Abstract
Ca2+ is an essential requirement in membrane fusion, acting through binding proteins such as calmodulin (CaM). Ca2+/CaM is required for early endosome fusion in vitro, however, the molecular basis for this requirement is unknown. An additional requirement for endosome fusion is the protein Early Endosome Antigen 1 (EEA1), and its recruitment to the endosome depends on phosphatidylinositol 3-phosphate [PI(3)P] and the Rab5 GTPase. Herein, we demonstrate that inhibition of Ca2+/CaM, by using either chemical inhibitors or specific antibodies directed to CaM, results in a profound inhibition of EEA1 binding to endosomal membranes both in live cells and in vitro. The concentration of Ca2+/CaM inhibitors required for a full dissociation of EEA1 from endosomal membranes had no effect on the activity of phosphatidylinositol 3-kinases or on endogenous levels of PI(3)P. However, the interaction of EEA1 with liposomes containing PI(3)P was decreased by Ca2+/CaM inhibitors. Thus, Ca2+/CaM seems to be required for the stable interaction of EEA1 with endosomal PI(3)P, perhaps by directly or indirectly stabilizing the quaternary organization of the C-terminal FYVE domain of EEA1. This requirement is likely to underlie at least in part the essential role of Ca2+/CaM in endosome fusion.
INTRODUCTION
It has been widely recognized that membrane fusion in the exocytic and endocytic pathways in cells is a Ca2+-dependent process (Hutton, 1986; Chamberlain et al., 1995; Emmanouilidou et al., 1999; Pryor et al., 2000; Chen et al., 2001), yet the specific calcium sensors that operate at each step have not been fully identified. In yeast, calmodulin (CaM) has been identified as a Ca2+ sensor required in the postdocking phase of vacuole fusion (Peters et al., 1998). Studies in mammalian cells have shown that endosome fusion in vitro requires calcium, and it has been hypothesized that calmodulin is a sensor for calcium in endosome fusion. (Colombo et al., 1997; Peters and Mayer, 1998; Huber et al., 2000; Mills et al., 2001) Evidence for a Ca2+/CaM requirement in endosome fusion is also supported by results in live cells. For example, CaM binding proteins have been detected in endosome-enriched fractions of liver (Enrich et al., 1988) and inhibitors of CaM profoundly inhibit transcytosis and transferrin recycling in polarized epithelial cells (Apodaca et al., 1994). Moreover, it has been hypothesized that pathogens such as Mycobacterium tuberculosis may disrupt normal phagosome progression by inhibition of Ca2+/CaM function (Russell, 2001). The target protein(s) for Ca2+/CaM in early endocytic trafficking, however, have not been identified.
The early endosomal protein EEA1 is a 170-kDa coiledcoil dimer that is crucial for endosome fusion in vitro (Mills et al., 1998; Simonsen et al., 1998; Christoforidis et al., 1999; Mills et al., 1999). The association of EEA1 with endosomes and subsequent endosome fusion events are regulated by the activities of phosphatidylinositol 3-kinase (PI 3-kinase) and the Rab5 GTPase. Both the PI 3-P binding FYVE domain and regions that interact with activated Rab5 are localized to the carboxy terminus of EEA1. A second Rab5 binding site as well as a C2H2 domain are present at the amino terminus of EEA1 (Simonsen et al., 1998). In addition, a potential CaM binding IQ-like motif is located just 5′ to the Rab5 interaction domain in the carboxy terminus of EEA1 (Mu et al., 1995). Recently, this IQ-like motif has been shown to bind CaM in a protein overlay assay (Mills et al., 2001).
To further examine the role of CaM and Ca2+/CaM in EEA1 function, we made use of the chemical inhibitors [N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamides] that are known to inhibit the interaction of Ca2+/CaM with protein targets, as well as of an inhibitory monoclonal antibody (mAb) that binds CaM and Ca2+/CaM. The effects of these reagents on the localization of EEA1 to endosomal membranes in live cells and in vitro was analyzed. Our results reveal a crucial requirement for Ca2+/CaM for the association of EEA1 with endosomal membranes. To determine the molecular basis for this requirement, the in vitro interaction of CaM with EEA1 and with diverse mutants of EEA1 was studied. Our findings reveal two separate and independent regions of EEA1 involved in CaM binding through both Ca2+-dependent and Ca2+-independent mechanisms. These observations suggest that the Ca2+/CaM requirement in early endosome fusion in mammalian cells is at least in part dependent on its regulatory interactions with EEA1.
MATERIALS AND METHODS
Materials
1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W7), N-(6-aminohexyl)-l-napthalenesulfonamide, and calmidazolium were purchased from Calbiochem (San Diego, CA). Wortmannin was purchased from Sigma-Aldrich (St. Louis, MO). Stock solution aliquots were prepared and stored in either dimethyl sulfoxide or water at -20°C; aliquots were thawed and used immediately before each experiment. Immobilized calmodulin was from Stratagene (San Diego, CA).
Plasmids and Recombinant Proteins
GstRab5c, RFP-Rab5Q79L,eGFP-EEA1wt, and eGFP-EEA1-1277-1411 were generated as described previously (Lawe et al., 2002). eGFP-EEA1 Q1289L/R1293G was constructed by polymerase chain reaction (PCR)-based mutagenesis of eGFP-EEA1wt and confirmed by sequencing. GFP-EEA1wt was digested with XhoI and EcoR1 to delete the amino-terminal 2498 base pairs of EEA1 and form eGFP-EEA1-835-1411. Murine myc-tagged VPS34 was cloned by reverse transcription-PCR amplification of 3T3-L1 adipocyte RNA and cloned from a 3T3-F442A adipocyte cDNA library by using methods described previously (Virbasius et al., 1996). GST-SARA-FYVE, comprised of residues 587 to 750 of the human SARA cDNA, was constructed by PCR from the full-length cDNA (gift from J. Wrana, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Canada). Purified recombinant protein production has been described previously (Lawe et al., 2002).
Cell Culture and Preparation of Cellular Extracts
COS-7 cells were maintained in Dulbeccco's modified Eagle's medium supplemented with 10% fetal calf serum. The cells were grown in 100-mm dishes and transfected using FuGENE (Roche Diagnostics, Indianapolis, IN). To prepare cytosolic extracts, cells were rinsed twice and swollen by incubation (10 min) with a 10-fold dilution of cytosol buffer (25 mM HEPES, pH 7.0, 125 mM potassium acetate, 2.5 mM magnesium acetate, 0.2 M sucrose, 1 mM dithiothreitol, 1 mM ATP, 5 mM creatine phosphate, 0.01 mg/ml creatine phosphokinase with 10 μg/ml leupeptin, 1 mM TAME, 1 mM 1,10-phenantroline) in water. Cells were then washed by addition of 10 ml of cytosol buffer, aspirated, and then scrapped into the remaining buffer (500 μl) and homogenized by repeated passage (30 times) through a 27-gauge needle. The cytosol was separated from particulate structures by centrifugation at 200,000 × g for 15 min and used in binding assays. 3T3-L1 cells were grown in 150-mm dishes and maintained and differentiated as described previously (Patki et al., 2001). Postnuclear supernatants were prepared as follows. Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and once with cytosol buffer. The cells were then scrapped into 2.5 ml of cytosol buffer and homogenized by repeated passage (7–10 times) through a 27-gauge needle. Postnuclear extracts were obtained by centrifugation at 1000 × g for 5 min to remove nuclei and unbroken cells and used in in vitro membrane association assays.
