Abstract
Rho GTPases are overexpressed in a variety of human tumors contributing to both tumor proliferation and metastasis. Recently, several studies demonstrate an essential role of transcriptional regulation in Rho GTPases-induced oncogenesis. Herein, we demonstrate that RhoA, Rac1, and Cdc42 promote the expression of cyclooxygenase-2 (COX-2) at the transcriptional level by a mechanism that is dependent on the transcription factor nuclear factor-κB (NF-κB), but not Stat3, a transcription factor required for RhoA-induced tumorigenesis. With respect to RhoA, this effect is dependent on ROCK, but not PKN. Treatment of RhoA-, Rac1-, and Cdc42-transformed epithelial cells with Sulindac and NS-398, two well-characterized nonsteroid antiinflammatory drugs (NSAIDs), results in growth inhibition as determined by cell proliferation assays. Accordingly, tumor growth of RhoA-expressing epithelial cells in syngeneic mice is strongly inhibited by NS-398 treatment. The effect of NSAIDs over RhoA-induced tumor growth is not exclusively dependent on COX-2 because DNA-binding of NF-κB is also abolished upon NSAIDs treatment, resulting in complete loss of COX-2 expression. Finally, treatment of RhoA-transformed cells with Bay11-7083, a specific NF-κB inhibitor, leads to inhibition of cell proliferation. We suggest that treatment of human tumors that overexpress Rho GTPases with NSAIDs and drugs that target NF-κB could constitute a valid antitumoral strategy.
INTRODUCTION
Rho GTPases are a multimember family of proteins involved in diverse cellular functions that relate to cell growth, development, apoptosis, tumorigenesis, and metastasis (Van Aelst and D'Souza-Schorey, 1997; Bar-Sagi and Hall, 2000; Aznar and Lacal, 2001a,b, 2003; Ridley, 2001; Schmitz et al. 2002). Rho proteins regulate transcription via several transcription factors that include SRF, NF-κB, E2F, Stat3, Stat5a, Pax6, FHL-2, Estrogen Receptor α/β, ELK, PEA3, ATF2, MEF2A, Max, and CHOP/GADD153 (Aznar and Lacal, 2001b).
When overexpressed, Rho GTPases are tumorigeneic and transform murine fibroblast to promote in vivo tumor growth and distant lung metastasis in syngeneic mice (Perona et al., 1993; van Leeuwen et al., 1995; del Peso et al., 1997). As well, they mediate many aspects of the oncogenicity of several oncogenes such as Ras, Met, EGFR, and IGFR (Qiu et al., 1995a,b; Nur-E-Kamal et al., 1999; Boerner et al., 2000; Sachdev et al., 2001). Overexpression or deregulation of the GTPase or some element of the Rho pathway has been reported for human breast, colon, head and neck squamous carcinomas; and testicular germ, ovarian, leukemias, osteosarcomas, gastric, thyroid papillary, prostate, and hepatocellular cancer, among others (reviewed in Aznar and Lacal, 2003).
The role of transcription in promoting the tumoral and metastatic phenotype of Rho GTPases is acquiring increased attention. We have recently described that activation of Stat3 is involved in transformation of human fibroblasts by oncogenic RhoA (Benitah et al., 2003). Furthermore, we have identified Stat5a as an essential component of RhoA-induced epithelial to mesenchymal transition and cell motility (Aznar et al., 2002). Transcription of cyclin D1 and the protooncogene c-myc takes place by a Rho-dependent mechanism that permits G1 entry (Danen et al., 2000; Chiariello et al., 2001; Welsh et al., 2001). Finally, an indirect role for both nuclear factor-κB (NF-κB) in RhoGEF-mediated tumorigenesis, and for FHL2 in Rho-dependent tumor progression of prostate cancer, has been proposed (Whitehead et al., 1999; Muller et al., 2002). With respect to the metastatic phenotype, transcription and expression of the uPAR gene is dependent on RhoA upon integrin signaling, and SRF is regulated by changes in actin dynamics to promote transcription of vinculin and actin, both necessary for the cytoskeletal changes essential to motility and invasion (Sotiropoulos et al., 1999; Muller et al., 2000, 2002; Psichari et al., 2002). However, little is known on the target genes regulated by these transcription factors that allow proper tumor progression in the context of Rho GTPases.
