Abstract
In view of the common regulatory mechanism that induces transcription of the mitotic phosphatase cdc25C and cyclin A at the beginning of S-phase, we investigated whether cdc25C was required for S-phase transit. Here, we show that in both nontransformed human fibroblasts and HeLa cells, cdc25C protein levels significantly increased concomitant with S-phase onset and cyclin A synthesis. Activity measurements on immunoprecipitates from synchronized HeLa cells revealed a sharp rise in cdc25C-associated phosphatase activity that coincided with S-phase. Microinjection of various antisense-cdc25C molecules led to inhibition of DNA synthesis in both HeLa cells and human fibroblasts. Furthermore, transfection of small interfering RNA directed against cdc25C specifically depleted cdc25C in HeLa cells without affecting cdc25A or cdc25B levels. Cdc25C RNA interference was also accompanied by S-phase inhibition. In cells depleted of cdc25C by antisense or siRNA, normal cell cycle progression could be re-established through microinjection of wild-type cdc25C protein but not inactive C377S mutant protein. Taken together, these results show that cdc25C not only plays a role at the G2/M transition but also in the modulation of DNA replication where its function is distinct from that of cdc25A.
INTRODUCTION
Cyclin-dependent kinases (cdks) play a central role in the regulation of eukaryotic cell cycle progression (Morgan, 1995). Regulatory mechanisms responsible for their controlled activation include combinatorial assembly of cdks with one of a number of different cyclins, activating phosphorylation of a conserved threonine residue (T161 in human cdk1/cdc2), inhibition by association with specific inhibitors, and inhibitory phosphorylation of a conserved tyrosine (and adjacent threonine) in the amino-terminal ATP-binding region. Ultimately cdk/cyclin complexes are rapidly activated by dephosphorylation of this tyrosine (and threonine), which is brought about by cdc25 dual specificity phosphatases (for review, see Nilsson and Hoffmann, 2000). Cdc25 phosphatases belong to the tyrosine phosphatase family. Their active site loop, containing the HCX5R motif, is similar to that of tyrosine phosphatases but their overall topology is different (Fauman et al., 1998).
In human cells, cdc25 proteins are encoded by a multigene family, consisting of cdc25A, cdc25B, and cdc25C (Sadhu et al., 1990; Galaktionov and Beach, 1991; Nagata et al., 1991). All three display more than 60% identity to each other within the carboxyl terminal, catalytic domain. The less-conserved amino terminal domains are multiply phosphorylated and harbor distinct regulatory and substrate specificity determinants (Hoffmann et al., 1994; Strausfeld et al., 1994; Gabrielli et al., 1997). Amino terminal domains are also subject to alternative splicing giving rise to two, three, and five variants of cdc25A, B, and C, respectively (Baldin et al., 1997; Bureik et al., 2000; Wegener et al., 2000). Cdc25A is required for G1/S transition (Jinno et al., 1994) and seems to be regulated in part by mitogenic signaling (Galaktionov et al., 1995).
More recent data suggest that in humans a stable form of cdc25A may also play a role in mitosis (Mailand et al., 2002) and checkpoint signaling (Zhao et al., 2002). Cdc25B is present in S- and G2-phase cells. Overexpression of cdc25B in mammalian cells rapidly induces mitotic entry, overriding most cell cycle checkpoints (Karlsson et al., 1999). Indeed cdc25B is required for the G2/M progression and a number of reports suggest that cdc25B is the initial activator of cdc2/cyclin B at mitotic entry (Gabrielli et al., 1996; Nishijima et al., 1997; Lammer et al., 1998).
Likewise cdc25C has been associated with mitotic activation. There is strong evidence that cdc25C is involved in mitotic entry by dephosphorylating T14 and Y15 of cdc2/cyclin B. Its inhibition through microinjection of antibodies blocks entry into mitosis (Millar et al., 1991; Seki et al., 1992). Cdc25C becomes phosphorylated and activated at mitosis by cdc2/cyclin B itself and a positive feedback loop has been proposed (Hoffmann et al., 1993; Izumi and Maller, 1993; Strausfeld et al., 1994). Indeed, cdc25C phosphorylated by cdc2/cyclin B is capable of inducing partial mitotic activation (Hoffmann et al., 1993; Strausfeld et al., 1994). However, cdc25C is also subject to other site-specific phosphorylation events at mitosis, particularly by polo-like kinase 1 (Plk1; Toyoshima-Morimoto et al., 2002) and undergoes pin1-dependent proline isomerization (Zhou et al., 2000). Moreover, cdc25C is a converging point of several signal transduction cascades and links the mitotic activation of cdc2/cyclin B to the DNA replication and repair checkpoint (for review, see Walworth, 2001). Muller and colleagues have reported that cdc25C gene transcription is restricted to the S- and G2-phase, and the underlying transcriptional regulation is the same as found for cyclin A (Zwicker et al., 1995). Cyclin A protein appears exclusively during S and G2 and is required for the initiation of DNA replication (Girard et al., 1991). In light of these parallels to cyclin A expression we have addressed the question whether cdc25C had a role during S-phase and provide experimental evidence that in human fibroblast and HeLa cells, active cdc25C protein is required for proper G1-S progression even in the presence of cdc25A.
MATERIAL AND METHODS
Cell Culture, Synchronization, and Cell Cycle Analysis
Nontransformed human fibroblasts Hs68 (CRL-1365) and HeLa cells were cultured in DMEM supplemented with 10% fetal calf serum. Hs68 fibroblasts were synchronized in G1 by serum deprivation and resynchronized for S and G2 using hydroxyurea (HU) as described elsewhere (Girard et al., 1992). HeLa cells were synchronized by blocking the cells with thymidine at G1/S as described elsewhere (Stein et al., 1998). Briefly, asynchronous HeLa cells were supplemented with 2 mM thymidine for 24 h. Cells were released from the thymidine block by incubation in PBS for 30 s and 5 min at 37°C followed by incubation in serum containing DME supplemented with 24 μM deoxycytidine for 15 min. Finally, cells were refed with fresh media supplemented with 24 μM deoxycytidine and grown for 12–14 h. At this point cells were refed with fresh media containing 2 mM thymidine for 24 h and subsequently released as described above. In some experiments mitotic cells were mechanically collected by mitotic shakes 10, 11, and 12 h after double thymidine block. Cell cycle distribution was determined by fluorescence-activated cell sorting (FACS). Cells were trypsinized, and nuclei were stained for DNA in PBS, 0.1% Triton X-100, 5% glycerol, and 50 μg/ml propidium iodide. Subsequently, fluorescence from propidium iodide–DNA complexes was determined using a FACScan flow cytometer and CellQuest analysis software (Becton Dickinson, Franklin Lakes, NJ).
Semiquantitative RT-PCR
Total RNA from synchronized or randomly growing Hs68 fibroblasts or HeLa cells was prepared using the RNeasy kit (Qiagen, Courtaboeuf, France). Total RNA, 0.25 μg, was reverse-transcribed using a RNA PCR kit (Perkin Elmer-Cetus, Boston, MA). The resulting cDNA was then subjected to PCR amplification in 100-μL reactions containing 10 mM Tris/Cl, pH 8.3, 1.5 mM MgCl, 50 mM KCl, 0.2 mM of each dATP, [α-32P]dCTP (50 mCi/mmol), dGTP, and dTTP, 0.5 μM forward oligonucleotide, GATGCCAGAGAACTTGAAC (annealing to nucleotides 858–866 of cdc25C), 0.5 μM reverse oligonucleotide, TGAAACCTAATCCATTCCC (annealing to nucleotides 1779–1797 of cdc25C), and 2.5 U of Taq polymerase. These oligonucleotides were designed to amplify a fragment in the 3′ region of cdc25C, which is common to all described cdc25C splice variants (Bureik et al., 2000; Wegener et al., 2000). Twenty-five amplification cycles with the following temperature profile were performed: denaturation at 95°C for 15 s, primer annealing at 55°C for 30 s, and primer elongation at 72°C for 2 min. These conditions allowed detection of cdc25C transcripts during all cell cycle stages and were in the linear range of amplification (our unpublished results). Reactions were analyzed on ethidium-bromide–containing 1.4% agarose gels or on 5% polyacrylamide gels followed by autoradiography.
Antibodies and Immunoblot Analyses
Antiserum was raised against the peptide MSTELFSSTREEGSSGSGPS corresponding to the N-terminus of cdc25C. Immunizations and subsequent affinity purification of the antibody N20 were performed as previously described (Turowski et al., 1999). This antibody was highly specific for in vitro–translated or recombinant cdc25C as well as the endogenous protein when used in immunoprecipitations or immunoblots (see also Figure 2F).
