Abstract
We have recently shown that the pancreatic bile salt–dependent lipase (BSDL) can be taken up by intestinal cells and transported to the blood circulation. This mechanism likely involves (specific) receptor(s) able to bind BSDL and located at the apical intestinal cell membrane. In this study, using Int407 human intestinal cells cultured to form a tight epithelium, we attempted to characterize (the) BSDL receptor(s). We found that an apical 50-kDa protein was able to bind BSDL. Further, we have demonstrated that Int407 cells expressed the lectin-like oxidized-LDL receptor (LOX-1), the upregulation of which by oxidized-LDL potentiates the transcytosis of BSDL, whereas carrageenan and to a lesser extent polyinosinic acid and fucoidan decrease the enzyme transcytosis. The mAb JTX92, which blocks the LOX-1 receptor function, also impaired the BSDL transcytosis. To confirm these results, the cDNA encoding the human intestinal receptor LOX-1 has been cloned, inserted into vectors, and transfected into Int407 cells. Overexpression of LOX-1 by these cells leads to a substantial increase in the BSDL transcytosis. Globally, these data support the view that LOX-1 could be an intestinal receptor for BSDL, which is implicated in the transcytosis of this enzyme throughout Int407 cells.
INTRODUCTION
The bile salt–dependent lipase (BSDL, EC 3.1.1.13), also referred to as carboxyl ester lipase or bile salt–stimulated lipase, is a lipolytic enzyme, synthesized and secreted by the exocrine pancreas (Lombardo, 2001). The secretion of this digestive enzyme requires the participation of the Grp94 molecular chaperone (Bruneau and Lombardo, 1995; Bruneau et al., 1998). Once in the lumen of the duodenum, BSDL, in concert with other pancreatic lipolytic enzymes and preduodenal lipase, acts to complete the digestion of dietary lipids. Indeed, upon activation by primary bile salts, BSDL catalyzes the duodenal hydrolysis of cholesterol esters into free cholesterol and fatty acids before their absorption by intestinal cells (Howles et al., 1996; Lombardo et al., 1980; Lombardo and Guy, 1980; Weng et al., 1999). Although controversial (Howles et al., 1996), BSDL could also participate in the intestinal free cholesterol absorption (Lopez-Candales et al., 1993), acting as a cholesterol transfer protein (Myers-Payne et al., 1995), or as a cholesterol reesterifying enzyme after cholesterol absorption by intestinal cells (Bhat and Brockman, 1982).
A fraction of BSDL, possibly associated with Grp94, reaches the vicinity of microvilli and upon binding to the surface of intestinal cells is internalized and transported up to the blood compartment (Bruneau et al., 1998, 2000a, 2000b, 2003). Consequently, BSDL has been reported in the plasma of normolipidemic patients (Lombardo et al., 1993) where it associates with apolipoprotein B-containing lipoproteins such as low-density lipoprotein (LDL), chylomicron and very-low-density lipoprotein (VLDL; Bruneau et al., 2003).
The pathway of BSDL throughout intestinal cells has been recently delineated using Int407 cells (Bruneau et al., 2001), which do not express BSDL. When added to the apical reservoir of Transwell-grown Int407 cells, BSDL was shown to first interact with the apical membrane. Further, BSDL forms clusters that are internalized via clathrin-coated pits. After endocytosis, BSDL is directed to a multivesicular compartment, and then the protein transited through the Golgi apparatus where it colocalized with the KDEL retrieval-receptor. Finally, enzymatically active intact BSDL that had experienced this transcytotic motion was released at the basolateral membrane level. The transit of BSDL throughout the Golgi compartment is compatible with the involvement of the enzyme in the assembly and secretion of chylomicrons (Kirby et al., 2002) and the association of BSDL with these lipoproteins after intestinal transcytosis (Bruneau et al., 2003).
Although a receptor for BSDL on intestinal cell membranes has not been identified, the initial interaction of the protein with CaCo-2 human intestinal cells is mediated by its binding to putative low-affinity and high-capacity binding sites (Huang and Hui, 1990). Structural elements of BSDL potentially implicated in its interaction with a receptor present at the apical membrane of enterocytes are multiple. These are as follows: 1) the heparin-binding site present on BSDL, which could recognize heparin-like molecules lining microvilli membranes (Bosner et al., 1988); 2) N-linked glycan structures that can bind to mannose/fucose lectin receptors (Sallusto et al., 1995); and 3) finally, O-linked mucin-type structures of the C-terminal domain of BSDL (Wang et al., 1995) susceptible to bind lectin-like receptor or ligands such as selectins. It is therefore conceivable that receptor(s) present at the apical surface of intestinal cells could be involved in BSDL uptake by enterocytes (Bruneau et al., 2001, 2003). The goal of this study was to characterize (a) receptor(s) involved in the transcytosis mechanism of BSDL using the human intestinal Int407 cells as model. Data support the view that the lectin-like oxidized-LDL receptor (LOX-1) is, at least partly, implicated in the transcytosis of BSDL throughout intestinal cells.
MATERIALS AND METHODS
Reagents
Human transferrin, FITC-conjugated goat anti-rabbit IgG antibodies, FITC-labeled antibodies to goat IgG, peroxidase-labeled antibodies to biotin, heparan sulfate from bovine intestine (∼7500 Da), dithiobissuccinimidyl propionate, lectins and ligands used in this study were obtained from Sigma (St. Louis, MO). Heparin sodium salt from bovine intestine (∼3000 Da) was from Fluka (L'Isle-D'Abeau-Chesnes, France). [35S]methionine (trans-35S label; >1000 Ci/mmol) was from ICN Biochemicals (Costa Mesa, CA). Affinity-purified rabbit polyclonal antibodies against human pancreatic BSDL (pAbL64) were homemade (Abouakil et al., 1988). An mAb (mAbJ28) to the fucosylated J28 epitope of BSDL (Mas et al., 1997) was a gift of Dr. M-J. Escribano (INSERM, Marseilles, France). Mouse monoclonal antibodies to transferrin receptor (CD71, clone H68.4) were from Zymed (San Francisco, CA). Rabbit polyclonal antibodies to LDL receptor and to Grp94 were from Progen (Heidelberg, Germany) and StressGen (Victoria, Canada), respectively. Goat polyclonal antibodies to the N-terminal (N-14) and to the C-terminal (E-19) domain of the lectin-like oxidized-LDL receptor (LOX-1) were from Santa Cruz Biotechnology (Santa Cruz, CA). A functional blocking antibody JTX92 was provided by Dr. T. Sawamura (Osaka, Japan). Human native LDLs were isolated from pooled fresh sera by sequential ultracentrifugation, dialyzed, sterilized by filtration, and stored under nitrogen (Augé et al., 1999). LDLs were acetylated (Ac-LDL) according to standard procedures (Goldstein et al., 1980). Mildly oxidized (Ox)-LDLs were prepared by UV-C irradiation (Augé et al., 1999). Human BSDL was isolated from human pancreatic juice (Lombardo et al., 1978). Porcine pancreatic Grp94 was a gift from Dr. C. Nicchitta (Durham, NC).
Human BSDL Immobilization
Human BSDL was immobilized on CNBr-activated Sepharose (1 mg of protein/0.5 g of wet-gel) as described (Bruneau and Lombardo, 1995). Before use, the gel was alternatively washed with basic (0.1 M sodium phosphate, pH 8.0) and acidic (0.1 M Mes, pH 5.5) buffers.
Cell Culture and Metabolic Labeling
Human intestinal Int407 cells (ECACC Nb:85051004) were cultured in Eagle's minimum essential medium (EMEM, Invitrogen, Cergy Pontoise, France), supplemented with 2 mM glutamine, 10% (vol/vol) fetal calf serum (FCS), 1% (vol/vol) essential amino acids. For each experiment, cells were plated at a density of 2 × 105 cells per well on 12-mm polycarbonate permeable supports (Transwell filter inserts, 0.4-μm pore sizes, Corning Costar, Issy-les-Moulineaux, France) for 5 d, to obtain a tight monolayer (Bruneau et al., 2001). Metabolic labeling of proteins was performed on cell grown in methionine-free DMEM for 45 min. Once starved, cells were pulse-labeled with [35S]methionine (20 μCi/ml) for 6 h and then washed with complete PBS.
Chemical Cross-linking
Int407 cells grown on Transwell inserts were washed with the FCS-free EMEM and incubated with BSDL (100 nM) for 15 min at 4°C. Cells were then washed with complete PBS and subjected to chemical cross-linking (30 min, 4°C) by the addition of 1 mM dithiobissuccinimidyl propionate (DSP) in PBS. The cross-linking reaction was terminated by addition of 10 mM glycine (10 min, 4°C), and cells were washed twice with cold PBS. Cells were then prepared either for immunofluorescence microscopy or for Western blotting. For immunofluorescence microscopy, after washing cells were fixed and incubated with PBS, bovine serum albumin 1% (PBS-BSA) for 30 min and further incubated for 120 min at room temperature with pAbL64 in PBS-BSA. Cells were washed and incubated with FITC-anti-IgG antibodies (diluted 1/100 in PBS-BSA). Finally, cells were washed with PBS, and filters were mounted on glass slides in the Dabco 10%, glycerol 50%, in PBS. Cells were photographed using a fluorescence microscope (BHR2-RFCA Olympus, Hamburg, Germany). For Western blotting, cells were harvested and lysed in lysis buffer (10 mM HEPES, pH 7.4, buffer, 200 mM NaCl, 2 mM EDTA, and 1.5% Triton X-100, 10 μg/ml leupeptin, 2 mM benzamidine, PMSF, Soya-bean trypsin inhibitor and β-phenyl propionate [2 mM each]). Cell lysate was cleared by centrifugation (20 min, 5000 × g, 4°C). The lysate was electrophoresed, and BSDL complexes were detected by Western blotting using pAbL64 antibodies.