Herein, we demonstrate that Rho GTPases induce cyclooxygenase-2 (COX-2) expression in epithelial cells by a NF-κB–dependent mechanism. COX-1 and COX-2 catalyze the synthesis of prostaglandins (Gupta and Dubois, 2001). Whereas COX-1 is constitutively expressed in most tissues and maintains housekeeping prostaglandin synthesis, COX-2 is inducible upon proinflammatory cytokines, growth factors, and oncogenes (Dubois, 2001; Gupta and Dubois, 2001). Accordingly, tumor cells that express COX-2 secrete proangiogenic factors stimulating tube formation and endothelial migration, contributing to the vascularization and growth of the tumor (Tsujii et al., 1998; Cao and Prescott, 2002). COX-2 is tumorigenic because its overexpression in the mammary glands itself causes malignant growth and metastasis in transgenic mice (Liu et al., 2001). At last, several human tumors including colon, breast, pancreas, lung, and squamous cell carcinoma of the head and neck display high levels of COX-2 protein (Tegeder et al., 2001).
COX-2 has acquired great interest as a potential target for the prevention and treatment of several human cancers. Nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit COX-2 are potent antitumoral and antimetastatic agents in vivo against several tumor models (Tegeder et al., 2001). However, these drugs display COX-2 independent effects that mainly affect activator protein-1, mitogen-activated protein kinase, and NF-κB (Tegeder et al., 2001). Consequently, the promiscuity of NSAIDs has led to the development of new COX-2 inhibitors, termed Coxibs (celecoxib and rofecoxib) with very selective COX-2 inhibitory capacity, albeit, their specificity has been recently challenged (Jones et al., 1999; Tegeder et al., 2001).
Herein, we provide evidence that indicates that treatment of Rho-bearing tumors with NSAIDs and drugs that target NF-κB may constitute a valid cancer therapy.
MATERIALS AND METHODS
Cell Culture, Transfections, and NSAIDs Treatment
Madin-Darby canine kidney (MDCK) epithelial cells, human colorectal carcinoma cells HT29 and DLD1 were cultured in DMEM supplemented with 10% fetal bovine serum and 1 mM glutamine. NIH3T3 fibroblasts were cultured in DMEM supplemented with 5% newborn calf serum and 1 mM glutamine. For transient expression assays, 2 × 105 cells were transfected in six-well dishes by LipofectAMINE Plus method as described by the manufacturer (Invitrogen, Carlsbad, CA). The amount of plasmidic DNA was kept constant at 3–5 μg/33-mm plate with the corresponding empty vector, and 0.5 μg of reporter was transfected where indicated. For stable expression, cells were transfected as indicated above and 48 h posttransfection selection was added. For pcDNAIIIb, RhoAQL, Rac1QL, Cdc42QL, Rac1N17, and Cdc42N17, selection was carried out with 750 μg/ml G418 (Sigma-Aldrich, St. Louis, MO). Sulindac and NS-398 were purchased from LKT Laboratories (Ann Arbor, MI) and Cayman Chemical (Ann Arbor, MI), respectively. Both NSAIDs were diluted to the indicated concentrations and medium was added fresh each 48 h. A 50 mM stock solution of Bay11-7083 (Calbiochem, San Diego, CA) was prepared and later used at a final concentration of 10 μM in DMEM.
Plasmids
PCDNAIIIB plasmid (Invitrogen) and derived expression vectors encoding for constitutively activated RhoA (QL), Rac1 (QL), and Cdc42Hs (QL) proteins and their wild-type versions have been described previously (Aznar et al., 2001). The HIV-Luc reporter that contains NF-κB-responsive elements has been described (Aznar et al., 2001). PRCCMV-p65 and pRCCMV-IκB A32/36S, wild-type and dominant negative pCEFL-Stat3 constructs, have been described previously (Aznar et al., 2001). COX-2-Luc reporter vector containing the promoter sequence spanning from nucleotide -1778 to +107 of human COX-2 gene was kindly provided by Dr. Muñoz Salas (Diaz-Cazorla et al., 1999). Wild-type and dominant negative (deltaF3) pRCCMV-FLAG-PKN constructs were kindly provided by Dr. Ono (Biosignal Research Center and Graduate School of Science and Technology, Kobe University, Japan). Wild-type and dominant negative (KD-IA) pCAG-myc-ROCK constructs were a kind gift of Dr. Narumiya (Department of Pharmacology, Kyoto University, Faculty of Medicine, Kyoto, Japan).