In Vitro Membrane Association Assay
Aliquots (100 μl) of postnuclear extract (containing ∼2.5 mg/ml total protein) were incubated with the chemical inhibitors or anti- CaM antibody as indicated in each figure legend. Cytosol and membranes were then separated by centrifugation at 200,000 × g for 15 min. The cytosol was removed, and the membrane pellets resuspended in 100 μl of cytosol buffer. Equal aliquots of cytosol and resuspended membrane were boiled in SDS sample buffer and analyzed by SDS-PAGE and immunoblotting with an anti-EEA1 polyclonal antiserum. In some experiments, the membrane pellet obtained from untreated postnuclear supernatant was resuspended, dispensed into 100-μl aliquots, and incubated with inhibitors or anti-CaM antibody. The release of EEA1 from the membrane into the buffer was monitored by immunoblotting of the pellet and supernatant obtained after a subsequent high-speed centrifugation.
Binding Assays
Binding of EEA1 from 3T3 cytosolic extracts to immobilized GST-Rab5c was performed as described previously (Lawe et al., 2002). To assess the binding of EEA1 constructs to CaM, COS-7 cells were transfected with the indicated enhanced green fluorescent protein (eGFP)-tagged EEA1 constructs described in each experiment and cytosolic extracts prepared as described above. Cytosolic extracts were supplemented with Ca2+ chelators or additional CaCl2 as indicated in each experiment. Extracts were then mixed with 25 μl of Sepharose beads or CaM-Sepharose beads previously washed in cytosol buffer containing 10 mg/ml bovine serum albumin. After 30 min at room temperature, beads were washed three times with cytosol buffer containing 0.1% Tween 20. Proteins bound to the beads were detected by immunoblotting with anti-GFP mAb (Zymed Laboratories, South San Francisco, CA).
Liposome Binding Assay
To measure the binding of EEA1 to phosphatidylinositol-3-phosphate [PI(3)P], 3T3-L1 cytosolic extracts were divided into two aliquots; one aliquot was treated with 100 μM W7, and the other was incubated with the W7 solvent dimethyl sulfoxide for 1 h at room temperature. Liposomes containing PI(3)P were prepared as described previously (Lawe et al., 2002). Aliquots of cytosol (100 μl containing approximately 3 mg/ml total protein) were incubated with 50-μl aliquots of suspended liposomes for 15 min at room temperature. The liposomes were collected by centrifugation at 13,200 × g for 10 min, resuspended in SDS-sample buffer, and analyzed by immunoblotting with anti-EEA1 antibody.
Immunoblotting
Filters were then incubated with anti-EEA1 polyclonal antibody or with anti-glutathione S-transferase (GST) as indicated. Bound antibody was detected using horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Promega, Madison, WI), which was detected by Renaissance enhanced luminol reagent (PerkinElmer Life Sciences, Boston, MA). Films were scanned using an Arcus II scanner and Photoshop 6.0 software. The intensity of each band in the digitized image was quantified using the histogram function on a rectangle of constant size placed within the center of each band. This method was found to be linear within 1 order of magnitude, assessed using serial dilutions of control samples (our unpublished data).
PI 3-Kinase Assays and Membrane PI(3)P Levels
COS-7 cells transiently transfected with myc-Vps34 were lysed in buffer A supplemented with 1% Triton X-100. Lysates were clarified by centrifugation at 100,000 × g for 15 min, and incubated with 10 μl of protein A-Sepharose beads bound to mouse anti-myc antibody. After 1 h, beads were washed three times and resuspended in 40 μl of assay buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 3.5 mM MgCl2, 0.5 mM EGTA) containing the inhibitors indicated in each experiment. Liposomes (0.2 mg/ml phosphatidylinositol/phosphatidylserine 1:1; Avanti Polar Lipids, Birmingham, AL) and 10 μM ATP supplemented with 12.5 μCi of [32P]ATP was then added, and after a further 10-min incubation lipids were extracted and separated by thin layer chromatography. PI 3-kinase activity in cytosolic extracts was measured as follows: cytosolic extracts of 3T3-L1 cells (100 μl) were incubated with the indicated inhibitors for 10 min and then supplemented with 20 μg of liposomes as described above. After 30 min at room temperature, liposomes were pelleted by centrifugation at 15,000 × g, resuspended, and spotted onto Hybond nitrocellulose membranes by using a dot-blotting manifold. To specifically detect PI(3)P, the nitrocellulose blot was then probed with 1.5 μg/ml GST-fusion protein of the FYVE domain of SARA in Tris-buffered saline/Tween 20 supplemented with 3% bovine serum albumin. Blots were then washed and probed with anti-GST antibody as described above. The PI(3)P content of membranes from postnuclear extracts of 3T3-L1 cells was measured as follows: postnuclear supernatants were prepared as described above, and incubated in the absence or presence of 100 nM Wortmannin or 100 μM W7 for 1 h at room temperature. Membranes were pelleted by centrifugation at 200,000 × g for 15 min, cytosol was removed, and membranes were resuspended in 50 μl of icecold cytosol buffer. Lipids were extracted by addition of 750 μl of MeOH/1 M HCl (1:1) and 380 μl of CHCl3. After vortexing for 2 min, the organic phase was separated, dried, resuspended in a small volume of Tris-buffered saline/Tween 20 and spotted onto Hybond nitrocellulose for probing with GST-SARA-FYVE protein as described above.
Fluorescence Microscopy
COS-7 cells were grown to 40–50% confluence on coverslips and, where indicated, transfected using calcium phosphate precipitation. Twenty-four hours posttransfection, cells were treated with inhibitors as indicated. In some experiments cells were incubated with Alexa 594-labeled transferrin (Molecular Probes, Eugene, OR) at a concentration of 25 μg/ml at 37°C for the indicated times. Coverslips were then washed twice with cold PBS, fixed in 4% formaldehyde for 10 min at 4°C, and permeabilized with 0.2% Triton X-100/PBS for 10 min at 4°C. Cells were then blocked with 1% fetal bovine serum in PBS for 30 min at 4°C and stained in the same buffer with a mAb directed to the NH2 terminus of EEA1 (Transduction Laboratories, Lexington, KY), or human antiserum to EEA1 (gift of Dr. Ban-Hoc Toh, Monash University Medical School, Victoria, Australia). Secondary antibodies coupled to Alexa 488 (Molecular Probes) were used to detect the primary antibody. Coverslips were imaged using a conventional wide-field microscope fitted with a 60- or 100-Å Nikon plan-apo objective. For live cell imaging, cells were transfected with eGFP-tagged constructs and imaged using highspeed microscopy (Patki et al., 2001). Stacks of 21 optical sections, spaced by 250 nm were acquired every 10 s for 20 continuous minutes. The haze originating from light sources outside the infocus plane of the cell was reduced by image restoration.
