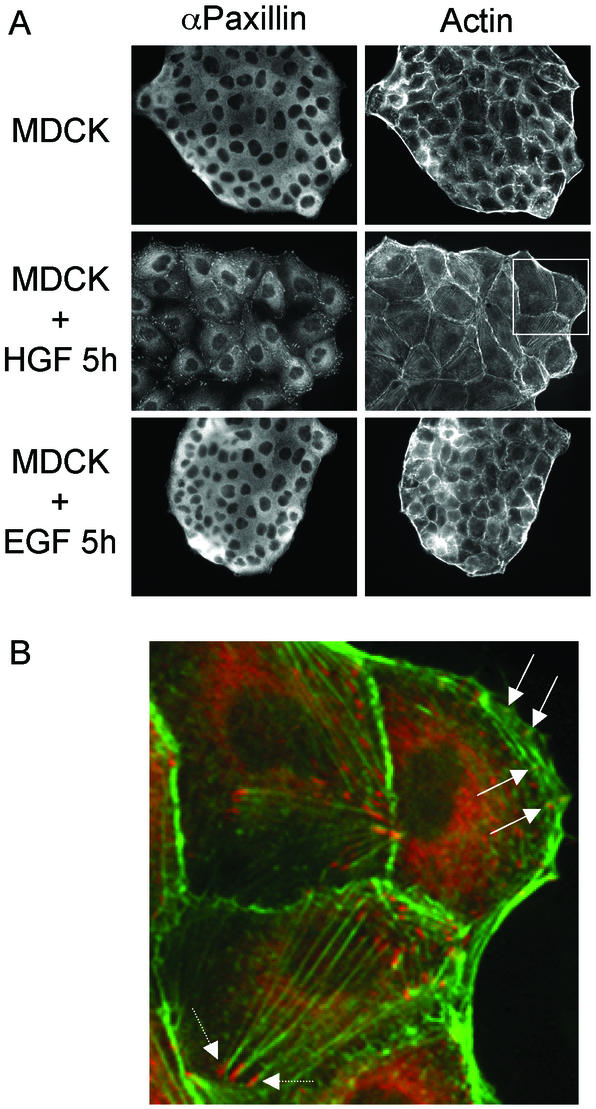
HGF promotes cell spreading through lamellipodia formation, reorganization of the actin cytoskeleton, and the formation of nascent focal complexes within the lamellipodia (Ridley et al., 1995; Royal et al., 2000). To define the requirements for the spreading of colonies of epithelial cells, we examined the changes that occur in response to HGF, which promotes cell spreading when compared with EGF, which fails to do so. In unstimulated MDCK cells, Paxillin was predominantly cytoplasmic (Figure 1A). After stimulation with HGF, a pool of Paxillin accumulated within focal contacts, with the remainder of Paxillin remaining in the cytoplasm, possibly in the Golgi (Figure 1A). At higher magnification, Paxillin is observed within focal complexes in the lamellipodia at the leading edge of colonies (Figure 1B, arrow) and within focal adhesions at the ends of actin stress fibers (Figure 1B, arrowhead). In contrast, EGF failed to promote the spreading of MDCK cells and Paxillin displayed a diffuse distribution within the cytoplasm (Figure 1A), similar to unstimulated MDCK cells (Figure 1A).
Figure 1.
HGF but not EGF promotes the relocalization of Paxillin to lamellipodia and to the ends of actin stress fibers. (A) MDCK cells were left untreated or stimulated with 5 U/ml HGF or 100 ng/ml EGF for 5 h and fixed. Cells were processed for indirect immunofluorescence by using αPaxillin/αmouse-CY3 and phalloidin-Alexa 488. (B) The αPaxillin and Phalloidin staining from the region highlighted in A were merged and enlarged. The solid arrow highlights Paxillin staining present within lamellipodia and the dotted arrow highlights Paxillin staining at the ends of actin stress fibers.
We have previously demonstrated that the stable overexpression of CrkII or CrkL in colonies of epithelial cells promotes lamellipodia formation, cell spreading, and breakdown of adherens junctions (Lamorte et al., 2002b). These are similar to the changes that occur after HGF stimulation (Ridley et al., 1995; Royal and Park, 1995; Potempa and Ridley, 1998; Royal et al., 2000; Figure 1). To understand the mechanism involved in Crk-mediated cell spreading, we compared the localization by indirect immunofluorescence of Paxillin and p130Cas, proteins associated with cell spreading and reorganization of the actin cytoskeleton and known to bind CrkII and CrkL (Feller, 2001). As shown above, in unstimulated cells, Paxillin displayed a diffuse cytoplasmic distribution in colonies of epithelial cells (Figure 1A), whereas in cells microinjected with CrkII expression plasmids, a pool of Paxillin relocalized to focal complexes present throughout the cell and within large lamellipodia at the edge of the colony (Figure 2A). Relocalization of Paxillin was also observed in MDCK cells microinjected with CrkL expression plasmids (Figure 2B). In contrast, there was no detectable relocalization of p130Cas to focal complexes in cells microinjected with CrkII (Figure 2C). Moreover, Paxillin failed to relocalize in cells microinjected with p130Cas expression plasmids (Figure 2D), consistent with the inability of p130Cas overexpression to promote cell spreading in MDCK cells (Lamorte et al., 2002b). Hence, the overexpression of CrkII or CrkL, as well as stimulation of colonies of MDCK cells with HGF, promotes the redistribution of Paxillin to focal complexes at the leading edge of spreading cells.
Functional Crk SH2 and SH3 Domains Are Required for Paxillin Relocalization
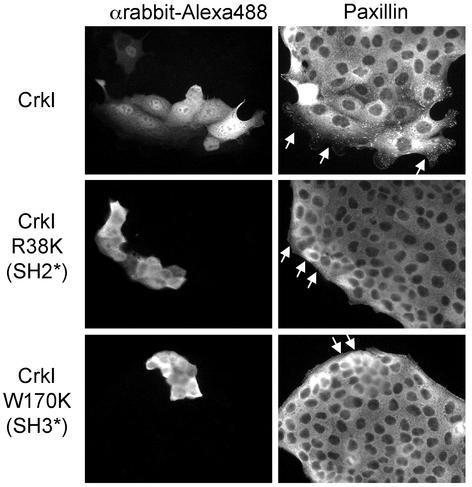
To define the requirements for Paxillin redistribution in response to Crk, plasmids encoding Crk proteins with a mutation in the SH2 (R38K) or amino-terminal SH3 (W170K) domain were microinjected into MDCK cells. CrkI, an alternatively spliced form of CrkII lacking the carboxy-terminal SH3 domain (Matsuda et al., 1992), promoted cell spreading and Paxillin redistribution to the leading edge (Figure 3). However, mutations within either the SH2 or SH3 domains of CrkI failed to promote cell spreading and Paxillin relocalization (Figure 3). Hence, although the carboxy-terminal SH3 domain of Crk is dispensable for cell spreading and the redistribution of Paxillin, both the SH2 and amino-terminal SH3 domains are required.
