Abstract
Insulin stimulates the movement of glucose transporter-4 (Glut4)–containing vesicles to the plasma membrane of adipose cells. We investigated the role of post-Golgi t-soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) in the trafficking of Glut4 in 3T3-L1 adipocytes. Greater than 85% of syntaxin 6 was found in Glut4-containing vesicles, and this t-SNARE exhibited insulin-stimulated movement to the plasma membrane. In contrast, the colocalization of Glut4 with syntaxin 7, 8, or 12/13 was limited and these molecules did not translocate to the plasma membrane. We used adenovirus to overexpress the cytosolic domain of these syntaxin's and studied their effects on Glut4 traffic. Overexpression of the cytosolic domain of syntaxin 6 did not affect insulin-stimulated glucose transport, but increased basal deGlc transport and cell surface Glut4 levels. Moreover, the syntaxin 6 cytosolic domain significantly reduced the rate of Glut4 reinternalization after insulin withdrawal and perturbed subendosomal Glut4 sorting; the corresponding domains of syntaxins 8 and 12 were without effect. Our data suggest that syntaxin 6 is involved in a membrane-trafficking step that sequesters Glut4 away from traffic destined for the plasma membrane. We speculate that this is at the level of traffic of Glut4 into its unique storage compartment and that syntaxin 16 may be involved.
INTRODUCTION
Insulin stimulates glucose transport in adipose and muscle cells by inducing the movement (“translocation”) of intracellular Glut4-containing vesicles to the cell surface (Pessin et al., 1999; Bryant et al., 2002). In the absence of insulin, >90% of the cellular Glut4 is intracellularly sequestered and has been localized to the endosomal system, the trans-Golgi network (TGN) and a tubulo-vesicular compartment present throughout the cell (Slot et al., 1991, 1997; Martin et al., 1994; Ploug et al., 1998; Ramm et al., 2000). In response to insulin, Glut4 levels in each of these regions are decreased, concomitant with the movement of Glut4 to the cell surface (Slot et al., 1991, 1997; Martin et al., 1994; Ploug et al., 1998; Ramm et al., 2000). Recent studies have put forward the notion that the majority of the insulin-responsive Glut4 is localized to a specialized compartment that is analogous to small secretory vesicles present in neuroendocrine tissues, termed Glut4 storage vesicles (GSVs) (reviewed in (Rea and James, 1997; Pessin et al., 1999; Bryant et al., 2002).
However, it is clear that the trafficking of Glut4 in adipocytes and muscle involves a complex interplay between multiple cellular compartments that include endosomes and the TGN in addition to the GSVs. Thus, for example, a considerable fraction of Glut4 is found colocalized with mannose-6-phosphate receptors (MPRs) and in response to insulin there seems to be a budding of Glut4-containing vesicles from this compartment (Martin et al., 2000; Ramm et al., 2000). In contrast, electron microscopic analysis predicts that the GSVs may move directly to the cell surface without the requirement for a budding step (Ramm et al., 2000). This latter observation suggests that adipocytes and muscle may contain the machinery required for the packaging of Glut4 within specialized transport containers (GSVs). This model is supported a wealth of data, including the demonstration that Glut4 is targeted to secretory vesicles in cardiomyocytes (Slot et al., 1997). Little is known about the biogenesis of the GSV compartment. Because Glut4 is present in both the endosomes and TGN, it has been speculated that GSVs may arise from either or both of these compartments (Rea and James, 1997).
Studies of the formation of secretory granules in neuroendocrine cells have resulted in a model for the biogenesis of secretory vesicles that involves the formation of an immature secretory granule, from which unwanted cargo molecules (such as MPRs) are removed by selective budding in an AP-1–dependent step (Urbe et al., 1998; Austin et al., 2000; Tooze et al., 2001). In this regard, it is interesting to note that the intracellular distribution of Glut4 overlaps considerably with AP-1 and MPRs (Martin et al., 2000; Ramm et al., 2000), suggesting that the biogenesis of GSVs or trafficking of Glut4 may share some common themes with secretory granule biogenesis.
Recent studies have begun to clarify the roles of different t-soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs)/syntaxin (STX) molecules in intracellular traffic (Jahn and Sudhof, 1999; Pelham, 2001; Teng et al., 2001). STX1-4 are predominantly plasma membrane localized and are thus likely to be mainly involved in traffic at the distal stage of the secretory pathway. In contrast, STX6, 7, 8, 12/13, and 16 have all been localized to either the TGN or endosomal network where they seem to regulate distinct aspects of endosomal traffic. Syntaxin 6 is involved in both TGN-to-endosome traffic and secretory granule biogenesis (Watson and Pessin, 2000; Wendler et al., 2001), STX7 is localized to late endosomes and is required for late endosome-lysosome fusion (Prekeris et al., 1999; Mullock et al., 2000; Nakamura et al., 2000), and STX8 has been localized to early endosomes but may also function in late endosome fusion (Prekeris et al., 1999; Subramanian et al., 2000). Syntaxin 12/13 mediates cycling of plasma membrane proteins via tubulo-vesicular recycling endosomes (Prekeris et al., 1998), and STX16 may function in early endosome/TGN traffic, perhaps in concert with syntaxin 6 (Tang et al., 1998; Mallard et al., 2002).
Although the SNARE proteins involved in the fusion of GSVs with the plasma membrane have been identified and studied (reviewed in Rea and James, 1997; Foster and Klip, 2000), the molecules involved in the intracellular trafficking of Glut4 remain obscure. We set out to determine the extent of overlap of syntaxins 6, 7, 8, and 12/13 with Glut4 in murine 3T3-L1 adipocytes and to determine whether these syntaxins play a specific role in either Glut4 trafficking or the biogenesis of the GSV compartment. We show that each of these syntaxins is present in Glut4 vesicles to some degree, but that only STX6 exhibited extensive overlap with Glut4. Immuno-isolation of Glut4-containing vesicles showed that ∼85% of the cellular STX6 was present in Glut4-containing vesicles. Consistent with this, we report that insulin results in a significant translocation of STX6 to the plasma membrane of adipocytes, reminiscent of the translocation of Glut4 and the insulin-responsive aminopeptidase (IRAP). To define the molecular role of these different syntaxins in Glut4 traffic, we constructed recombinant adenoviruses to express the cytosolic domains of syntaxins 6, 8, or 12 in 3T3-L1 adipocytes. We show that overexpression of STX8 or 12/13 in adipocytes was without effect on cell surface transferrin receptor levels, basal or insulin-stimulated glucose transport, and ACRP30 secretion. In contrast, cells overexpressing STX6 exhibit increased levels of basal transport, but the ability of insulin to stimulate glucose transport and Glut4 translocation were not compromised. Such data prompt the hypothesis that the intracellular sequestration of Glut4 involves STX6. To test this directly, we examined the ability of Glut4 to be reinternalized from the plasma membrane of insulin-stimulated cells overexpressing STX cytosolic domains. We show that overexpression of STX6 significantly slowed the ability of Glut4 to be reinternalized from the cell surface. These data suggest a model in which Glut4 traffic to the specialized storage compartment involves an STX6-dependent event. Finally, we show that STX6 is associated with STX16 in 3T3-L1 adipocytes. Like STX6, STX16 exhibits a high degree of colocalization with Glut4 but seems to be localized to a biochemically distinct compartment. We show that STX16 is a phosphoprotein and that the phosphorylation of STX16 declines upon insulin-stimulation, suggesting that STX16 may be a crucial site of regulation of Glut4 traffic in adipose cells.
MATERIALS AND METHODS
Materials
Antibodies specific for Glut4 were those described in Brant et al. (1993), and monoclonal anti-IRAP serum was provided by Professor Morris J. Birnbaum (University of Pennsylvania, Philadelphia, PA) (Garza and Birnbaum, 2000). Fluorescent secondary antibodies were from Sigma Chemical (Poole, Dorset, United Kingdom), and porcine insulin was the kind gift of Dr. Gillian Danielson (Novo Nordisk, Baagsvaerd, Denmark). Pansorbin was from Calbiochem (San Diego, CA). All other reagents were as described in Millar et al. (1999, 2000). Syntaxin 1A and 4 cDNAs were generously provided by Professor David E. James (Garvan Institute, Sydney, Australia), and STX6 cDNA was generously provided by Dr. Jeff Pessin (University of Iowa, Iowa City, IA). Anti-STX4 was from Chemicon International (Temecula, CA). Anti-STX16 is described in Tang et al. (1998).
Antibody Productionx
cDNAs encoding the cytosolic domains of rat syntaxins 6 and 8 and mouse STX12 (residues 1–234 of STX6, 1–213 of STX8, and 1–247 of STX12) were amplified by polymerase chain reaction, TOPO cloned, and fully sequenced on both strands before subcloning into pQE30 to allow the bacterial production of hexahistidine-tagged recombinant protein. After purification on Ni2+-NTA-agarose columns the proteins were used to produce rabbit polyclonal antibodies (Diagnostics Scotland, Law Hospital, Carluke, Lanarkshire, United Kingdom). The antibodies exhibited specific cross-reactivity with the cognate STX and did not exhibit cross-reactivity to any other recombinant STX tested (STX1A, 2, 3, 4, 6, 7, 8, and 12/13; our unpublished data).