Figure 2.
cdc25C activity transiently increases during S-phase. (A). HeLa cells were synchronized before S-phase by double thymidine block. At different times after washing out thymidine, cells were lysed and analyzed by Western blot for the expression of cdc25A (top panel) and cdc25C. Protein extracts were quantitated using Bradford assay (B), and equal amounts of cell extract were loaded for each lane. Western blots show typical examples obtained in four different experiments. Alternatively, HeLa cells were synchronized by double thymidine block or nocodazole and at different times after release were lysed for cdc25C activity measurements or fixed for analysis of BrdU incorporation. cdc25C activity was assessed by immunoprecipitation under nondenaturing conditions. Release of phosphate from 3-OMFP was measured by the associated increase in fluorescence. 3-OMFP phosphatase activity of cdc25C immunoprecipitates isolated at different times after release from double thymidine block. The time points correspond to those analyzed by Western blot in A. (C) The incorporation of BrdU in cells at the same time points. (D) A similar analysis of cdc25C activity in cells released from a nocodazole block and the corresponding data for BrdU incorporation (E). Inset in F: a Western blot analysis of the immunoprecipitate corresponding to the 6-h time point in A and B. Immunoprecipitates made with either C20 or N20 anti-cdc25C antibodies were blotted for the presence of cdc25C (top), cdc25A (middle) and cdc25B (bottom). Histograms are the mean values obtained in three different experiments. Activity is expressed in arbitrary fluorescence units compared against the levels in double thymidine blocked cells, which are essentially the same as those detected in an assay without immunoprecipitate.
Total extracts of HeLa or Hs68 cells were electrophoretically separated on 10% SDS-polyacrylamide gels and immunoblotted as described (Turowski et al., 1999). Primary antibodies against cdc25A (sc7389, Santa Cruz Biotechnology, Santa Cruz, CA), cdc25B (sc326, Santa Cruz Biotechnology, and clone 23, Transduction Laboratories, Becton Dickinson, San Diego, CA), cdc25C (C20: sc327, Santa Cruz Laboratories, and N20), anti-cdk2 (M2, sc163, Santa Cruz Laboratories), and cyclin A (Girard et al., 1991) were used at 1:500. Antitubulin ascites (clone DM1A; Blose et al., 1984) was used at 1:10,000.
Immunoprecipitation and Phosphatase Activity Measurement
Active cdc25C was immunoprecipitated from double thymidine synchronized HeLa cells using anti-cdc25C-C20 or N20 antibodies. Briefly, 5 μg anti-cdc25C were incubated with 20 μL protein G-sepharose (AP-Biotech, Amersham, UK) for 2 h at 4°C. Synchronized HeLa cells from a 100-mm culture dish were washed twice in ice-cold PBS, scraped into PBS, and collected by centrifugation at 1000 × g for 5 min. Cells were lysed in RIPA buffer (50 mM Tris/Cl, pH 8.0, 50 mM NaCl, 1.0 mM EDTA, 0.5% sodium deoxycholate, 1.0% Triton X-100, 50 nM calyculin A, 2 μg/ml aprotinin, 4 μM leupeptin, 1 mM PMSF, 3 μM pepstatin), and the soluble lysate was precleared with protein G-Sepharose for 30 min at 4°C. Subsequently 20 μL of antibody-protein G-Sepharose complex was added to lysate containing 500 μg of protein. After incubation for 2 h, antibody-protein G complexes were collected by centrifugation and washed twice with RIPA buffer, twice with RIPA containing 400 mM NaCl, twice in TBS (50 mM Tris/Cl, pH 7.5, 150 mM NaCl), and twice in TBS containing 1.0 mM EDTA before activity measurements were made.
Cdc25C activity was measured using 3-O-methyl fluorescein phosphate (3-OMFP) essentially as described by Gottlin et al. (1996). 3-OMFP was resuspended at a final concentration of 150 μM in 50 mM Tris/Cl, pH 8.0, 50 mM NaCl, 5 mM DTT, 0.1% (wt/vol) BSA, 1.0 mM EDTA. Pellets of immunoprecipitated cdc25C beads were resuspended in 300 μL of 3-OMFP assay buffer with and without 2 mM sodium orthovanadate. After incubation at 30°C for 30 min fluorescence was measured on a Wallac Victor2 fluorescence plate reader (Perkin Elmer-Cetus Life Sciences, Boston, MA) using 489-nm excitation and recording at 531 nm. Experiments were done in duplicates and repeated at least three times. Fluorescent emissions values were corrected for background activity, which was immunoprecipitated from HeLa cells double blocked in thymidine and which generally gave the same values as immunoprecipitates without antibody.
Microinjection and Immunofluorescence
Cdc25C antisense oligonucleotides AS1 (TGAGAAGAGTTCCGTAGACAT), AS2 (CCTTGAATTTTTCCACCTGCT), the corresponding sense oligonucleotides S1 and S2, and cdc25B antisense oligonucleotide (CCGGCCCCGCCGCGATGGAGGTGC) were synthesized using phosphorothioate modification of end bases to increase in vivo half-life and purified by reversed-phase chromatography (Eurogentec, Seraing, Belgium). For the sense and antisense cdc25C expression vectors p25Cs and p25Cas, the open reading frame (nucleotides 211-1632) from human cdc25C cDNA (Sadhu et al., 1990) was subcloned into pJ3 (Morgenstern and Land, 1990).
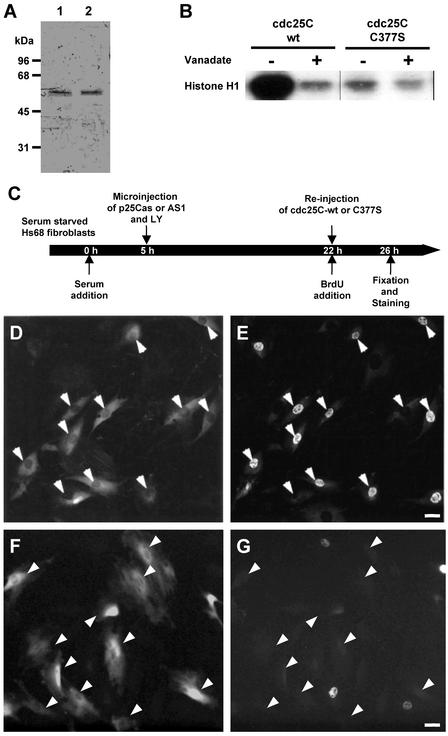
Synchronized HS68 or HeLa cells were microinjected as described previously (Lamb and Fernandez, 1997). Before injection, plasmids, oligonucleotides, and proteins were diluted into injection buffer containing 0.5 mg/ml inert marker antibodies (to act as a marker for injected cells). Plasmids were injected at <100 copies per cell (ca. 10 nM in the needle) and oligonucleotides at concentrations of <5 nM. Higher concentrations tended to affect S-phase nonspecifically. Cdc25C-wt and C377S proteins were at a concentration of ca. 0.2 mg/ml after dilution into marker-containing injection buffer. Cells were microinjected at indicated times throughout G1 or early S-phase and further subcultured in presence of bromodeoxyuridine (BrdU). At various time points after microinjection, cells were fixed and stained for incorporated BrdU, DNA, and microinjection marker (Girard et al., 1991). Cells were mounted and photographed as described elsewhere (Turowski et al., 1999).
Recombinant cdc25C Protein
Human Cdc25C subcloned into the T7-inducible bacterial expression vector pRK171 was a kind gift of Paul Russell (Scripps Research Institute, La Jolla, CA). Recombinant cdc25C was expressed using BL21 (DE3) bacteria transformed with the pRK171-hcdc25C construct and cdc25C-containing inclusion bodies isolated as previously described (Strausfeld et al., 1991). Instead of the renaturation procedure described before, inclusion bodies were solubilized in guanidinium-containing buffer, and proteins were renatured by quick dilution. After overnight dialysis cdc25C was further separated from contaminants and improperly folded protein by anion exchange chromatography on a Mono Q column. Details of this procedure will be published elsewhere and are available on request. The plasmid pRK171-hcdc25C was mutated using the Quickchange Mutagenesis kit (Stratagene, La Jolla, CA) and mutant oligonucleotides (top strand: ATAATCATCGTGTTCCACAGTGAGTTCTCCTCAGAGAGGG and the corresponding lower strand oligonucleotide). The mutated construct was verified by sequencing and encoded human cdc25C with a single cysteine to serine change at amino acid position 377 (cdc25C-C377S). Subsequently, the C377S protein was expressed and purified in the same way as wild-type protein. Specific phosphatase activities were measured using paranitrophenylphosphate (pNPP) as a substrate (Gottlin et al., 1996). Wild-type cdc25C had a specific activity of 46 nmol/min/mg, whereas the C377S mutant protein displayed no activity toward pNPP at all.