Biotinylation of Apical Cell Surface Proteins
Monolayers of Int407 cells cultured on Transwell inserts, were washed with PBS and incubated for 1 h at 4°C with 1 mg/ml N-hydroxysuccinimide-long chain-biotin (NHS-LC-biotin, Pierce, Rockford, IL) in sodium bicarbonate, pH 8.0 buffer added in the apical reservoir. At the end of the incubation, the biotinylation agent was removed and the reaction stopped with 0.1 M glycine (4°C, 15 min), cells were washed with PBS and lysed in the Sepharose-immobilized-BSDL column loading buffer (5 mM Mes pH6.0 buffer, 0.5% Nonidet P-40, 0.1 M NaCl and protease inhibitors) by sonication (15 s, 4 W). The lysate was centrifuged (20 min, 5000 × g, 4°C) and chromatographed on the Sepharose-immobilized BSDL column.
Effects of Various Ligands on the Transepithelial Movement of BSD
Int407 cells were cultured on Transwell inserts, washed twice with FCS-free EMEM, and preincubated with ligands, lectins, or antibodies in FCS-free medium for 1 h in an apical reservoir. Cells were incubated for another 3 h with ligands, lectins, or antibodies in the presence of pancreatic BSDL (100 nM) in the apical reservoir, whereas fresh FCS-free EMEM medium was added in the basolateral reservoir. Alternatively, a preincubation of BSDL (100 nM) with ligands, lectins, or antibodies, followed by the incubation of the putative complexes thus formed, with cells in the apical reservoir was performed. At the end of the incubation apical and basolateral media were collected and used for determination of BSDL (activity and Western blotting).
PAGE and Western Blotting
Gel electrophoreses (SDS-PAGE) were performed according to Laemmli (1970). After SDS-PAGE, proteins were electrophoretically transferred onto a nitrocellulose membrane (Burnette, 1981). Western blottings were performed using pAbL64 antibodies to BSDL. The antigen-antibody complexes were detected using chemiluminescence (Roche, Mannheim, Germany). Quantitations of protein were performed by scanning fluororadiogram using NIH Image program.
Enzyme Assays
The BSDL activity was determined on 4-nitrophenyl hexanoate (Gjellesvik et al., 1992).
One-cycle Immunoprecipitation and Recapture of LOX-1 by BSDL
Radiolabeled 35S-labeled proteins eluted from the Sepharose-immobilized BSDL column were used for immunoprecipitation. For one-cycle immunoprecipitation the eluted material was incubated overnight (4°C) in presence 20 μg of antibodies to LOX-1 receptor (antibodies N-14 and E-19, 1/1 by vol mix). Prewashed protein A- and protein G-Sepharose (5 mg each) were added to antibody-antigen complexes and incubated under agitation (4 h, 4°C). The antigen-antibody protein-A/G complexes were then recovered by centrifugation (10,000 × g, 20 min). The pellet was washed twice successively with the washing buffer (10 mM Tris/HCl buffer, pH 7.4, 25 mM EDTA, and 1% Triton X-100), with the washing buffer supplemented with 1 M NaCl, with the last buffer supplemented with 0.1% SDS and with 10 mM Tris/HCl buffer, pH 7.4, containing 5 mM EDTA. Recapture of immunoprecipitated material was performed using E-19 and N-14 antibodies to LOX-1 receptor coupled with the gel of the Seize primary immunoprecipitation kit (Pierce, Brebières, France). For this purpose radiolabeled cell proteins were subjected to immunoprecipitation with E-19–or N-14–coupled gel. After elution the immunoprecipitated material was recaptured by incubation with BSDL (100 nM) or without BSDL (control) for 4 h, and additional pAbL64 antibodies to BSDL were added and incubated overnight (4°C). Protein A-Sepharose (10 mg) prewashed with the binding buffer was incubated with receptor-ligand antibody complexes for another 4 h. At the end of incubation, these complexes were recovered by centrifugation (10,000 × g, 15 min, 4°C). The pellet was then washed twice with the washing buffer (10 mM Tris/HCl, pH 7.4, buffer, 25 mM EDTA, and 1% Triton X-100), twice with the washing buffer supplemented with 1 M NaCl, and twice again with 10 mM Tris/HCl, pH 7.4, 5 mM EDTA.
Pellets obtained in both experiments were transferred into the Laemmli's sample buffer (Laemmli, 1970), boiled for 2 min, centrifuged, and electrophoresed on SDS-PAGE. Gels were stained with Coomassie blue R250, destained in ethanol/acetic acid water (2/3/35 by volume), and autoradiographed using BioMax MS FILM (Eastman Kodak, Rochester, NY).
Reverse Transcription and PCR
Total mRNA was isolated with Trizol (Invitrogen) from Int-407 cells. RNA (1 μg) was reverse-transcribed (RT) with oligo-dT primers using the M-MLV reverse transcriptase (Promega, Madison, WI), following manufacturer instructions. RT material (5 μl) was amplified by PCR with Taq DNA polymerase (Ozyme, St. Quentin/Yvelines, France) using forward primer, (5′-TCTATCATTCTTAgCTTgAATTTggAAATg-3′) and reverse primer, (5′-TCACTgTgCTCTTAggTTTgCCTT-3′) specific to LOX-1 (Genset, Evry, France). For PCR, 30 cycles were used at 94°C for 40 s, 55°C for 1 min, and 72°C for 1 min. The RT-PCR fragment (∼0.9 kb) was then cloned in the pCR-II TOPO plasmid (Invitrogen) and sequenced. The sequence of this transcript matched totally that of the cDNA encoding human LOX-1 receptor (Adachi et al., 1997). This cDNA was subcloned into pBK vector (Stratagene, Amsterdam, The Netherlands) and named pBK-LOX-1 and alternatively into pEGFP-N1 (Clontech, Saint Quentin Yvelines, France), without fusion of LOX-1 with the EGFP, to give pLOX-1.
Expression of LOX-1 Receptor
Stable transfection of Int407 cells was performed using a lipopolyamine-mediated transfection procedure according to the manufacturer (Lipofectamine, Invitrogen). The selection of stable clones was performed for 3 weeks in selection EMEM-medium with G418 (500 μg/ml medium). The surviving cells were trypsinized and cloned by end-limiting dilution method. Stably transfected clones were maintained under the same selection conditions. Two positive clones, one referred to as Int-407 clone 14 (cells transfected with pLOX-1) and another referred to as Int-407 clone 2 (cells transfected with pBK-LOX-1) were selected for further experiments.
Flow Cytometry
Detection of LOX-1 at the surface of Int407 cells was carried out by indirect fluorescence. Cells were released from culture plates by treatment with nonenzymatic cell dissociation solution (Sigma) for 15 min at 37°C. All subsequent steps were carried out at 4°C. The cells were washed three times in PBS, fixed with 2% paraformaldehyde in PBS for 10 min, and washed extensively with PBS-BSA. Int407 cells were incubated with E-19 antibodies to the extracellular domain of the LOX-1 receptor for 1 h, washed three times with PBS, and finally incubated for 30 min with FITC-labeled secondary antibodies. The cells were then washed, resuspended in Isoflow buffer, and analyzed on an EPICS Profile II flow cytometer (Coulter, Hialeah, FL).
RESULTS
Interaction of Pancreatic BSDL with Int407 Cell Membrane Proteins
In a recent study, we have shown that BSDL is transported from the apical to basolateral pole of Int407 cells. This pancreatic protein seems to be specifically internalized by receptor-mediated endocytosis via clathrin-coated pits and seems to be excluded from other endocytic pathways such as those involving caveolae (Bruneau et al., 2001). The intestinal human cell line Int407 constitute a potent epithelial intestinal cell model to characterize a putative receptor involved in the transcytosis of BSDL.
We have first attempted to demonstrate the association of BSDL with proteins present at the apical membrane of Int407 cells. For this purpose, cell monolayers were grown on a Transwell insert to obtain a tight epithelium and incubated with human BSDL for 15 min at 4°C. At the end of the incubation period, cells were exhaustively washed with complete PBS and subjected to chemical cross-linking with DSP. In control experiments, DSP was omitted. Once the cross-linking reaction was terminated, cells were washed with complete PBS and incubated with pAbL64 antibodies, followed by FITC-labeled anti-IgG and examined for immunofluorescence. As shown in Figure 1A, no fluorescence can be detected on control cell membranes, whereas a strong reactivity was obtained with cells that have experienced the chemical cross-linking. Thus BSDL can be coupled to proteins present at the membrane of Int407 cells. These cells were then harvested and lysed, and proteins were resolved by SDS-PAGE. After transfer onto nitrocellulose membranes, blots were probed with pAbL64. As shown in Figure 1B, BSDL can be detected in cell lysates independently of the presence of the cross-linker. However, when DSP was present in the incubation of cells with BSDL, two extra bands with lower migration than BSDL were detected. These bands correspond to proteins of ∼200 and 150 kDa. Although the upper band could correspond to cross-linked BSDL dimers, that migrating at 150 kDa might be formed of human BSDL monomer (100 kDa) coupled to a protein of ∼50 kDa.
Figure 1.
Cross-linking of BSDL with apical membrane protein. (A) Int407 cells grown on Transwell insert were incubated with BSDL (100 nM), extensively washed, subjected to chemical cross-linking in the presence (+) or absence (–) of cross-linker (DSP), and once again extensively washed with PBS. Cells were then treated for immunofluorescence analysis using pAbL64 as primary antibodies and FITC anti-rabbit IgG as second antibodies. (B) Int407 cells incubated as in A were lysed, and the cleared homogenate was then analyzed on SDS-PAGE and Western blotting using pAbL64 and chemiluminescence Western blotting kit.