RESULTS
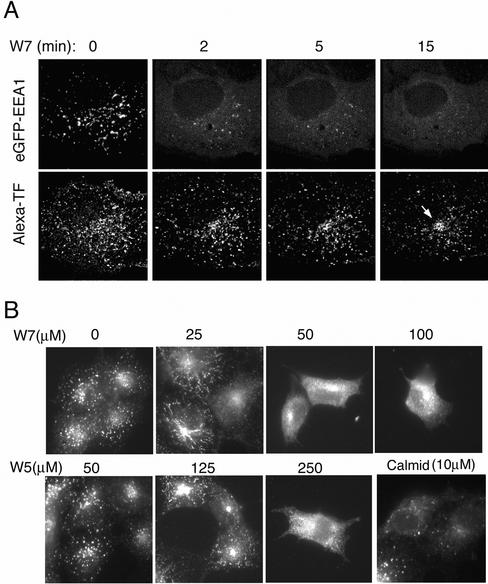
Sulfonamide-derivative calmodulin inhibitors such as W7 bind with high affinity to the hydrophobic surface of calmodulin that is specifically exposed upon Ca2+ binding (Nishikawa and Hidaka, 1982). We have analyzed the effects of W7 in live cells expressing an EEA1 construct tagged with GFP, which has previously been shown to behave in a manner similar to endogenous EEA1 (Lawe et al., 2002). To simultaneously monitor endosome content, cells were incubated with Alexa-594–labeled transferrin for 10 min at 37°C before addition of the inhibitor. The fluorescence signal throughout the three-dimensional volume of the cell was captured in stacks of 21 optical sections, which were acquired every 10 s for 15 min. The out-of-focus blur in each optical section was restored by image deconvolution, and each stack of 21 optical sections projected onto a single two-dimensional (2D) image (Patki et al., 2001). Figure 1A illustrates projections of stacks taken at the times indicated after addition of W7. Before addition of the inhibitor, EEA1 was localized to vesicular structures that concentrated toward the juxtanuclear region. Transferrin was localized to structures containing EEA1 as well as to non-EEA1 labeled endosomes. Within 5 min of addition of W7, the EEA1 endosomal signal became much more diffuse, indicating its relocalization to the cytoplasm. Transferrin remained contained in endosomes and eventually accumulated in a tight juxtanuclear region potentially corresponding to the recycling endosome.
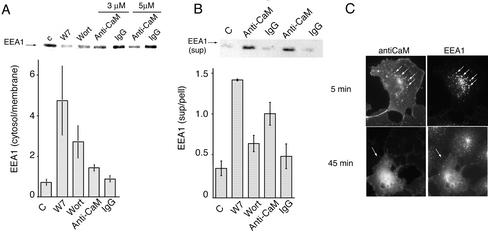
Figure 1.
(A) Effects of CaM inhibitors on the localization of GFP-EEA1 and Alexa 594-transferrin in COS-7 cells. Cells expressing GFP-EEA1 were incubated with Alexa 594-transferrin for 10 min and washed once before addition of 100 μM W7. Stacks of 21 optical sections were collected every 30 s imaging alternatively with lasers tuned to 488- and 594-nm wavelengths. After image restoration, stacks were projected into a single 2D image. Projections for the times indicated are shown. The arrow indicates the concentration of transferrin in the recycling endosome after 15 min of treatment with W7. (B) Effects of diverse CaM inhibitors on endogenous EEA1 localization. COS-7 cells were treated with the indicated inhibitors at the concentrations indicated above each panel. After 10 min, cells were fixed and stained with human antibody to EEA1 and imaged by epifluorescence.
To determine the specificity of the effect of W7 on EEA1 localization, the dose dependency of its effect on endogenous EEA1 was compared with that of a less active analog, W5, and a structurally unrelated Ca2+/CaM inhibitor, cal-midazolium. W7 was at least 5 times more potent than W5 in causing a decrease in the endosomal steady-state localization of EEA1, and calmidazolium was effective at lower doses (Figure 1B). This pharmacological profile is consistent with the hypothesis that these inhibitors affect EEA1 dynamics through their inhibitory actions on Ca2+/CaM.
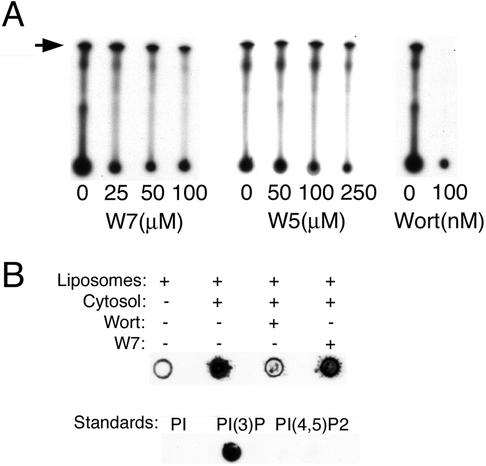
The localization of EEA1 to endosomes is dependent on the presence of PI(3)P generated through the activity of mammalian PI-3 kinases, including VPS34 (Siddhanta et al., 1998). The possibility that Ca2+/CaM inhibition might lead to the observed changes in EEA1 localization through a nonspecific inhibition of the catalytic activity of VPS34 or other PI 3-kinases was tested. First, the catalytic activity of HA-VPS34 was measured in immunopreciptates of the enzyme incubated with liposomes containing phosphatidylinositol (PI) and ATP (Figure 2A). W5 and W7 had no significant effect on the catalytic activity of VPS34, even at concentrations higher than those at which they caused a full redistribution of EEA1 in live cells (Figure 1B). In contrast, wortmannin completely inhibited this activity. To test the effects of Ca2+/CaM inhibitors on total PI 3-kinase activity in cell extracts, cytosolic extracts from 3T3-L1 cells were treated with W7 and incubated with liposomes containing PI for 30 min. The liposomes were then pelleted by centrifugation, spotted on nitrocellulose paper, and the blot analyzed with a probe specific for PI(3)P, composed of the FYVE domain of the protein SARA fused to GST. The amount of probe bound was determined using antibodies to GST and horseradish peroxidase-coupled anti-rabbit secondary antibodies. Although wortmannin caused a pronounced inhibition of PI(3)P synthesis by cytosolic extracts, W7 was without effect (Figure 2B). Thus, the effects of Ca2+/CaM inhibitors on EEA1 do not seem to be attributable to direct inhibitory effects on the catalytic activity of Vps34 nor any other PI 3-kinase isoform.
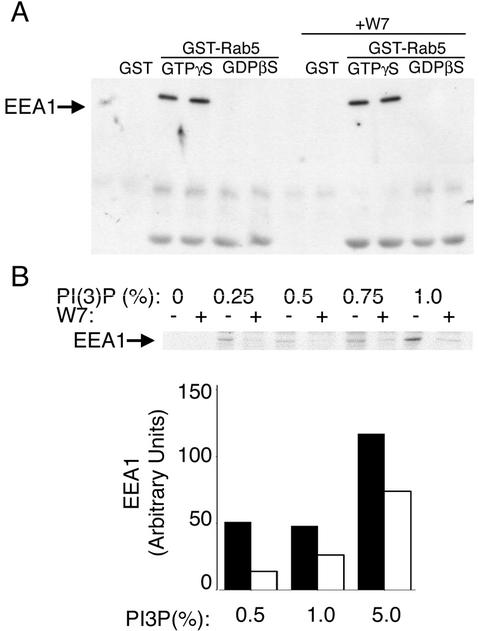
Figure 2.