Figure 3.
Crk mutants lacking functional SH2 or SH3 domains fail to promote cell spreading and the relocalization of Paxillin to focal complexes. Expression plasmids (100 ng/μl) encoding CrkI, CrkI R38K, and CrkI W170K were microinjected together with rabbit immunoglobulin G (0.6 μg/μl) into the nuclei of MDCK cells. Cells were fixed after a 5-h incubation and double stained with αrabbit-Alexa Fluor 488 and αPaxillin/αmouse-CY3. Arrows indicate microinjected cells.
Crk-stimulated Paxillin Redistribution Is Rac Dependent
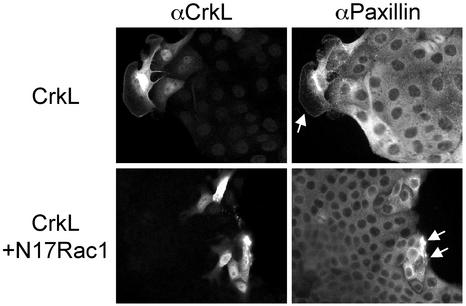
The spreading of colonies of epithelial cells in response to HGF requires the coordinated regulation of Rho GTPases and is inhibited by the expression of a mutant Rac1 protein unable to bind guanine nucleotides (N17Rac1) (Ridley et al., 1995; Royal et al., 2000). The involvement of Rac1 in Crk-induced Paxillin relocalization was examined by coinjecting cells with plasmids that express CrkL and dominant negative Rac1 (N17Rac1). Consistent with the ability of N17Rac1 to inhibit Crk-dependent lamellipodia formation and cell spreading (Lamorte et al., 2002b; Figure 4), Paxillin failed to relocalize to focal complexes in cells microinjected with CrkL and N17Rac1 (Figure 4).
Figure 4.
Rac is required for CrkII-induced Paxillin redistribution. CrkL plasmids (50 ng/μl) were coinjected into the nuclei of MDCK cells with vector (20 ng/μl) or N17 Rac1 (20 ng/μl). Cells were fixed after a 5-h incubation and double stained with αCrkL/αrabbit AlexaFluor488 and αPaxillin/αmouse-CY3. Arrows indicate microinjected cells.
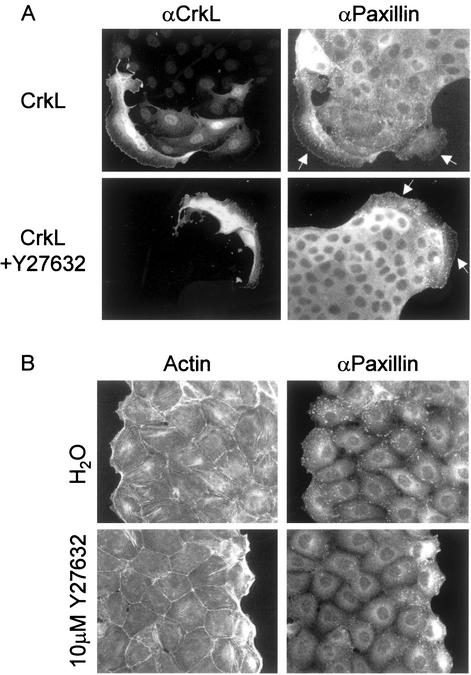
Although Paxillin redistribution to focal adhesions is RhoA-dependent (Manser et al., 1997), pharmacological inhibition of Rho-Kinase with 10 μM Y27632 (Uehata et al., 1997) did not inhibit CrkL-induced Paxillin relocalization nor did it inhibit the formation of lamellipodia or cell spreading (Figure 5A). Dominant negative mutants of RhoA could not be used because they promote HGF-independent cell spreading and dispersal in MDCK cells (Ridley et al., 1995). Y27632 inhibited HGF-induced actin stress fiber formation (Figure 5B), confirming that Y27632 inhibited Rho-kinase activity. Consistent with the localization of Paxillin to the ends of actin stress fibers in cells stimulated with HGF (Figure 1B), the presence of Paxillin-containing focal adhesions within the interior of HGF-stimulated colonies was significantly reduced in cells treated with Y27632 (Figure 5B). However, Y27632 did not inhibit HGF-stimulated relocalization of Paxillin within lamellipodia in cells at the edge of the colony (Figure 5B).
Figure 5.
Rho-Kinase is required for HGF-dependent Paxillin relocalization to the ends of actin stress fibers but is dispensable for HGF- and Crk-dependent Paxillin redistribution to focal complexes. (A) CrkL expression plasmids (50 ng/μl) were microinjected into the nuclei of MDCK cells pretreated for 30 min with H2O or 10 μM Y27632. After a 5-h incubation, cells were fixed and double stained with αCrkL/αrabbit-Alexa488 and αPaxillin/αmouse-CY3. Arrows indicate microinjected cells. (B) MDCK cells were treated with H2O or 10 μM Y27632 for 30 min before stimulation with 5 U/ml HGF for 5 h. After fixation, cells were processed for indirect immunofluorescence by using αPaxillin/αmouse-CY3 and phalloidin-Alexa 488.
CrkII Associates with Paxillin/GIT2/β-PIX Complexes upon Overexpression
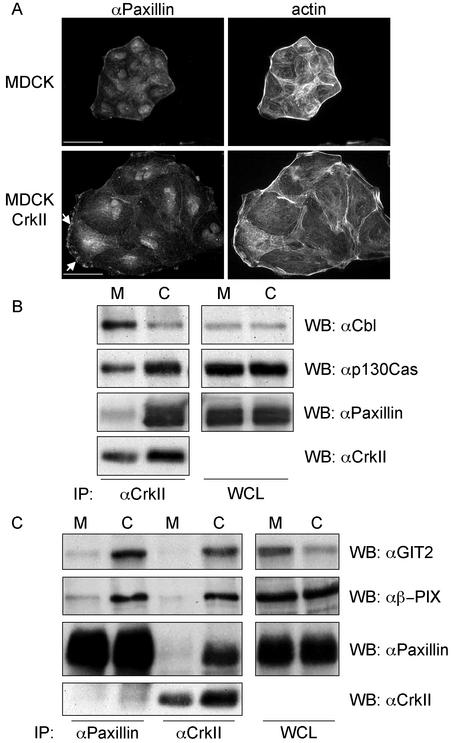
MDCK cell lines that overexpress CrkII display enhanced cell spreading in the absence of HGF stimulation (Lamorte et al., 2002b; Figure 6A). Moreover, in these cell lines, Paxillin was localized to insoluble complexes within the lamellipodia that are retained after solubilization with CSK buffer (Figure 6A). The Crk SH2 domain and SH3 domains interact with multiple proteins (Feller, 2001). We have previously shown that in MDCK cells, Crk associates with several phosphotyrosine containing proteins, including Paxillin and p130Cas and that its association with these proteins as well as with Cbl and Gab1 are increased after HGF stimulation (Lamorte et al., 2002b). To establish whether the overexpression of CrkII enhanced the coupling of Crk with specific tyrosine phosphorylated proteins, CrkII was immunoprecipitated from MDCK and MDCK cells overexpressing CrkII, and Western blotted with Paxillin, p130Cas, and Cbl antibodies (Figure 6B). Although the binding of Cbl to CrkII was decreased in MDCK cells overexpressing CrkII (Figure 6B), enhanced binding of Paxillin and p130Cas to CrkII was observed in MDCK cells overexpressing CrkII compared with control cells (Figure 6B).