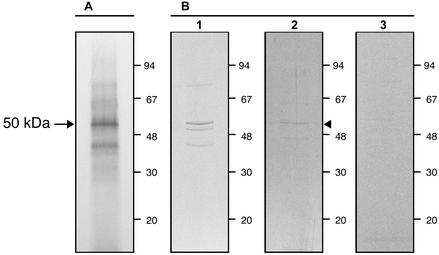
To confirm these results and to further attempt to locate the 50-kDa protein, Int407 cells were grown in the presence of [35S]methionine and lysed. The cell lysate was clarified by centrifugation and chromatographed on an affinity column made of BSDL-immobilized on Sepharose beads to isolate proteins having an affinity for the enzyme. After elution of unbound material and washing out of the column with the lysis buffer, bound proteins were tentatively eluted, first with 0.1 M sodium acetate, pH 4.0, buffer (buffer A, Figure 2A) and second with the buffer A supplemented with Nonidet P-40 (1%; buffer B, Figure 2A). 35S-methionine–labeled material, eluted with buffer A and B, was pooled to give pool 1 and pool 2, respectively, then concentrated, analyzed on SDS-PAGE, and autoradiographed. Alternatively, the buffer B supplemented with 3 mg/ml heparin was used for elution and eluted fractions were pooled to give pool 3. As shown on Figure 2B, one band representing 30% of the material and migrating at ∼50 kDa was detected in fractions eluted with buffer B, along with two other bands around 30 kDa (pool 2, lane 2). However only the 50-kDa protein was eluted with buffer B supplemented with heparin (pool 3, lane 3). No trace of this material was detected in the material eluted with buffer A (pool 1, lane 1). Therefore, BSDL seems to form a complex with a 50-kDa protein, and the complex, which is insensitive to acidic pH, is dissociated in the presence of detergent or heparin. To further determine whether this 50-kDa protein locates at the apical membrane of Int407 cells, a biotinylation of cell surface proteins was performed on cells cultured in Transwell inserts to form a tight epithelium. After biotinylation, cells were extensively washed and lysed, and the cleared lysate was chromatographed on the affinity column. The bound material was eluted with buffer B and analyzed by Western blotting using peroxidase-conjugated antibodies to biotin. Although many cell surface proteins were biotinylated (unpublished data), only three proteins interacted with immobilized BSDL (Figure 2B, lane 4). The two proteins with the lower migration (∼90 and 150 kDa) could be aggregates of the third one, the migration of which coincides with the 50-kDa protein. Because only apical proteins can be biotinylated under conditions used, one has to conclude that the 50-kDa protein that binds BSDL is a protein located at the apical membrane of Int407 cells. This protein could represent a specific receptor involved in the uptake of BSDL by intestinal cells (Huang and Hui, 1990; Bruneau et al., 2001).
Figure 2.
Characterization of a putative intestinal receptor for BSDL. Int407 cells were metabolically labeled with [35S]methionine (20 μCi/ml) for 6 h and lysed. Radiolabeled cell lysate was chromatographed on Sepharose-immobilized-BSDL column preequilibrated with the washing buffer (5 mM MES pH 6.0, buffer). After loading of radiolabeled proteins, the column was washed with 5 mM MES, pH 6.0, buffer with 0.5% Nonidet P-40, 0.1 M NaCl, and 1 mM EDTA to eliminate unbound material and with the washing buffer. Bound material was then eluted with 0.1 M sodium acetate, pH 4.0, buffer (Buffer A, pool 1) or with buffer A supplemented with 1% Nonidet P-40 (Buffer B, pool 2). Eluted radioactive material was concentrated and electrophoresed on SDS-PAGE (1% SDS, 7.5% polyacrylamide) before autoradiography. (A) Chromatogram of the material eluted from the Sepharose-immobilized BSDL column loaded with 35S-methionine–labeled Int407 cell proteins. (B) SDS-PAGE and autoradiogram of the material bound on Sepharose immobilized-BSDL column; lane 1, material eluted with buffer A (pool 1); lane 2, material eluted with buffer B (pool 2). Lane 3 displays the radiolabeled material eluted with buffer B supplemented with 3 mg/ml heparin. Lane 4 shows the material eluted with buffer B after biotinylation of apical cell surface proteins, revealed using chemiluminescence Western blotting kit with peroxidase-conjugated antibiotin antibodies.
BSDL Transcytosis and Ligand Competition
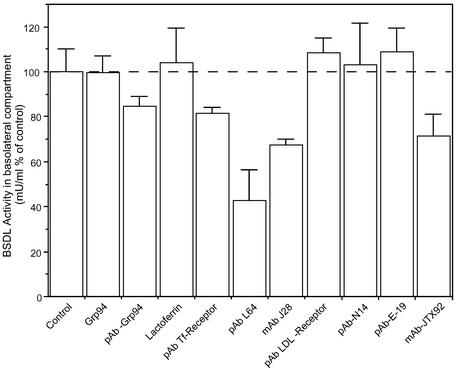
We furthered this study by determining ligand specificity of the intestinal receptor of BSDL. The enzyme has been shown to associate with the molecular chaperone Grp94 (Bruneau et al., 1998, 2000b) and with transferrin (Erlanson-Albertsson et al., 1985) in pancreatic and duodenal fluids. Therefore, BSDL may be taken up by receptors that internalize Grp94 or transferrin. To determine whether these companion proteins are involved in the BSDL uptake by and transcytosis throughout Int407 cells, we incubated the human enzyme with either Grp94 (10 μg/ml) or transferrin (10 μg/ml) in FCS-free medium for 30 min at 37°C to restore in vitro eventual complexes. Then the mixtures were added in the apical reservoir and incubated for 3 h; thereafter the amount of BSDL that had transcytosed up to the bottom reservoir was determined by recording the enzyme activity. Any of the ligands used had an effect on the BSDL activity. As illustrated in Figure 3 and in the conditions used, neither Grp94 nor transferrin affected the amount of transcytosed BSDL. Also, a 30-min preincubation of Int407 cells with 10 μg/ml antibodies specific to Grp94 or to transferrin receptor (which is expressed by Int407 cells; unpublished data) had no significant effect on BSDL transcytosis. Only specific polyclonal pAbL64 and monoclonal mAbJ28 antibodies to BSDL significantly decrease the transcytosis of the enzyme throughout Int407 cells.
Figure 3.
Effect of proteins and antibodies on BSDL transcytosis. Int407 cells were grown on Transwell inserts to obtain a tight epithelium. BSDL (10 μg/ml or 100 nM) was independently incubated for 30 min (37°C under gentle agitation) in the culture medium with 10 μg/ml Grp94 or transferrin. Alternatively antibodies (10 μg/ml) were preincubated with cells for 30 min in the culture medium. Then cell culture medium of the apical reservoir was removed and replaced by fresh medium containing BSDL and Grp94 or transferrin, whereas BSDL (100 nM) was directly added into the apical compartment of cells preincubated with either antibodies. After 3-h incubation, the medium of the bottom reservoir was removed, and the amount of BSDL evaluated from its activity. Values are expressed as percent of control performed in the absence of companion protein or ligand (control) or in the presence of unrelated antibodies (control not shown). Values are means ± SD of at least three independent experiments.
These data indicated that under our experimental conditions, the transcytosis of BSDL may neither implicate the Grp94 protein and the Grp94-receptor (Wassenberg et al., 1999) nor be mediated via the transferrin receptor (Dautry-Varsat et al., 1983; Marsh et al., 1995). Therefore, only antibodies to BSDL impaired the transcytosis of the protein possibly by binding to structural elements involved in the interaction of the protein with its intestinal receptor.
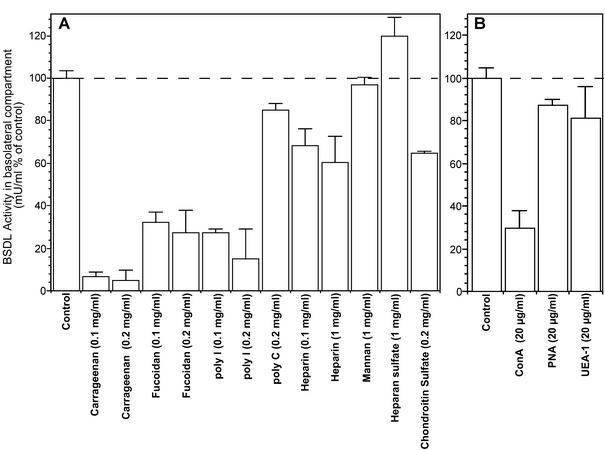
These structural elements can be the N- and O-linked glycans of BSDL, which bore epitopes recognized by pAbL64 and mAbJ28 and that carry out oligomannosidic and fucosylated structures (Sugo et al., 1993; Mas et al., 1993; Wang et al., 1995, 1997). The heparin-like binding site of the enzyme (Wang and Hartsuck, 1993) could also be implicated. We thus investigated whether these structural elements are involved and further attempted to characterize ligand specificity of the BSDL putative intestinal receptor. For this purpose the effects of various ligands, which have been shown to hinder glycoprotein interaction with specific receptors or to inhibit oxidized-LDL binding to class A and B scavenger receptors and to the lectin-like Ox-LDL receptor (LOX-1; Moriwaki et al., 1998; Shirai et al., 1999), were examined. First of all, to rule out the possibility that BSDL interacted with the cell via the mannose/fucose lectin receptor (Avraméas et al., 1996), we performed competition experiments using mannan as a competitor (Arnold-Schild et al., 1999). No inhibitory effect was observed using this ligand at a concentration of 1 mg/ml (Figure 4A), whereas 0.3 mg/ml mannan was shown to inhibit completely the uptake of FITC-dextran by the mannose/fucose lectin receptor of dendritic cells (Sallusto et al., 1995). Heparin (0.1 and 1 mg/ml) partially decreased the amount of BSDL present in the bottom reservoir, whereas neither heparan sulfate (1 mg/ml) nor chondroitin sulfate (200 μg/ml) had important effect on BSDL transcytosis (Figure 4A). Other carbohydrate compounds used at lower concentration (100 μg/ml) such as kappa-carrageenan (a polysaccharide extracted from the lichen Eucheuma cottonii and composed predominantly of sulfated galactose residues) quite totally annihilates the transcytosis of BSDL. Fucoidan (a polysaccharide extracted from the brown algae Fucus vesiculosus and composed predominantly of sulfated fucose residues) at comparable concentration is a little less efficient than carrageenan in inhibiting the enzyme transcytosis. Polyanionic nucleic acid such as polycytidylic acid had also no effect, albeit the four-stranded polyinosinic acid had an inhibitory effect at either 100 or 200 μg/ml. This study was completed by determining the effects of various lectins on BSDL transcytosis. For this purpose monolayers of Int407 cells were preincubated with lectin (20 μg/ml in the culture medium) applied for 1 h in the apical reservoir. The culture medium was then removed, and BSDL (100 nM) was added with lectin in the apical reservoir. After 3-h incubation the amount of transcytosed BSDL was determined in the basolateral reservoir. As shown on Figure 4B, only concanavalin A (ConA), which primarily recognizes oligomannosidic structures, decreased by some 70% the transcytosis of BSDL, whereas peanut agglutinin (PNA) and Ulex europeaus I lectin (UEA-I) had a poor effect. These two lectins bound to O-linked glycans and to fucose residues α (1–2) linked to galactose, respectively.