Cell Culture
3T3-L1 fibroblasts were grown and differentiated into adipocytes as described previously (Millar et al., 1999, 2000). Cells were used between passages 2 and 12 and at days 7–12 postdifferentiation. Before experiments, cells were incubated in serum-free media for 2 h. For phosphorylation analysis, cells were incubated in serumfree, phosphate-free DMEM (Invitrogen, Paisley, Scotland) containing 0.25 mCi/ml 32Pi (Amersham Biosciences, Cardiff, United Kingdom) for 2 h. These conditions are sufficient to equilibrate the ATP pool of 3T3-L1 adipocytes (our unpublished data; Gibbs et al., 1986) before stimulation with insulin or treatment with wortmannin.
Adenovirus Production and Infection
cDNAs encoding the soluble cytosolic domains of STX6 (corresponding to amino acid residues 1–234) 8 (corresponding to amino acid residues 1–213), and 12 (corresponding to amino acid residues 1–247) were generated by polymerase chain reaction, subcloned into the vector pCRII (Invitrogen), and fully sequenced on both strands. The constructs were then subcloned into the pShuttle CMV vector, linearized with PmeI, and cotransformed with pAdeasy into Eshcerichia coli strain BJ5183 by electroporation. Recombinants were selected and amplified in E. coli DH5α. The chosen clones were linearized with PacI to expose the inverted terminal repeats and allow viral packaging when transfected in human embryonic kidney 293 cells. Large-scale amplification and titer of viral stocks was performed as outlined in He et al. (1996). In some cases, large-scale viral production was performed commercially by Q-BIOgene (Illkirch Cedex, France).
For infection of 3T3-L1 adipocytes, cells were washed in serumfree DMEM containing 5 mg/ml bovine serum albumin and then incubated in the same media containing virus at a typical multiplicity of infection (m.o.i.) of 50:1 overnight. The next day, the viral containing media was aspirated and replaced with normal media (DMEM containing 10% fetal bovine serum) and the cells were used 24 h later. In our hands, this procedure routinely resulted in >90% of the cells on a dish being infected (our unpublished data). For most of the assays described herein, we infected cells at day 6 postdifferentiation and thus assayed at day 8 (unless otherwise stated).
Glut4 Vesicle Immunoadsorption
Plates (10 cm) of 3T3-L1 cells were incubated in serum-free media for 2 h before washing in ice-cold HES buffer (225 mM sucrose, 25 mM HEPES, 1 mM EDTA, pH 7.4). Cells were homogenized as described and centrifuged to produce a low-density microsomal (LDM) fraction as outlined in Morris et al. (1998). NaCl (1 M) was then added to these fractions to a final concentration of 100 mM. Samples for immunoadsorption were split into two, and to one-half was added Staphylococcus aureus cells loaded with anti-Glut4, the other cells loaded with random rabbit IgG. The LDM and adsorbant were mixed by gentle end-over-end rotation at 4°C for 2 h, the cells collected, and washed in HES buffer three times as described above. After the final wash, bound vesicles were released by incubation in homogenization buffer containing 0.5% Thesit (Roche Diagnostics, Indianapolis, IN) as described previously (Morris et al., 1998).
SDS-PAGE and Immunoblotting
SDS-PAGE and immunoblotting were performed exactly as described in Millar et al. (1999, 2000). Quantification of immunoblot signals was performed as outlined in using a Bio-Rad (Hercules, CA) scanner and associated software. In all cases, multiple exposures of x-ray film were analyzed to be certain of studying immunoreactive signals within the linear range of both the detection antibodies and film.
Immunoprecipitation
Plates of 3T3-L1 adipocytes after experimental manipulation (32Pilabeling, insulin-stimulation, etc.) were washed three times in icecold HPFEV buffer (50 mM HEPES, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 2 mM EDTA, 2 mM sodium orthovanadate, pH 7.4). Cells were then lysed in ice-cold HPFEV containing 2% Thesit (Roche Diagnostics) supplemented with Complete Proteinase Inhibitor tablets. After incubation on ice for 10 min, cells were triturated and transferred to microfuge tubes and incubated a further 10 min on ice. Tubes were then centrifuged at 50,000 × g to pellet insoluble material, and the supernatant used for immunoprecipitations. To immunoprecipitate STX6, 8, or 12, the equivalent of 7.5 μg of anti-STX antibody was used per 10-cm plate of cells. To immunoprecipitate STX16, we used 5 μg of anti-STX16 per 10-cm plate of cells. Immunoprecipitations were performed as outlined in (Gibbs et al., 1986).
Subcellular Fractionation and Iodixanol Gradient Centrifugation
3T3-L1 adipocytes (either untreated or stimulated with 1 μM insulin for 30 min) were washed twice in ice-cold HES buffer (20 mM HEPES, 1 mM EDTA, 225 mM sucrose, pH 7.4), and scraped into HES supplemented with a protease-inhibitor cocktail (Roche Diagnostics). The cells were homogenized and subjected to a differential centrifugation procedure to prepare plasma membrane, heavy and light microsome fractions, as outlined in Millar et al. (1999). Light microsomes (LDMs) contain the majority of the Glut4 that traffics to the cell surface in response to insulin. Heavy microsomes (HDMs) contain dense material such as endoplasmic reticulum (Piper et al., 1991). For iodixanol gradient centrifugation, the homogenate was centrifuged at 41,000 × g for 20 min at 4°C, and the supernatant from this step further centrifuged at 180,000 × g for 1 h to pellet light microsomal membranes, including the majority of the insulinresponsive Glut4 compartments. This pellet was resuspended in HES and iodixanol (OptipPrep; Nycodenz-Pharma, Oslo, Norway) added to 14% and the tube gently mixed. This was centrifuged in a near-vertical rotor for 1 h at 295,000 × g and fractions collected from the bottom of the tube as outlined in Hashiramoto and James (2000) and Maier and Gould (2000). This method has previously been used in our laboratory to separate Glut4 within GSV and TGN/endosome compartments (Maier and Gould, 2000).
Deoxyglucose Transport
2-Deoxy-d-glucose (deGlc) transport was assayed in 12-well plates of cells. Briefly, cells were rinsed in Krebs-Ringer-phosphate (KRP) buffer at 37°C three times and then covered with 0.5 ml of the same with or without 10 μM cytochalasin B. Insulin was added at the concentrations and times shown in the figures. Transport was initiated by the addition of an aliquot of deGlc such that the final concentration was 50 μM, with 0.25 μCi/well. Transport was assayed for 3 min (1 min for insulin reversal experiments), after which cells were rapidly washed in ice-cold phosphate-buffered saline, air-dried, and then solubilized in 1% Triton X-100. The radioactivity associated with the cells was determined by liquid scintillation spectrophotometry.
For insulin reversal experiments, cells were stimulated with 100 nM insulin for 20 min, and then rapidly washed into KRM buffer at pH 6.0 (as KRP except MES replaced the sodium phosphate). Cells were gently washed in KRM every 2 to 3 min before a brief wash in KRP and subsequent assay in KRP as outlined above.
Other Assays
Adipsin secretion was assayed as outlined previously (Millar et al., 2000) and was quantified using an antibody against murine adipsin kindly supplied by Dr. Jess Miner (University of Nebraska, Lincoln, NE). ACRP30 secretion was assayed in a similar manner by using an anti-peptide antibody raised against the C-terminal 15 amino acids of murine protein (Zymed Laboratories, South San Francisco, CA). Cell surface transferrin receptor levels were determined exactly as outlined in Millar et al. (2000).
RESULTS
Subcellular Distribution of Syntaxins in 3T3-L1 Adipocytes
To evaluate the functional role (if any) of syntaxins 6, 7, 8, and 12/13 in the trafficking of Glut4, we first examined the crude subcellular distribution of these molecules in basal and insulin-stimulated 3T3-L1 adipocyte subcellular fractions (Figure 1A). Consistent with previous studies, both Glut4 and IRAP were predominantly expressed in intracellular membranes in the absence of insulin, but exhibited translocation to the plasma membrane in response to acute insulin treatment. Surprisingly, we also observed a similar translocation phenomena for STX6. Quantification of several experiments of this type revealed that STX6 consistently exhibited a fold translocation to the plasma membrane of a similar order to that exhibited by Glut4 and IRAP (Figure 1B). STX7, 8, and 12/13 did not significantly change their subcellular distribution in response to insulin. In our hands, the signal obtained from the STX7 antibody was markedly weaker than any of the other antibodies used; hence, this molecule was not studied further.
Figure 1.