Gene Expression Analysis
Cells (2 × 105) were transfected with the indicated plasmids. Forty-eight hours after transfection protein extracts were prepared by lysis with the commercially available Reporter lysis buffer (Promega, Madison, WI). Protein (0.5–2 μg) was assayed for luciferase activity by using a commercial kit as described by the manufacturer (Promega). Transfection efficiencies were corrected by detection of the expressed proteins by Western immunoblotting and with a constitutive RSV5-CAT reporter vector as indicated previously (Aznar et al., 2001).
Western Blot Assays and Antibodies
For protein expression assays, cells were transfected with the corresponding plasmids and incubated in DMEM 0.5% fetal bovine serum or 10% fetal bovine serum where indicated for the next 48 h. Preparation of the samples was carried out as described previously (Benitah et al., 2003). After transfer of proteins to Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, MA), the blots were incubated with the corresponding antibodies, and immunocomplexes were visualized by enhanced chemiluminescence detection (Amersham Biosciences, Piscataway, NJ) by using either an anti-rabbit and anti-mouse antibody conjugated to peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). α-COX-2 and α-Cdc42 monoclonal antibodies were purchased to BD Biosciences (San Jose, CA). α-COX1, α-p65, and α-RhoA were purchased to Santa Cruz Biotechnology. Anti-Rac1 was purchased to Upstate Biotechnology (Lake Placid, NY). Anti-Stat3 and anti-phosphoStat3 (Tyr 705) were purchased to Cell Signaling Technology (Beverly, MA). Mouse monoclonal anti-phospho-p44/42 mitogen-activated protein kinase (Thr202/Tyr204) and phospho-MEK1 were purchased from New England Biolabs (Beverly, MA). Anti-FLAG and anti-myc antibodies to detect the expression of PKN and ROCK were purchased from Santa Cruz Biotechnology.
Electrophoretic Mobility Shift Assays (EMSAs)
For EMSA assays, cells were either transfected with the corresponding plasmids or indicated treatments and incubated in appropriate medium for 24–36 h. Nuclear extracts were obtained as described previously (Benitah et al., 2003). Briefly, 2 μg of nuclear protein was incubated with 0.1 ng of κB probe (5000 cpm) or with unlabeled probe and subjected to electrophoresis (80 V, 45 min) on a nondenaturing 4% acrylamide/bisacrylamide gel (29:1) (Bio-Rad, Hercules, CA). For gel supershift analysis, the nuclear extract was incubated for 10 min (room temperature) with anti-p65 or anti-p50 (Santa Cruz Biotechnology) in ice before addition of the labeled probe. For nonspecific competition, Stat3-binding element hSIE from the c-fos promoter was used.
Anchorage-independent Growth in Soft Agar
Cells (3 × 103; MDCK or RhoAQL stable clones) in 60-mm dishes were trypsinized and resuspended in fresh medium. Anchorage-independent growth assay was performed as described previously by plating 5000 cells/60-mm dish (Aznar et al., 2001). After 3 wk of incubation the medium was absorbed, 500 μl of 0.005% crystal violet was added and incubated for 1 h at 37°C. Plates were then washed once with 1× phosphate-buffered saline and visualized under a microscope.