Figure 4.
Effect of various ligands on BSDL transcytosis. (A) Int407 cells were cultured as described in the legend of Figure 3. Cell culture medium of the apical reservoir was removed at time 0 and replaced by media containing BSDL (100 nM) and ligands at the final concentration as indicated. (B) Int407 cells were separately preincubated for 1 h with each lectin (20 μg/ml), and after removal of the culture medium, Int407 cells were further incubated for 3 h with fresh medium containing BSDL (100 nM), and lectin was applied in the apical reservoir. (Con A, concanavalin A; PNA, peanut agglutinin; UEA-1 = Ulex Europeaus-1 lectin). After 3-h incubation, medium of the bottom reservoir of each insert was removed, and the amount of transcytosed BSDL was evaluated from its activity. Values are expressed as percentage of control performed in the absence of ligand (control) and are means ± SD of at least three independent experiments.
These data suggest that the uptake of BSDL that precedes its transcytosis throughout Int407 cells did not involve the mannose/fucose lectin receptor, which is implicated in the macropinocytosis of glycosylated macromolecules (Arnold-Schild et al., 1999). Nevertheless, it seems evident that carbohydrate structures of BSDL are implicated in the interaction of the enzyme with its receptor. Furthermore, the inhibition of BSDL transcytosis promoted by reagents used above also suggest that class A or B scavenger receptors or LOX-1 could be, at least partly, implicated in the BSDL uptake by Int407 cells. Many facts indicate that these receptors could be actually involved in BSDL interaction with intestinal cell membranes. First, scavenger receptors, in general, have been shown to bind polysaccharides (Hampton et al., 1991). Second, class B scavenger receptors were located in intestine (Cai et al., 2001) where they catalyze cholesterol uptake (Hauser et al., 1998). Third, BSDL, which is involved in cholesterol absorption (Lombardo, 2001), is also susceptible to associate with lipoprotein structures such as LDL (Caillol et al., 1997), receptors of which are also expressed by intestinal cells (Fong et al., 1995).
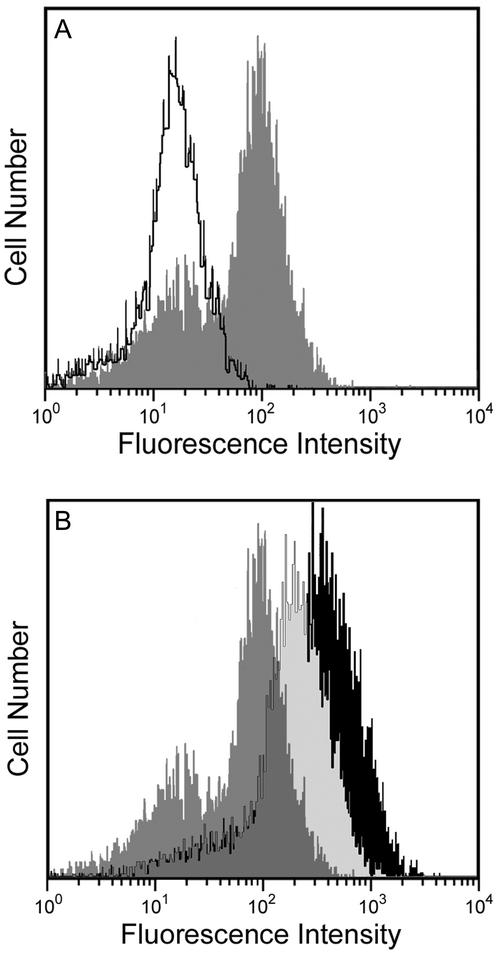
All these results suggest that the putative intestinal receptor of BSDL is a 50-kDa protein that recognizes glycan structures. This receptor could be a lectin-like receptor. The 50-kDa receptor LOX-1 has been characterized as such lectin-like receptor (Metha and Li, 2002); therefore we focused on this receptor. First, we determined whether the intestinal cell line Int407 synthesized LOX-1 and expressed the receptor at their cell surface. The cell surface expression of this receptor was analyzed by flow cytometry using E-19 antibodies to the extracellular domain of LOX-1 receptor. As shown in Figure 5A, compared with control in which these primary antibodies were omitted, a net increase in fluorescence was observed when Int407 cells were treated with E-19 antibodies. This result strongly suggests that LOX-1 receptor is expressed at the apical surface of Int407 grown to form a tight epithelium. Second, the radioactive material eluted with the buffer B in Figure 2 has been further immunoprecipitated with a vol/vol mixture of E-19 and N-14 antibodies to LOX-1. The material thus immunoprecipitated was resolved on SDS-PAGE and autoradiographed. As shown in Figure 6A, a main band representing a protein of ∼50 kDa can be detected, suggesting that this protein interacting with the Sepharose-immobilized BSDL is actually LOX-1. Finally, recapture experiment was performed (Figure 6B). When radiolabeled cell proteins were immunoprecipitated with antibodies to LOX-1 (E-19 and N-14 coupled to Seize gel), eluted, and analyzed on SDS-PAGE, three main bands were detected (Figure 6B, lane 1). The material eluted, using pH 2.8 glycine buffer and neutralized with Tris/HCl buffer (pH 9.5) to avoid denaturation as specified in the protocol of the immunoprecipitation kit, was further recaptured by incubation with BSDL and material susceptible to bind BSDL was then immunoprecipitated with pAbL64. Results of this recapture cycle showed that only one protein of ∼50 kDa can be specifically isolated (Figure 6B, lane 2, arrowhead). These data indicated that the 50-kDa protein immunoprecipitated with antibodies to LOX-1 recognized BSDL. Control omitting BSDL in recapture experiment did not allow isolating the 50-kDa protein (Figure 6B, lane 3).
Figure 5.
Flow cytometry analysis of LOX-1 antigenic sites on Int407 cells. Cultured Int407 cells were released from culture plates by nonenzymatic dissociation solution and exhaustively washed with PBS. Cells were incubated with the E-19 antibodies directed to the extracellular domain of LOX-1, followed by two washes with PBS and then incubated with FITC-labeled anti-goat IgG antibodies. After washing, cells were suspended in Isoflow buffer and analyzed by flow cytometry. Controls were performed by omitting E-19 primary antibodies. (A) The open peak represents the histogram of control Int407 cells, and the solid peak is representative of histograms obtained with Int407 cells incubated with E-19 antibodies. (B) Int407 cells transfected with plasmids including the cDNA sequence encoding LOX-1 were also analyzed by flow cytometry as described above, using E-19 antibodies. The shaded peak represents the histogram obtained with the wild-type Int407 cells; the light-shaded and the black peak represent the histogram obtained with clone 14 and clone 2 of transfected Int407 cells, respectively.
Figure 6.
One-cycle immunoprecipitation and recapture of LOX-1 by BSDL.Int407 cells were grown on Transwell inserts to obtain a tight epithelium, starved in methionine-free DMEM, and pulse-labeled with [35S]methionine (20 μCi/ml, 6 h). Cells were then lysed, and lysate was clarified by centrifugation (20 min, 10,000 × g,4°C). (A) The clarified cell lysate was chromatographed on Sepharose-immobilized BSDL column (see Figure 2); then the material eluted with buffer B was immunoprecipitated with antibodies (N-14 and E-19, 1-to-1 mix) to LOX-1 receptor and resolved on SDS-PAGE, followed by autoradiogram. (B) Radiolabeled proteins of Int407 cells were subjected to the two-cycle immunoprecipitation procedure using E-19 and N-14 antibodies to LOX-1 coupled to the Seize gel. The radiolabeled material bound to antibodies was eluted and either separated on SDS-PAGE and autoradiographed (lane 1) or recaptured by incubation with BSDL (100 nM, 4 h), and the material interacting with the enzyme was immunoprecipitated with antibodies to BSDL (pAbL64) and analyzed on SDS-PAGE as above (lane 2). Control experiment was performed by omitting BSDL in the recapture experiment (lane 3).