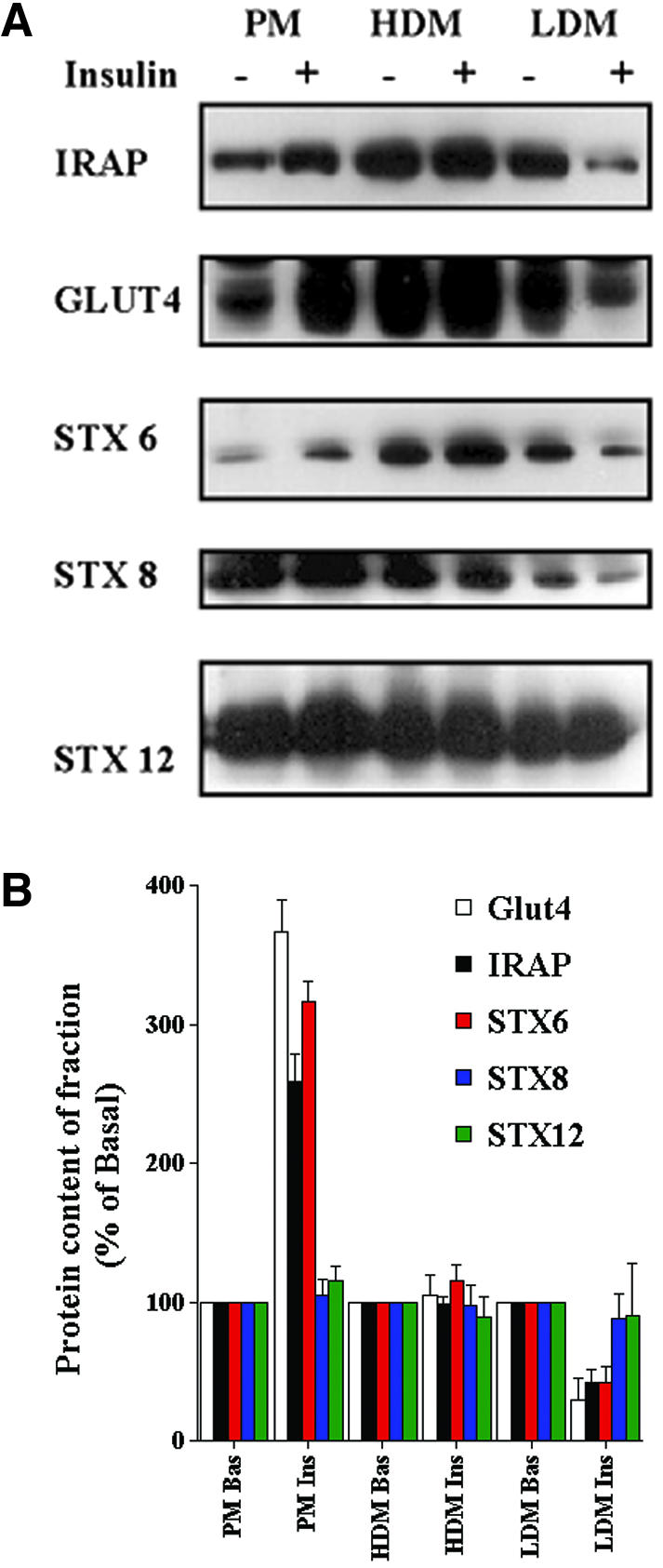
Subcellular distribution of t-SNAREs in adipocytes and the effect of insulin. 3T3-L1 adipocytes were treated with or without 100 nM insulin for 30 min. Cells were homogenized and plasma membrane (PM), HDM and LDM fractions were separated as described in text. SDS-PAGE was performed using equal quantities of protein (15 μg) from each fraction. The distribution of the indicated proteins was then studied by immunoblotting. (A) Data from a single representative experiment. (B) Quantification of data from four independent fractionation experiments of this type (presented relative to basal cells for each fraction, mean + SD). The insulinstimulated increases in plasma membrane levels of Glut4, IRAP, and STX6 are all significant (p <0.05 compared with control cells), as are the corresponding decreases in the LDM fraction (p <0.05).
Colocalization of Syntaxins with Glut4 in 3T3-L1 Adipocytes
We next set out to examine the extent of overlap of these different syntaxins with Glut4 by using immuno-isolation of Glut4-containing vesicles and subsequent immunoblotting of the fractions. The data (Figure 2) illustrate that depletion of intracellular membranes of Glut4 quantitatively depletes IRAP from the intracellular membrane fraction. This is consistent with previous data and demonstrates the efficiency of the immuno-isolation technique in our hands. As shown, both STX8 and 12/13 are present to a limited degree in Glut4 vesicles. Averaging data from four experiments of this type, we found that 15 + 4% of STX8 and 31 + 7% of STX12/13 were present in Glut4-containing vesicles. Thus, although these proteins clearly do exhibit some colocalization with Glut4, the extent of this is rather modest. In contrast, 87 + 9% of the cellular STX6 was present within Glut4-containing vesicles (Figure 2).
Figure 2.
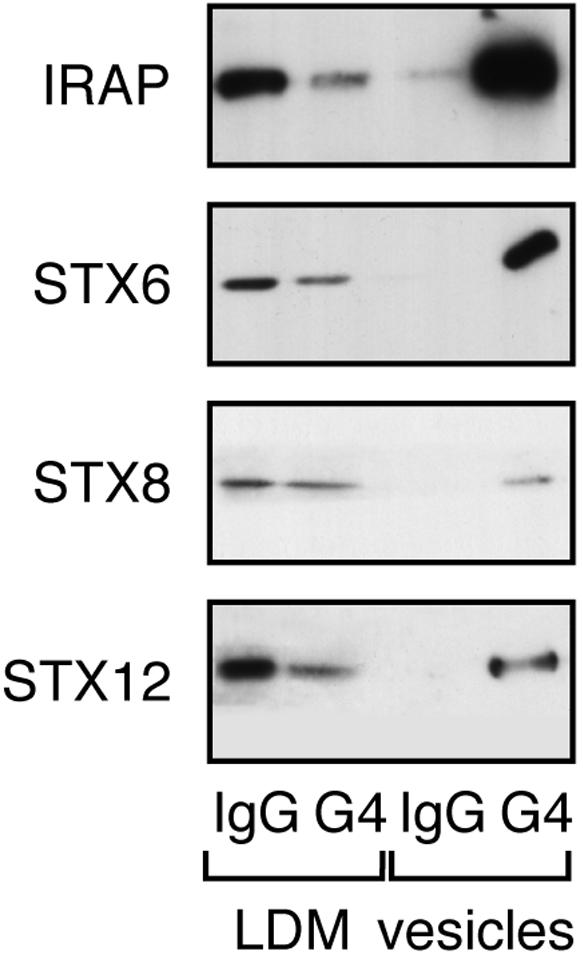
Colocalization of t-SNAREs in Glut4 vesicles. GLUT4 vesicles were isolated from 3T3-L1 adipocyte postnuclear supernatants as described in text. anti GLUT4 (7.5 μg) or anti-rabbit Ig/10-cm plate cell homogenate was used. LDM (1.9%) and 5% of vesicles were used for SDS-PAGE and subsequent immunoblotting with the antibodies shown. The four lanes of each immunoblot are (from left to right): LDM IgG, LDM recovered after immunoadsorption by using rabbit IgG; LDM G4, LDM recovered after immunoadsorption by using anti GLUT4; Ves IgG, vesicle fraction recovered after immunoadsorption by using rabbit IgG; and Ves G4, vesicle fraction recovered after immunoadsorption by using anti GLUT4. The data shown are from a representative experiment, repeated three further times with similar results. For quantification, see text. Note that Glut4 is not observed in the Ves G4 lane, as in agreement with other studies (Cain et al., 1992; Mastik et al., 1994; Ross et al., 1998), in our hands most of Glut4 remains associated with the antibody under the conditions used to solubilize the vesicles.
Recombinant Adenovirus Delivery of Cytosolic SNARE Domains in Adipocytes
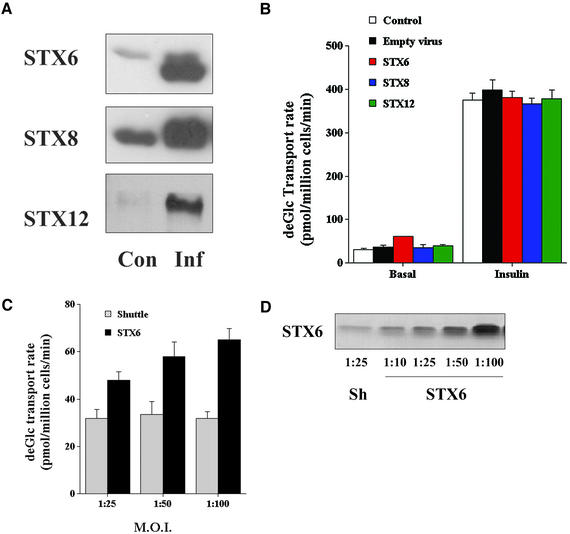
In an attempt to further determine the functional role of STX6 in 3T3-L1 adipocytes, we used recombinant adenovirus to overexpress the cytosolic domains of STX6, 8, or 12/13 in 3T3-L1 adipocytes. We reasoned that these cytosolic domains could act as “poison proteins” and thus inhibit the function of the endogenous molecules, as has been shown to be the case for plasma membrane localized STX1A and STX4 in this and other systems (Volchuk et al., 1996; Olson et al., 1997; Tellam et al., 1997). The adenoviruses constructed were found to drive high-level overexpression of each of the SNARE cytosolic proteins in 3T3-L1 adipocytes. In control experiments, we observed >90% infection by using these viruses at an m.o.i. of 50:1; hence, this value was used in most of the studies shown. We routinely observed 12- to 16-fold overexpression of STX6 or 12/13 cytosolic domain in cells infected at an m.o.i. of 50:1 on day 6 postdifferentiation and assayed at day 8; STX8 consistently gave by comparison somewhat lower levels of expression of approximately fourfold (Figure 3A). We therefore examined the effect of overexpression of these different cytosolic domains on a range of membrane trafficking events in 3T3-L1 adipocytes. We consistently found that infection with empty virus, STX8, or STX12 had no discernible effect on either basal or insulinstimulated glucose transport, regardless of infection day (Figure 3B). The data in Figure 3B are for cells infected on day 6 postdifferentiation and assayed on day 8. Similar data were observed for cells infected on day 2, day 4, or in cells multiply infected on day 2, 4, and 6; in each case, we then assayed transport on day 8 (our unpublished data). Similar infections with STX6 encoding virus had no significant effect on insulin-stimulated deGlc transport (Figure 3B). In contrast, we consistently observed a small but statistically significant increase in basal deGlc transport in cells overexpressing the STX6 cytosolic domain. The effect on basal transport rate was dependent upon the m.o.i. (Figure 3, C and D), indicating that it is a consequence of overexpression of the STX6 domain overexpression and not a nonspecific effect of virus infection, because infection with similar m.o.i. with an empty virus was without effect (Figure 3, C and D).