RNA Interference
The small interference RNA (siRNA) duplexes were 21 base pairs. They were selected from within the open reading frames of cdc25A, B, and C ∼70 base pairs downstream of the ATG at the next double adenine. The sequences of the coding strands of the human cdc25A, B, and C siRNA duplexes were AAGGCGCUAUUUGGCGCUUCA, AAGAGCGAGGCGGGCAGUGGA, and AAAAAUGUUGCCUCGAUCUUUC, respectively. The control siRNA used was ACCCGCGCCGAGGUGAAGUU, targeting enhanced green fluorescent protein (Cortez et al., 2001). siRNA were synthesized and annealed by Dharmacon Research Inc, (Lafeyette, CO). For RNA interference siRNA duplexes were resuspended in RNAse-free water at 20 μM and transfected as described elsewhere (Liu and Erikson, 2002). Alternatively, cells were transfected using calcium phosphate (Alessi et al., 1996). Cells were incubated in the presence of the antisense RNA for 24 h. Cells were washed and further cultured in the absence or presence of BrdU for 12–24 h before they were processed for immunofluorescence, microinjection, or Western blotting. To determine transfection efficiency, cells were transfected with siRNA directed against GFP coupled to fluorescein. After 24-h transfection, cells were washed once in PBS, and the proportion of cells positive for fluorescein was determined.
RESULTS
Cell Cycle Analysis of cdc25C Expression and Activity
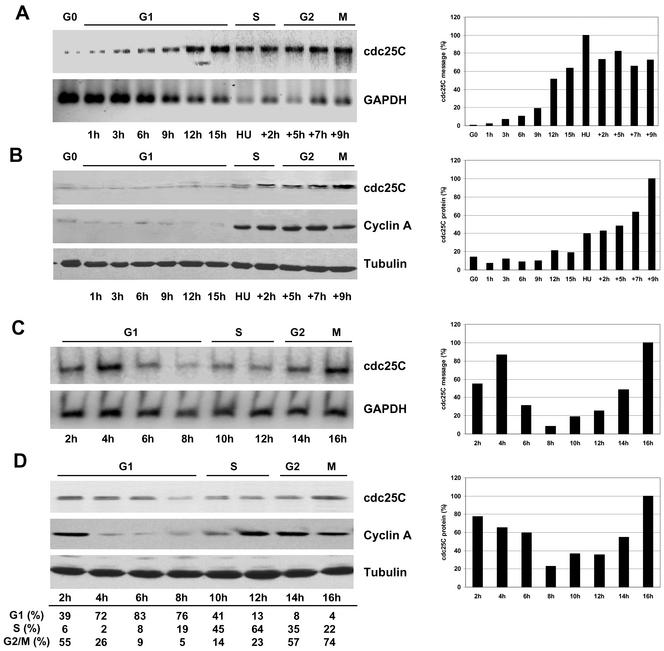
Human fibroblasts Hs68 were synchronized by serum starvation and RNA was isolated at various times after serum stimulation during the cell division cycle. RT-PCR analysis revealed an ca. 5–10-fold increase of cdc25C message levels during S- and G2-phase (Figure 1A). This observation is in line with previous reports showing that human cdc2, cyclin A, and cdc25C genes are subject to S/G2-specific transcription (Lucibello et al., 1995; Zwicker et al., 1995). For cdc2, transcriptional changes are without consequence on steady state protein levels (McGowan et al., 1990), whereas for cyclin A, protein and transcript levels are tightly linked (Pines and Hunter, 1990). We analyzed whether S/G2-specific transcription of human cdc25C was also linked to G1/S-phase specific protein induction (Figure 1B). Low amounts of cdc25C were detected in quiescent cells and throughout the majority of G1. At the G1/S transition, coinciding with the appearance of cyclin A (Girard et al., 1991), cdc25C protein levels markedly increased. The amount of cdc25C further increased throughout S and G2, reaching maximal levels in late G2 and mitosis. To discount that this induction of cdc25C was due only to growth factor readdition after serum starvation rather than regulated by the cell cycle, Hela cells were synchronized mechanically (by mitotic shake), and cdc25C levels were analyzed at various times after replating (Figure 1, C and D). Cdc25C message levels were high as cells entered G1 and reached a maximum 4 h after the mitotic shake. Thereafter message levels dropped markedly to attain a relative minimum in late G1, just before S-phase. During S and G2 message levels again increased strongly similarly to those observed in Hs68 cells (Figure 1C). Cdc25C protein levels paralleled these message levels (Figure 1D). They were high in mitosis and decreased throughout the following G1 phase to reach a minimum in late G1. At S-phase entry, concomitant with cyclin A synthesis, we again observed a major increase in cdc25C. The G1 decrease in cdc25C protein levels observed in HeLa cells was found in four independent experiments with cells synchronized by either mitotic shake or nocodazole. Taken together from these data, it appeared that G1 fibroblasts entering the cell cycle from a quiescent state hardly contain any cdc25C, and notable de novo synthesis of RNA and cdc25C protein occurred at the G1/S-phase boundary. Likewise in cycling HeLa cells, we observed a clear decrease of cdc25C during G1 and induced expression at the G1/S boundary.
Figure 1.
S/G2-specific expression of cdc25C in human cell lines. (A and B) Hs68 fibroblasts were synchronized by serum starvation for G1 and resynchronized for S and G2 using hydroxyurea (HU). Under these conditions, HS68 cells were in G1 from 0 to 16 h after refeeding and in case of resynchronization using HU, cells were in S during the first 4 h after release from HU block, and in G2 in the following 5 hours, with a peak of mitosis (M) at around 9 h after HU release (Girard et al., 1992). (A) Total RNA from HS68 fibroblasts was isolated at the indicated time points, and 0.25 μg was subjected to RT-PCR using specific primers for cdc25C or GAPDH as detailed in MATERIALS AND METHODS. Subsequently, levels of cdc25 and GAPDH messages were revealed by PAGE and autoradiography. Radioactivity of cdc25C RT-PCR products was recorded, normalized to the corresponding GAPDH values, and expressed as percent of maximal measured value (histogram on the right). (B) Cells were lysed at the indicated time points, and ∼40 μg of total cellular proteins was separated electrophoretically and immunoblotted for cdc25C (top panel), cyclin A to indicate S-phase onset (middle), and tubulin as loading control (bottom). Densitometric quantification of cdc25C protein levels, normalized to the corresponding tubulin levels, is shown on the right (expressed as percentage of maximal measured value). (C and D) Mitotic HeLa cells were collected by mitotic shake and recultured. At the indicated times RNA (C) and protein (D) levels were analyzed as detailed for A and B. The cell cycle phases indicated below the mitotic release times in D, were determined by FACS for the same cell sample as analyzed.
As shown in Figure 2A, thymidine-blocked HeLa cells (TT) contained steady state levels of cdc25C similar to those observed in G1 cells. However, on release from the thymidine block, cdc25C levels rapidly decreased for 60 min before de novo synthesis occurred, followed by a consistent increase in cdc25C levels over the next 8 h. In contrast, levels of cdc25A remained constant during the same period. We next asked if these changes in cdc25C expression were also reflected by changes in cdc25C activity. Considering the low abundance of cdc25C, we chose to immunoprecipitate the protein from synchronized human fibroblasts or HeLa cells and to measure activity using the highly sensitive fluorescent substrate 3-OMFP (Gottlin et al. 1996). As shown in Figure 2B, as HeLa cells were released from a thymidine block, no cdc25C activity was observed during the first 4 h. However, cdc25C activity increased sharply 5–6 h after thymidine release before falling again. This peak in cdc25C activity coincided with maximal S-phase activity as determined by BrdU incorporation (Figure 2C). A similar result was obtained in HeLa cells released from a nocodazole block (Figure 2D). Again the peak of cdc25C activity coincided with the peak of S-phase activity (Figure 2E). Cdc25C phosphatase activity at S-phase constituted only a fraction of that measurable at mitosis. Typically, cdc25C immunoprecipitated from M-phase extracts (blocked and released from nocodazole) and measured under the same conditions as for S-phase showed 3–5-fold higher activity than peak S-phase samples (our unpublished results). However, this increase in activity at S-phase reverted to background levels as cells entered G2. As shown in Figure 2F, the phosphatase activity measured was specific to cdc25C because our cdc25C antibodies quantitatively immunoprecipitated cdc25C but not even traces of cdc25A or B. A similar increase in cdc25C activity was also detected in synchronized HS68 cells, with the peak of cdc25C activity detectable 18.5 h after refeeding (corresponding to mid–S-phase, our unpublished results). These data show that cdc25C is specifically synthesized at S-phase entry in refed cells and in cycling cells. In addition, this de novo synthesis of cdc25C is accompanied by a peak in cdc25C activity, which also coincides with maximal DNA synthesis. Interestingly, our observation that this activity was restricted to S-phase decreasing as cells transit into G2, strongly suggests regulation by posttranslational modification because the levels of cdc25C protein continue to increase, whereas the S-phase activity declines (see also DISCUSSION).