(A) Effect of CaM inhibitors on PI 3-kinase activity. COS-7 cells were transfected with myc-tagged Vps34, lysed, and immunoadsorbed with anti-myc antibody immobilized on Sepharose beads. Beads were washed and incubated with [γ-32P]ATP and liposomes in the presence of the concentrations of inhibitor indicated under each lane. Lipids were separated by TLC and analyzed by autoradiography. The position of PI(3)P on the chromatogram is indicated by the arrow. (B) Postnuclear extracts from 3T3 cells were prepared as described in MATERIALS AND METHODS and incubated with wortmannin (Wort) or W7 at 100 nM or 100 μM final concentration, respectively, for 1 h. Cytosol was separated by high-speed centrifugation and incubated with PS/PI liposomes for 30 min. Liposomes were pelleted and spotted on nitrocellulose, which was probed with GST-SARA-FYVE and antibodies to GST. The specificity of the probe is depicted in the bottom row, in which 1 μg of the indicated lipids was spotted.
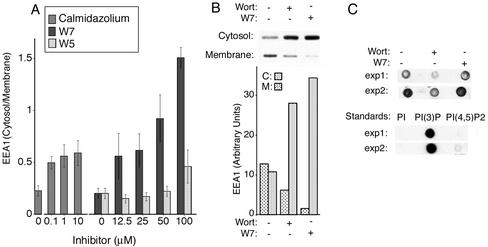
To further rule out that the effects of CaM inhibitors might be due to targets in the PI 3-kinase pathway that may not be reflected in catalytic activity assays (e.g., kinase targeting to the endosomal membrane), we measured the endogenous levels of PI(3)P in membranes and compared these to the distribution of EEA1. In postnuclear extracts prepared in the presence of ATP and an ATP-regenerating system, EEA1 was detected in both high-speed supernatant (cytosol) and pellet (membrane) fractions obtained after centrifugation (Figure 3, A and B). Incubation of the postnuclear extracts with CaM inhibitors for 15 min resulted in a redistribution of EEA1 into cytosolic fraction, with a pharmacological profile similar to that seen in intact cells, and consistent with a specific inhibition of Ca2+/CaM (Figure 3A). Longer incubation with wortmannin and W7 for 60 min before centrifugation resulted in a more pronounced increase in the proportion of EEA1 distributed in the cytoplasmic fraction by both inhibitors, where W7 consistently caused a more pronounced redistribution than wortmannin (Figure 3B). The levels of PI(3)P in chloroform/methanol extracts of the postnuclear pellets was measured in replicates from experiments similar to that shown in Figure 3B, and are shown in Figure 3C. Endogenous levels of PI(3)P were decreased by wortmannin by ∼60%, but were not detectably decreased by W7. Thus, CaM inhibitors cause a redistribution of EEA1 from endosomal membranes to the cytosol under conditions in which the membrane levels of PI(3)P are not detectably affected. These results indicate that the point at which CaM inhibitors disrupt the steady-state distribution of EEA1 is downstream of the PI 3-kinase pathway.
Figure 3.
Effects of the Ca2+/CaM inhibitor W7 on the association of EEA1 with cell membranes and on the membrane levels of PI(3)P. (A) Dose response of EEA1 to Ca2+/CaM inhibitors: A postnuclear supernatant from 3T3 cells was obtained as described in MATERIALS AND METHODS, dispensed into aliquots, and incubated for 15 min with CaM inhibitors at the concentrations indicated. Cytosol was separated from membranes by high-speed centrifugation. The membrane pellet was resuspended in cytosol buffer to the same volume as the cytosol and equal volume aliquots analyzed by SDS-PAGE and immunoblotting with antibodies to EEA1 and chemiluminescence. The exposed films were scanned and the intensity of each band quantified. The ratio of the cytosol and membrane intensities was plotted. Bars represent the mean, and lines the SE of three independent experiments. (B) The experiment was performed as described in A with no additions, 100 nM wortmannin (Wort), or 100 μM W7 for 60 min. The insets illustrate the immunoblots of the membrane and cytosolic extracts. Plotted are the intensities of each band. This experiment was repeated five times with similar results (C) The resuspended membrane pellets were extracted with chlorophorm/methanol as described in MATERIALS AND METHODS. Extracted lipids were spotted on nitrocellulose and probed with GST-SARA-FYVE as described in MATERIALS AND METHODS and in Figure 2. Two experiments are illustrated, with their corresponding standards. This experiment was repeated four times with similar results.
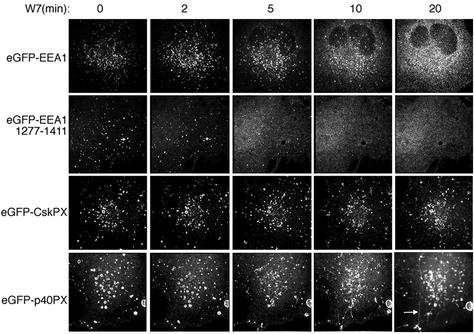
Because PI 3-kinases remain active in the presence of W7 it would be expected that the distribution of proteins other than EEA1 that bind to PI(3)P with high affinity would remain unchanged upon inhibition of CaM function. The PX domains of p40phox and the protein kinase CISK have been shown to interact with PI(3)P and to localize to endosomes (Cheever et al., 2001; Ellson et al., 2001; Kanai et al., 2001; Song et al., 2001; Virbasius et al., 2001; Xu et al., 2001; Yu and Lemmon, 2001). We compared the effects of W7 on the localization of eGFP-tagged constructs of full-length EEA1, of the C-terminal domain of EEA1, and of the PX domains of p40Phox and CISK. Although W7 caused a rapid and almost complete redistribution of full-length and C-terminal EEA1 from endosomes to cytosol, it did not result in a comparable redistribution of the PX domain constructs (Figure 4), as predicted from its lack of effect on PI(3)P levels. Interestingly, in several cells the eGFP-p40PX domain probes could be seen distributed on tubules that emanated from vesicular endosomes toward the cell periphery (Figure 4, lower left, arrow and Video 1). Tubular extensions occurred within the time frame during which EEA1 dissociated from the endosome. Tubulation of endosomes has also been observed in response to wortmannin in cells in which the association of EEA1 with endosomes is sensitive to this inhibitor (Shpetner et al., 1996). Although the role of these tubular endosomal extensions in normal endosomal function is unclear, it is possible that they may have an important role in trafficking.
Figure 4.
Effect of Ca2+/CaM inhibition on full-length EEA1, the C termini of EEA1, and the p40Phox and CISK PX domains in live cells. COS-7 cells were transfected with the construct indicated on the left and exposed to 100 μM W7. Stacks of 21 optical sections were obtained every 20 s, restored, and projected into single 2D images. Projections for the times indicated on top of each panel are shown. Arrow in bottom right panel indicates the formation of tubules in response to W7. The complete set of images for the p40Phox construct can be seen as a QuickTime video (Video 1).
Although it is widely accepted that W7 interacts specifically with Ca2+/CaM, nonspecific interactions of this inhibitor with other potential targets cannot be ruled out. Thus, we sought to complement these experiments with the use of a potent mAb raised against CaM (Sacks, 1994). Increasing concentrations of this antibody added to postnuclear extracts of cells caused a progressive redistribution of EEA1 into the cytoplasmic fraction (Figure 5A). Based on the reported total cellular concentration of CaM of 30–40 μM, the concentration in these postnuclear extracts was estimated to be 6–8 μM. Thus, EEA1 redistributed into the cytosol in direct proportion to concentrations of the antibody that approached the concentration of CaM in the extract. To determine the effects of anti-CaM antibody in the absence of cytoplasmic CaM, postnuclear extracts were centrifuged, the cytosol removed, and pellets resuspended in cytosol buffer containing anti-CaM antibody, equal amounts of control IgG, W7, or wortmannin. After 1 h at room temperature, membranes were collected by centrifugation, and the release of EEA1 into the supernatant analyzed by SDS-PAGE and immunoblotting with anti-EEA1 antibody (Figure 5B). Anti-CaM antibody, W7, and wortmannin, but not IgG control, significantly increased the release of EEA1 into the buffer.