Figure 6.
Altered cell morphology in MDCK cells overexpressing CrkII correlates with increased CrkII/Paxillin/GIT2/β-PIX coupling. (A) MDCK and MDCK cells overexpressing CrkII were grown on glass coverslips in DMEM containing 10% FBS for 48 h. Cells were solubilized with 0.25× CSK buffer for 10 min and fixed in formaldehyde. Cells were stained with αPaxillin/αmouse-CY3 and phalloidin-Alexa 488. The bar represents 25 μm. (B and C) MDCK and MDCK cells overexpressing CrkII were serum starved for 6 h and lysed. Cell lysate (2 mg) was used for immunoprecipitation with CrkII or Paxillin antibodies. The immunoprecipitates were washed and associated proteins together with 25 μg of whole cell lysate were resolved by SDS-PAGE. Proteins on the gel were transferred to a nitrocellulose membrane, immunoblotted with αp130Cas, αCbl, αPaxillin, and αCrk. M and C refer to MDCK and MDCK cells overexpressing CrkII, respectively.
The formation of a complex of Paxillin with GIT2 and β-PIX is promoted in a Rac-dependent manner in fibroblasts (Brown et al., 2002). Because both cell spreading and the redistribution of Paxillin in cells microinjected with CrkL is dependent on Rac, we established whether the coupling of GIT2 and β-PIX with Paxillin was enhanced in MDCK cells overexpressing CrkII. The association of Paxillin with GIT2 and β-PIX in MDCK cells overexpressing CrkII was greatly enhanced over the levels of these proteins that coimmunoprecipitated with Paxillin in control MDCK cells (Figure 6C). Consistent with the ability of Crk to bind Paxillin (Birge et al., 1993), the association of Crk with Paxillin, GIT2 and β-PIX was also increased in cells overexpressing CrkII (Figure 6C). This suggests that increased expression of CrkII promotes an increased association of Paxillin with GIT2 and β-PIX.
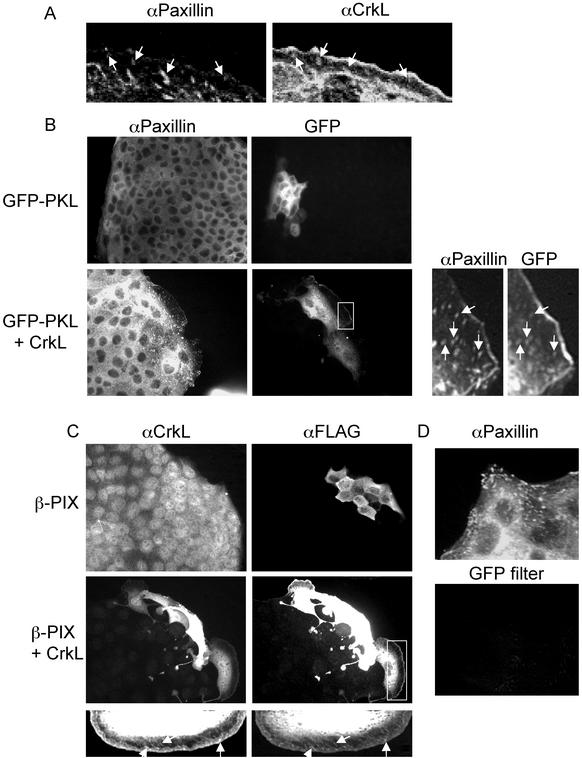
To establish whether the enhanced assembly of a Crk/Paxillin complex in cells overexpressing CrkII promotes the localization of Crk to focal complexes, MDCK cell colonies were microinjected with CrkL and the colocalization of CrkL with endogenous Paxillin was examined by indirect immunofluorescence. Although the majority of CrkL displayed a diffuse cytoplasmic distribution after microinjection (Figures 2B and 7A), CrkL localized to focal complexes at the edge of the lamellipodia and showed some colocalization with endogenous Paxillin (Figure 7A). Similarly, although no punctate GFP-PKL or β-PIX was observed in cells microinjected with vector (Figure 7, B and C), some colocalization of GFP-PKL with Paxillin (Figure 7B) and β-PIX with CrkL (Figure 7C) was observed in cells microinjected with CrkL expression plasmids. The colocalization of GFP-PKL with Paxillin was specific, because noninjected cells displaying punctate Paxillin localization did not display any staining when visualized with fluorescent excitation filters specific for GFP (Figure 7D).
Figure 7.
CrkL, Paxillin, PKL, and β-PIX colocalize to focal complexes in cells microinjected with CrkL. (A) The nuclei of MDCK cells were microinjected with CrkL expression plasmids (50 ng/μl), fixed 5 h later, and double stained with αCrkL/αrabbit Alexa Fluor 488 and αPaxillin/αmouse-CY3. (B) The nuclei of MDCK cells were microinjected with GFP-PKL expression plasmids (20 ng/μl) and vector (50 ng/μl) or CrkL expression plasmids (50 ng/μl). After a 5-h incubation, cells were fixed and stained with αPaxillin/αmouse-CY3. (C) The nuclei of MDCK cells were microinjected with FLAG-β-PIX expression plasmids (20 ng/μl) and vector (50 ng/μl) or CrkL expression plasmids (50 ng/μl). After a 5-h incubation, cells were fixed and stained with αFLAG/αmouse-CY3 and αCrkL/αrabbit Alexa Fluor 488. (D) Noninjected cells from B that contained punctate Paxillin staining were excited with a GFP-specific filter and photographed. Arrows indicate colocalization.