Antisense Depletion of cdc25C in Human Cells
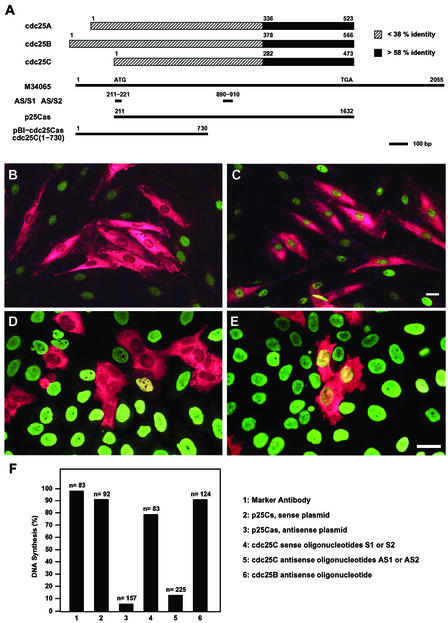
With low levels in G1, induction during S and maximal levels during G2/M, the observed cdc25C expression pattern is reminiscent of that seen with cyclin A (Pines and Hunter, 1990). Cyclin A has been shown to be required for S-phase initiation (Girard et al., 1991), whereas no such role has so far been described for cdc25C. To examine if the induction of cdc25C protein expression and activity was associated with a potential role of cdc25C at S-phase, we chose to specifically interfere with its protein resynthesis using different antisense approaches (Figure 3A). On the one hand we used the expression constructs p25Cs and p25Cas, containing the full-length open reading frame of human cdc25C under the control of an SV40 promoter in sense or antisense orientation, respectively. Alternatively, because mammalian cdc25s share strong sequence identity within their catalytic domain, antisense oligonucleotides targeting the most specific and divergent regions of cdc25C were designed. The sequences of single-stranded DNA oligonucleotides AS1 and AS2 were unique to cdc25C and absent from cdc25A or B. To act as controls, oligonucleotides encoding the sense strands were used.
Figure 3.
Microinjection of antisense-cdc25C prevents S-phase entry. (A) cdc25A, B, and C protein sequences are schematically aligned to each other. Numbers correspond to the amino acids of the published sequences (GenBank accession numbers M81933, M81934, and M34065, respectively). The catalytic domains (black) display more than 58% identity, the amino terminal domains (white) <38% identity. The cDNA of cdc25C (Sadhu et al., 1990; M34065) is depicted underneath to illustrate the position of the antisense and sense (control) oligonucleotides AS/S1 and AS/S2 and the fragments used for the antisense vectors p25Cas. The positions in base pairs are given relative to M34065. (B and C) Hs68 fibroblasts were synchronized by serum starvation and refed with medium containing serum and BrdU. Ten hours after serum readdition, cells were microinjected with cdc25C antisense AS1 (B) or sense S1 (C) oligonucleotide, together with inert rabbit IgG. Cells were fixed 22 h after refeeding (when >95% of cells have initiated or completed DNA synthesis) and double-stained for the rabbit marker antibodies (red) and BrdU incorporation (green). (D and E). Mitotic HeLa cells were obtained using thymidine followed by nocodazole treatment. Three hours after relieving the mitotic block, cells were microinjected with oligonucleotides AS1 (D) or S1 (E) and marker antibody. BrdU was added and the cells were cultured for another 12 h before fixation. Shown are merged micrographs of cells double-stained for marker antibody (red) and BrdU (green). Scale bars, 10 μm. The histogram in F summarizes the data obtained from four to six independent sets of microinjection experiments performed with synchronized Hs68 fibroblasts. The experimental outline was as described in B and C, except that p25Cs (2) and p25Cas (3) were injected in the first 5 h after the refeed to allow efficient expression. In all cases cells were fixed 22–24 h after the refeed. Values in columns 4 (and 5) represent data from experiments performed with either oligonucleotides S1 or S2 (or AS1 or AS2). Percent of DNA synthesis refers to the percentage of microinjected cells that have incorporated BrdU. n = number of injected cells.
To analyze the effect of cdc25C antisense tools on S-phase transition, HS68 fibroblasts were synchronized by serum deprivation and microinjected at different times after entry into G1. Cells were fixed and analyzed 20–22 h later when most cells have completed DNA replication (Girard et al., 1992). Transition through S-phase was assessed by monitoring BrdU incorporation. Microinjection of oligonucleotides AS1 or AS2 effectively prevented BrdU incorporation, whereas microinjection of either control oligonucleotides S1 or S2 did not affect S-phase transition (Figure 3, B, C, and F). Notably, microinjection of oligonucleotide AS1 still blocked S-phase entry when injected as late as 15 h after serum stimulation (Table 1), which corresponded to the time in late G1 just before the marked increase in cdc25C protein (Figure 1B). Similar S-phase inhibition was observed in cells microinjected with the antisense-cdc25C expressing construct p25Cas (Figure 3F). Microinjection of either the sense construct p25Cs or marker antibodies had no effect on DNA synthesis. When microinjected into synchronized HeLa cells p25Cas, AS1 or AS2 efficiently blocked S-phase as well (Figure 3, D and E). This inhibition of S-phase was specific to antisense cdc25C and was not observed using antisense cdc25B. Neither microinjection of an antisense-cdc25B RNA expressing construct nor cdc25B antisense oligonucleotides prevented S-phase transition in Hs68 or HeLa cells (Figure 3F and our unpublished results).
Table 1.
Inhibition of DNA synthesis by antisense-cdc25C oligonucleotides in mid- and late-GI
| Microinjection | No. of cells injected | Number of cell positive for BrdU | % cells inhibited |
|---|---|---|---|
| Sense cdc25C oligonucleotide S1 after 10 h | 106 | 98 | 8 |
| Sense cdc25C oligonucleotide S1 after 15 h | 36 | 34 | 6 |
| Antisense cdc25C oligonucleotide S1 after 10 h | 168 | 17 | 90 |
| Antisense cdc25C oligonucleotide S1 after 15 h | 103 | 31 | 70 |
Background DNA synthesis was 97%. Hs68 fibroblasts were synchronized by serum starvation. In mid- and late-G1, at 10 or 15 h after the refeed, cells were microinjected with marker antibodies and sense (S1) or antisense oligonucleotide (AS1). After injection cells were further cultured in the presence of BrdU and fixed 22 h after refeeding. Subsequently, cells were stained for microinjection marker (to give the number of cells injected) and for DNA synthesis using anti-BrdU (to give the number of cells that have entered S-phase). None of the oligonucleotides inhibited DNA synthesis significantly when microinjected into S-phase cells, i.e., 18 to 22 h after the refeed. Numbers represent mean values of two to four independent experiments
SiRNA to cdc25C Specifically Suppresses cdc25C Expression
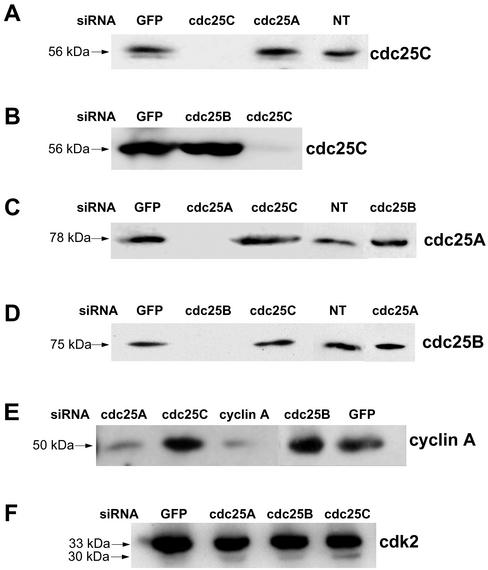
To further confirm the specific requirement for cdc25C expression for S-phase, we exploited the recently described siRNA technique (Elbashir et al., 2001). To specifically target cdc25C expression and deplete cdc25C protein in HeLa cells, we designed siRNA with a sequence located within the open reading frame of human cdc25C. Control siRNA duplexes targeting cdc25A, cdc25B, cyclin A, or enhanced green fluorescence protein (EGFP; Cortez et al., 2001) were also used. Asynchronously growing HeLa cells were transfected with siRNA targeting human cdc25 proteins or EGFP and after 36–48 h were harvested for Western blotting. Transfection of control siRNA targeting a sequence in EGFP did not affect protein levels of cdc25A, B, or C (Figure 4). Transfection of siRNA targeting cdc25A, B, or C caused a specific and complete downregulation of each protein targeted (Figure 4). Strikingly, the transfection of siRNA targeting each of the three cdc25 isoforms did not affect the steady state protein levels of the other two. As shown, in cells transfected with siRNA to cdc25C, the levels of cdc25A (A) or cdc25B (B) were the same as those in nontransfected cells (NT) or cells transfected with siRNA to GFP. Densitometric analyses of many such immunoblots revealed that the changes in cdc25A or B were 5% or less in cells transfected with siRNA targeting cdc25C. That the level of cdc25A or cdc25B protein remained unchanged with respect to nontransfected cells after depletion of cdc25C is noteworthy because it implies that cells did not compensate for the lack of cdc25C by upregulating cdc25A or B. Similar results were obtained when HeLa cells were synchronized before the siRNA transfection. Cdc25C levels could be effectively knocked out within 12 h after release from a double thymidine block when using cdc25C siRNA, again suggesting that de novo synthesis of cdc25C occurs at S-phase. From these data we concluded that any result obtained with cdc25C siRNA was specifically due to the suppression of cdc25C protein expression and not to unspecific effects on cdc25A or B. Indeed when cells were transfected with siRNA to cdc25A and B, we again observed a similar isoform specific knockdown of either cdc25A (Figure 4C) or cdc25B (Figure 4D). In none of the cases where a cdc25 isoform was targeted did we see changes in the levels of the other cdc25 isoforms when compared with GFP siRNA-transfected or nontransfected cells (Figure 4, A–D).