Figure 5.
Anti-CaM antibody causes a release of EEA1 from endosomal membranes. (A) Postnuclear supernatant from 3T3 cells was obtained as described in MATERIALS AND METHODS, and aliquots were incubated without or with 100 nM wortmannin (Wort), 100 μM W7, or the indicated concentration of nonimmune mouse IgG (IgG) or anti-CaM IgG (anti-CaM) for 60 min. Membranes and cytosol were separated by high-speed centrifugation and analyzed by SDS-PAGE and immunoblotting with antibodies to EEA1 and chemiluminescence. The exposed films were scanned and the intensity of each band quantified and plotted. Inset illustrates the immunoblot from the membrane fractions. Bars represent the mean and lines the SE of the mean from three independent experiments. (B) The membrane fraction from 3T3 cells was isolated by high-speed centrifugation, resuspended in cytosol buffer, and incubated without or with 100 nM wortmannin (Wort), 100 μM W7, or 5 μM nonimmune mouse IgG (IgG) or anti-CaM IgG (Anti-CaM) for 60 min at room temperature. The membrane pellet was separated from supernatant and equal quantities of the resulting fractions immunoblotted for EEA1. Immunoblots were scanned, and the intensities of the bands quantified. Plotted are the ratios of supernatant and pellet. Bars represent the mean and lines the SE of the mean from four independent experiments. Inset illustrates a Western blot of the supernatant from one experiment. (C) Anti-CaM antibody was microinjected into COS-7 cells, which were fixed within 5 min of injection (top) or after 45 min postinjection. Injected antibody was detected with anti-mouse secondary antibodies and endogenous EEA1 with human anti-EEA1 antiserum and antihuman secondary antibodies. Arrows in top panels point to sites of colocalization of the two probes and in lower panels point to the injected cell.
The effect of microinjection of the anti-CaM mAb on EEA1 localization in cells was also analyzed (Figure 5C). In cells fixed within 5–10 min after microinjection with low antibody concentrations, the injected anti-CaM antibody localized predominantly at the cell periphery and to vesicular structures that also contained endogenous EEA1. This result is consistent with previous reports in which CaM has been found associated with endosomal fractions (Enrich et al., 1988). When cells were fixed longer after injection, EEA1 displayed a more diffuse cytoplasmic localization and depletion from peripheral endosomes was observed in at least 50% of the injected cells (Figure 5C, lower panels). These results indicate that EEA1 containing endosomes are enriched in CaM relative to other membrane structures and that inhibition of CaM by titration with a specific mAb negatively affects EEA1 binding to membranes.
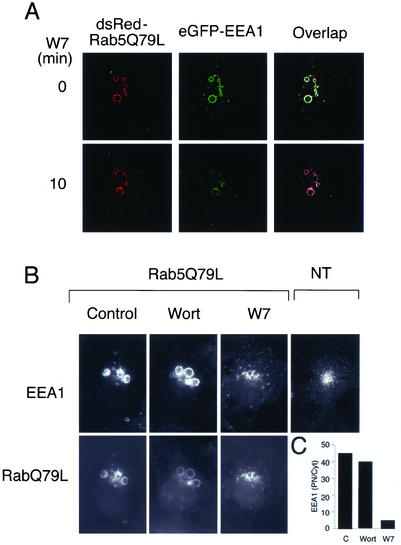
In addition to the FYVE domain, which mediates multivalent binding to PI(3)P (Dumas et al., 2001), the C termini of EEA1 contains a region that interacts with the Rab5 GTPase. Cells expressing a persistently activated form of Rab5, dsRed-RabQ79L, display greatly enlarged endosomes, to which the majority of transfected eGFP-EEA1 (Figure 6A) or endogenous cellular EEA1 (Figure 6B) is associated at steady state. Exposure to W7 for ∼10 min resulted in a decrease in the amount of transfected eGFP-EEA1 (Figure 6A) or endogenous cellular EEA1 (Figure 6B) associated with Rab5Q79L-containing endosomes and a decrease in their size. The decrease in the amount of eGFP-EEA1 associated with dsRed-Rab5Q79-containing endosomes occurred with a time course that parallels the decrease in the size of such endosomes (Figure 6A). The association of EEA1 with dsRed-Rab5Q79-containing endosomes is not greatly decreased by treatment for 15 min with wortmannin (Figure 6B), perhaps due to a high level of PI(3)P generated by wortmannin-insensitive kinases or to a slow turnover of the pool of PI(3)P bound to EEA1. The much larger effect of W7 compared with wortmannin suggests that Ca2+/CaM inhibition causes either the dissociation of EEA1 from existing PI(3)P or inhibits its association with persistently activated Rab5.
Figure 6.
(A) Effects of W7 on eGFP-EEA1 and endogenous EEA1 association to enlarged endosomes containing persistently activated Rab5. COS-7 cells were cotransfected with dsRed-Rab5Q79L and eGFP-EEA1. Cells expressing both constructs were localized and imaged as described in Figure 1. Stacks of 21 optical sections were obtained every 30 s, restored, and projected into single 2D images. Two time points are illustrated. The overlap of the red and green signal is displayed as pure white in regions of equal pixel intensity. (B) COS-7 cells were transfected with dsRed-Rab5Q79L, and after 36 h treated for 10 min with 100 nM wortmannin (Wort) or 100 μM W7. Cells were fixed and endogenous EEA1 was detected using a human antiserum, and Alexa 488 secondary antibodies. Cells shown express similar levels of Rab5Q79L based on fluorescence intensity. A nontransfected cell (NT) is shown for comparison. (C) The fluorescence intensity of EEA1 on the endosomes containing Rab5Q79L and on the cytoplasmic region surrounding the endosomes was quantified and the ratio plotted.
To distinguish between these possibilities, the binding of EEA1 to Rab5 was measured directly using cytoplasmic extracts from 3T3 cells and recombinant GST-Rab5 immobilized on glutathione beads. Binding of endogenous EEA1 to beads loaded with Rab5-GTPγS, but not to beads loaded with Rab5GDPβS or GST alone was observed. Addition of W7 to the cytosol 60 min before the incubation with beads did not impair the binding of EEA1 to Rab5GTPγS (Figure 7A). Thus, the decreased interaction between EEA1 and Rab5Q79L-enriched endosomes seems not to be due to a disruption in the interaction between Rab5 and EEA1. To test the possibility that the failure of EEA1 to associate with the endosomal membrane was due to a disruption of its binding to endosomal phosphoinositide, cytoplasmic extracts were treated without or with W7 and then incubated with liposomes containing increasing concentrations of PI(3)P. A significant decrease in the binding of cytosolic EEA1 to such endosomes was observed in response to W7. The inhibitory effect of W7 was greater at lower PI(3)P concentrations, suggesting that inhibition of Ca2+/CaM reduces the affinity of EEA1 binding to the liposome surface (Figure 7B). This decreased affinity, in the context of the endosomal concentration of PI(3)P, could result in the dramatic displacement of EEA1 from the endosomal membrane observed in intact cells.