Figure 3.
Adenoviral delivery of cytosolic t-SNARE domains. (A) 3T3-L1 adipocytes were infected with adenovirus designed to overexpress the cytosolic domains of STX6, 8, or 12, or empty virus at an m.o.i. of 50:1 on day 6 postdifferentiation. Forty-eight hours later, cell lysates were prepared and immunoblotted with antibodies to the indicated STX. As shown, the virus drives high-level overexpression of each of the cognate STX cytosolic domains. These data were derived from the same cells used in the experiment shown in B. (B) Cells infected as described above were incubated in serum-free media for 2 h and deGlc uptake assayed in cells incubated with or without 1 μM insulin for 30 min. The data shown (from an experiment repeated four times) are the mean and SD of triplicate determinations at each point. There are no significant effects on insulin-stimulated transport rates. Basal transport in cells expressing STX6 was significantly elevated compared with control cells (p <0.03). (C) Illustration of elevated basal rates of transport observed upon overexpression of the STX6 cytosolic domain vary with m.o.i. The increased value of basal transport compared with empty vector-infected cells was significant at each m.o.i. (p < 0.03). (D) Representative immunoblots of whole cell lysates from 3T3-L1 adipocytes infected with either empty vector (Sh) or STX6 at the indicated m.o.i.
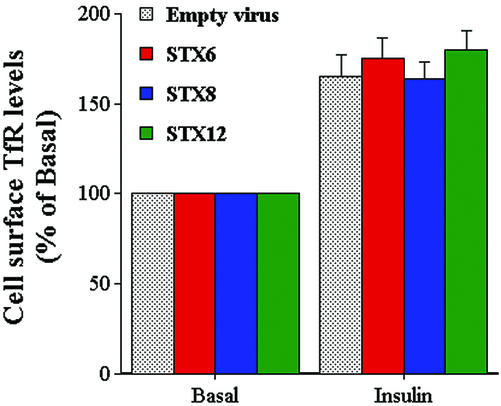
We also examined the rate of secretion of adipsin and ACRP 30 from cells infected with STX6 or empty virus. No effect on the rate of secretion of these proteins (either in the presence of absence of insulin) was observed upon expression of the STX6 cytosolic domain (Perera, Clarke, and Gould, unpublished data). Similarly, we found that basal and insulin-stimulated levels of cell surface transferrin receptors were unaltered by overexpression of any of the SNARE proteins used herein (Figure 4).
Figure 4.
Effect of STX cytosolic domain overexpression on transferrin receptor levels at the surface of 3T3-L1 adipocytes. 3T3-L1 adipocytes were infected with either empty virus or virus expressing the cytosolic domains of STX6, 8, and 12 on day 6 and assayed 48 later. Before assay, cells were incubated in serum-free media for 2 h and cell surface transferrin receptor levels assayed in cells incubated with or without insulin for 1 h. Note that both basal and insulin-stimulated levels of transferring-receptor (TfR) were similar among all groups, i.e., viral infection per se had no effect on TfR levels. Moreover, expression of STX cytosolic domains was without effect on basal or insulin-stimulated cell surface TfR levels. The experiment was repeated three times with similar results.
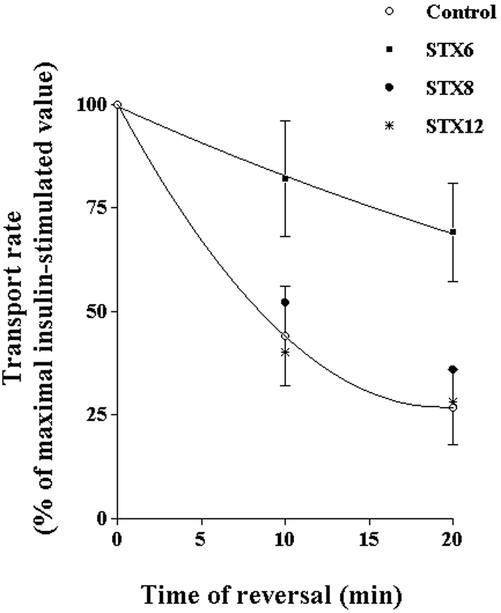
We reasoned that the increased levels of basal deGlc transport shown in Figure 3 could reflect a reduced ability of Glut4 to traffic from the endosomal system into its unique storage compartment. Such a defect would result in increased levels of Glut4 within recycling endosomes and thus increased levels at the cell surface. To test this hypothesis further, we examined the ability of deGlc transport to return to basal levels after insulin withdrawal in cells expressing the different SNARE protein cytosolic domains. The result of a typical experiment is shown in Figure 5. As shown, in this experiment the rate at which insulin-stimulated deGlc transport returns to basal levels is significantly slower in cells overexpressing STX6 compared with either empty virus-infected cells or cells expressing STX8 or 12/13. Such data provide compelling evidence that perturbation of STX6 function modulates Glut4 trafficking.
Figure 5.
STX6 cytosolic domain slows the reversal of insulinstimulated deGlc transport. 3T3-L1 adipocytes were infected with either empty virus or virus expressing the cytosolic domains of STX6, 8, and 12 on day 6 and assayed 48 later. Before assay, cells were incubated in serum-free media for 2 h. After transfer to a heated-plate incubator, cells were stimulated with 100 nM insulin for 20 min, and then either assayed immediately or washed in KRM to induce reversal of insulin-stimulated deGlc transport for the times shown. DeGlc was assayed as described in text, and the data shown are mean values of triplicate determinations (+ SD, for clarity only the curves and errors for STX6 and control cells are shown). The data shown are the average values from six independent experiments (mean + S.D.). Analysis of the data showed that the rate of reversal of insulin-stimulated deGlc transport was significantly slower in cells overexpressing STX6 cytosolic domains compared with empty virus-infected cells, or cells expressing either STX8 or STX12 (*p <0.02 at all time points for each data set).
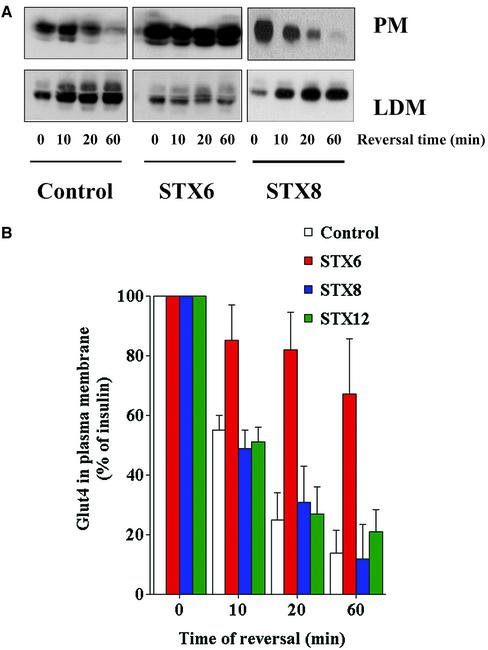
The effect of STX6 could be explained by either a decreased rate of endocytosis of Glut4 from the plasma membrane or by a reduced rate of sequestration away from the recycling endosomal system into the GSVs. To address this question, we examined the distribution of Glut4 in cells at times after insulin withdrawal. Analysis of purified plasma membrane or LDM fractions (Figure 6A) showed that in control cells, or cells expressing the cytosolic domains of STX8 or STX12, the levels of Glut4 in the plasma membrane steadily fall as a function of time after insulin withdrawal. In contrast, in cells overexpressing the cytosolic domain of STX6 the rate of Glut4 decline in the plasma membrane is markedly impaired. The data from several experiments is quantified in Figure 6B, which clearly reveal a statistically significant reduction in the rate of loss of Glut4 from the plasma membrane fraction upon insulin withdrawal in cells expressing the cytosolic domain of STX6 compared with control cells, or cells expressing the cytosolic domain of STXs 8 or 12. Similarly, analysis of the LDM fraction demonstrated that the appearance of Glut4 in this fraction after insulin withdrawal is also compromised in cells expressing the STX6 domain (Figure 6A). Such data argue strongly that the trafficking of Glut4 is impaired in cells that overexpress the STX6 cytosolic domain. Again, the effect was specific, because other STX domains were without effect. Note that in control experiments not shown herein, we found no evidence for altered Glut4 levels in the HDM fraction (our unpublished data).