Figure 4.
si-RNA specifically deplete cells of cdc25A, B, or C. Asynchronous HeLa cells were grown to ca. 50% confluence. They were then transfected with siRNA duplexes targeting EGFP (acting as a control, lanes GFP), cdc25A, cdc25B, or cdc25C. Thirty-six hours thereafter cells were harvested and total protein extracts were prepared. Samples were quantified by Bradford protein assay, and ∼20 μg of total cellular proteins was electrophoretically separated and transferred onto nitrocellulose. Equal loading between different lanes on the same blots was verified by Ponceau Red-coloration of the membranes. Subsequently, membranes were immunodecorated for cdc25C (A and B), cdc25A (C), cdc25B (D), cyclin A (E), or cdk2 (F). NT, nontransfected cells showing the endogenous levels of cdc25 proteins in these cells. Arrows in F are the positions of the slower-migrating active form or fast-migrating inactive form of cdk2.
Cyclin A and cdk2 were also examined after cdc25C siRNA transfection: Cyclin A was strongly reduced in cells transfected with siRNA to cyclin A as expected, but unchanged in cells transfected with cdc25C (Figure 4E). We also examined the phosphorylation state of cdk2 as a potential substrate for cdc25C at S-phase. HeLa cells were depleted of cdc25C by siRNA, and cdk2 was examined by Western blot using long gels run at pH 8.6. This has previously been shown to allow immunodetection of two bands for cdk2: the top band being the dephosphorylated active form and the lower band corresponding to the phosphorylated, inactive form (Dulic et al., 1993). As shown in Figure 4F, mainly the 33-kDa top band was detectable in control cells transfected with siGFP. When cells are depleted of cdc25C, the majority of cdk2 still resolves as the slow-migrating, active form, but a small proportion now clearly resolves in the fast migrating form. Membranes were reprobed for cdc25C to ensure that effective knockdown of cdc25C had occurred (our unpublished results). These results suggest that under these conditions, cdk2 was not a direct target for cdc25C. In support of this we did not find significant changes in cdk activity in cyclin A or cdk2 immunoprecipitates isolated from cdc25C depleted cells (our unpublished results).
SiRNA to cdc25C Inhibits DNA Synthesis
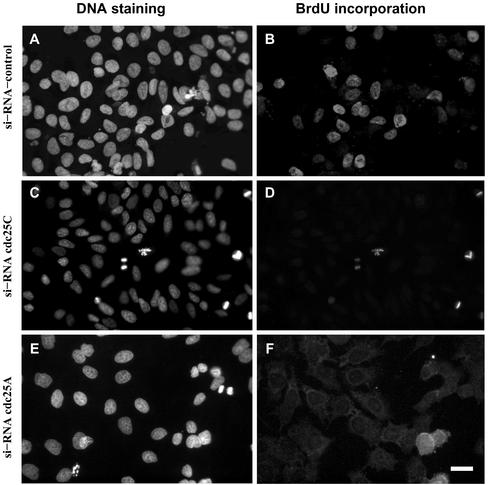
We next examined the consequences of cdc25C siRNA on DNA synthesis. As shown in Figure 5, HeLa cells transfected with cdc25C siRNA failed to incorporate BrdU during the following 24-h period. Little or no BrdU staining was seen in cells transfected with siRNA to cdc25C (Figure 5, C and D). The only cells positive for BrdU were in mitosis. In comparison, control cells transfected with EGFP siRNA showed BrdU staining in ∼40% of cells (Figure 5, A and B). In all experiments the levels of transfection were very high (ca. 95%), as assessed using a fluorescently tagged siRNA targeting GFP. Transfections for shorter periods of time before BrdU addition resulted in a higher background levels of BrdU incorporation, most likely reflecting the half-life of the cdc25C protein present at the time of transfection. A similar failure to undergo DNA synthesis was also observed in cells transfected with cdc25A (Figure 5, E and F) but not cdc25B siRNA (our unpublished results), consistent with their previously reported functions at G1/S and mitosis, respectively (Jinno et al., 1994; Gabrielli et al., 1996).
Figure 5.
SiRNA duplexes targeting cdc25A and cdc25C block DNA synthesis in Hela cells. Asynchronous HeLa cells were grown to ca. 30% confluence and then transfected with control siRNA targeting EGFP (acting as control; A and B), cdc25C (C and D), or cdc25A (E and F). Twentyfour hours after transfection, cells were pulsed for 24 h with BrdU and then fixed. Subsequently, the transfected cells were costained for DNA (A, C, and E) and incorporated BrdU (B, D, and F). Scale bar, 10 μm.
Cdc25C Protein Injection into cdc25C-depleted Cells Restores S-Phase
Although the described cdc25C-depletion experiment seemed to specifically target cdc25C with respect to the other cdc25 isoforms, there remained the possibility that they interfered with another as yet unidentified cellular process. To further specify the role of cdc25C at S-phase entry, functional rescue experiments were performed. Human wild-type (wt) cdc25C was expressed in Escherichia coli and purified to apparent homogeneity (Figure 6A, lane 1). For catalysis all dual-specificity protein phosphatases depend on an essential cysteine residue in their active site (see Fauman et al., 1998 and references therein). We mutated the catalytic cysteine in human cdc25C (C377) to serine to produce cdc25C-C377S. The C377S protein was expressed and purified in a way similar to that of the wt protein (Figure 6A, lane 2). Phosphatase activity of both proteins was assessed using inactive cdc2/cyclin B complexes immunoprecipitated from S/G2 HeLa cells.
Figure 6.
Active cdc25C restores S-phase in cdc25C-antisense cells. Wild-type cdc25C and mutant cdc25C-C377S were expressed in E. coli and purified to apparent homogeneity. (A) Approximately 0.5 μg of wild-type (lane 1) and C377S protein (lane 2) were separated on 10% SDS-PAGE. Purity was assessed by Coomassie Blue staining. The molecular masses of marker proteins in kilodaltons are indicated at the left. (B). To confirm the activity of purified cdc25C proteins, low-activity cdc2/cyclin B1 complexes were immunopreciptated from early G2 HeLa cells and incubated with purified cdc25C WT or catalytically inactive cdc25C-C377S in the presence or absence of 2 mM sodium vanadate. Subsequently, histone H1 kinase activity was measured. Shown is a typical autoradiograph of histone H1 phosphorylation induced by the activation of cdc2. (C–G) As schematized in C Hs68 fibroblasts were synchronized by serum starvation. Five hours after serum readdition, cells were microinjected with p25Cas together with Lucifer Yellow (LY). Twenty-two hours after refeeding, injected cells were identified by the presence of LY in the cytoplasm and reinjected with active, recombinant cdc25C-wt (D and E), or inactive, recombinant cdc25C-C377S (F and G). Immediately after the second injection cells were further cultured in the presence of BrdU for 4 h. Cells were then fixed and stained for BrdU incorporation (E and G) and rabbit marker antibodies (D and F; included during the second injection). Arrowheads depict microinjected cells. Scale bars, 10 μm.
As shown in Figure 6B, wild-type cdc25C but not C377S mutant protein strongly activated the histone H1 kinase activity of cdc2/cyclin B in a vanadate-sensitive manner, showing the functional integrity of these proteins. Hs68 fibroblasts were microinjected with p25Cas together with Lucifer Yellow to act as an injection marker. Twenty-two hours after injection when cells were blocked before S-phase, fluorescent cells were reinjected with wt or C377S cdc25C, and subsequent DNA synthesis was monitored (Figure 6, D–G). Active, wild-type cdc25C specifically restored the ability to enter S-phase (Figure 6, D and E; Table 2). In contrast, inactive cdc25C-C377S could not relieve the S-phase block in antisense cdc25C-depleted cells (Figure 6, F and G; Table 2), suggesting that cells need active cdc25C phosphatase to enter S-phase. Similar results were also obtained in cell microinjected with antisense DNA oligonucleotides (Table 2).
Table 2.