Figure 7.
Effects of Ca2+/CaM inhibition on EEA1 association with activated Rab5 or PI(3)P. (A) Cytosolic extracts of 3T3 cells were incubated with nothing or with 100 μM W7 for 60 min and then incubated with GST alone or recombinant GST-tagged Rab5 immobilized on glutathione-Sepharose. GST-Rab5 was loaded with GTPγS or GDPβS as indicated. Bound proteins were analyzed by SDS-PAGE and immunoblotting with antibodies to EEA1. The position of EEA1 on the gel is indicated. Duplicates of each condition were done in each experiment, and three experiments with similar results were obtained. (B) Cytosolic extracts of 3T3 cells were incubated with nothing or with 100 μM W7 for 60 min and incubated with PS/PI liposomes containing increasing concentrations of PI(3)P as indicated above each lane. Liposomes were pelleted and analyzed by immunoblotting with antibodies to EEA1. The exposed films were scanned and the intensity of each band quantified. The inset represents the immunoblot from one experiment, and the graph represents the results from a different experiment. Similar results were obtained in three independent experiments.
The pronounced effect of CaM inhibition to cause displacement of EEA1 from the lipid bilayer surface both in vivo and in liposome binding assays suggest that CaM might directly interact with EEA1. In fact, a direct association of Ca2+/CaM with the IQ-like motif at the C termini of EEA1 has been proposed based on overlay assays of GST-fusion constructs of the EEA1 C termini in the presence of high Ca2+ concentrations (Mills et al., 2001). To determine whether this interaction might be directly involved in the interaction of EEA1 with the lipid bilayer in vivo, point mutations were introduced in residues crucial to IQ domain structure, and cells expressing wild-type GFP-tagged EEA1 were compared with those expressing the IQ domain mutant (eGFPEEA1Q1289L/R1293G) at similar levels. As previously reported by Stenmark et al. (1996), this mutation in EEA1 did not detectably impair its association with endosomes (our unpublished data) and suggests that the IQ-like motif may not be the single target of CaM or Ca2+/CaM in EEA1.
To identify additional regions within EEA1 required for Ca2+/CaM-dependent bilayer interactions, we analyzed the binding of wild-type EEA1, of the Q1289L/R1293G mutation, and of deletion constructs of EEA1 to CaM immobilized on Sepharose beads. Cytosolic extracts prepared from COS-7 cells overexpressing each construct were incubated with CaM-Sepharose, or Sepharose beads alone. eGFP-tagged full-length EEA1 bound to CaM-Sepharose, but not to Sepharose control beads (Figure 8A). The Ca2+ chelators BAPTA and EGTA markedly, but not completely, impaired the binding of EEA1 to CaM-Sepharose. Further addition of 2 μM Ca2+ to the cytosolic extract did not augment the binding of EEA1, and higher concentrations of Ca2+ resulted in a pronounced increase in nonspecific binding, as assessed by the similarly increased binding of EEA1 to both CaM-Sepharose and Sepharose control beads (our unpublished data). The low calcium concentrations required for EEA1 binding to CaM are similar to those reported to be required for in vitro endosome fusion (Peters and Mayer, 1998; Holroyd et al., 1999).
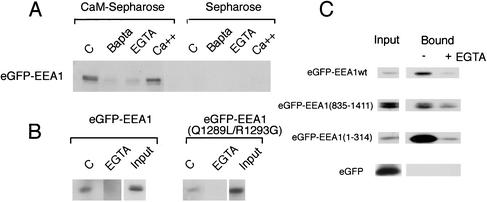
Figure 8.
Association of GFP-tagged wild-type and mutant EEA1 with immobilized CaM. (A) The cytoplasmic extract from COS-7 cells expressing GFP-EEA1wt was incubated in the absence (C) or presence of 10 mM BAPTA, 10 mM EGTA or with an additional 2 μM CaCl2 for 30 min at room temperature. The extract was then incubated with CaM-Sepharose or Sepharose alone as described in MATERIALS AND METHODS. Bound proteins were analyzed by immunoblotting with antibody to GFP. (B) Cytoplasmic extracts from COS-7 cells expressing either eGFP-EEA1 or eGFP-EEA1 containing mutations in the IQ-like motif (eGFPEEA1Q1289L/R1293G) were incubated in the presence or absence of EGTA. Binding to immobilized CaM was then determined as described in A. The concentration of the eGFP constructs in 20% of the starting cell extract (input) is shown. (C) Cytosol from cells expressing either GFP alone, GFP-EEA1wt, or EEA1 deletion constructs comprised of the amino acids indicated were incubated with or without EGTA and assessed for binding to CaM-Sepharose as described in A.
The binding of eGFP-EEA1Q1289L/R1293G to CaM-Sepharose was only slightly decreased compared with wild-type eGFP-EEA1 (Figure 8B), and the Ca2+-sensitivity of binding was largely preserved. In contrast, deletion of the N terminus of EEA1 [eGFP-EEA1(835–1411)] greatly decreased Ca2+-dependent binding to CaM-Sepharose (Figure 8C, compare ratios of input to bound). Further deletion of the N terminus to amino acid 1277 had no further effect, and neither of these truncations induced nonspecific binding to Sepharose beads alone (our unpublished data). These results suggest the presence of a Ca2+/CaM binding site within the N-terminal half of EEA1. To further test this hypothesis, a GFP-construct containing the first 314 amino acids of the EEA1 N terminus was analyzed. This construct bound avidly to CaM-Sepharose, but not Sepharose alone, in a Ca2+-dependent manner (Figure 8C). Thus, at least two separate domains in EEA1 are capable of mediating Ca2+-dependent and Ca2+-independent CaM binding interactions.
DISCUSSION
A crucial requirement for Ca2+ and CaM in endosome fusion in vitro and in vivo has been previously documented, but the precise mechanism for this requirement has not been fully determined. In this manuscript, we identify EEA1 as a major Ca2+/CaM-sensitive component of the endosome fusion machinery, which may at least in part explain the actions of CaM inhibition on this process.
EEA1 binding to endosomal membranes is known to depend on its C-terminal FYVE domain, which directly binds to PI(3)P on the endosomal surface. In addition, EEA1 interacts with the activated small GTPase Rab5, and expression of persistently active Rab5 causes a marked increase in EEA1 binding to endosomes and endosome enlargement. The observations made herein indicate that Ca2+/CaM also plays a critical role in the binding of EEA1 to the endosome surface. This requirement may reflect additional, novel interactions between EEA1, CaM, and yet unidentified targets on the endosomal surface. Alternatively, the effect of Ca2+/CaM may be to regulate the conformation of EEA1 to effectively interact with endosomal Rab5 or PI(3)P. The results presented herein suggest the latter, based principally from the observed inhibition of EEA1 binding to liposomes containing PI(3)P upon inhibition of Ca2+/CaM.