Paxillin Mutants Impair Crk-dependent Lamellipodia Formation and Cell Spreading
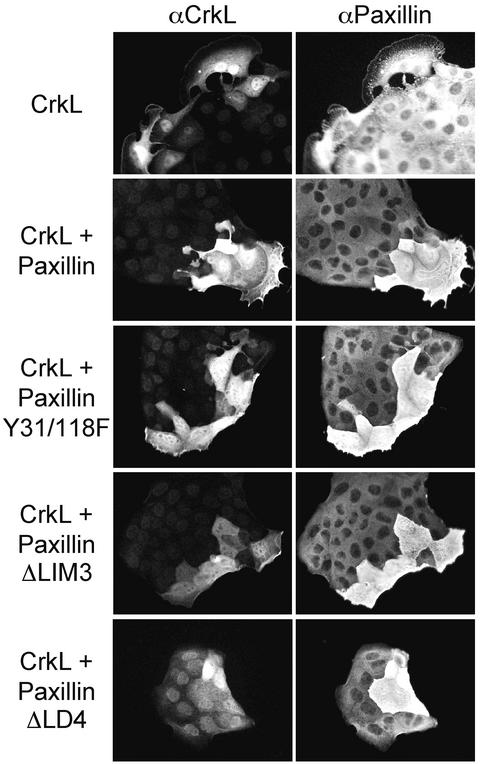
To examine the potential contribution of Paxillin to Crk-mediated lamellipodia formation and cell spreading, Paxillin mutants were coinjected with CrkL into MDCK cells. The ΔLIM3 mutant lacks the LIM3 domain (amino acids 444–494) and displays significantly reduced targeting to focal adhesions (Brown et al., 1996). The Y31/118F mutant contains tyrosine to phenylalanine mutations at residues 31 and 118, which represent Crk SH2 binding sites (Petit et al., 2000). The ΔLD4 mutant lacks the LD4 domain (amino acids 263–282) and fails to bind PKL/GIT2 (Turner et al., 1999). The microinjection of wild-type Paxillin did not impair CrkL-stimulated lamellipodia formation (Figure 8). In contrast, the microinjection of the ΔLIM3, Y31/118F, or ΔLD4 mutants diminished the effects of CrkL on lamellipodia formation and cell spreading (Figure 8) while promoting enhanced membrane ruffling for the Y31/118F and ΔLIM3 mutants (Figure 8). These effects were observed in >50% of injected colonies (Table 1).
Figure 8.
Paxillin mutants lacking Crk SH2-binding sites, the LIM3 domain or the LD4 domain impair Crk-stimulated lamellipodia formation and cell spreading. The nuclei of MDCK cells were microinjected with CrkL expression plasmids (50 ng/μl) and vector (A), wild-type Paxillin (B), PaxillinY31/118F (C), PaxillinΔLIM3 (D), or PaxillinΔLD4 (E), each at 100 ng/μl. Cells were fixed 5 h later and double stained with αCrkL/αrabbit Alexa Fluor 488 and αPaxillin/αmouse-CY3.
Table 1.
Effect of Paxillin on CrkL-dependent lamellipodia
| Plasmid | % Lamellipodia and cell spreading | SD |
|---|---|---|
| Wt Paxillin | 80.6 | 9.8 |
| Paxillin ΔCrk | 37.6 | 13.3 |
| Paxillin ΔLIM3 | 44.8 | 4.5 |
| Paxillin ΔLD4 | 47.9 | 5.0 |
DISCUSSION
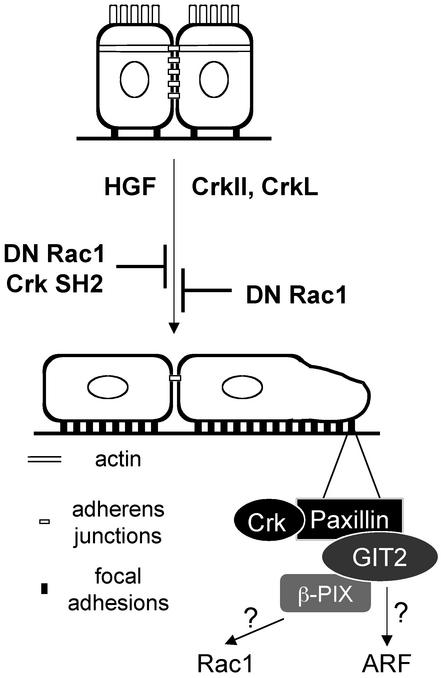
We have previously demonstrated that the CrkII and CrkL adapter proteins are required for the spreading of epithelial colonies and the breakdown of adherens junctions in response to HGF (Lamorte et al., 2002b). Despite a growing interest in the Crk adapter proteins as modulators of cell spreading and migration, the role of Crk in these processes is not completely defined. The goal of our study was to determine the mechanisms by which Crk adapter proteins regulate these cellular processes. Our results show that in colonies of epithelial cells, Crk promotes the redistribution of a pool of Paxillin from the cytoplasm to focal complexes within developing lamellipodia. Paxillin redistribution and the formation of focal complexes is dependent on Rac activity and correlates with an increase in the formation of a multiprotein complex containing Crk and Paxillin, as well as an ARF-GAP, GIT2, and a Rac1 exchange factor, β-PIX (Figure 9). Paxillin mutants that fail to bind Crk or fail to associate with GIT2 inhibit Crk-dependent lamellipodia formation, supporting a role for this multiprotein complex in lamellipodia formation and cell spreading, processes critical for cell migration (Figure 9).
Figure 9.
Overexpression of Crk adapter proteins in MDCK cells promotes lamellipodia formation and cell spreading, mirroring the response of cells at the edge of the colony to HGF stimulation. Similarly, overexpression of Crk or stimulation of MDCK cells with HGF promotes the redistribution of Paxillin to focal contacts throughout the cell and within lamellipodia. In cells overexpressing CrkII, the assembly of a Crk/Paxillin/GIT2/β-PIX complex that relocalizes to focal complexes at the leading edge contributes to lamellipodia formation and cell spreading, possibly by influencing the activities of the Rac and ARF GTPases.
Paxillin plays an important role in focal adhesion signaling (Turner, 2000) and is critical for efficient cell spreading and motility (Hagel et al., 2002). In colonies of epithelial cells, Paxillin is predominantly localized to a cytosolic compartment (Figure 1A). However, unlike Vinculin (Lamorte et al., 2002b), Paxillin is not detected within established focal adhesions present at the edge of the colony (Figure 1A). In response to HGF, Paxillin redistributes to newly forming focal adhesions at the ends of actin stress fibers and to focal complexes within lamellipodia at the leading edge of the colony (Figure 1B). Similarly, the relocalization of Paxillin to membrane ruffles was observed in mIMCD-3 cells in response to HGF (Liu et al., 2002b). In contrast, EGF, which fails to stimulate the formation of large lamellipodia or the spreading of epithelial cell colonies, fails to promote the redistribution of Paxillin (Figure 1A), demonstrating that the relocalization of Paxillin correlates with cell spreading. Consistent with the ability of the Crk adapter protein to promote lamellipodia formation and cell spreading in colonies of epithelial cells (Lamorte et al., 2002b; Figure 2), the microinjection of Crk expression plasmids promotes the redistribution of Paxillin and Vinculin into focal complexes throughout the cell and within developing lamellipodia (Figure 2; our unpublished data). Noninjected cells surrounding the injected cells also display Paxillin relocalization (Figures 2, 3, and 5), indicating that the Crk-dependent loss of adherens junctions (Lamorte et al., 2002b) would favor the spreading of neighboring cells and subsequently, the redistribution of Paxillin to focal contacts.