DNA synthesis in antisense-cdc25C injected cells following cdc25C protein microinjection.
| Microinjection | No. of cells reinjected | Number of cell positive for BrdU | % cells positive for BrdU |
|---|---|---|---|
| Cdc25C-wt into antisense plasmid p25as cells | 22 | 18 | 82 |
| Cdc25C-wt into antisense oligo AS1 cells | 49 | 30 | 62 |
| Cdc25C-C377S into antisense oligo AS1 cells | 42 | 3 | 7 |
Background DNA synthesis levels was 96%. Hs68 fibroblasts were synchronised by serum starvation. Five hours after serum readdition cells were microinjected with p25Cas or oligonucleotide AS1 and Lucifer Yellow (LY). Twenty-two hours after refeeding, injected cells were identified by the presence of LY in the cytoplasm and reinjected with active cdc25C-wt, inactive cdc25C-C377S or cyclin A. Immediately after the second injection cells were further cultured in the presence of BrdU for 4 h. Cells were then fixed and stained for rabbit marker antibodies (for the number of cells reinjected) and BrdU incorporation (for cells that have entered S-phase). Total results from two independent experiments are shown. DNA synthesis in antisense-injected, but not re-injected, surrounding cells was 14% (for p25as) and 15% (for AS1)
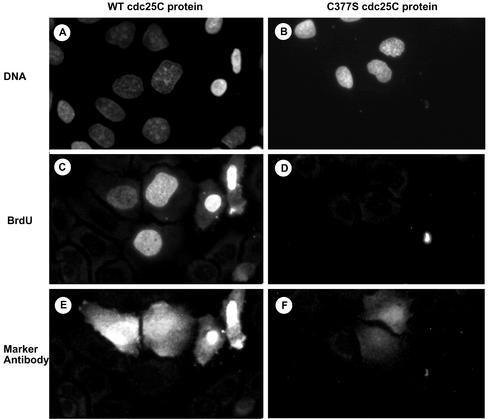
Functional rescue experiments were also performed in cells depleted of cdc25C through RNA interference. As shown in Figure 7 and in confirmation of the results shown in Figure 6, microinjection of active cdc25C into Hela cells effectively overcame the cell-cycle block induced by cdc25C siRNA because injected cells underwent DNA synthesis within 4 h (Figure 7, A, C, and E; Table 3). Again the microinjection of inactive cdc25C-C377S mutant protein was unable to restore DNA synthesis (Figure 7, B, D, and F; Table 3).
Figure 7.
Active cdc25C overcomes the block of DNA synthesis in siRNA-cdc25C–transfected cells. Asynchronously growing HeLa cells were transfected with siRNA duplexes targeting cdc25C. Thirtysix hours after transfection, cells were microinjected with either active cdc25C-wt protein (A, C, and E) or the inactive C377S mutant protein (B, D, and F). Cells were further cultured in the presence of BrdU for 4 h before fixation. Subsequently cells were costained for DNA (A and B), BrdU (C and D), and rabbit marker antibodies (E and F; included in the microinjection solution). Scale bar, 10 μm.
Table 3.
DNA synthesis in cdc25C siRNA-transfected cells following cdc25C protein microinjection.
| Microinjection | No. of cells injected | Number of cell positive for BrdU | % of cells rescued |
|---|---|---|---|
| Cdc25C-wt | 84 | 76 | 90 |
| Cdc25C-C377S | 77 | 5 | 6 |
| Marker antibodies | 90 | 5 | 5 |
Background level of DNA synthesis was 5%. Asynchronously growing HeLa cells were transfected with siRNA duplexes targeting cdc25C. Thirty-six hours after transfection, cells were microinjected with active cdc25C-wt protein, inactive C377S mutant protein, or microinjection marker antibodies alone. Cells were further cultured in the presence of BrdU for 4 h before fixation. Subsequently cells were co-stained for rabbit marker antibodies (for the number of cells injected) and BrdU (for the number of cells that have entered S-phase). Numbers represent mean values from four independent experiments
Taken together, these data show that the failure to progress through S-phase in human nontransformed cells after ablation cdc25C results from the specific lack of cdc25C activity. This activity is required despite the presence of unaltered levels of cdc25A, suggesting a function distinct from that of cdc25A.
DISCUSSION
Cdc25C mRNA was originally described to be expressed primarily in the G2-phase of the cell cycle (Sadhu et al., 1990; Jinno et al., 1994). Studies based on more sensitive PCR technology showed that cdc25C transcripts appear from late G1 onward and that high levels are already reached in S-phase (Lucibello et al., 1995). We observed a similar expression pattern in synchronized human fibroblasts Hs68 and HeLa cells. This stringently controlled, cell cycle-dependent transcription, which is also found for the cdc2 and cyclin A genes, is the result of repression by a cell-cycle–dependent element during G1 (Zwicker et al., 1995). Cdc25C protein levels were reported to fluctuate very little during cell cycle progression, but a certain increase has always been observed at S-phase (Millar et al., 1991; Patel et al., 1999).
We report here that in highly synchronized Hs68 fibroblasts cdc25C protein levels were markedly induced at the G1/S transition, subsequent to the rise in mRNA levels and parallel to cyclin A accumulation. Moreover, we found a similar induction at the G1/S transition of postmitotically synchronized HeLa cells or HeLa cells released from a thymidine block. Changes in cdc25C protein levels were not as marked as those found for cyclin A, especially in cycling HeLa cells (Figure 1D). Also, de novo cdc25C protein synthesis in refed Hs68 occurred significantly later than the induction of the RNA (Figure 1, A and B). This raises the possibility that cdc25C unlike cyclin A is additionally regulated on the translational level. However, in contrast to the rapid protein degradation typically seen for cyclins (Morgan, 1995), the decrease of cdc25C after mitosis was slow.
We present experimental evidence that cdc25C displays significant phosphatase activity coinciding with DNA synthesis. Activity started to be detected at cell cycle times when protein levels also markedly increased. However, activity induction was much stronger than the increase in protein. Moreover cdc25C phosphatase activity was restricted to S-phase despite protein levels continually rising throughout G2. Therefore and in analogy to mitotic activation of cdc25C, posttranslational modification appears to regulate its S-phase–specific activity as well. It will be interesting to determine whether cdc25C activity at S-phase is modulated by direct posttranslational modifications such as phosphorylation as is the case in mitosis (Strausfeld et al., 1994), changes in conformation such as isomerization (Zhou et al., 2000), or binding to 14.3.3 (Peng et al., 1997). We used the broad specificity phosphatase substrate 3-OMFP principally because of its acute sensitivity. However, like pNPP, 3-OMFP presents the drawback of giving no indication about the nature of the in vivo substrate. Current experiments are underway to determine which proteins associate with cdc25C during S-phase with the objectives of determining the intracellular target activated at S-phase.
The marked increase in cdc25C protein levels and activity at the S-phase entry led us to examine whether there was functional requirement for this mitotic phosphatase as early as S-phase. For this we chose to interfere with cdc25C expression using antisense approaches. A well-documented problem with the use of antisense molecules is that nonspecific effects can cause artifacts (for review, see Branch, 1998). The antisense oligonucleotides were designed for stability and specificity with a length of ∼20 base pairs, phosphorothioate modifications at their ends, and by avoiding any of the sequence motifs previously described to induce nonspecific antiproliferative effects. Furthermore, we used a number of different antisense oligonucleotides and vectors covering distinct regions of cdc25C, and all of them similarly affected entry into S-phase, whereas neither sense cdc25C nor antisense cdc25B molecules induced such effects. Because mammalian cdc25 gene products display a high degree of homology and cdc25A appears as an essential regulator of G1 progression and/or S-phase entry (Jinno et al., 1994; Blomberg and Hoffmann, 1999; Sexl et al., 1999), it was important to discount the possibility that cdc25C antisense molecules affected cdc25A nonspecifically. For this we used siRNA to specifically inhibit cdc25C expression (Elbashir et al., 2001). SiRNA-induced suppression of cdc25C was accompanied by a block at G1/S in the presence of unchanged levels of cdc25A or B proteins, proving that only cdc25C was targeted but not cdc25A or B.
We also show that the effects of cdc25C antisense or siRNA molecules on G1-S transition could be specifically attributed to a lack of cdc25C phosphatase activity because microinjection of active cdc25C phosphatase was required to restore S-phase transit. This role of cdc25C is distinct from that of cdc25A since cdc25A levels were unaffected in cdc25C-depleted cells. In addition we have observed that, unlike siRNA to cdc25C that left levels of cyclin A unchanged, siRNA to cdc25A resulted in a strong reduction in cyclin A levels (Figure 4E), further supporting that cdc25A and C have distinct roles at S-phase and that in human cells, cdc25A cannot substitute for cdc25C at S-phase. Finally, we showed that cdc25A ablation like cdc25C also arrested cells before S-phase. This arrest was not due to nonspecific effects of siRNA because siRNA targeting cdc25B had no effect on S-phase entry while ablating cdc25B expression. A similar lack of effect was observed when cells were transfected with siRNA targeting human MEF2 (our unpublished results).
Previous studies have concluded that the principal cdc25 phosphatase active at S-phase entry is cdc25A (Jinno et al., 1994) with the two other isoforms being attributed roles at mitosis (Millar et al., 1991; Gabrielli et al., 1996; Lammer et al., 1998). Importantly, antisense cdc25B or cdc25C oligonucleotides affected mitotic transit when microinjected into G2 cells (Lamb, N.J.C., unpublished result) demonstrating that they also inhibited previously described functions of these two phosphatases. A role of cdc25C during S-phase appears unprecedented, but a number of technical and/or functional reasons could explain why such a role may have been systematically overlooked in previous studies. First, cdc25C being required at two points in the cell cycle is more difficult to demonstrate than with a single cell cycle function, especially because most previous studies concentrated essentially on mitosis using synchronized cells in S and G2 (Millar et al., 1991; Hoffmann et al., 1993; Strausfeld et al., 1994; Gabrielli et al., 1996). Second, our initial results of S-phase arrest after cdc25C depletion were obtained in Hs68 fibroblasts, microinjected early in G1 when they contained little or no cdc25C protein. In contrast, in HeLa cells low cdc25C levels were only observable during a short time in late G1 (Figures 1 and 2). We also show that cdc25C is degraded during the first 60 min after thymidine release, and this brief window may well have been overlooked in previous studies. Lastly, some of the multiple phosphorylation sites implicated in the mitotic activation of cdc25C (Hoffmann et al., 1993; Strausfeld et al., 1994; Lammer et al., 1998) may not be restricted to mitosis, because we have recently observed phosphorylation of some “mitotic” sites as early as S-phase using phosphorylation site-specific antibodies (our unpublished observations).