To define the involvement of LOX-1 in the BSDL transcytosis, monolayers of Int407 cells were incubated for 1 h with polyclonal antibodies (N-14 or E-19) directed against LOX-1 or with mAb JTX92 (described as a blocking antibody for the LOX-1 receptor function) or with antibodies to the LDL-receptor applied in the apical reservoir. Cells were then washed and BSDL added in fresh medium containing each antibodies. After 3-h incubation under standard conditions, the amount of transcytosed BSDL was determined by recording the enzyme activity in the basolateral reservoir. As shown on Figure 3, although antibodies to the LDL receptor or to LOX-1 (N-14 or E-19) were ineffective in inhibiting the transcytosis of the enzyme, mAb JTX92 decreased the transport of BSDL throughout Int407 cells.
These data demonstrated that the LOX-1 receptor is actually expressed at the apical surface of Int407 cells and that LOX-1 may interact with BSDL and could be involved in the transcytosis of BSDL.
LOX-1 Is Implicated in BSDL Transcytosis throughout Int407 Cells
Because the expression of LOX-1 may be upregulated by Ox-LDL and by atherogenic components of oxidized lipoproteins (Aoyama et al., 1999, 2000), we examined the effects of incubation of Int407 cells with native and modified LDL (40 μg/ml), on the amount of transcytosed BSDL. For this purpose, Int407 cells were incubated for 24 h with LDL (native or modified), and then the apical reservoir medium containing LDL was withdrawn, and fresh medium containing BSDL (100 nM) was added and incubated for another 3 h before recording BSDL in the bottom reservoir. As illustrated in Figure 7, Ox-LDL does increase the amount of BSDL detected in the bottom reservoir, whereas neither native LDL nor Ac-LDL promote such effect. This result suggested that Ox-LDL increases the rate of the BSDL transcytosis possibly by an enhancement of the expression of the LOX-1 receptor.
Figure 7.
Effect of Int407 cells preincubation with native and modified LDL on BSDL transcytosis. Int407 cells were grown on Transwell inserts to obtain a tight epithelium. Cells were then independently incubated for 24 h with native or modified LDL (40 μg/ml) in the cell culture medium. Then cell culture medium of the apical reservoir was removed and replaced by media containing BSDL (100 nM). After another 3-h incubation, medium of the bottom reservoir was removed and the amount of transcytosed BSDL evaluated from Western blottings (B) or from its activity (C). For Western blotting (A, only a typical experiment is shown), proteins present in the bottom reservoir (50 μl of medium were separated on SDS-PAGE and electrotransferred onto nitrocellulose membranes). Membranes were then probed with pAbL64. After visualization, quantitation of protein bands was performed using the NIH Image program. Values are expressed as percent of control performed in the absence of LDL preincubation (control). Values are means ± SD of at least three independent experiments.
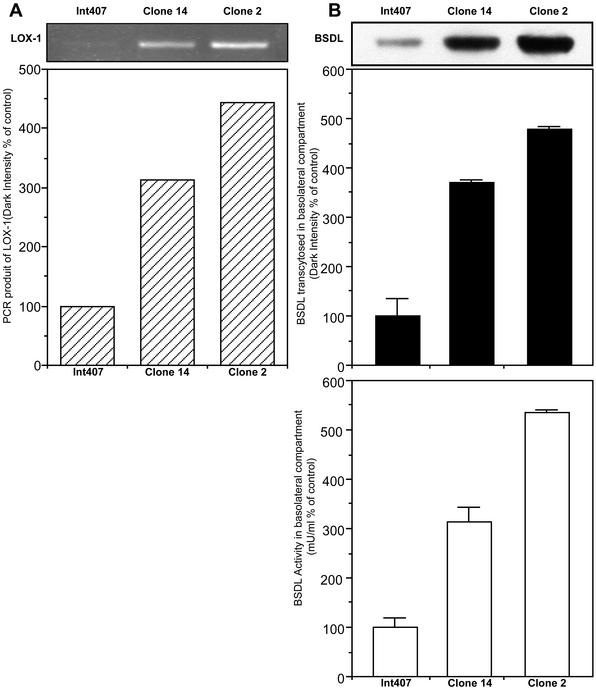
We next examined the effect of Ox-LDL on the expression of lox-1 RNA transcripts in Int-407 cells. For this goal, intestinal cells were grown for 24 h in the absence or in the presence of Ox-LDL (40 μg/ml); at the end of the incubation period, cells were washed and harvested, and RNA was extracted. RNA was then used as matrix for RT-PCR using a pair of oligonucleotide primers specific for human LOX-1. Products were further analyzed on agarose gel (Figure 8). Under conditions used and even though at least three attempts were done, it was quite difficult to detect RT-PCR product from RNA of Int407 cells grown in absence of Ox-LDL, whereas a 0.9-kb transcript was clearly obtained after the RT-PCR using RNA extracted from cells incubated with Ox-LDL. This transcript shown an open reading frame of 933 base pairs, the sequence of which (unpublished data) matched totally that of human LOX-1 (Adachi et al., 1997; Sawamura et al., 1997). This cDNA was then subcloned into two eukaryotic expression vectors, pEGFP-N1 and pBK-CMV under the control of SV40 and CMV promoter to give pLOX-1 and pBK-LOX-1 vectors, respectively. Int407 cells were transfected with these expression vectors and then selected by end-dilution, and two cell clones, one referred to as Int-407 clone 14 (cells transfected with pLOX-1) and another referred to as Int-407 clone 2 (cells transfected with pBK-LOX-1), were also used. These two clones expressed different amount of LOX-1 transcripts as assessed by RT-PCR (Figure 9A). Flow cytometry experiments demonstrated (see Figure 5B) that the cell surface expression of LOX-1 is higher in transfected Int407 cells than in the wild-type cell. Furthermore, clone 2 fluorescence is a little higher than that of clone 14, suggesting that the amount of LOX-1 receptor expressed at the surface is higher in clone 2 than in clone 14. Therefore, these two selected clones were grown on Transwell inserts until a tight epithelium was reached and assayed for BSDL transcytosis under standard conditions. As shown in Figure 9B, compared with wild Int407 control cells, transfected cells allow a significant increase in BSDL transcytosis, by 300 and 500% by Int407 clone 14 and clone 2, respectively. Therefore, amount of BSDL that had transcytosed is directly proportional to the amount of RT-PCR transcripts (compare Figure 9, A and B) and to the amount of LOX-1 receptors expressed at the cell surface (see Figure 5B). These data confirmed that LOX-1 receptor participates in the BSDL transcytosis. We further examined the BSDL transcytosis under conditions in which the apical reservoir was buffered between pH 4 and pH 8. No modification in BSDL transcytosis occurs under these conditions (our unpublished results).
Figure 8.
RT-PCR using RNA extracted from Int407 cells. Int407 cells were cultured until a tight epithelium was formed. and then the culture medium was replaced with fresh medium without or with Ox-LDL (40 μg/ml) and further incubated for 24 h. At the end of the incubation, RNA was extracted and amplified using sense and antisense primers designed to cover the full-length sequence of LOX-1. Amplification products were then separated on 1% agarose gel. Lane 1, ladder; lane 2, cDNA from mock-treated Int407 cells; lane 3, cDNA from Ox-LDL–treated Int407 cells.
Figure 9.
Transcytosis of BSDL through Int407 cells overexpressing LOX-1. Int407 cells were transfected with expression vectors containing cDNA coding for the human LOX-1 receptor. Two Int407 cell clones (clone 14 and clone 2) were selected. (A) The amount of LOX-1 mRNA transcript expressed by either wild-type, clone 14 and clone 2 transfected Int407 cells was determined by RT-PCR and densitometric quantification of bands. (B) Wild-type and clone 14– and clone 2–transfected Int407 were cultured in Transwell insert until a tight epithelium was obtained. Cells were further incubated in the presence of BSDL (100 nM) applied in the apical reservoir. After 3 h of incubation, the amount of transcytosed BSDL present in the bottom reservoir was measured from Western blotting using pAbL64 antibodies or BSDL activity. Values are expressed relative to the amount of BSDL that moved through wild-type Int407 cells and taken as 100%. Values are means of at least three independent experiments. RT-PCR analysis and Western blotting as shown are representative of typical experiments.
Bidirectional Transport of BSDL
We next wondered whether the transport of BSDL may be bidirectional. For this purpose BSDL (100 nM) was applied to apical or basolateral surface of Int407 cells. As assessed by Western blotting and activity, after 3 h at 37°C intact BSDL applied to apical or basolateral cell surfaces was transported in both luminal and abluminal directions across Int407 cell monolayers. However, when monolayers were incubated at 4°C, the transport of BSDL was largely reduced (Figure 10). The same relative decrease in BSDL transcytosis rate at 4 vs. 37°C was also observed with LOX-1–transfected Int407 cells (unpublished data). Thus, transepithelial flux of BSDL did not occur by passive diffusion through intercellular tight junctions or monolayer leaks. These data indicate that BSDL can enter both apically and basolaterally directed transcytosis pathway in Int407cells. Measurements of band intensities and of activities suggest that the apically directed transport pathway was as efficient than the basolaterally directed transport. Finally, we sought to determine whether the transport of BSDL from the basolateral to the apical compartment also depended upon LOX-1. First, at 37°C this abluminal transport was increased in the same proportion as the luminal transport in Int407 cells overexpressing LOX-1 compared with wild-type cells (unpublished data). This motion was also reduced when recorded at 4°C. Second, we furthered this study using LOX-1 inhibitors as described in Figure 4. Data indicated that, as the apical-to-basolateral transport of BSDL, the basolateral-to-apical transport of the enzyme is also inhibited by carrageenan (0.1 mg/ml, 95 ± 1% inhibition), fucoidan (0.1 mg/ml, 82 ± 5% inhibition), and Con A (20 μg/ml, 90.3 ± 15.5% inhibition), whereas heparin still remained a poor inhibitor (1 mg/ml, 38.3 ± 15.6% inhibition). These data strongly support that LOX-1 mediates BSDL transport in both apical-to-basolateral and basolateral-to-apical directions.