Rac but Not Rho-kinase Is Required for Crk-dependent Paxillin Relocalization
There are several distinct classes of cell-matrix adhesions. Focal adhesions localize to the ends of actin stress fibers on the basal surface of the cell and their formation is dependent on RhoA activity (Ridley and Hall, 1992), whereas focal complexes are generally smaller in size, localize within lamellipodia or filopodia, and are Rac1 or Cdc42 dependent, respectively (Nobes and Hall, 1995). Pretreatment of cells with a pharmacological inhibitor of Rho-kinase, Y27632 (Uehata et al., 1997), blocked HGF-stimulated actin stress fiber formation and Paxillin relocalization in cells within the interior of the colony, consistent with a requirement for RhoA activity in Paxillin relocalization and tyrosine phosphorylation (Barry and Critchley, 1994; Manser et al., 1997; Clark et al., 1998). In contrast, Y27632 failed to inhibit the extensive relocalization of Paxillin observed in response to HGF in cells at the periphery of the colony, indicating that HGF-dependent Paxillin relocalization is differentially regulated. Notably, in response to HGF, cells at the edge of the colony develop large lamellipodia that contain Rac-dependent focal complexes (Figure 5B). The pretreatment of cells with Y27632 failed to inhibit Crk-induced lamellipodia formation and Paxillin relocalization to focal complexes (Figure 5A), indicating that pathways downstream of Rho-Kinase are dispensable for these events, implicating a possible role for Rac in Crk-dependent Paxillin relocalization. In support of this, we have previously shown that CrkII overexpression enhances the basal activity of Rac in MDCK cells (Lamorte et al., 2002b). Moreover, dominant negative mutants of Rac1 inhibit Crk-dependent Paxillin relocalization as well as lamellipodia formation and spreading of cells at the edge of the colony (Figure 4; Lamorte et al., 2002b). Hence, the overexpression of Crk mirrors the response of cells at the edge of the colony to HGF, further supporting a role for Crk adapter proteins in HGF-mediated epithelial-mesenchymal transitions.
Enhanced Assembly and Association with CrkII of a Multiprotein Paxillin/GIT2/β-PIX Complex
Using Crk mutant proteins, we have shown that Crk-dependent cell spreading and Paxillin relocalization requires both an intact Crk SH2 domain and an intact amino terminal Crk SH3 domain (Figure 3). This indicates that the association of the Crk SH2 domain with tyrosine phosphorylated proteins and the Crk SH3 domain with proline-rich domain containing proteins is required to initiate signals that promote lamellipodia formation, cell spreading, and Paxillin relocalization. Paxillin that is present within focal adhesions and at the cell periphery is tyrosine phosphorylated at Y31 and Y118 (Nakamura et al., 2000; West et al., 2001). These phosphorylated tyrosine residues form consensus binding sites for the Crk SH2 domain (Petit et al., 2000; Schaller and Schaefer, 2001). Consistent with this, HGF stimulation enhances Crk/Paxillin coupling (Lamorte et al., 2002b). Moreover, in cells overexpressing CrkII, the association of CrkII with Paxillin is enhanced (Figure 6, B and C) and after microinjection, CrkL relocalizes to Paxillin containing focal complexes present within lamellipodia (Figure 7A).
In addition to its ability to associate with Crk, Paxillin acts as a scaffold for other proteins, including GIT2/PKL, a member of the ARF-GAP family (Turner et al., 1999), which also includes GIT1, PAP/PAG3, ASAP1, and ACAP1/2 (Turner et al., 2001). GIT2/PKL binds β-PIX (Turner et al., 1999), a Rac1 exchange factor (Bagrodia et al., 1998; Manser et al., 1998), and β-PIX binds PAK (Bagrodia et al., 1998; Manser et al., 1998). Together, this complex is thought to act in a synergistic manner to recruit PAK to focal complexes (Manser et al., 1998) where it could promote focal complex disassembly (Manser et al., 1997) and participate in Rac-dependent actin reorganization (Obermeier et al., 1998), thereby promoting cell spreading. In support of this, Drosophila PAK is involved in dorsal closure, together with Rac1 and Cdc42 (Harden et al., 1996).
We provide evidence that CrkII overexpression enhances the levels of a Paxillin/GIT2/β-PIX complex in cells (Figure 6C) and in turn these proteins localize to focal complexes in cells microinjected with CrkL expression plasmids (Figure 7). Paxillin/GIT2/β-PIX complexes are present within CrkII immunoprecipitates in stable cell lines overexpressing CrkII (Figure 6C), indicating that CrkII associates with this multiprotein complex. Due to poor specificity of available PAK sera, we were unable to detect endogenous PAK within the Paxillin/GIT2/β-PIX complex in MDCK cells overexpressing CrkII. However, from the tight association observed between PAK and β-PIX, we would predict that PAK is recruited to this complex. Because the activation of Rac and Cdc42 enhances the association of PKL with Paxillin (Brown et al., 2002), the enhanced association of the Paxillin/GIT2/β-PIX multiprotein complex in cells overexpressing CrkII is consistent with the elevated levels of Rac activity observed in these cells (Lamorte et al., 2002b). Similarly, V12Rac stimulates the redistribution of a related ARF-GAP, GIT1/APP1, to focal complexes (Zhao et al., 2000; Matafora et al., 2001).