Our data imply that a significant difference must exist between mice and humans with respect to cdc25C. In mice, data from gene knockouts suggests that cdc25C has a nonessential and redundant role (Chen et al., 2001). We have shown here that in both normal and transformed human cells, cdc25C, in addition to cdc25A, is required for entry into S-phase. These functions are clearly nonredundant because human cells blocked in the absence of cdc25C have wild-type levels of cdc25A and vice versa. Analysis of the protein product from the mouse cdc25C gene suggests that it differs significantly from its human counterpart. This is particularly notable with respect to the cytoplasmic anchoring region required for binding cdc25C to 14.3.3 and the putative NLS (amino acid region 200–250 in human cdc25C, absent in mouse cdc25C). In addition, key sites for cell cycle checkpoint kinase signaling cascades are also absent in mouse cdc25C. Murine cdc25C protein shows many similarities with the alternatively spliced forms of human cdc25C identified in human cancer cells (Bureik et al., 2000; Wegener et al., 2000). These forms are thought to promote deregulated proliferation due to the absence of key N-terminal regulatory sequences. It is therefore possible that in mice, the key functions of cdc25 phosphatases have been taken over by cdc25A with cdc25C playing a dispensable role similar to the divergent forms seen in human cancers. In addition, there is also a possibility that, unlike the analysis in living organisms where the time scale after knock-out allows establishing compensatory mechanism(s), antisense and siRNA targeting studies leave no time for such compensation to develop.
The finding that cdc25C is specifically synthesized and required at S-phase, raises the question of what role it plays. Our data show that cdc25C activity is induced during S-phase with a peak coinciding closely with maximal DNA synthesis, suggesting that cdc25C functions in the course of DNA replication. However, very much as for cyclin A (Girard et al., 1991), cells lacking cdc25C do not initiate S-phase because we did not even observe a punctate BrdU staining. There is accumulating evidence of cellular mechanisms to measure the presence of essential cell cycle components well before they are required. For instance centrosomes need to be present in G1 for the cell to commit to S-phase (Hinchcliffe et al., 2001). Therefore the lack of cdc25C could be detected by a sensor mechanism, such as the S-phase checkpoint. Our results also show that catalytically active cdc25C is required to enter S-phase, whereas inactive cdc25C polypeptide is not sufficient to relieve the S-phase block. Thus this checkpoint would rather measure phosphatase activity than the mere presence of protein. We are currently examining the status of the human checkpoint kinases in cdc25C-depleted cells with the objective of determining whether they are involved in detecting the absence of cdc25C activity. Most importantly, the requirement for active cdc25C to enter S-phase suggests a role in the activation of a cdk-cyclin complex, and future work will concentrate on the identification of such a complex. Cdk2 could be a candidate molecule: its activating role in S-phase induction is well documented (Ekholm and Reed, 2000). However, we did not find significant changes in either cdk2 activity or its phosphorylation status when cells were depleted of cdc25C by siRNA. Cdc25A has been implicated in the activation of cdk2 (Blomberg and Hoffmann, 1999), but a mechanism involving direct dephosphorylation of cdk2 by cdc25A has been challenged (Sexl et al., 1999). Cdc25A and/or C may still dephosphorylate a specific subpopulation of cdk2 for a precise function and such subtle changes may not be readily observable with the currently available techniques. Yet another possible function of cdc25C upstream of cyclin A synthesis seems unlikely because cyclin A is solely regulated by transcription (Pines and Hunter, 1990), and cdc25C transcription underlies the same mechanism. Alternatively, cdc25C could modulate the half-life of cyclins or other cell cycle proteins at S-phase. Indeed the finding that cdc25C is specifically lost in cycling late G1 HeLa cells suggests that a degradation mechanism plays a key function in modulating G1 to S-phase progression. A recent study has shown that hEmi1, originally identified as an inhibitor of APC20 ubiquitination in mitosis, is also transcriptionally induced at S-phase entry where it inhibits APCcdh1, and consequently promotes cyclin A accumulation (Hsu et al., 2002). It will be interesting to investigate whether cdc25C is implicated in this mechanism either as a target for the APC or in modulating the activation of hEmi1.
In conclusion, we demonstrate that like cdc25A, but unlike cdc25B, cdc25C is necessary for G1-S transition in transformed and nontransformed human cells. This previously unidentified function sheds new light on the biology of cdc25 phosphatases, like a number of recent reports that implicate cdc25B in mitotic induction (Nishijima et al., 1997; Lammer et al., 1998; Karlsson et al., 1999) or cdc25A during G2/M transition (Mailand et al., 2002). Taking into account the increasing number of cdc25 splice variants found in mammals (Baldin et al., 1997; Bureik et al., 2000; Wegener et al., 2000), the original roles assigning respectively cdc25A, B, and C to G1/S, S/G2, and G2/M progression and the proposed substrates for these different dual specificity phosphatases need to be reexamined. It appears feasible that more than one cdc25 phosphatase is implicated in the control of a given cell cycle transition and that they are imbedded in several parallel and hierarchical networks.
Acknowledgments
We thank Dr. Paul Russell for the cdc25C-containing plasmids BSK1 and pRK171, Cosette Rebouissou for help with FACS analyses, and Djamila Pavlovic for cell culture; Drs. Pete Adamson and Rob Blaber for critical reading of the manuscript; and Dr. Jean-Marc Brondello for scientific discussions concerning cdk2 activation. This work was supported by grants from Hoechst Marion Roussel (now Aventis), the EEC (Grant BIO4-CT96–0517 and Biomed 2, no. BMHa-CT98–3328) and the Association pour la Recherche contre le Cancer (contract 4459).
Abbreviations used: BrdU, 5 bromo-deoxyuridine; cdk, cyclin-dependent kinase; PMSF, phenymethlysulphonyl fluoride; H1, histone 1.