Figure 10.
Bidirectional transcytosis of BSDL through Int407 cells. BSDL (100 nM) was applied either in the apical reservoir to determine the apical-to-basolateral transcytosis or in the basolateral reservoir to determine the basolateral-to-apical transcytosis. Cells were incubated for 3 h at 37°C or at 4°C as mentioned. Analyses were performed by Western blotting using 50-μl sample collected from apical or basolateral reservoir, further quantitated by densitometry and activity determination (total unit recovered in each compartment i.e., mU/ml corrected for the total volume of culture medium in each reservoir). Western blotting shows a typical experiment and histogram values are means ± SD of at least three independent experiments.
DISCUSSION
In the early 1960s, Gallo and coworkers detected BSDL in the rat intestinal cells (Gallo and Treadwell, 1963), a result that was confirmed by us in human (Lechêne de la Porte et al., 1987) and rat (Bruneau et al., 1998). We have further shown that BSDL is present on microvilli, in the endosomal compartment, and in interdigitations of the basolateral membrane of enterocytes (Bruneau et al., 1998). Recently, using Int407 human intestinal cells, we have shown that pancreatic BSDL formed clusters, in association with clathrin-coated pits at the apical surface, which were then internalized. Subsequent to internalization, coated vesicle-containing BSDL fuse with a nocodazole-sensitive multivesicular compartment. The protein then transited through the Golgi apparatus and the intact enzymatically active protein is further released at the basolateral level (Bruneau et al., 2001).
We have further demonstrated that the circulating BSDL originated from the pancreatic secretion via the intestinal transcytosis (Bruneau et al., 2003). Very few macromolecules traverse the epithelial barrier intact by passive diffusion (Harada et al., 1994a, 1994b). However some macromolecules are efficiently transported across epithelial cells. This transcellular pathway of macromolecular transport termed transcytosis is most often receptor-mediated, as exemplified by the mechanism for the mucosal secretion of polymeric IgA by the polymeric immunoglobulin receptor (pIgA-R, Mostov and Blobel, 1983) and IgG by the MHC-class-I-related Fc receptor (FcRn; Dickinson et al., 1999). Macromolecular transcellular transport can occur by fluid-phase or non–receptor-mediated endocytosis, but this pathway is inefficient, and most of endocytosed cargo is delivered to lysosomes for degradation (Futter et al., 1996). Therefore, we can suggest that BSDL interacted with an apical receptor likely located in clathrin-coated pits, before its motion through intestinal cells. Although a receptor for BSDL in intestinal cells has not been identified, binding studies using a heterologous system (i.e., human intestinal CaCo-2 cells and porcine BSDL) have shown that the initial interaction of the enzyme with these cells is mediated by the binding of BSDL to putative low-affinity and high-capacity binding sites at the cell surface (Huang and Hui, 1990). In the present study we have attempted to characterize this receptor.
It has been hypothesized that the heparin-binding site present on BSDL (Fält et al., 2001) could be involved in such binding (Bosner et al., 1988). As shown here, heparin at concentration up to 1 mg/ml only partially impaired the uptake of BSDL by the cells (as assessed by the amount of enzyme activity recorded in the basal reservoir of Transwell inserts). This suggests that the enzyme could interact, albeit with a poor avidity, with brush-border membrane-associated, heparin-like molecules before internalization. These heparin-like molecules could be part of proteoglycans. However, the lack of heparan sulfate effect indicated that these proteoglycans are not related to the syndecan family, which is composed of transmembrane heparan sulfate proteoglycans (Mali et al., 1990) described as alternative receptors or coreceptors for the uptake of lipoprotein lipase-bridged atherogenic lipoproteins (Williams et al., 1992; Fuki et al., 1997). Other sulfated glycans such as chondroitin sulfate are also poorly efficient in inhibiting the transcytosis of BSDL throughout Int407 cells. Also internalization of the enzyme via the transferrin receptor (present at the cell surface of Int407 cells), which is clathrin dependent (Hansen et al., 1993), may not represent a way for BSDL to associate with clathrin-coated pits of the apical membrane of enterocytes because neither transferrin nor antibodies to transferrin-receptor modulate the BSDL motion through intestinal cells.
Another possibility was the involvement of the N-linked glycan structure of BSDL (Mas et al., 1993). This structure, which is highly variable (Sugo et al., 1993) may help BSDL to interact with mannose/fucose lectin receptor (Avraméas et al., 1996). The molecular chaperone Grp94, once associated with BSDL (Bruneau et al., 1998) can also be internalized via this receptor and macropinocytosis (Arnold-Schild et al., 1999; Wassenberg et al., 1999). Mannose/fucose lectin receptor seems to be not involved in BSDL transcytosis because mannan, a specific competitor of this receptor (Arnold-Schild et al., 1999), does not impair BSDL transcytosis. However, the competitive effect of Con A, a lectin that recognizes the oligomannosidic core of N-linked glycans, suggests that the N-glycosylation of BSDL could be involved.
Finally, the O-glycosylated C-terminal mucin-like tail of BSDL (Wang and Hartsuck, 1993; Wang et al., 1995; Mas etal., 1997), which differs between species both in the amount of repeated identical sequences (Sbarra et al., 1998) and in glycosylation (Wang and Hartsuck, 1993), could also be involved in the transcytosis of the enzyme. The use of heterologous systems, e.g., binding of BSDL to human CaCo-2 cells (Huang and Hui, 1990) and transcytosis of rat BSDL through human Int407 cells (Bruneau et al., 2001) and the lack of effect of PNA allowed us to rule out in first instance this assumption. Antibodies to BSDL (pAbL64 and mAbJ28) impaired transcytosis throughout Int407 cells and steric hindrance may be advocated to explain this effect.
Many receptors could be involved in BSDL uptake and transcytosis throughout Int407 cells (Ramprasad et al., 1995; Adachi et al., 1997; Dhaliwal and Steinbrecher, 1999; Shirai et al., 1999; Cai et al., 2001; Lobo et al., 2001; Werder et al., 2001; Gillotte-Taylor et al., 2001). Among them the lectin-like oxidized LDL receptor or LOX-1, a 50-kDa protein detected in nearly all tissues examined (Sawamura et al., 1997) has retained our attention. Although carrageenan and polyinosinic acid abolished Ox-LDL binding to LOX-1, fucoidan, chondroitin sulfate and polycytidylic acid are less effective (Moriwaki et al., 1998). A unique feature of LOX-1 is the presence of a lectin-like domain in a conserved C-terminal domain, which is essential for Ox-LDL binding (Chen et al., 2001b). As mentioned above, the fact that Con A, which is specific for the N-linked glycans of BSDL, blocked the transcytosis of this latter protein indicated that a lectin-type receptor could be involved in the transcytotic mechanism of BSDL.
However, we cannot rule out the binding of Con A to LOX-1, which is also a N-glycosylated protein. From current results it seems obvious that the BSDL receptor of Int407 cells is partially blocked by mAb to LOX-1, upregulated by Ox-LDL as is LOX-1 (Aoyama et al., 1999; Metha and Li, 2002), the mRNA of which can be easily detected upon stimulation of Int407 cells by Ox-LDL. The implication of LOX-1 in BSDL transcytosis is confirmed by the overexpression of LOX-1 in Int407 cells transfected with the cDNA encoding the human LOX-1. This overexpression leads to a substantial increase in the amount of transcytosed enzyme. Taken as a whole, data demonstrated that the transcytosis of BSDL throughout intestinal cells implicates LOX-1. A striking result is the decrease in BSDL transcytosis upon fucoidan treatment of Int407 cells, because fucoidan does not compete with Ox-LDL for binding to LOX-1 (Moriwaki et al., 1998). No clear explanation can be done to this result; however, it is possible that the interaction of BSDL with LOX-1 involves fucose residues of BSDL, an interaction that should be impaired by nonsulfated fucose residues of fucoidan, whereas the interaction between LOX-1 and the protein moiety of Ox-LDL (Moriwaki et al. 1998) could involve ionic and hydrophilic interactions and not actual LOX-1 lectin-binding properties (Chen et al., 2001a, 2001b; Shi et al., 2001). Clearly, if fucose residues of BSDL are involved in the interaction of the enzyme with the LOX-1 receptor, they should not be α(1–2) linked to galactose residues as UEA-1 lectin did not impair BSDL transcytosis. A way to conciliate the binding of BSDL to heparin-like molecules lining the brush border membrane (see Fält et al., 2001; Bosner et al., 1988) and the implication of LOX-1 would be that BSDL first bind to heparin-like molecules lining intestinal cells and then are transferred to LOX-1 with which the enzyme associates by mean of glycannic determinants. Once bound to LOX-1, BSDL could form clusters that are taken up via clathrin-coated pits of intestinal cells (Bruneau et al., 2001).
The mechanism of LOX-1–mediated BSDL transport in Int407 cells possibly differs from that defined for the pIgA-R (Mostov et al., 1980, 2000; Mostov and Blobel, 1983; Apocada et al., 1994) but instead resembles that of FcRn (Dickinson et al., 1999). As observed for this latter receptor, the bidirectional transcytosis of BSDL through Int407 cells suggests that LOX-1 may reenter the cell for return to the opposite membrane. Thus unlike pIgA-R, which is committed to only one round of dimeric IgA transport, the same LOX-1 molecule could transport BSDL for multiple rounds in both luminal and abluminal direction. Nevertheless, contrary to FcRn, which first binds its ligand within the acidic endosome after fluid phase uptake (Dickinson et al., 1999), as suggested by cross-linking experiments, LOX-1 should rather bind BSDL at the cell surface as pIgA-R does with IgA (Mostov and Blobel, 1983; Apocada et al., 1994). As a consequence, IgG transport across epithelial cells by FcRn is dependent on the pH and sensitive to bafilomycin A1, which collapses pH gradient in intracellular vesicles, whereas transport of BSDL across Int407 cells did not depend upon pH (our unpublished results) and is insensitive to bafilomycin A1 (Bruneau et al., 2001). Of course the exact mechanism of BSDL transcytosis and the pathway of LOX-1 receptor through Int407 cells need further studies. Another question concerns the release of BSDL from LOX-1 once the complex reached the basolateral domain of intestinal cell membrane. One possibility is that intestinally neo-synthesized lipoprotein particles such as chylomicrons (Hui and Howles, 2002; Bruneau et al., 2003) may help the enzyme to dissociate from LOX-1. However, the involvement of LOX-1 in the BSDL transcytosis does not exclude that of other receptor(s).