Members of the ARF family of small GTP binding proteins have been implicated in the reorganization of the actin cytoskeleton. ARFs regulate membrane traffic between endosomes and the Golgi (Chavrier and Goud, 1999). Moreover, ARF1 has been reported to mediate the recruitment of Paxillin to focal adhesions in fibroblasts (Norman et al., 1998), and ARF6 promotes the relocalization of Rac1 to the plasma membrane (Radhakrishna et al., 1999; Zhang et al., 1999; Boshans et al., 2000). Several ARF-GAP proteins associate with focal adhesion protein complexes, suggesting that these proteins and their associated ARF GTPases are important regulators of signaling pathways during cell spreading and migration (de Curtis, 2001). Although dominant negative mutants of ARF1 or ARF6 impaired HGF-stimulated cell spreading, their comicroinjection with Crk failed to inhibit Crk-stimulated cell spreading and Paxillin relocalization (Lamorte and Park, submitted), suggesting that these proteins may act upstream or in a pathway parallel to Crk. Hence, the increased assembly of a Paxillin/GIT2/β-PIX complex after CrkII overexpression, together with the Crk-dependent recruitment of these proteins to focal complexes (Figure 7), supports a role for this complex in Crk-dependent lamellipodia formation and cell spreading. Consistent with this, mutants of Paxillin that fail to associate with Crk (Y31/118F), or GIT2 (ΔLD4), or do not target to focal adhesions (ΔLIM3), impaired CrkL-dependent lamellipodia formation and cell spreading (Figure 8). With the exception of cells microinjected with PaxillinΔLD4, cells microinjected with the other Paxillin mutants displayed elevated membrane ruffling (Figure 8) consistent with Rac activation. Hence, both the association of Crk with Paxillin/GIT2 complexes and the targeting of Crk/Paxillin complexes to focal complexes are required for the ability of Crk to stimulate lamellipodia formation and cell spreading. In a similar manner, expression of a PaxillinY31/118F mutant inhibited the migration of NBT-II bladder carcinoma cells on collagen type I (Petit et al., 2000) and PaxillinΔLD4 inhibited IGF-1-dependent cell spreading and lamellipodia formation (Turner et al., 1999). Moreover, CHO.K1 cells overexpressing PaxillinΔLD4 are defective in directed motility (West et al., 2001), and overexpression of the LD4 motif perturbs directed motility (Turner et al., 1999; Zhao et al., 2000). Thus, the coupling of Crk proteins with Paxillin and the assembly of Paxillin/GIT2/β-PIX complexes may represent an important mechanism for cell spreading and migration, enabling the localization and activation of downstream pathways such as Rac1, sustaining lamellipodia formation and cell spreading. However, additional mechanisms for activating Rac1 and promoting lamellipodia formation, involving p130Cas/Crk and/or Gab1/Crk complexes must exist as not all cells microinjected with the Paxillin mutants failed to promote Crk-dependent lamellipodia formation (Table 1). Moreover, HGF-dependent lamellipodia formation and cell spreading are not inhibited by the microinjection of the different Paxillin mutants (our unpublished data). Thus, the coupling of Crk with Paxillin is dispensable for HGF-dependent cell spreading, suggesting that additional pathways can compensate for the loss of these signals.
The binding of the Crk SH2 domain to Paxillin would enable the recruitment of Crk to Paxillin-containing focal contacts, possibly targeting Crk SH3 binding proteins to focal complexes and promoting localized Rac activation. In support of this, DOCK180, a Crk amino-terminal SH3 binding protein, functions as a two-component Rac1 exchange factor through its interaction with ELMO (Brugnera et al., 2002). Furthermore, the coexpression of p130Cas, CrkII, and DOCK180 promotes the spreading of single cells and the accumulation of these complexes to focal adhesions (Kiyokawa et al., 1998b). We have described the formation of a distinct complex involving Crk/Paxillin/GIT2/β-PIX that may behave similarly (Figure 9). Although CrkII/p130Cas complex formation is enhanced in cells overexpressing CrkII, p130Cas does not detectably relocalize to focal contacts in cells overexpressing Crk (Figure 2C). However, we cannot exclude a role for Crk/p130Cas interactions in lamellipodia formation and cell spreading. Moreover, the ability of CrkII/p130Cas coupling to regulate cell migration and invasion (Klemke et al., 1998; Cho and Klemke, 2000; Spencer et al., 2000) indicates that these complexes may have a similar role in enhancing the invasiveness of MDCK cells (Lamorte et al., 2002a).
In conclusion, our results identify a novel role for Crk in promoting the relocalization of Paxillin to focal complexes. Both Rac activation and the targeting of Crk/Paxillin complexes to focal complexes are essential for lamellipodia formation and cell spreading in cells overexpressing Crk adapter proteins (Figure 9). Recruitment of Paxillin binding proteins, such as GIT2- and GIT2-associated proteins (β-PIX and PAK) to these focal complexes enables lamellipodia formation and cell spreading, possibly through the regulation of Rac and ARF activity (Figure 9). These results provide further insights into the mechanisms involved in the regulation of epithelial-mesenchymal transitions, events critical for tumor cell migration and metastasis.
Acknowledgments
We thank James Casanova, John Groffen, Alan Hall, Bruce Mayer, Michel Tremblay, George Vande Woude, and Arthur Weiss for reagents provided in this study. L.L. is a recipient of a Canadian Institutes of Health Research studentship. M.P. is a recipient of a Canadian Institutes of Health Research scientist award. S.R. is a recipient of a McGill University Health Centre Research Institute studentship. V.S. is a recipient of a Cancer Research Society postdoctoral fellowship. This research was supported by an operating grant to M.P. from the Canadian Breast Cancer Research Initiative with money from the Canadian Cancer Society and National Institutes of Health grant GM-47607 (to C.T.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0497. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-08-0497.
Abbreviations used: ARF, ADP-ribosylation factor; EM, epithelial-mesenchymal; FBS, fetal bovine serum; GAP, GTPase activating protein; GTPase, guanosine triphosphatase; HGF, hepatocyte growth factor; MDCK, Madin-Darby canine kidney; PBS, phosphate-buffered saline; SH2, Src homology 2; SH3, Src homology 3.