References
- Alessi, D.R., Andjelkovic, M., Caudwell, B., Cron, P., Morrice, N., Cohen, P., and Hemmings, B.A. (1996). Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541-6551. [PMC free article] [PubMed] [Google Scholar]
- Baldin, V., Cans, C., Superti-Furga, G., and Ducommun, B. (1997). Alternative splicing of the human CDC25B tyrosine phosphatase. Possible implications for growth control? Oncogene 14, 2485-2495. [DOI] [PubMed] [Google Scholar]
- Blomberg, I., and Hoffmann, I. (1999). Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell Biol. 19, 6183-6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose, S.H., Meltzer, D.I., and Feramisco, J.R. (1984). 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J. Cell Biol. 98, 847-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch, A.D. (1998). A good antisense molecule is hard to find. Trends Biochem. Sci. 23, 45-50. [DOI] [PubMed] [Google Scholar]
- Bureik, M., Rief, N., Drescher, R., Jungbluth, A., Montenarh, M., and Wagner, P. (2000). An additional transcript of the cdc25C gene from A431 cells encodes a functional protein. Int. J. Oncol. 17, 1251-1258. [DOI] [PubMed] [Google Scholar]
- Chen, M.S., Hurov, J., White, L.S., Woodford-Thomas, T., and Piwnica-Worms, H. (2001). Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol. Cell Biol. 21, 3853-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez, D., Guntuku, S., Qin, J., and Elledge, S.J. (2001). ATR and ATRIP: partners in checkpoint signaling. Science 294, 1713-1716. [DOI] [PubMed] [Google Scholar]
- Dulic, V., Drullinger, L.F., Lees, E., Reed, S.I., and Stein, G.H. (1993). Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc. Natl. Acad. Sci. USA 90, 11034-11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm, S.V., and Reed, S.I. (2000). Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12, 676-684. [DOI] [PubMed] [Google Scholar]
- Elbashir, S.M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., and Tuschl, T. (2001). Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- Fauman, E.B., Cogswell, J.P., Lovejoy, B., Rocque, W.J., Holmes, W., Montana, V.G., Piwnica-Worms, H., Rink, M.J., and Saper, M.A. (1998). Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell 93, 617-625. [DOI] [PubMed] [Google Scholar]
- Gabrielli, B.G., Clark, J.M., McCormack, A.K., and Ellem, K.A. (1997). Hyperphosphorylation of the N-terminal domain of Cdc25 regulates activity toward cyclin B1/Cdc2 but not cyclin A/Cdk2. J. Biol. Chem. 272, 28607-28614. [DOI] [PubMed] [Google Scholar]
- Gabrielli, B.G., De Souza, C.P., Tonks, I.D., Clark, J.M., Hayward, N.K., and Ellem, K.A. (1996). Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J. Cell Sci. 109, 1081-1093. [DOI] [PubMed] [Google Scholar]
- Galaktionov, K., and Beach, D. (1991). Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell 67, 1181-1194. [DOI] [PubMed] [Google Scholar]
- Galaktionov, K., Jessus, C., and Beach, D. (1995). Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 9, 1046-1058. [DOI] [PubMed] [Google Scholar]
- Girard, F., Strausfeld, U., Cavadore, J.-C., Russel, P., Fernandez, A., and Lamb, N.J.C. (1992). cdc25 is a nuclear protein expressed constitutively throughout the cell cycle in nontransformed mammalian cells. J. Cell Biol. 118, 785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard, F., Strausfeld, U., Fernandez, A., and Lamb, N.J. (1991). Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 67, 1169-1179. [DOI] [PubMed] [Google Scholar]
- Gottlin, E.B., Xu, X., Epstein, D.M., Burke, S.P., Eckstein, J.W., Ballou, D.P., and Dixon, J.E. (1996). Kinetic analysis of the catalytic domain of human cdc25B. J. Biol. Chem. 271, 27445-27449. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe, E.H., Miller, F.J., Cham, M., Khodjakov, A., and Sluder, G. (2001). Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291, 1547-1550. [DOI] [PubMed] [Google Scholar]
- Hoffmann, I., Clarke, P.R., Marcote, M.J., Karsenti, E., and Draetta, G. (1993). Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 12, 53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, I., Draetta, G., and Karsenti, E. (1994). Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 13, 4302-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, J.Y., Reimann, J.D., Sorensen, C.S., Lukas, J., and Jackson, P.K. (2002). E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat. Cell Biol. 4, 358-366. [DOI] [PubMed] [Google Scholar]
- Izumi, T., and Maller, J.L. (1993). Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol. Biol. Cell 4, 1337-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno, S., Suto, K., Nagata, A., Igarashi, M., Kanaoka, Y., Nojima, H., and Okayama, H. (1994). Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 13, 1549-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, C., Katich, S., Hagting, A., Hoffmann, I., and Pines, J. (1999). Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J. Cell Biol. 146, 573-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, N.J.C., and Fernandez, A. (1997). Microinjection of antibodies into mammalian cells. Methods Enzymol. 283, 72-83. [DOI] [PubMed] [Google Scholar]
- Lammer, C., Wagerer, S., Saffrich, R., Mertens, D., Ansorge, W., and Hoffmann, I. (1998). The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J. Cell Sci. 111, 2445-2453. [DOI] [PubMed] [Google Scholar]
- Lincoln, A.J., Wickramasinghe, D., Stein, P., Schultz, R.M., Palko, M.E., De Miguel, M.P., Tessarollo, L., and Donovan, P.J. (2002). Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat. Genet. 30, 446-449. [DOI] [PubMed] [Google Scholar]
- Liu, X., and Erikson, R.L. (2002). Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc. Natl. Acad. Sci. USA 99, 8672-8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucibello, F.C., Truss, M., Zwicker, J., Ehlert, F., Beato, M., and Muller, R. (1995). Periodic cdc25C transcription is mediated by a novel cell cycle-regulated repressor element (CDE). EMBO J. 14, 132-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand, N., Podtelejnikov, A.V., Groth, A., Mann, M., Bartek, J., and Lukas, J. (2002). Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 21, 5911-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan, C.H., Russell, P., and Reed, S.I. (1990). Periodic biosynthesis of the human M-phase promoting factor catalytic subunit p34 during the cell cycle. Mol. Cell Biol. 10, 3847-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, J.B.A., Blevitt, J., Gerace, L., Sadhu, K., Featherstone, C., and Russel, P. (1991). p55cdc25 is a nuclear protein required for the initiation of mitosis in human cells. Proc. Natl. Acad. Sci. USA 88, 10500-10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D.O. (1995). Principles of cdk regulation. Nature 374, 131-134. [DOI] [PubMed] [Google Scholar]
- Morgenstern, J.P., and Land, H. (1990). A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 18, 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice, W.G., Brunn, G.J., Wiederrecht, G., Siekierka, J.J., and Abraham, R.T. (1993). Rapamycin induced inhibition of p34cdc2 kinase activation is associated with G1/S-phase growth arrest in T lymphocytes. J. Biol. Chem. 268, 3734-3738. [PubMed] [Google Scholar]
- Morris, M.C., and Divita, G. (1999). Characterization of the interactions between human cdc25C, cdks, cyclins and cdk-cyclin complexes. J. Mol. Biol. 286, 475-487. [DOI] [PubMed] [Google Scholar]
- Nagata, A., Igarashi, M., Jinno, S., Suto, K., and Okayama, H. (1991). An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol. 3, 959-968. [PubMed] [Google Scholar]
- Nilsson, I., and Hoffmann, I. (2000). Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4, 107-114. [DOI] [PubMed] [Google Scholar]
- Nishijima, H., Nishitani, H., Seki, T., and Nishimoto, T. (1997). A dual-specificity phosphatase Cdc25B is an unstable protein and triggers p34(cdc2)/cyclin B activation in hamster BHK21 cells arrested with hydroxyurea. J. Cell Biol. 138, 1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R., Holt, M., Philipova, R., Moss, S., Schulman, H., Hidaka, H., and Whitaker, M. (1999). Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J. Biol. Chem. 274, 7958-7968. [DOI] [PubMed] [Google Scholar]
- Peng, C.Y., Graves, P.R., Thoma, R.S., Wu, Z., Shaw, A.S., and Piwnica-Worms, H. (1997). Mitotic and G2 checkpoint control: regulation of 14–3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501-1505. [DOI] [PubMed] [Google Scholar]
- Pines, J., and Hunter, T. (1990). Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature 346, 760-763. [DOI] [PubMed] [Google Scholar]
- Sadhu, K., Reed, S.I., Richardson, H., and Russel, P. (1990). Human homolog of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc. Natl. Acad. Sci. USA 87, 5139-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, T., Yamashita, K., Nishitani, H., Takagi, T., Russell, P., and Nishimoto, T. (1992). Chromosome condensation caused by loss of RCC1 function requires the cdc25C protein that is located in the cytoplasm. Mol. Biol. Cell 3, 1373-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexl, V., Diehl, J.A., Sherr, C.J., Ashmun, R., Beach, D., and Roussel, M.F. (1999). A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene 18, 573-582. [DOI] [PubMed] [Google Scholar]
- Stein, G.S., Stein, J.L., Lian, J.B., Last, T.J., Owen, T.A., and McCabe, L. (1998). Synchronization of normal diploid and transformed mammalian cells. In: Cell Biology: A Laboratory Manual, ed. J.E. Celis. San Diego: Academic Press, 253-260.
- Strausfeld, U., Fernandez, A., Capony, J.-P., Girard, F., Lautredou, N., Derancourt, J., Labbé, J.-C., and Lamb, N.J.C. (1994). Activation of p34cdc2 protein kinase by microinjection of human cdc25C in mammalian cells. J. Biol. Chem. 269, 5989-6000. [PubMed] [Google Scholar]
- Strausfeld, U., Labbé, J.-C., Fesquet, D., Cavadore, J.-C., Picard, A., Sadhu, K., Russel, P., and Dorée, M. (1991). Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature 351, 242-245. [DOI] [PubMed] [Google Scholar]
- Takizawa, C.G., and Morgan, D.O. (2000). Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12, 658-665. [DOI] [PubMed] [Google Scholar]
- Toyoshima-Morimoto, F., Taniguchi, E., and Nishida, E. (2002). Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 3, 341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski, P., Myles, T., Hemmings, B.A., Fernandez, A., and Lamb, N.J.C. (1999). Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell 10, 1997-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth, N.C. (2001). DNA damage: Chk1 and Cdc25, more than meets the eye. Curr. Opin. Genet. Dev. 11, 78-82. [DOI] [PubMed] [Google Scholar]
- Wegener, S., Hampe, W., Herrmann, D., and Schaller, H.C. (2000). Alternative splicing in the regulatory region of the human phosphatases CDC25A and CDC25C. Eur. J. Cell Biol. 79, 810-815. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Watkins, J.L., and Piwnica-Worms, H. (2002). Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. USA 99, 14795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X.Z., Kops, O., Werner, A., Lu, P.J., Shen, M., Stoller, G., Kullertz, G., Stark, M., Fischer, G., and Lu, K.P. (2000). Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol. Cell 6, 873-83. [DOI] [PubMed] [Google Scholar]
- Zwicker, J., Lucibello, F.C., Wolfraim, L.A., Gross, C., Truss, M., Engeland, K., and Muller, R. (1995). Cell cycle regulation of the cyclin A, cdc25C and cdc2 genes is based on a common mechanism of transcriptional repression. EMBO J. 14, 4514-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]