Cell Cytometry and Cell Proliferation Assays
For cell proliferation assays, 1500 cells were seeded in 24-well dishes and 24 h later the indicated drugs were added to fresh medium. At the indicated time points, cells were washed, fixed on 1% glutaraldehyde (500 μl) for 30 min, and washed three times with 1× phosphate-buffered saline. Once all time points were collected, 500 μl of 0.1% crystal violet was added to cells for 30 min and then washed as described above. To obtain the incorporated crystal violet 500 μl of 10% acetic acid was added for 10 min and was later collected and read at a wavelength of 595 nm. For cell cytometry analysis, 2 × 105 cells were plated on 60-mm dishes and were treated with sulindac or NS-398 for the indicated time. For FACSSCAN analysis, the protocol was followed as described previously (Embade et al., 2000). Adhered cells were trypsinized and the cell membrane was permeabilized with 70% ethanol, spun, and resuspended in propidium iodide.
In Vivo Tumorigenic Assay and NSAIDs Treatment
Cells (2 × 106) were trypsinized and resuspended in 100 μl of fresh DMEM medium. Cells were injected subcutaneously in the limb and tumor growth was monitored twice a week for 90 d. Tumor volume was determined using the following equation: V = (Dxd2)/2. When tumors had reached a volume of 0.1 cm3, 3 mg/kg NS-398 was injected intraperitoneally three times a week during 9 wk. Tumor volume was measured at 2-d intervals during the treatment.
Prostaglandin E2 (PGE2) Quantification
Cells (5000) were plated on 24-well dishes and 24 h later they were treated with Bay11-7083 (10 μM) at the indicated time intervals (6, 8, 12, and 24 h) to collect all the supernatants at the same time of analysis. The amount of PGE2 was measured using the commercial kit PGE2 EIA kit-monoclonal (Cayman Chemical) as described by the manufacturer. Medium (50 μl) was collected and mixed in a PGE2 monoclonal antibody-coated 96-well dish and incubated overnight for 18 h at 4°C. The wells were then washed five times and incubated with Ellman's reagent in the dark for 90 min at room temperature. The assay was read at a single wavelength of 405 nm.
RESULTS
Oncogenic RhoA, Rac1, and Cdc42 Induce the Expression of COX-2 in NIH3T3, HT29, and MDCK Cells
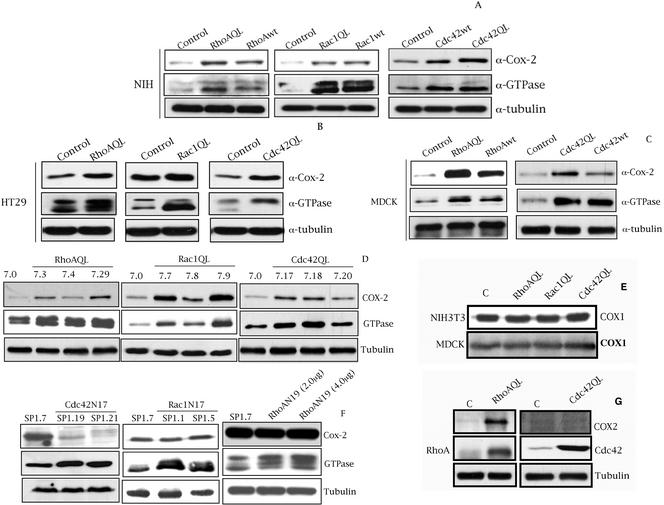
We ectopically expressed pCDNAIIIb or derived expression vectors encoding for RhoA, Rac1, and Cdc42 in NIH3T3 fibroblasts, HT29 human colorectal carcinoma cells, and MDCK epithelial cells. Wild-type and oncogenic version of all three GTPases induced high levels of COX-2 in NIH3T3 with respect to pcDNAIIIb control transfected cells (Figure 1A). In addition, oncogenic RhoA, Rac1, and Cdc42 (QL) increase the high endogenous level of COX-2 in HT29 compared with empty vector transfected cells (Figure 1B). With respect to MDCK cells, transient expression of both RhoA and Cdc42 (QL and wt) induced COX-2 expression when compared with MDCK-pcDNAIIIb cells (Figure 1C). However, we were not able to obtain significant transient expression of Rac1QL in MDCK cells as determined by Western immunoblot, nor Rac1-dependent effects such as SRF or NF-κB transcriptional activation. Thus, we sought to verify whether Rac1QL regulates COX-2 levels in MDCK cells by stable expression. In this sense, we generated MDCK stable transfectants of pcDNAIIIb or its derived vector encoding for Rac1QL, and verified the level of COX-2 expression. We were able to select six independent Rac1QL-expressing clones that exhibited increased levels of Rac1QL and that induced high COX-2 expression of which three representative ones are shown (Figure 1D). Additionally, we generated MDCK stable transfectants of vectors encoding for RhoA and Cdc42QL, and tested for the level of COX-2 expression for three independent clones of each GTPase. Two representative RhoAQL clones SP7.3, SP7.4, and a mass culture (SP7.29) that express different amounts of oncogenic RhoA, exhibit differential expression of COX-2 with respect to MDCK control cells, SP7.0. The same effect was observed with two independent Cdc42-expressing clones, SP7.18 and SP7.17, and a mass culture (SP7.20). Thus, RhoA, Rac1, and Cdc42 (QL) induce high levels of COX-2 also in MDCK cells.