References
- Bagrodia, S., Taylor, S.J., Jordon, K.A., Van Aelst, L., and Cerione, R.A. (1998). A novel regulator of p21-activated kinases. J. Biol. Chem. 273, 23633–23636. [DOI] [PubMed] [Google Scholar]
- Barry, S., and Critchley, D. (1994). The RhoA-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C-delta to focal adhesions. J. Cell Sci. 107, 2033–2045. [DOI] [PubMed] [Google Scholar]
- Birchmeier, C., and Gherardi, E. (1998). Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 8, 404–410. [DOI] [PubMed] [Google Scholar]
- Birge, R.B., Fajardo, J.E., Reichman, C., Shoelson, S.E., Songyang, Z., Cantley, L.C., and Hanafusa, H. (1993). Identification and characterization of a high-affinity interaction between v-Crk and tyrosinephosphorylated Paxillin in CT10-transformed fibroblasts. Mol. Cell. Biol. 13, 4648–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshans, R.L., Szanto, S., van Aelst, L., and D'Souza-Schorey, C. (2000). ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol. Cell. Biol. 20, 3685–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, B., Valles, A.M., and Edme, N. (2000). Induction and regulation of epithelial-mesenchymal transitions. Biochem. Pharmacol. 60, 1091–1099. [DOI] [PubMed] [Google Scholar]
- Brown, M.C., Perrotta, J.A., and Turner, C.E. (1996). Identification of LIM3 as the principal determinant of Paxillin focal adhesion localization and characterization of a novel motif on Paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 135, 1109–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M.C., West, K.A., and Turner, C.E. (2002). Paxillin-dependent Paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol. Biol. Cell 13, 1550–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnera, E., Haney, L., Grimsley, C., Lu, M., Walk, S.F., Tosello-Trampont, A.C., Macara, I.G., Madhani, H., Fink, G.R., and Ravichandran, K.S. (2002). Unconventional Rac-GEF activity is mediated through the Dock180 ELMO complex. Nat. Cell Biol. 4, 574–582. [DOI] [PubMed] [Google Scholar]
- Burridge, K., Turner, C.E., and Romer, L.H. (1992). Tyrosine phosphorylation of Paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 119, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier, P., and Goud, B. (1999). The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11, 466–475. [DOI] [PubMed] [Google Scholar]
- Cho, S.Y., and Klemke, R.L. (2000). Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 149, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, E.A., King, W.G., Brugge, J.S., Symons, M., and Hynes, R.O. (1998). Integrin-mediated signals regulated by members of the rho family of GTPases. J. Cell Biol. 142, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis, I. (2001). Cell migration: GAPs between membrane traffic and the cytoskeleton. EMBO Rep. 2, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare, A., Paris, S., Albertinazzi, C., Dariozzi, S., Andersen, J., Mann, M., Longhi, R., and de Curtis, I. (2000). p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nat. Cell Biol. 2, 521–530. [DOI] [PubMed] [Google Scholar]
- Feller, S.M. (2001). Crk family adaptors-signalling complex formation and biological roles. Oncogene 20, 6348–6371. [DOI] [PubMed] [Google Scholar]
- Feller, S.M., Knudsen, B., and Hanafusa, H. (1994). c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 13, 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixman, E.D., Fournier, T.M., Kamikura, D.M., Naujokas, M.A., and Park, M. (1996). Pathways downstream of Shc and Grb2 are required for cell transformation by the tpr-Met oncoprotein. J. Biol. Chem. 271, 13116–13122. [DOI] [PubMed] [Google Scholar]
- Franco, M., Peters, P.J., Boretto, J., van Donselaar, E., Neri, A., D'Souza-Schorey, C., and Chavrier, P. (1999). EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 18, 1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, B., Bershadsky, A., Pankov, R., and Yamada, K.M. (2001). Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell. Biol. 2, 793–805. [DOI] [PubMed] [Google Scholar]
- Gotoh, T., Hattori, S., Nakamura, S., Kitayama, H., Noda, M., Takai, Y., Kaibuchi, K., Matsui, H., Hatase, O., Takahashi, H., and et al. (1995). Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol. Cell. Biol. 15, 6746–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagel, M., George, E.L., Kim, A., Tamimi, R., Opitz, S.L., Turner, C.E., Imamoto, A., and Thomas, S.M. (2002). The adaptor protein Paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22, 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Harden, N., Lee, J., Loh, H.Y., Ong, Y.M., Tan, I., Leung, T., Manser, E., and Lim, L. (1996). A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol. Cell. Biol. 16, 1896–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmeryckx, B., van Wijk, A., Reichert, A., Kaartinen, V., de Jong, R., Pattengale, P.K., Gonzalez-Gomez, I., Groffen, J., and Heisterkamp, N. (2001). Crkl enhances leukemogenesis in BCR/ABL P190 transgenic mice. Cancer Res 61, 1398–1405. [PubMed] [Google Scholar]
- Jackson, T.R., Brown, F.D., Nie, Z., Miura, K., Foroni, L., Sun, J., Hsu, V.W., Donaldson, J.G., and Randazzo, P.A. (2000). ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J. Cell Biol. 151, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa, E., Hashimoto, Y., Kobayashi, S., Sugimura, H., Kurata, T., and Matsuda, M. (1998a). Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 12, 3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa, E., Hashimoto, Y., Kurata, T., Sugimura, H., and Matsuda, M. (1998b). Evidence that DOCK180 up-regulates signals from the CrkII-p130(Cas) complex. J. Biol. Chem. 273, 24479–24484. [DOI] [PubMed] [Google Scholar]
- Klemke, R.L., Leng, J., Molander, R., Brooks, P.C., Vuori, K., and Cheresh, D.A. (1998). CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 140, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, A., Hashimoto, S., Yano, H., Nagayama, K., Mazaki, Y., and Sabe, H. (2000). A new Paxillin-binding protein, PAG3/Papalpha/KIAA0400, bearing an ADP-ribosylation factor GTPase-activating protein activity, is involved in Paxillin recruitment to focal adhesions and cell migration. Mol. Biol. Cell 11, 1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamorte, L., Rodrigues, S., Naujokas, M., and Park, M. (2002a). Crk synergizes with epidermal growth factor for epithelial invasion and morphogenesis and is required for the met morphogenic program. J. Biol. Chem. 277, 37904–37911. [DOI] [PubMed] [Google Scholar]
- Lamorte, L., Royal, I., Naujokas, M., and Park, M. (2002b). Crk adapter proteins promote an epithelial-mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol. Biol. Cell 13, 1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger, D.A., and Horwitz, A.F. (1996). Cell migration: a physically integrated molecular process. Cell 84, 359–369. [DOI] [PubMed] [Google Scholar]
- Laukaitis, C.M., Webb, D.J., Donais, K., and Horwitz, A.F. (2001). Differential dynamics of alpha 5 integrin, Paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol. 153, 1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Loijens, J.C., Martin, K.H., Karginov, A.V., and Parsons, J.T. (2002a). The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol. Biol. Cell 13, 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z.X., Yu, C.F., Nickel, C., Thomas, S., and Cantley, L.G. (2002b). Hepatocyte growth factor (HGF) induces ERK-dependent Paxillin phosphorylation and regulates Paxillin/FAK association. J. Biol. Chem. 9, 9. [DOI] [PubMed] [Google Scholar]
- Manabe Ri, R., Kovalenko, M., Webb, D.J., and Horwitz, A.R. (2002). GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J. Cell Sci. 115, 1497–1510. [DOI] [PubMed] [Google Scholar]
- Manser, E., Huang, H.Y., Loo, T.H., Chen, X.Q., Dong, J.M., Leung, T., and Lim, L. (1997). Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 17, 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser, E., Loo, T.H., Koh, C.G., Zhao, Z.S., Chen, X.Q., Tan, L., Tan, I., Leung, T., and Lim, L. (1998). PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1, 183–192. [DOI] [PubMed] [Google Scholar]