Figure 6.
STX6 cytosolic domain perturbs Glut4 reinternalization. (A) Pairs of 10-cm plates of 3T3-L1 adipocytes, treated as outlined in the legend to Figure 5, were homogenized and PM and LDM fractions were separated as described in text. SDS-PAGE was performed using 10% from each fraction. The distribution of the indicated proteins was then studied by immunoblotting. Shown are fractions obtained after insulin-stimulation and reversal for 0, 10, 20, and 60 min. The quantification of this data is presented in B as mean + SD from the three experiments. The rate of Glut4 removal from the plasma membrane is impaired in cells overexpressing STX6 (p < 0.05 at all time points).
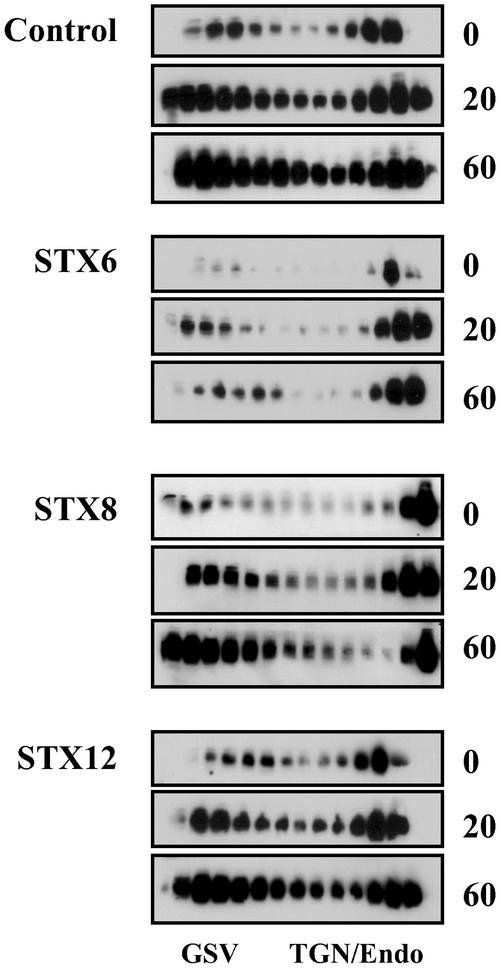
To resolve whether STX6 modulated trafficking between the endosomal system/TGN and the GSV compartment, we examined the distribution of Glut4 in intracellular membranes by iodixanol gradient analysis at different times after insulin withdrawal. This approach has been shown to effectively separate two distinct populations of Glut4, that within the TGN/endosomes and Glut4 within the specialized GSVs (Hashiramoto and James, 1999; Maier and Gould, 2000). Hashiramoto and James (2000) also showed that the TGN/endosomal fraction is in dynamic communication with the endosomal system, arguing that these fractions can be considered to be intracellular destinations of reinternalized Glut4. The data are presented in Figure 7. Inspection of the immunoblots clearly reveals two phenomena: the first is that in cells overexpressing the cytosolic domain of STX6, the amount of Glut4 present within both fractions on the gradient (i.e., the sum of Glut4 internalized) is less than that observed in control cells, or in cells overexpressing the cytosolic domains of STX8 or STX12. These data support and extend the data of Figure 6 and suggest that reinternalization of Glut4 from the plasma membrane is impaired in cells overexpressing STX6. Further inspection of Figure 7 also suggests a qualitative different in the distribution of Glut4 among the two pools resolved by this technique. In cells expressing STX6, the amount of Glut4 reaching the GSV compartment 60 min after insulin withdrawal is clearly significantly less than that observed in control cells, or cells expressing any of the other STX cytosolic domains. Such data suggest that subendosomal sorting of Glut4 may be impaired in cells overexpressing STX6.
Figure 7.
STX6 cytosolic domain perturbs Glut4 endosomal sorting. The LDM fractions from cells treated as described in the legend to Figure 6 were subjected to iodixanol gradient analysis. Fractions were collected from the bottom of the gradient (left of figure), and SDS-PAGE was performed using 10% of each fraction. The distribution of Glut4 was then determined by immunoblotting, and the position of the GSV and TGN/endosomal Glut4 peaks are indicated. The representative experiment shown was repeated twice with qualitatively similar data.
Syntaxin 6 and 16 Coimmunoprecipitate
We set out to identify the proteins that may be associated with STX6 in 3T3-L1 adipocytes. We immunoprecipitated STX6 from lysates of 3T3-L1 adipocytes and probed the lysates for syntaxins 4, 7, 8, 12/13, and 16. The results of this kind of analysis are presented in Figure 8A. As shown, we consistently recovered STX16 in the STX6 immunoprecipitates. We therefore set out to determine whether STX16 was also present within Glut4 vesicles. As illustrated in Figure 8B, we consistently found that >85% of STX16 was present within Glut4 vesicles isolated by immunoadsorption. Vesicle isolation approaches such as those used in Figure 2 cannot distinguish between different pools of intracellular Glut4. To further probe the distribution of STX16, and to compare it with that of STX6, we performed iodixanol gradient analysis to resolve Glut4 within the TGN/endosomes and Glut4 within the specialized GSVs (Figure 8C). Analysis of the distribution of Glut4 in these fractions revealed the typical presence of Glut4 within these two distinct fractions. Although STX6 and STX16 were present in both fractions, we consistently found higher levels of STX6 in the TGN/endosome fractions. In contrast, STX16 was mainly present in the GSV fraction (Figure 8C). Such data offer the hypothesis that these two SNAREs populate distinct Glut4 compartments within the endosomal system.
Figure 8.
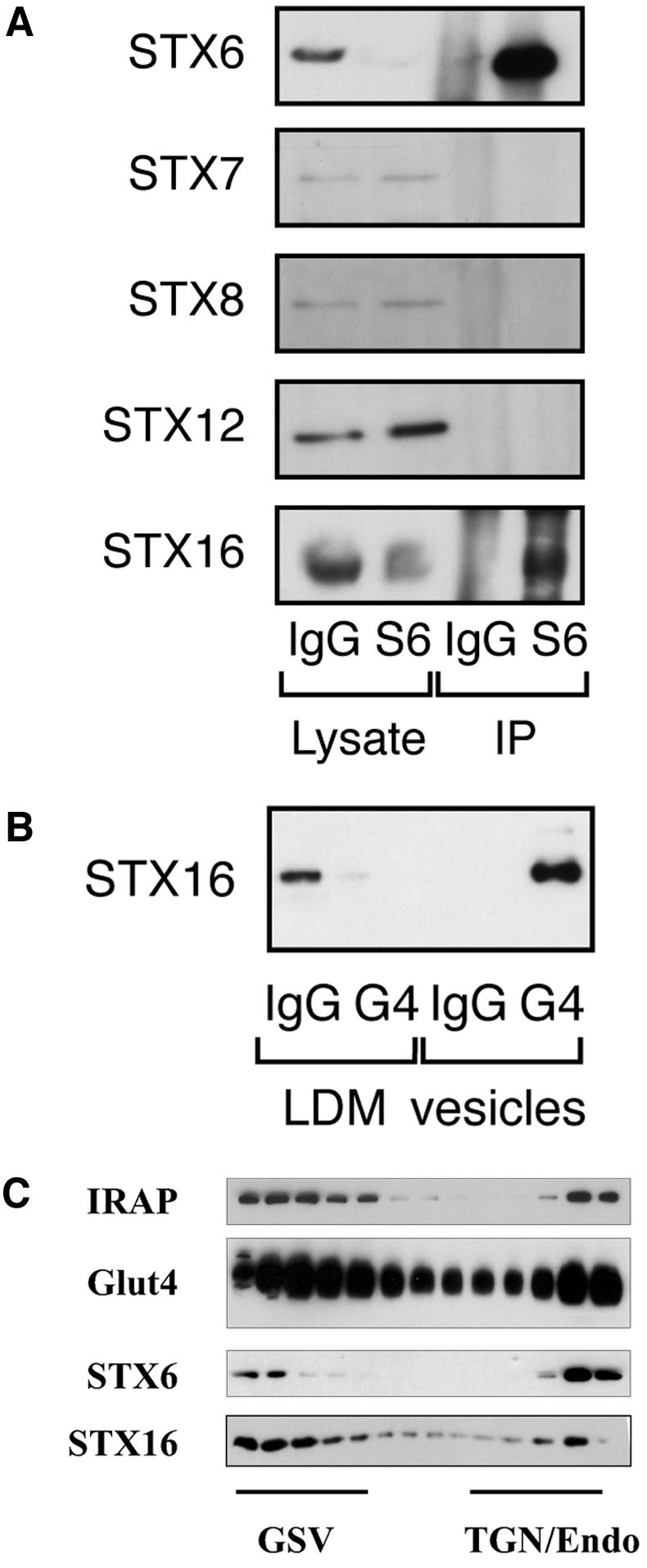
STX6 binds STX16. (A) Syn 6 was immunoprecipitated from lysates of 3T3-L1 adipocytes, as described in text. Anti-syntaxin 6 (mouse monoclonal) and anti-mouse Ig were used at 7.5 μg for cell lysates of a 10-cm dish. The representative immunoblot shown compares the lysate after IP with STX6 or IgG, and the corresponding immunoprecipitated material. In this figure, the lysate corresponds to 2.5% of a 10-cm plate of cells, and the IP corresponds to 15% of the total immunoprecipitate (i.e., 15% of the IP from a single 10-cm plate). (B) Presence within Glut4 vesicles of STX16 (see legend to Figure 2). (C) Iodixanol gradient profile of LDM fractions from basal (nonstimulated) adipocytes immunoblotted for Glut4, STX6, and STX16. The position of the GSV and TGN/endosomal peaks is shown. The representative experiment shown was repeated twice with qualitatively similar data.