Figure 1.
Rho GTPases induce the expression of COX-2 in NIH3T3, HT29, DLD-1, and MDCK cells. (A) Transient expression of RhoA, Rac1, and Cdc42 (wt and QL) induce the expression of COX-2 in NIH3T3 cells compared with empty vector (pcDNAIIIb) transfected cells. (B) Transient expression of RhoAQL, Rac1QL, and Cdc42QL increases the expression of COX-2 in HT29 human colorectal cell line, compared with HT29-pcDNAIIIb transfected cells. (C) Transient expression of wild-type and oncogenic RhoA and Cdc42 induces the expression of COX-2 in MDCK epithelial cells compared with empty vector transfected cells. For A, B, and C, all cell lines were transfected as indicated in MATERIALS AND METHODS and whole cell lysates were obtained 48 h posttransfection. (D) Stable MDCK-RhoAQL, Rac1QL, and Cdc42QL cell lines exhibit high expression of COX-2 with respect to stable MDCK-pcDNAIIIB cells. (E) RhoAQL, Rac1QL, and Cdc42QL do not stimulate the expression of COX-1 in MDCK (same extracts as in D) or NIH3T3 cells (same extracts as in A). (F) Stable HT29 transfectants of dominant negative Cdc42 (N17) (left), but not Rac1N17 (middle), display a complete loss of COX-2 expression compared with control empty vector transfected parental cell line. Transient expression of dominant negative RhoA (N19) does not inhibit COX-2 expression in HT29 cells (right). (G) RhoAQL, but not Cdc42, induces the expression of COX-2 in human colorectal cell line DLD1, which lack endogenous expression of COX-2. Equal loading was verified with anti-tubulin in all blots. All experiments were performed at least three independent times.
In addition, we verified that this effect was specific to COX-2 and not the constitutively expressed isoform COX-1 (Figure 1E). SP7.0 (MDCK-pcDNAIIIb control) and cells that express high levels of each GTPase, SP7.29 (RhoAQL), SP7.9 (Rac1QL), and SP7.18 (Cdc42QL) were used to verify COX-1 expression. As seen in Figure 1E the levels of COX-1 remain unchanged upon Rho GTPases expression in MDCK cells. The same results were obtained with all RhoAQL-, Rac1QL-, and Cdc42QL-expressing clones and mass cultures with identical results (our unpublished data). As well, no change in COX-1 expression was observed upon transient expression of RhoAQL, Rac1QL, or Cdc42QL in NIH3T3 cells compared with empty vector transfected cells (Figure 1E).