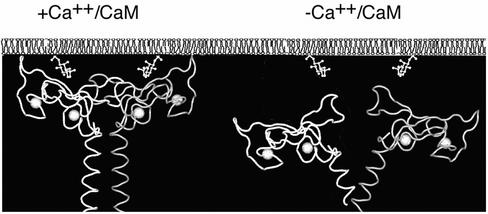
Although the precise mode by which Ca2+/CaM might control the interaction between EEA1 and PI(3)P is not clear, the recently solved crystal structure of the C termini of EEA1 complexed to PI(3)P head group (Dumas et al., 2001), as well as by new examples of mechanisms of interaction of Ca2+/CaM with protein targets (Hoeflich and Ikura, 2002) suggest possible mechanisms. The crystal structure of the C termini of EEA1 reveals a parallel coiled-coil dimer that terminates abruptly in a splayed FYVE domain (Figure 9). Notably, the quaternary structural arrangement of the EEA1 FYVE domain includes an extensive interface involving nonconserved residues of the FYVE domain structure. This interface is unusual in that, beyond displaying a high degree of complementarity between the two interacting surfaces, there are no amino acid residues that stabilize it through conventional electrostatic or hydrophobic interactions. Thus, a rather complex interface seems to be held together solely through weak van der Waals forces (Dumas et al., 2001). This conformation suggests that additional energetic contributions may be required for stabilization of the dimer interface and the FYVE domain quaternary structure. The heptad repeats that immediately precede the FYVE domain may provide such additional stabilization, which may be further enhanced by external elements such as CaM. This stabilization may be crucial in enabling the bivalent interaction of EEA1 with the PI(3)P head group, and thereby increasing the affinity of EEA1 for PI(3)P on the endosomal membrane surface (Figure 9).
Figure 9.
Model depicting hypothetical effect of CaM on the structure of the C termini of EEA1 and consequent binding to PI(3)P on membranes. Solid bars represent the coiled-coil region of each monomer, leading into the splayed FYVE domains.
Elucidating the precise mechanism of CaM function requires information on the mechanism(s) of CaM interaction with EEA1. Previous results have shown that biotinylated CaM can bind in an overlay assay to a GST-fusion protein containing the EEA1 C termini, but not to a similar construct containing a deletion of the IQ motif (Mills et al., 2001). Under these conditions, binding was enhanced by the presence of large quantities (100 μM) of Ca2+ or Zn2+ in the overlay buffer. These results suggested that the IQ motif in EEA1 directly binds Ca2+/CaM. In the present study, the interaction of EEA1 from cytosolic extracts with CaM-Sepharose or Sepharose alone as a control was measured. Fulllength EEA1 and a construct harboring point mutations in residues critical for the conformation of the IQ motif bound comparably to CaM-Sepharose (Figure 8B). These observations suggested that Ca2+/CaM binding to EEA1 is not restricted to the C-terminal IQ domain. Direct analysis of truncation constructs of EEA1 reveals two independent regions capable of CaM binding. One site is located within the first 314 amino acids of the N terminus of EEA1, and the other within the 134 amino acids of the C termini. Although the N-terminal binding site seems to bind exclusively to Ca2+/CaM but not to CaM, both full-length EEA1 and constructs containing the C-terminal region display significant Ca2+-insensitive CaM binding (Figure 8). The detection of Ca2+-independent CaM binding by the C termini in our studies but not in those reported by Mills et al. (2001) may be explained by the differences in the concentrations of Ca2+ used in each study, and inherent to the different techniques used.
The extent of binding of EEA1 C-terminal constructs to CaM-Sepharose was similar to the binding of full-length EEA1 to CaM-Sepharose in the presence of chelators, suggesting a major contribution of the N-terminal binding site in Ca2+/CaM binding, and the existence of a Ca2+-independent CaM binding contribution at the C termini. Notably, binding of the IQ domain point mutant of EEA1 to CaM-Sepharose was not fully inhibited by Ca2+ chelators, suggesting CaM binding to the C termini on sites additional to the IQ motif. Complex mechanisms for binding of both apo-CaM and Ca2+/CaM to a homodimer have a precedent in the small conductance Ca2+ activated potassium channel SK2 (Schumacher et al., 2001).
A previous study (Mills et al., 2001) has shown that large concentrations of CaM in the presence of 100 μM Ca2+ antagonize the binding of PI(3)P-containing liposomes and of Rab5-GTP to immobilized EEA1. This particular result suggests that Ca2+/CaM might be a negative regulator of EEA1 association with the endosomal surface. This model is inconsistent with results presented in this manuscript, in which Ca2+/CaM seems to be a requirement for EEA1 binding to endosomes in vitro and in intact cells. However, in the study by Mills et al. (2001) addition of a large concentration of Ca2+/CaM is reported not to impair the binding of EEA1 to endosomal membranes in a broken cell system. This result suggests that under conditions that better represent the intracellular environment Ca2+/CaM is not a negative regulator of EEA1 association with the endosomal surface. Moreover, biochemical studies of endosome fusion in vitro have shown a profound inhibitory effect of CaM inhibitors and Ca2+ chelators on fusion (Gorvel et al., 1991; Colombo et al., 1997; Mills et al., 1998, 2001). Thus, most experimental results are consistent with a model in which Ca2+/CaM is a positive regulator of EEA1 binding to the endosomal surface and of endosome fusion.
A decrease in the endosomal binding of EEA1 is observed in response to wortmannin, to Ca2+/CaM inhibitors and to dominant-negative Rab5 constructs, supporting a model in which EEA1 is a crucial element in early endosome fusion operating downstream of PI 3-kinase and Rab5 in a CaM-dependent manner. Interestingly, however, at the cellular level these three manipulations lead to different phenotypes: cells expressing dominant-negative mutants of Rab5 contain small endosomes and display a block in transferrin uptake, whereas cells treated with wortmannin or with CaM inhibitors contain tubulated and enlarged endosomes, normal transferrin uptake, but decreased transferrin recycling. These observations suggest that the results of in vitro early endosome fusion assays reflect the collective fusion events taking place in the uptake and recycling phases in the endosomal system. Because manipulations that impair the association of EEA1 with endosomes in intact cells result mainly in defective recycling, this molecule may in fact control fusion events in the recycling portion more than in the initial phases of the endosome fusion pathway. More extensive analysis of specific molecular elements that regulate endosome fusion in vitro in intact cells will be required to fully understand the functional architecture of the endocytic pathway. Nevertheless, the results presented here explain the molecular basis for the observed requirement for Ca2+/CaM in endosome fusion in vitro and in intact cells.
Supplementary Material
Acknowledgments
We acknowledge the helpful discussions with Dr. Akira Hayakawa, and the gift of anti-CaM antibody from Dr. David Sacks. This work was funded by National Institutes of Health grants DK60564 and DK54479 (to S.C.).
The online version of this article contains video material for some figures. Online version is available at silvia.corvera@umassmed.edu.