Acknowledgments
We are indebted to Dr. C. Nicchitta (Duke University, Durham, NC) and to Dr. T Sawamura (Osaka, Japan) for its kind gift of porcine pancreatic Grp94 and functional blocking antibodies to LOX-1 receptor, respectively. Our special thanks also go to Dr. A. Salvayre (INSERM U-466, Toulouse, France) for the preparation and gift of native and modified human LDL and to Dr. M-J. Escribano (INSERM U-260, Marseille, France) for the release of mAbJ28 antibody. This work was supported by institutional funding from the Institut National de la Santé et de la Recherche Médicale (Paris, France) and by the Université de la Méditerranée.
Accepted March 2003. Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0544. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-08-0544.
References
- Abouakil, N., Rogalska, E., Bonicel, J., and Lombardo, D. (1988). Purification of pancreatic carboxylic-ester hydrolase by immunoaffinity and its application to the human bile-salt-stimulated lipase. Biochim. Biophys. Acta 961, 299–308. [DOI] [PubMed] [Google Scholar]
- Adachi, H., Tsujimoto, M., Arai, H., and Inoue, K. (1997). Expression cloning of a novel scavenger receptor from human endothelial cells. J. Biol. Chem. 272, 31217–31220. [DOI] [PubMed] [Google Scholar]
- Aoyama, T., Fujiwara, H., Masaki, T., and Sawamura, T. (1999). Induction of lectin-like oxidized LDL receptor by oxidized LDL and lysophosphatidylcholine in cultured endothelial cells. J. Mol. Cell. Cardiol. 31, 2101–2114. [DOI] [PubMed] [Google Scholar]
- Aoyama, T., Chen, M., Fujiwara, H., Masaki, T., and Sawamura, T. (2000). LOX-1 mediates lysophosphatidylcholine-induced oxidized LDL uptake in smooth muscle cells. FEBS Lett. 467, 217–220. [DOI] [PubMed] [Google Scholar]
- Apodaca, G., Katz, L.A., and Mostov, K.E. (1994). Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J. Cell Biol. 125, 67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold-Schild, D., Hanau, D., Spehner, D., Schmid, C., Rammensee, H-G., De la Salle, H., and Schild, H. (1999). Cutting edge, receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 162, 3757–3760. [PubMed] [Google Scholar]
- Augé, N., Nikolova-Karakashian, M., Carpentier, S., Parthasarathy, S., Negre-Salvayre, A., Salvayre, R., Merrill, A.H., Jr., and Levade, T. (1999) Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J. Biol. Chem. 274, 21533–21538. [DOI] [PubMed] [Google Scholar]
- Avraméas, A., McIlroy, D., Hosmalin, A., Autran, B., Debré, P., Monsigny, M., Roche, A.C., and Midoux, P. (1996). Expression of a mannose/fucose membrane lectin on human dendritic cells. Eur. J. Immunol. 26, 394–400. [DOI] [PubMed] [Google Scholar]
- Bhat, S.G., and Brockman, H.L. (1982). The role of cholesteryl ester hydrolysis and synthesis in cholesterol transport across rat intestinal mucosal membrane. A new concept. Biochem. Biophys. Res. Commun. 109, 436–492. [DOI] [PubMed] [Google Scholar]
- Bosner, M.S., Gulick, T., Riley, D.J.S., Spilburg, C.A., and Lange, L.G., III. (1988). Receptor-like function of heparin in the binding and uptake of neutral lipids. Proc. Natl. Acad. Sci. USA 85, 7438–7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau, N., Bendayan, M. Gingras, D., Ghitescu, L., Levy, E., and Lombardo, D. (2003). Circulating bile salt-dependent lipase originates from the pancreas via intestinal transcytosis. Gastroenterology 124, 470–480. [DOI] [PubMed] [Google Scholar]
- Bruneau, N., and Lombardo, D. (1995). Chaperon function of a Grp 94-related protein for folding and transport of the pancreatic bile salt-dependent lipase. J. Biol. Chem. 270, 13524–13533. [DOI] [PubMed] [Google Scholar]
- Bruneau, N., Lombardo, D., and Bendayan, M. (1998). Participation of Grp94 related protein in secretion of pancreatic bile salt dependent lipase and in internalization by the intestinal epithelium. J. Cell Sci. 111, 2665–2679. [DOI] [PubMed] [Google Scholar]
- Bruneau, N., Lombardo, D., and Bendayan, M. (2000a). The affinity binding sites of pancreatic bile salt-dependent lipase in pancreatic and intestinal tissues. J. Histochem. Cytochem. 48, 267–276. [DOI] [PubMed] [Google Scholar]
- Bruneau, N., Lombardo, D., Levy, E., and Bendayan, M. (2000b). Roles of molecular chaperones in pancreatic secretion and their involvement in intestinal absorption. Microsc. Res. Tech. 49, 329–345. [DOI] [PubMed] [Google Scholar]
- Bruneau, N., Nganga, A., Bendayan, M., and Lombardo, D. (2001). Transcytosis of pancreatic bile salt-dependent lipase through human Int407 intestinal cells. Exp. Cell Res. 271, 94–108. [DOI] [PubMed] [Google Scholar]
- Burnette, W.N. (1981). “Western Blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose an radiographic detection with antibody and radiodinated protein A. Anal. Biochem. 112, 195–203. [DOI] [PubMed] [Google Scholar]
- Cai, S.F., Kirby, R.J., Howles, P.N., and Hui, D.Y. (2001). Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J. Lipid Res. 42, 902–909. [PubMed] [Google Scholar]
- Caillol, N., Pasqualini, E., Mas, E., Valette, A., Verine, A., and Lombardo, D. (1997). Pancreatic bile salt dependent lipase activity in serum of normolipidemic patients. Lipids 32, 1147–1153. [DOI] [PubMed] [Google Scholar]
- Chen, M., Inoue, K., Narumiya, S., Masaki, T., and Sawamura, T. (2001a). Requirements of basic amino acid residues within the lectin-like domain of LOX-1 for the binding of oxidized low-density lipoprotein. FEBS Lett. 499, 215–219. [DOI] [PubMed] [Google Scholar]
- Chen, M., Narumiya, S., Masaki, T., and Sawamura, T. (2001b). Conserved C-terminal residues within the lectin-like domain of LOX-1 are essential for oxidized low-density-lipoprotein binding. Biochem. J. 355, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat, A., Ciechanover, A., and Lodish, H.F. (1983). pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 80, 2258–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal, B.S., and Steinbrecher, U.P. (1999). Scavenger receptors and oxidized low density lipoproteins. Clin. Chim. Acta 286, 191–205. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson, C., Sternby, B., and Johannesson, U. (1985). The interaction between human pancreatic carboxylester hydrolase (bile-salt-stimulated lipase of human milk) and lactoferrin. Biochim. Biophys. Acta 829, 282–287. [DOI] [PubMed] [Google Scholar]
- Fält, H., Hernell, O., and Blackberg, L. (2001). Do human bile salt stimulated lipase and colipase-dependent pancreatic lipase share a common heparin-containing receptor? Arch. Biochem. Biophys. 386, 188–194. [DOI] [PubMed] [Google Scholar]
- Fong, L.G., Fujishima, S.E., Komaromy, M.C., Pak, Y.K., Ellsworth, J.L., and Cooper, A.D. (1995). Location and regulation of low-density lipoprotein receptors in intestinal epithelium. Am. J. Physiol. 269, G60–G72. [DOI] [PubMed] [Google Scholar]
- Fuki, I.V., Kuhn, K.M., Lomazov, I.R., Rothman, V.L., Tuszynski, G.P., Iozzo, R.V., Swenson, T.L., Fisher, E.A., and Williams, K.J. (1997). The syndecan family of proteoglycans. Novel receptors mediating internalization of atherogenic lipoproteins in vitro. J. Clin. Invest. 100, 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter, C.E., Pearse, A., Hewlett, L.J., and Hopkins, C.R. (1996). Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 132, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, L.L., and Treadwell, C.R. (1963). Localization of cholesterol esterase and cholesterol in mucosal fractions of rat small intestine. Proc. Soc. Exp. Biol. Med. 114, 69–72. [DOI] [PubMed] [Google Scholar]
- Gillotte-Taylor, K., Boullier, A., Witztum, J.L., Steinberg, D., and Quehenberger, O. (2001). Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 42, 1474–1482. [PubMed] [Google Scholar]
- Gjellesvik, D.R., Lombardo, D., and Walther, B.T. (1992). Pancreatic bile salt-dependent lipase from cod (Gadus morhua): purification and properties. Biochim. Biophys. Acta 1124, 123–134. [DOI] [PubMed] [Google Scholar]
- Goldstein, J.L., Ho, Y.K., Brown, M.S., Innerarity, T.L., and Mahley, R.W. (1980). Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta very low-density lipoproteins. J. Biol. Chem. 255, 1839–1848. [PubMed] [Google Scholar]
- Hansen, S.H., Sandvig, K., and van Deurs, B. (1993). Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J. Cell Biol. 123, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton, R.Y., Golenbock, D.T., Penman, M., Krieger, M., and Raetz, C.R. (1991). Recognition and plasma clearance of endotoxin by scavenger receptors. Nature 352, 342–344. [DOI] [PubMed] [Google Scholar]