As shown in Figure 1B, HT29 human colorectal carcinoma cells show high endogenous level of COX-2 compared with other cell systems. We next verified whether Rho GTPases are involved in the expression of COX-2 in the human colorectal cancer-derived cell line HT29. To that end, we attempted to generate HT29 stable transfectants that express either dominant negative Rac1 (N17), Cdc42 (N17), RhoA (N19), or control empty vector (pcDNAIIIb). As observed in Figure 1F, expression of Cdc42N17 induced a drastic reduction of COX-2 expression, whereas Rac1N17 expression had no effect on COX-2 levels. The same result was obtained with transient expression of Cdc42N17 in HT29 cells, although due to a transfection efficiency of ∼35%, we did not observed a full inhibition of COX-2 expression (our unpublished data). Although HT29 cells can transiently express high levels of dominant negative RhoA (N19), we were not able to obtain viable clones that expressed dominant negative RhoA (N19) in a stable manner. Thus, we expressed RhoA in transient transfection experiments. RhoAN19 did not affect the expression of COX-2 in HT29 cells (Figure 1F). As controls of dominant negative activity for each GTPase, we verified that expression of RhoAN19, Rac1N17 and Cdc42N17 in HT29 cells inhibited the activation of NF-κB activity by Ost, Vav1, and Dbl, respectively, as described previously (Montaner et al. (1998)) (our unpublished data).
Because HT29 have a high level of endogenous COX-2 expression, we next investigated whether Rho GTPases were able to regulate COX-2 expression in another human colorectal cancer-derived cell line such as DLD-1, with low levels of expression of Rho GTPases and which completely lacks endogenous COX-2 expression. As shown in Figure 1G, RhoA efficiently induced the expression of COX-2 in DLD1 cells when expressed ectopically. In contrast, Cdc42 (Figure 1G), and Rac1 (our unpublished data) failed to do so. Thus, these results suggest that Rho GTPases can modulate COX-2 expression in human colon cancer. However, each GTPase analyzed in our work seems to have differential contribution or mechanisms to effect regulation of COX-2.
Rho-A-, Rac1-, and Cdc42-induced Expression of COX-2 Is Dependent on the NF-κB Transcription Factor
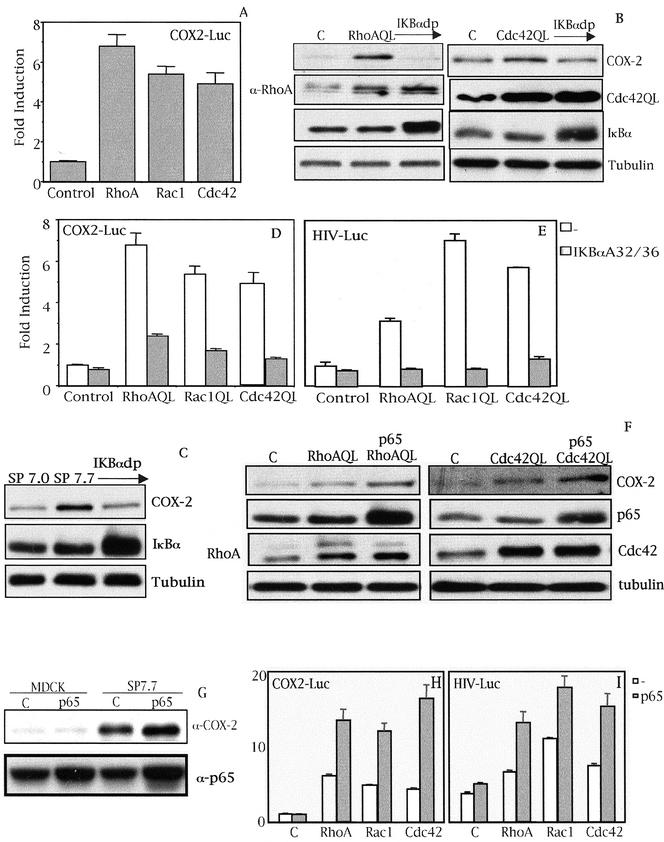
Analysis of the promoter of human COX-2 revealed several putative binding sites for transcription factors whose activity is modulated by Rho GTPases. These include NF-κB, SRF, C/EBPβ, AP-1, c-Myc, and STATs. To quantify the extent of transcription of the cox-2 gene under Rho signaling a reporter vector termed COX2-Luc containing the cox-2 promoter region spanning from bases -1772 to +106 was generated (Diaz-Cazorla et al., 1999). Transient transfection of 0.5 μg of COX2-Luc into 7.0 (MDCK-pcDNAIIIB), 7.3 (MDCK-RhoAQL), 7.9 (MDCK-Rac1QL), and 7.18 (MDCK-Cdc42QL) clones was carried out and 48 h posttransfection luciferase activity was measured. All three RhoA, Rac1, and Cdc42 (QL) induced transcription of the cox-2 promoter compared with empty vector transfected cells (Figure 2A).