Finally, we examined the phosphorylation status of syntaxins 6 and 16 in these cells, and the effect of insulin on this phosphorylation. We found no evidence for STX6 phosphorylation in either basal or insulin-stimulated cells (our unpublished data). In contrast, STX16 was found to be a phosphoprotein, and acute insulin treatment (1 μM insulin for 30 min), reduced this phosphorylation by ∼50% (Figure 9).
Figure 9.
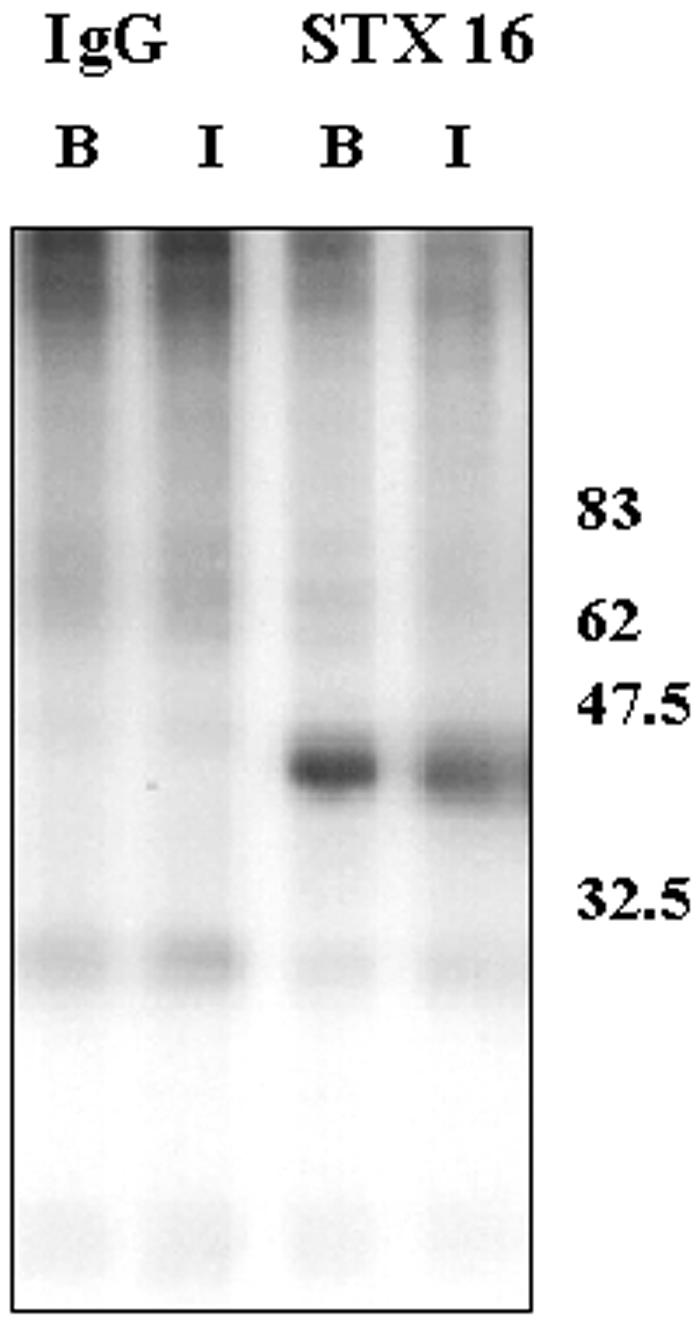
STX16 is a phosphoprotein. The figure shows the autoradiograph of STX16 immunoprecipitated from 3T3-L1 adipocytes labeled with 32Pi and treated acutely with or without 1 μM insulin for 30 min. Also shown are corresponding immunoprecipitates with random IgG. The experiment was repeated with similar results.
DISCUSSION
Many studies have shown that the itinerary of Glut4 within the intracellular membranes of adipocytes is a complex, dynamic, and highly regulated process (Bryant et al., 2002; Zeigerer et al., 2002). Although much is now known regarding the morphology of Glut4-containing compartments and the mechanism by which Glut4 vesicles fuse with the plasma membrane (Bryant et al., 2002), we still know remarkably little about the biogenesis of the Glut4 storage vesicles, or the processes by which Glut4 traffics from the endosomes and/or TGN to such a compartment. In an attempt to address these kinds of questions, we studied the intracellular distribution and function of endosomal t-SNAREs on Glut4 traffic.
We have shown that of the endosomal/TGN t-SNAREs examined (STX6, 7, 8, and12/13) only STX6 exhibited a significant degree of colocalization within Glut4 vesicles with >85% of the cellular compliment of STX6 within Glut4 vesicles (Figure 2). Furthermore, STX6 exhibited insulin-stimulated translocation to the plasma membrane, and the magnitude of this response approached that observed for Glut4 or IRAP (Figure 1). Such data argue that at least a proportion of intracellular STX6 is present within the fraction of Glut4-containing vesicles that translocate to the cell surface upon insulin challenge.
The mere presence of a protein within a given intracellular location does not provide any clue as to function. To address the functional role of STX6, 8, or 12, we generated a panel of recombinant adenoviruses designed to overexpress the cytosolic domains of these proteins in the anticipation that they would act as poison proteins and perturb the function of the endogenous molecules. This approach proved particularly useful and allowed very high levels of overexpression of STX cytosolic domain in 3T3-L1 adipocytes (Figure 3A). Despite the presence of very high levels of these proteins, we consistently observed no effect on the maximal rate of insulin-stimulated deGlc transport in cells overexpressing STX6, 8, or 12/13 (Figure 3B). This lack of an effect was evident regardless of the time of infection (relative to the time of onset of Glut4 expression during differentiation) and despite repeated infections every 2 d during the period over which Glut4 expression becomes activated and reaches a maximum (days 2–8).
In contrast, we consistently observed that the rate of deGlc transport in the absence of insulin was higher in cells expressing STX6 (Figure 3B) and that this effect was dependent upon the m.o.i. (Figure 3C). Such data could be interpreted to imply a role of STX6 in the trafficking of Glut4 within the endosomal system of adipocytes. If STX6 were involved in (for example) the withdrawal of Glut4 from the endosomal system into the unique Glut4 storage compartment, and then perturbation of STX6 function may be expected to result in modest increases in cell surface Glut4 levels because more Glut4 resides in the recycling endosomes for longer periods. We reasoned that if this were the case, then the rate of decline of insulin-stimulated deGlc transport after insulin withdrawal should be slower in STX6-overexpressing cells. Indeed, we hypothesized that the effect should be even more manifest because under these conditions there is substantially more Glut4 attempting to return to the GSV compartment. As shown in Figure 5, this was found to be the case, and STX6 cytosolic domain overexpression slowed the reversal of insulin-stimulated deGlc transport. This effect was specific for STX6 cytosolic domain, because neither STX8 nor STX12/13 induced this effect.
STX8 is predicted to function in late endosome fusion (Prekeris et al., 1999), and thus would perhaps not be expected to play a major role in Glut4 traffic. In contrast, STX12/13 has been localized to tubulo-vesicular endosomes and implicated in traffic to the plasma membrane from the endosomal system (Prekeris et al., 1998). Because the assays for these kinds of trafficking events are not established in adipocytes, it remains unclear whether the lack of effects of overexpression of STX8 or STX12/13 reflect a genuine lack of a role of this particular syntaxin or simply an inability of the overexpressed protein to function as an inhibitor of the endogenous molecule. However, we note that regardless of this point, the effect of overexpression of STX6 were specific to that particular SNARE and were not recapitulated by overexpression of other SNAREs to a similar level.