References
- Apodaca, G., Enrich, C., and Mostov, K.E. (1994). The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in Madin-Darby canine kidney cells. J. Biol. Chem. 269, 19005-19013. [PubMed] [Google Scholar]
- Chamberlain, L.H., Roth, D., Morgan, A., and Burgoyne, R.D. (1995). Distinct effects of alpha-SNAP, 14-3-3 proteins, and calmodulin on priming and triggering of regulated exocytosis. J. Cell Biol. 130, 1063-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever, M.L., Sato, T.K., de Beer, T., Kutateladze, T.G., Emr, S.D., and Overduin, M. (2001). Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol. 3, 613-618. [DOI] [PubMed] [Google Scholar]
- Chen, Y.A., Scales, S.J., Duvvuri, V., Murthy, M., Patel, S.M., Schulman, H., and Scheller, R.H. (2001). Calcium regulation of exocytosis in PC12 cells. J. Biol. Chem. 276, 26680-26687. [DOI] [PubMed] [Google Scholar]
- Colombo, M.I., Beron, W., and Stahl, P.D. (1997). Calmodulin regulates endosome fusion. J. Biol. Chem. 272, 7707-7712. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., McBride, H.M., Burgoyne, R.D., and Zerial, M. (1999). The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621-625. [DOI] [PubMed] [Google Scholar]
- Dumas, J.J., Merithew, E., Sudharshan, E., Rajamani, D., Hayes, S., Lawe, D., Corvera, S., and Lambright, D.G. (2001). Multivalent endosome targeting by homodimeric EEA1. Mol. Cell 8, 947-958. [DOI] [PubMed] [Google Scholar]
- Ellson, C.D., et al. (2001). PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox). Nat. Cell Biol. 3, 679-682. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou, E., Teschemacher, A.G., Pouli, A.E., Nicholls, L.I., Seward, E.P., and Rutter, G.A. (1999). Imaging Ca2+ concentration changes at the secretory vesicle surface with a recombinant targeted cameleon. Curr. Biol. 9, 915-918. [DOI] [PubMed] [Google Scholar]
- Enrich, C., Bachs, O., and Evans, W.H. (1988). A 115 kDa calmodulin-binding protein is located in rat liver endosome fractions. Biochem. J. 255, 999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel, J.P., Chavrier, P., Zerial, M., and Gruenberg, J. (1991). rab5 controls early endosome fusion in vitro. Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- Hoeflich, K.P., and Ikura, M. (2002). Calmodulin in action: diversity in target recognition and activation mechanisms. Cell 108, 739-742. [DOI] [PubMed] [Google Scholar]
- Holroyd, C., Kistner, U., Annaert, W., and Jahn, R. (1999). Fusion of endosomes involved in synaptic vesicle recycling. Mol. Biol. Cell. 10, 3035-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, L.A., Fialka, I., Paiha, K., Hunziker, W., Sacks, D.B., Bahler, M., Way, M., Gagescu, R., and Gruenberg, J. (2000). Both calmodulin and the unconventional myosin Myr4 regulate membrane trafficking along the recycling pathway of MDCK cells. Traffic 1, 494-503. [DOI] [PubMed] [Google Scholar]
- Hutton, J.C. (1986). Calcium-binding proteins and secretion. Cell Calcium 7, 339-352. [DOI] [PubMed] [Google Scholar]
- Kanai, F., Liu, H., Field, S.J., Akbary, H., Matsuo, T., Brown, G.E., Cantley, L.C., and Yaffe, M.B. (2001). The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol. 3, 675-678. [DOI] [PubMed] [Google Scholar]
- Lawe, D.C., A. Chawla, E. Merithew, J. Dumas, W. Carrington, K. Fogarty, L. Lifshitz, R. Tuft, D. Lambright, and S. Corvera. (2002). Sequential roles for phosphatidylinositol 3-phosphate and Rab5 in tethering and fusion of early endosomes via their interaction with EEA1. J. Biol. Chem. 277, 8611-8617. [DOI] [PubMed] [Google Scholar]
- Mills, I.G., Jones, A.T., and Clague, M.J. (1998). Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr. Biol. 8, 881-884. [DOI] [PubMed] [Google Scholar]
- Mills, I.G., Jones, A.T., and Clague, M.J. (1999). Regulation of endosome fusion. Mol. Membr. Biol. 16, 73-79. [DOI] [PubMed] [Google Scholar]
- Mills, I.G., Urbe, S., and Clague, M.J. (2001). Relationships between EEA1 binding partners and their role in endosome fusion. J. Cell Sci. 114, 1959-1965. [DOI] [PubMed] [Google Scholar]
- Mu, F.T., Callaghan, J.M., Steele-Mortimer, O., Stenmark, H., Parton, R.G., Campbell, P.L., McCluskey, J., Yeo, J.P., Tock, E.P., and Toh, B.H. (1995). EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 270, 13503-13511. [DOI] [PubMed] [Google Scholar]
- Nishikawa, M., and Hidaka, H. (1982). Role of calmodulin in platelet aggregation. Structure-activity relationship of calmodulin antagonists. J. Clin. Investig. 69, 1348-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki, V., Buxton, J., Chawla, A., Lifshitz, L., Fogarty, K., Carrington, W., Tuft, R., and Corvera, S. (2001). Insulin action on GLUT4 traffic visualized in single 3T3–l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 12, 129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, C., and Mayer, A. (1998). Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396, 575-580. [DOI] [PubMed] [Google Scholar]
- Pryor, P.R., Mullock, B.M., Bright, N.A., Gray, S.R., and Luzio, J.P. (2000). The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 149, 1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, D.G. (2001). Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell. Biol. 2, 569-577. [DOI] [PubMed] [Google Scholar]
- Sacks, D.B. (1994). Alteration of calmodulin-protein interactions by a monoclonal antibody to calmodulin. Biochim. Biophys. Acta 1206, 120-128. [DOI] [PubMed] [Google Scholar]
- Schumacher, M.A., Rivard, A.F., Bachinger, H.P., and Adelman, J.P. (2001). Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 410, 1120-1124. [DOI] [PubMed] [Google Scholar]
- Siddhanta, U., McIlroy, J., Shah, A., Zhang, Y., and Backer, J.M. (1998). Distinct roles for the p110alpha and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J. Cell Biol. 143, 1647-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., Lippe, R., Christoforidis, S., Gaullier, J.M., Brech, A., Callaghan, J., Toh, B.H., Murphy, C., Zerial, M., and Stenmark, H. (1998). EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494-498. [DOI] [PubMed] [Google Scholar]
- Song, X., Xu, W., Zhang, A., Huang, G., Liang, X., Virbasius, J.V., Czech, M.P., and Zhou, G.W. (2001). Phox homology domains specifically bind phosphatidylinositol phosphates. Biochemistry 40, 8940-8944. [DOI] [PubMed] [Google Scholar]
- Shpetner, H., Joly, M., Hartley, D., and Corvera, S. (1996). Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol. 132, 595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., Aasland, R., Toh, B.-H., and D'Arrigo, A. (1996). Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 271, 24048-24054. [DOI] [PubMed] [Google Scholar]
- Virbasius, J.V., Guilherme, A., and Czech, M.P. (1996). Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J. Biol. Chem. 271, 13304-13307. [DOI] [PubMed] [Google Scholar]
- Virbasius, J.V., Song, X., Pomerleau, D.P., Zhan, Y., Zhou, G.W., and Czech, M.P. (2001). Activation of the Akt-related cytokine-independent survival kinase requires interaction of its phox domain with endosomal phosphatidylinositol 3-phosphate. Proc. Natl. Acad. Sci. USA 98, 12908-12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., Hortsman, H., Seet, L., Wong, S.H., and Hong, W. (2001). SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat. Cell Biol. 3, 658-666. [DOI] [PubMed] [Google Scholar]
- Yu, J.W., and Lemmon, M.A. (2001). All phox homology (PX) domains from Saccharomyces cerevisiae specifically recognize phosphatidylinositol 3-phosphate. J. Biol. Chem. 276, 44179-44184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.