- Harada, E., Hashimoto, Y., and Syuto, B. (1994a). Orally administered spermine induces precocious intestinal maturation of macromolecular transport and disaccharidase development in suckling rats. Comp. Biochem. Physiol. A Physiol. 109, 667–673. [DOI] [PubMed] [Google Scholar]
- Harada, E., Hashimoto, Y., and Syuto, B. (1994b). Precocious cessation of intestinal macromolecular transport and digestive enzymes development by prostaglandin E2 in suckling rats. Comp. Biochem. Physiol. Physiol. 109, 245–253. [DOI] [PubMed] [Google Scholar]
- Hui, D.Y., and Howles, P.N. (2002). Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J. Lipid Res. 43, 2017–2030. [DOI] [PubMed] [Google Scholar]
- Hauser, H. et al. (1998). Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry 37, 17843–17850. [DOI] [PubMed] [Google Scholar]
- Howles, P., Carter, C., and Hui, D.Y. (1996). Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene targeted mice. J. Biol. Chem. 271, 7196–7202. [DOI] [PubMed] [Google Scholar]
- Huang, Y., and Hui, D.Y. (1990). Metabolic fate of pancreas derived cholesterol esterase in intestine: an in vitro study using Caco-2 cells. J. Lipid Res. 31, 2029–2037. [PubMed] [Google Scholar]
- Kirby, R.J., Zheng, S., Tso, P., Howles, P.N., and Hui, D.Y. (2002). Bile salt-stimulated carboxyl ester lipase influences lipoprotein assembly and secretion in intestine: a process mediated via ceramide hydrolysis. J. Biol. Chem. 277, 4104–4109. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lechêne de la Porte, P., Abouakil, N., Lafont, H., and Lombardo, D. (1987). Subcellular localization of cholesterol ester hydrolase in the human intestine. Biochim. Biophys. Acta 920, 237–246. [DOI] [PubMed] [Google Scholar]
- Lobo, M.V., Huerta, L., Ruiz-Velasco, N., Teixeiro, E., De la Cueva, P., Celdran, A., Martin-Hidalgo, A., Vega, M.A., and Bragado, R. (2001). Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J. Histochem. Cytochem. 49, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Lombardo, D. (2001). Bile salt-dependent lipase: its pathophysiological implications. Biochim. Biophys. Acta 1533, 1–28. [DOI] [PubMed] [Google Scholar]
- Lombardo, D., Fauvel, J., and Guy, O. (1980). Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. I. Action on carboxyl esters, glycerides and phospholipids. Biochim. Biophys. Acta 611, 136–146. [DOI] [PubMed] [Google Scholar]
- Lombardo, D., and Guy, O. (1980). Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim. Biophys. Acta 611, 147–155. [DOI] [PubMed] [Google Scholar]
- Lombardo, D., Guy, O., and Figarella, C. (1978). Purification and characterization of a carboxyl ester hydrolase from human pancreatic juice. Biochim. Biophys. Acta 527, 142–149. [DOI] [PubMed] [Google Scholar]
- Lombardo, D., Montalto, G., Roudani, S., Mas, E., Laugier, R., Sbarra, V., and Abouakil, N. (1993). Is bile salt-dependent lipase concentration in serum of any help in pancreatic cancer diagnosis? Pancreas 8, 581–588. [DOI] [PubMed] [Google Scholar]
- Lopez-Candales, A., Bosner, M.S., Spilburg, C.A., and Lange, L.G. (1993). Cholesterol transport function of pancreatic cholesterol esterase: directed sterol uptake and esterification in enterocytes. Biochemistry 32, 12085–12089. [DOI] [PubMed] [Google Scholar]
- Mali, M., Jaakkola, P., Arvilommi, A.M., and Jalkanen, M. (1990). Sequence of human syndecan indicates a novel gene family of integral membrane proteoglycans. J. Biol. Chem. 265, 6884–6889. [PubMed] [Google Scholar]
- Marsh, E.W., Leopold, P.L., Jones, N.L., and Maxfield, F.R. (1995). Oligomerized transferrin receptors are selectively retained by a luminal sorting signal in a long-lived endocytic recycling compartment. J. Cell Biol. 129, 1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas, E., Crotte, C., Lecestre, D., Michalski, J.C., Escribano, M.J., Lombardo, D., and Sadoulet, M.-O. (1997). The oncofetal J28 epitope involves fucosylated O-linked oligosaccharide structures of the fetoacinar pancreatic protein. Glycobiology 7, 745–752. [DOI] [PubMed] [Google Scholar]
- Mas, E., Abouakil, N., Roudani, S., Franc, J-L., Montreuil, J., and Lombardo, D. (1993). Variation of the glycosylation of human pancreatic bile-salt-dependent lipase. Eur. J. Biochem. 216, 807–812. [DOI] [PubMed] [Google Scholar]
- Metha, J.L. and Li, D. (2002). Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J. Am. Coll. Cardiol. 39, 1429–1435. [DOI] [PubMed] [Google Scholar]
- Moriwaki, H., Kume, N., Sawamura, T., Aoyama, T., Hoshikawa, H., Ochi, H., Nishi, E., Masaki, T., and Kita, T. (1998). Ligand specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 18, 1541–1547. [DOI] [PubMed] [Google Scholar]
- Mostov, K.E., and Blobel, G. (1983). Transcellular transport of polymeric immunoglobulin by secretory component: a model system for studying intracellular protein sorting. Ann. NY Acad. Sci. 409, 441–451. [DOI] [PubMed] [Google Scholar]
- Mostov, K.E., Kraehenbuhl, J.P., and Blobel, G. (1980). Receptor-mediate dtranscellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc. Natl. Acad. Sci. USA 77, 7257–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov, K., Verges, M., and Altschuler, Y. (2000). Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12; 483–490. [DOI] [PubMed] [Google Scholar]
- Myers-Payne, S.C., Hui, D.Y., Brockman, H.L., and Schroeder, F. (1995). Cholesterol esterase: a cholesterol transfer protein. Biochemistry 34, 3942–3947. [DOI] [PubMed] [Google Scholar]
- Ramprasad, M.P., Fischer, W., Witztum, J.L., Sambrano, G.R., Quehenberger, O., and Steinberg, D. (1995). The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc. Natl. Acad. Sci. USA 92, 9580–9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto, F., Cella, M., Danieli, C., and Lanzavecchia, A. (1995). Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura, T. et al. (1997). An endothelial receptor for oxidized low-density lipoprotein. Nature 386, 73–77. [DOI] [PubMed] [Google Scholar]
- Sbarra, V., Bruneau, N., Mas, E., Hamosh, M., Lombardo, D., and Hamosh, P. (1998). Molecular cloning of the bile salt-dependent lipase of ferret lactating mammary gland: an overview of functional residues. Biochim. Biophys. Acta 1393, 80–89. [DOI] [PubMed] [Google Scholar]
- Shi, X., Niimi, S., Ohtani, T., and Machida, S. (2001). Characterization of residues and sequences of the carbohydrate recognition domain required for cell surface localization and ligand binding of human lectin-like oxidized LDL receptor. J. Cell Sci. 7, 1273–82. [DOI] [PubMed] [Google Scholar]
- Shirai, H., Murakami, T., Yamada, Y., Doi, T., Hamakubo, T., and Kodama, T. (1999). Structure and function of type I and II macrophage scavenger receptors. Mech. Ageing Develop. 111, 107–121. [DOI] [PubMed] [Google Scholar]
- Sugo, T., Mas, E., Abouakil, N., Endo, T., Escribano, M-J., Kobata, A., and Lombardo, D. (1993). The structure of N-linked oligosaccharides of human pancreatic bile-salt-dependent lipase. Eur. J. Biochem. 216, 799–805. [DOI] [PubMed] [Google Scholar]
- Wang, C.S., and Hartsuck, J.A. (1993). Bile salt-activated lipase. A multiple function lipolytic enzyme. Biochim. Biophys. Acta 1166, 1–19. [DOI] [PubMed] [Google Scholar]
- Wang, C.S., Dashti, A., Jackson, K.W., Yeh, J.C., Cummings, R.D., and Tang, J. (1995). Isolation and characterization of human milk bile salt-activated lipase C-tail fragment. Biochemistry 34, 10639–10644. [DOI] [PubMed] [Google Scholar]
- Wassenberg, J.J., Dezfulian, C., and Nichitta, C.V. (1999). Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J. Cell Sci. 112, 2167–2175. [DOI] [PubMed] [Google Scholar]
- Weng, W., Li, L., van Bennekum, A.M., Potter, S.H., Harrison, E.H., Blaner, W.S., Breslow, J.L., and Fisher, E.A. (1999). Intestinal absorption of dietary cholesteryl ester is decreased but retinyl ester absorption is normal in carboxyl ester lipase knockout mice. Biochemistry 38, 4143–4149. [DOI] [PubMed] [Google Scholar]
- Werder, M., Han, C.H., Wehrli, E., Bimmler, D., Schulthess, G., and Hauser, H. (2001). Role of scavenger receptors SR-BI and CD36 in selective sterol uptake in the small intestine. Biochemistry 40, 11643–11650. [DOI] [PubMed] [Google Scholar]
- Williams, K.J., Fless, G.M., Petrie, K.A., Snyder, M.L., Brocia, R.W., and Swenson, T.L. (1992). Mechamisms by which lipoprotein lipase alters cellular metabolism of lipoprotein (a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J. Biol. Chem. 267, 13284–13292. [PubMed] [Google Scholar]