The data of Figure 5 clearly implicate STX6 in the trafficking of Glut4. Using subcellular fractionation and immunoblotting, we found that Glut4 levels at the plasma membrane of cells overexpressing the STX6 cytosolic domain decline much more slowly after insulin withdrawal than was the case in empty-virus infected cells, or cells overexpressing the cytosolic domains of STX8 or STX12 (Figure 6, A and B). Similarly, analysis of the LDM fraction, the crude intracellular fraction which houses the majority of the Glut4 mobilized in response to insulin, reveals that the appearance of Glut4 in this fraction after insulin withdrawal is significantly compromised in cells expressing the STX6 domain compared with the other STXs (Figure 6A). One interpretation of these data is that the endocytosis of Glut4 from the plasma membrane is significantly slowed by the overexpression of STX6 cytosolic domain. This hypothesis would be consistent with the observed increases in deGlc transport in the absence of insulin shown in Figure 3B. It is clear from the inspection of the data from three independent experiments that Glut4 is still capable of endocytosis in cells overexpressing STX6 (Figure 6B). However the half time is slowed from ∼10 min in empty-virus infected cells to >1 h, indicating not a quantitative trafficking block, but a partial inhibition. Such a partial effect could readily explain the small increases in basal deGlc transport, because the rate of Glut4 trafficking to and from the cell surface is slow; hence, a large accumulation of Glut4 at the surface of unstimulated adipocytes would not be expected. Rather, the effect is much more manifest upon conditions of insulin reversal, when high levels of Glut4 are being resequestered.
The GSV compartment, in addition to housing the insulinmobilized pool of Glut4 may also function to withdraw Glut4 from the recycling endosomal system and thus ensure levels of Glut4 at the cell surface are kept low (Rea and James, 1997; Byrant et al., 2002). The impairment in Glut4 trafficking revealed by Figure 6 could arise if the sorting of Glut4 to the specialized insulin-sensitive GSV compartment within the cells is impaired. This would have the effect of promoting elevated Glut4 levels within the endosomal system, and as such account for elevated levels of Glut4 at the plasma membrane. To resolve whether STX6 modulated trafficking between the endosomal system/TGN and the GSV compartment, we examined the distribution of Glut4 in intracellular membranes by iodixanol gradient analysis at different times after insulin withdrawal. The data are presented in Figure 7. Inspection of the immunoblots clearly shows that in cells overexpressing the cytosolic domains of STX6, the appearance of Glut4 within the GSV fraction resolved by this gradient as a function of time after insulin withdrawal is impaired. In contrast, Glut4's ability to reenter the GSV fractions after insulin withdrawal in cells overexpressing STX8 or STX12 cytosolic domains is clearly not impaired compared with control (empty virus-infected) cells. Such data provide support for the hypothesis that STX6 may regulate a trafficking step that controls Glut4 sorting between early endosomes and either late endosomes/TGN or early endosomes and the GSVs (or both). A future challenge will be to pinpoint this distinct site of defective traffic. Whether endocytosis from the plasma membrane is impaired (as is suggested by the data of Figure 6) or whether this is a consequence of defective sorting of Glut4 from early endosomes into a storage compartment cannot at present be definitively answered. Nonetheless, the data clearly implicate STX6 in Glut4 sorting during the trafficking from the plasma membrane to the GSVs.
It is important also to note that evidence of trafficking of Glut4 between the two intracellular membrane fractions identified by iodixanol gradient analysis has not been provided either in this study or in the literature. Hence, it is possible that STX6 function may be in the direct sorting to the GSV fraction from the plasma membrane, rather than from the TGN/endosomal fractions revealed by iodixanol gradient analysis. Further analysis, probably using the power of electron microscopy, will be required to definitively address this point. Indeed, because the existence of a specialized GSV compartment is somewhat controversial, it is worth noting at this stage that our data can equally well be interpreted within the model proposed by others, in which two dynamic cycles of Glut4 traffic lie at the heart of Glut4 intracellular sequestration. In this alternative model, STX6 may function in a trafficking step between the fast cycle (early endosomal) and the slow cycle (recycling endosomes/TGN). Perturbation of STX6 function would then result in more Glut4 within the recycling endosomes and slower Glut4 resequestration upon insulin withdrawal. Hence, our data points to a key role for STX6 in Glut4 trafficking at a stage between early endosomes and either (or both) of GSVs or a slowly recycling endosomal pool and is consistent with either model of Glut4 trafficking.
In an attempt to resolve how STX6 functions in Glut4 traffic, we sought to identify other t-SNAREs that may associate with it in cellular lysates. Figure 8 revealed that of the endosomal/TGN t-SNAREs examined, STX16 consistently associated with STX6. Consistent with this, we subsequently observed that >85% of STX16 was present within Glut4 vesicles, suggesting that STX6 and STX16 may exhibit overlapping distributions. To further resolve this overlap, we separated intracellular membranes on iodixanol gradients to partially resolve the specialized GSV compartment and Glut4 within the TGN/endosomes. We found that STX6 and STX16 exhibit distinct profiles, with STX16 enriched in the GSVs and STX6 enriched in the TGN/endosome fractions (Figure 8C). Consistent with this, we and others have found that STX16 exhibits insulin-stimulated translocation to the plasma membrane (HKIP and GWG, unpublished; see also (Bryant et al., 2002). The precise degree of overlap of STX6 and STX16 will require detailed electron microscopic studies to definitively resolve, but nonetheless, our albeit crude subcellular fractionation procedures do suggest that these molecules are on different compartments.
Because STX6 and STX16 seem to populate distinct Glut4-containing compartments, it is tempting to speculate that the interaction of these two t-SNAREs could mediate traffic between the TGN and the GSVs. Indeed, an interaction between these two SNAREs has previously been proposed (Mallard et al., 2002). Even more intriguing is the observation that STX16 is a phosphoprotein and that acute insulin stimulation decreases the phosphorylation of STX16 (Figure 9). Studies of other SNAREs have suggested that (de)phosphorylation of the t-SNAREs could be regulated the formation of a functional SNARE complex (Marash and Gerst, 2001; Gurunathan et al., 2002). Such studies raise the possibility that the phosphorylation status of STX16 may act to regulate traffic into or out of the GSV compartment. Because insulin signaling is known to involve a range of kinases and phosphatases, we reasoned it possible that phosphorylation of SNARE proteins may play a role in regulating the function of such complexes. The data presented herein offer the hypothesis that phosphorylation of STX16 is required for efficient sorting of Glut4 away from the rapidly recycling compartment; insulin-stimulation reduces this, and thus facilitates Glut4 recycling to and from the cell surface. Identifying this site, and studying its mechanistic significant will be of great interest.
On the basis of data present herein, we offer the following model. Traffic of Glut4 between the endosomes and the GSV compartment is regulated by/requires STX6. If this function is impaired, Glut4 sorting to the GSVs is perturbed. STX16 may act as the cognate t-SNARE for this transport step. Both STX6 and STX16 exhibit insulin-dependent translocation to the cell surface, presumably because of their presence in Glut4 vesicles in either (or both) of endosomes and GSVs. It is presently not clear whether the two STXs are translocated as a complex or in separate vesicles. These data place STX6 at the heart of Glut4 traffic and suggest that this t-SNARE may function early in the endocytic compartment during the delivery of Glut4 to deeper (later) compartments. Consistent with this hypothesis, we find that overexpression of the cytosolic domain of STX6 perturbs transferrin and LDL trafficking in HeLa cells, resulting in both ligands accumulating in early endosomes. Thus, we propose that syntaxin 6 functions in trafficking steps from early endosomes.
Acknowledgments
We thank Jeff Pessin (University of Iowa) for the STX6 cDNA, David E. James (Garvan Institute, Sydney, Australia) for cDNAs, and Dr Jess Miner (University of Nebraska) for the antiadipsin antiserum. We are grateful to Drs. Andy Baker, Tim Palmer, and Billy Sands (University of Glasgow) for help with the adenoviral work and particularly to Clare Miller for expert technical assistance. Finally, we thank all members of the Gould laboratory for helpful discussions. This work was supported by grant 17/C12621 from the Biotechnology and Biological Sciences Research Council and grant 060629 from the Wellcome Trust (to G.W.G.), a Diabetes Research and Wellness Fellowship (to L.H.C.), and by the Association of Commonwealth Universities UK (studentship to H.K.I.P.).
Abbreviations used: DeGlc, 2-deoxy-d-glucose; Glut, glucose transporter; GSV, Glut4 storage vesicle; HDM, high-density microsomal fraction; LDM, low-density microsomal fraction; m.o.i., multiplicity of infection; MPR, mannose-6-phosphate receptor; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; STX, syntaxin; TGN, trans-Golgi network.