- Maroun, C.R., Holgado-Madruga, M., Royal, I., Naujokas, M.A., Fournier, T.M., Wong, A.J., and Park, M. (1999). The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 19, 1784–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matafora, V., Paris, S., Dariozzi, S., and de Curtis, I. (2001). Molecular mechanisms regulating the subcellular localization of p95-APP1 between the endosomal recycling compartment and sites of actin organization at the cell surface. J. Cell Sci. 114, 4509–4520. [DOI] [PubMed] [Google Scholar]
- Matsuda, M., Tanaka, S., Nagata, S., Kojima, A., Kurata, T., and Shibuya, M. (1992). Two species of human CRK cDNA encode proteins with distinct biological activities. Mol. Cell. Biol. 12, 3482–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaki, Y., et al. (2001). An ADP-ribosylation factor GTPase-activating protein Git2-short/KIAA0148 is involved in subcellular localization of Paxillin and actin cytoskeletal organization. Mol. Biol. Cell 12, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, K., Yano, H., Uchida, H., Hashimoto, S., Schaefer, E., and Sabe, H. (2000). Tyrosine phosphorylation of Paxillin alpha is involved in temporospatial regulation of Paxillin-containing focal adhesion formation and F-actin organization in motile cells. J. Biol. Chem. 275, 27155–27164. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Nolan, K.M., Barrett, K., Lu, Y., Hu, K.Q., Vincent, S., and Settleman, J. (1998). Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 12, 3337–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, J.C., Jones, D., Barry, S.T., Holt, M.R., Cockcroft, S., and Critchley, D.R. (1998). ARF1 mediates Paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J. Cell Biol. 143, 1981–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier, A., Ahmed, S., Manser, E., Yen, S.C., Hall, C., and Lim, L. (1998). PAK promotes morphological changes by acting upstream of Rac. EMBO J. 17, 4328–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, F., Price, L., Schweitzer, J., Collard, J.G., and D'Souza-Schorey, C. (2001). An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20, 4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit, V., Boyer, B., Lentz, D., Turner, C.E., Thiery, J.P., and Valles, A.M. (2000). Phosphorylation of tyrosine residues 31 and 118 on Paxillin regulates cell migration through an association with CRK in NBT-II cells. J. Cell Biol. 148, 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa, S., and Ridley, A.J. (1998). Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol. Biol. Cell 9, 2185–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont, R.T., Claing, A., Vitale, N., Perry, S.J., and Lefkowitz, R.J. (2000). The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J. Biol. Chem. 275, 22373–22380. [DOI] [PubMed] [Google Scholar]
- Radhakrishna, H., Al-Awar, O., Khachikian, Z., and Donaldson, J.G. (1999). ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci. 112, 855–866. [DOI] [PubMed] [Google Scholar]
- Randazzo, P.A., Andrade, J., Miura, K., Brown, M.T., Long, Y.Q., Stauffer, S., Roller, P., and Cooper, J.A. (2000). The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 97, 4011–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien, P.W., and Horvitz, H.R. (2000). CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2, 131–136. [DOI] [PubMed] [Google Scholar]
- Reichman, C.T., Mayer, B.J., Keshav, S., and Hanafusa, H. (1992). The product of the cellular crk gene consists primarily of SH2 and SH3 regions. Cell Growth Differ. 3, 451–460. [PubMed] [Google Scholar]
- Ridley, A.J., Comoglio, P.M., and Hall, A. (1995). Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell. Biol. 15, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., and Hall, A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Royal, I., Lamarche-Vane, N., Lamorte, L., Kaibuchi, K., and Park, M. (2000). Activation of Cdc42, Rac, PAK, and Rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 11, 1709–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal, I., and Park, M. (1995). Hepatocyte growth factor induced scatter of MDCK cells requires phosphatidylinositol 3-kinase. J. Biol. Chem. 270, 27780–27787. [DOI] [PubMed] [Google Scholar]
- Santy, L.C., and Casanova, J.E. (2001). Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 154, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry, S.K., and Burridge, K. (2000). Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 261, 25–36. [DOI] [PubMed] [Google Scholar]
- Schaller, M.D., and Schaefer, E.M. (2001). Multiple stimuli induce tyrosine phosphorylation of the Crk-binding sites of Paxillin. Biochem. J. 360, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, K.S., Graus-Porta, D., Leng, J., Hynes, N.E., and Klemke, R.L. (2000). ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J. Cell Biol. 148, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker, M., Gherardi, E., Perryman, M., and Gray, J. (1987). Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327, 239–242. [DOI] [PubMed] [Google Scholar]
- ten Hoeve, J., Morris, C., Heisterkamp, N., and Groffen, J. (1993). Isolation and chromosomal localization of CRKL, a human crk-like gene. Oncogene 8, 2469–2474. [PubMed] [Google Scholar]
- Turner, C.E. (2000). Paxillin and focal adhesion signalling. Nat. Cell Biol 2, E231–E236. [DOI] [PubMed] [Google Scholar]
- Turner, C.E., Brown, M.C., Perrotta, J.A., Riedy, M.C., Nikolopoulos, S.N., McDonald, A.R., Bagrodia, S., Thomas, S., and Leventhal, P.S. (1999). Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 145, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C.E., West, K.A., and Brown, M.C. (2001). Paxillin–ARF GAP signaling and the cytoskeleton. Curr. Opin. Cell Biol. 13, 593–599. [DOI] [PubMed] [Google Scholar]
- Uchida, H., Kondo, A., Yoshimura, Y., Mazaki, Y., and Sabe, H. (2001). PAG3/Papalpha/KIAA0400, a GTPase-activating protein for ADP-ribosylation factor (ARF), regulates ARF6 in Fcgamma receptor-mediated phagocytosis of macrophages. J. Exp. Med. 193, 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata, M., et al. (1997). Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994. [DOI] [PubMed] [Google Scholar]
- Uemura, N., and Griffin, J.D. (1999). The adapter protein Crkl links Cbl to C3G after integrin ligation and enhances cell migration. J. Biol. Chem. 274, 37525–37532. [DOI] [PubMed] [Google Scholar]
- Webb, D.J., Parsons, J.T., and Horwitz, A.F. (2002). Adhesion assembly, disassembly and turnover in migrating cells - over and over and over again. Nat. Cell Biol. 4, E97–E100. [DOI] [PubMed] [Google Scholar]
- Weidner, K.M., Behrens, J., Vandekerckhove, J., and Birchmeier, W. (1990). Scatter Factor: Molecular characteristics and effect on the invasiveness of epithelial cells. J. Cell Biol. 111, 2097–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner, K.M., Sachs, M., and Birchmeier, W. (1993). The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J. Cell Biol. 121, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, K.A., Zhang, H., Brown, M.C., Nikolopoulos, S.N., Riedy, M.C., Horwitz, A.F., and Turner, C.E. (2001). The LD4 motif of Paxillin regulates cell spreading and motility through an interaction with Paxillin kinase linker (PKL). J. Cell Biol. 154, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, T., and Waterman-Storer, C.M. (2001). Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795–3803. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Calafat, J., Janssen, H., and Greenberg, S. (1999). ARF6 is required for growth factor- and rac-mediated membrane ruffling in macrophages at a stage distal to rac membrane targeting. Mol. Cell. Biol. 19, 8158–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z.S., Manser, E., Loo, T.H., and Lim, L. (2000). Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol. 20, 6354–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H., Naujokas, M.A., and Park, M. (1994). Receptor chimeras indicate that the Met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth/scatter factor. Cell Growth Differ 5, 359–366. [PubMed] [Google Scholar]