References
- Austin, C., Hinners, I., and Tooze, S.A. (2000). Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem. 275, 21862-21869. [DOI] [PubMed] [Google Scholar]
- Brant, A.M., Jess, T.J., Milligan, G., Brown, C.M., and Gould, G.W. 1993. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem. Biophys. Res. Commun. 192, 1297-1302. [DOI] [PubMed] [Google Scholar]
- Bryant, N.J., Govers, R., and James, D.E. (2002). Regulated trafficking of the glucose transporter, Glut4. Nat. Rev. Mol. Cell. Biol. 3, 267-277. [DOI] [PubMed] [Google Scholar]
- Byrant, N.J., Govers, R., and James, D.E. (2002). Regulated transport of the glucose transporter GLUT4. Mol. Cell. Biol. 3, 267-277. [DOI] [PubMed] [Google Scholar]
- Cain, C.C., Trimble, W.S., and Lienhard, G.E. (1992). Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J. Biol. Chem. 267, 11681-11684. [PubMed] [Google Scholar]
- Foster, L.J., and Klip, A. (2000). Mechanism and regulation of GLUT4 vesicle fusion in muscle and fat cells. Am. J. Physiol. Cell. Physiol. 279, C877-C890. [DOI] [PubMed] [Google Scholar]
- Garza, L.A., and Birnbaum, M.J. (2000). Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J. Biol. Chem. 275, 2560-2567. [DOI] [PubMed] [Google Scholar]
- Gibbs, E.M., Allard, W.J., and Lienhard, G.E. (1986). The glucose transporter in 3T3–L1 adipocytes is phosphorylated in response to phorbol ester but not in response to insulin. J. Biol. Chem. 261, 16597-16603. [PubMed] [Google Scholar]
- Gurunathan, S., Marash, M., Weinberger, A., and Gerst, J.E. (2002). t-SNARE phosphorylation regulates endocytosis in yeast. Mol. Biol. Cell 13, 1594-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiramoto, M., and James, D. E. 1999. Segregation and characterisation of the intracellular Glut4 compartments in 3T3–L1 adipocytes. Diabetes 48 (Suppl 1), 0952. [Google Scholar]
- Hashiramoto, M., and James, D.E. (2000). Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3–L1 adipocytes. Mol. Cell. Biol. 20, 416-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T.-C., Zhou, S., Da Costa, L.T., Yu, J., Kinzler, K.W., and Vogelstein, B. (1996). A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95, 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, R., and Sudhof, T.C. (1999). Membrane fusion and exocytosis. Annu. Rev. Biochem. 68, 863-911. [DOI] [PubMed] [Google Scholar]
- Maier, V.H., and Gould, G.W. (2000). Long-term insulin treatment of 3T3–L1 adipocytes results in mis-targeting of GLUT4: implications for insulin stimulated glucose transport. Diabetologia 43, 1273-1281. [DOI] [PubMed] [Google Scholar]
- Mallard, F., Tang, B.L., Galli, T., Tenza, D., Saint-Pol, A., Yue, X., Antony, C., Hong, W., Goud, B., and Johannes, L. (2002). Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marash, M., and Gerst, J.E. (2001). t-SNARE dephosphorylation promotes SNARE assembly and exocytosis in yeast. EMBO J. 20, 411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S., Millar, C.A., Lyttle, C.T., Meerloo, T., Marsh, B.J., Gould, G.W., and James, D.E. (2000). Effects of insulin on intracellular Glut4 vesicles in adipocytes: evidence for a secretory mode of regulation. J. Cell Sci. 113, 3427-3438. [DOI] [PubMed] [Google Scholar]
- Martin, S., Reaves, B., Banting, G., and Gould, G.W. (1994). Analysis of the co-localization of the insulin-responsive glucose transporter (GLUT4) and the trans Golgi network marker TGN38 within 3T3–L1 adipocytes. Biochem. J. 300, 743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastik, C.C., Aebersold, R., and Lienhard, G.E. (1994). Characterisation of a major protein in GLUT4 vesicles. J. Biol. Chem. 269, 6089-6092. [PubMed] [Google Scholar]
- Millar, C.A., Meerloo, T., Martin, S., Hickson, G.R.X., Shimwell, N.J., Wakelam, M.J.O., James, D.E., and Gould, G.W. (2000). Adipsin and the glucose transporter GLUT4 traffic to the cell surface via independent pathways in adipocytes. Traffic. 1, 141-151. [DOI] [PubMed] [Google Scholar]
- Millar, C.A., Sherwan, A., Hickson, G.R.X., James, D.E., and Gould, G.W. (1999). Differential regulation of secretory compartments containing the insulin-responsive glucose transporter, GLUT4, in 3T3–L1 adipocytes. Mol. Biol. Cell 10, 3675-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, N., Ross, S.A., Lane, W.S., Moestrup, S.K., Petersen, C.M., Keller, S.R., and Lienhard, G.E. (1998). Sortillin is the major 110 kDa protein in GLUT4 vesicles from adipocytes. J. Biol. Chem. 273, 3582-3587. [DOI] [PubMed] [Google Scholar]
- Mullock, B.M., et al. (2000). Syntaxin 7 is localised to late endosome compartments, associates with Vamp 8 and is required for late endosome-lysosome fusion. Mol. Biol. Cell 11, 3137-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N., Yamamoto, A., Wada, Y., and Futai, M. (2000). Syntaxin 7 mediates endocytic trafficking to late endosomes. J. Biol. Chem. 275, 6523-6529. [DOI] [PubMed] [Google Scholar]
- Olson, A.L., Knight, J.B., and Pessin, J.E. (1997). Syntaxin 4, VAMP2, and/or VAMP3/Cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 17, 2425-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H.R.B. (2001). SNAREs and specificity of membrane fusion. Trends Cell Biol. 11, 99-101. [DOI] [PubMed] [Google Scholar]
- Pessin, J.E., Thurmond, D.C., Elmendorf, J.S., Coker, K.J., and Okada, S. (1999). Molecular basis of insulin stimulated GLUT4 vesicle trafficking: Location! Location! Location! J. Biol. Chem. 274, 2593-2596. [DOI] [PubMed] [Google Scholar]
- Piper, R.C., Hess, L.J., and James, D.E. (1991). Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am. J. Physiol. 260, C570-C580. [DOI] [PubMed] [Google Scholar]
- Ploug, T., van Deurs, B., Ai, H., Cushman, S.W., and Ralston, E. (1998). Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 142, 1429-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., Klumpermann, J., Chen, Y.A., and Scheller, R.H. (1998). Syntaxin 13 mediates cycling of plasma membrane proteins via tubulo-vesicular recycling endosomes. J. Cell Biol. 143, 957-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., Yang, B., Oorschot, V., Klumpermann, J., and Scheller, R.H. (1999). Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol. Biol. Cell 10, 3891-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm, G., Slot, J.W., James, D.E., and Stoorvogel, W. (2000). Insulin recruits GLUT4 from specialised VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes. Mol. Biol. Cell 11, 4079-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea, S., and James, D.E. (1997). Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes 46, 1667-1677. [DOI] [PubMed] [Google Scholar]
- Ross, S.A., Keller, S.R., and Lienhard, G.E. (1998). Increased intracellular sequestration of the insulin-regulated aminopeptidase upon differentiation of 3T3–L1 cells. Biochem. J. 330, 1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot, J.W., Garruti, G., Martin, S., Oorschot, V., Posthuma, G., Kraegen, E.W., Laybutt, R., Thibault, G., and James, D.E. (1997). Glucose transporter (GLUT-4) is targeted to secretory granules in rat atrial cardiomyocytes. J. Cell Biol. 137, 1243-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot, J.W., Geuze, H.J., Gigengack, S., Lienhard, G.E., and James, D.E. (1991). Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 113, 123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, V.N., Loh, E., Horstmann, H., Habermann, A., Xu, Y., Cole, J., Griffith, G., and Hong, W. (2000). Preferential association of syntaxin 8 with the early endosome. J. Cell Sci. 113, 997-1006. [DOI] [PubMed] [Google Scholar]
- Tang, B.L., Low, Y.H., Lee, S.S., Tan, E.H., and Hong, W. (1998). Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem. Biophys. Res. Commun. 242, 673-679. [DOI] [PubMed] [Google Scholar]
- Tellam, J.T., Macaulay, S.L., McIntosh, S., Hewish, D.R., Ward, C.W., and James, D.E. (1997). Characterization of Munc-18c and Syntaxin-4 3T3–L1 adipocytes. J. Biol. Chem. 272, 6179-6186. [DOI] [PubMed] [Google Scholar]
- Teng, F.Y.H., Wang, Y., and Tang, B.L. (2001). The syntaxins. Genome biology. 2, 3012.1-3012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze, S.A., Martens, G.J.M., and Huttner, W.B. (2001). Secretory granule biogenesis: rafting to the SNARE. Trends Cell Biol. 11, 116-122. [DOI] [PubMed] [Google Scholar]
- Urbe, S., Page, L.J., and Tooze, S.A. (1998). Homotypic fusion of immature secretory granules during maturation in a cell-free assay. J. Cell Biol. 143, 1831-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk, A., Wang, Q., Ewart, H.S., Liu, Z., He, L., Bennett, M.K., and Klip, A. (1996). Syntaxin 4 in 3T3–L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol. Biol. Cell 7, 1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, R.T., and Pessin, J.E. (2000). Functional co-operation of two independent targeting domains in syntaxin 6 is required for its efficient localisation in the trans Golgi network of 3T3–L1 adipocytes. J. Biol. Chem. 275, 1261-1268. [DOI] [PubMed] [Google Scholar]
- Wendler, F., Page, L., Urbe, S., and Tooze, S.A. (2001). Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol. Biol. Cell 12, 1699-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer, A., Lampson, M.A., Karylowski, O., Sabatini, D.D., Adensik, M., Ren, M., and McGraw, T.E. (2002). Glut4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell 13, 2421-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]