Figure 2.
(facing page). Rho GTPase-dependent expression of COX-2 is at the transcriptional level and dependent on NF-κB. (A) RhoA, Rac1, and Cdc42 (QL) induce the transcription of the proximal region of the cox-2 promoter (-1772 to +106) in MDCK cells. Stable cell lines of RhoAQL, Rac1QL, and Cdc42QL (7.3, 7.9, and 7.18, respectively) were transfected with COX2-Luc reporter vector (0.5 μg), and luciferase activity was measured 48 h posttransfection. Data shown represents a single experiment performed in triplicate ± SD. (B) IκBαS32/36A inhibits RhoA- and Cdc42QL-induced COX-2 expression. Transient transfection of 2 μg of IκBαS32/36A (IκBdp) together with 1 μg of pcDNAIIIb, RhoAQL, or Cdc42QL was carried out in MDCK cells and extracts for Western blot analysis were obtained 48 h posttransfection. (C) IκBαS32/36A inhibits Rac1QL-induced COX-2 expression in MDCK cells. MDCK-pcDNAIIIb or clone SP7.9 (MDCK-Rac1QL) were transiently transfected with IκBαS32/36A (2 μg), and Western blot analysis was carried out 48 h posttransfection. Equal loading was verified with an antitubulin antibody. (D) IκBαS32/36A inhibits transcription of the cox-2 promoter induced by RhoAQL, Rac1QL, and Cdc42QL. COX2-Luc (0.5 μg) and IκBαS32/36A (1.0 μg) were transiently cotransfected in 7.0 (MDCK-pcDNAIIIb), 7.3 (MDCK-RhoAQL), 7.9 (MDCK-Rac1QL), and 7.18 (MDCK-Cdc42QL), and luciferase activity was measured 48 h posttransfection (E) Expression of IκBαS32/36A leads to a functional inhibition of NF-κB transcriptional activity in MDCK cells. Same experiment as in D was carried out with 0.5 μg of HIV-luc reporter instead of COX-2-luc, and luciferase activity was measured 48 h posttransfection. Data shown in D and E represent a single experiment performed in triplicate ± SD. (F) Overexpression of p65 augments COX-2 expression in RhoAQL- and Cdc42QL-expressing cells. MDCK cells were transfected with 2.0 μg of p65 together with pcDNAIIIB (1.0 μg) (referred as control cells) RhoAQL (1.0 μg) or Cdc42QL (1.0 μg), and extracts were obtained 48 h posttransfection. (G) p65 potentiates COX-2 expression in SP7.7 (Rac1QL-MDCK) clone without any effect in parental MDCK cells. p65 (2.0 μg) was transfected in MDCK-pcDNAIIIB control cells, or in SP7.7 cells and extracts were obtained 48 h posttransfection. Expression of p65 was verified in F and G with an anti-p65 antibody. Equal loading was determined with anti-tubulin. (H) Expression of p65 produces a synergism in Rho-mediated transcription of the cox-2 promoter. (I) Expression of p65 in MDCK cells together with RhoAQL, Rac1QL, or Cdc42QL leads to a functional increase of NF-κB transcriptional activity. For parts H and I transfection was carried out as indicated in parts D and E, but cotransfecting p65 rather than IκBαS32/36A. Transfection efficiencies in D, E, H, and I were normalized using an RSV5-CAT reporter (0.5 μg) transfected along with the indicated plasmids. All experiments were performed four times with similar results.
It has been reported that NF-κB regulates COX-2 expression under a variety of circumstances such as inflammation, hypoxia, bacterial infections, or colorectal cancers (Crofford et al., 1997; Schmedtje et al., 1997; Lim et al., 2001). Thus, we verified whether this transcription factor played any role in the induction of COX-2 by Rho GTPases. First, we transiently expressed dominant positive IκBα that carries serine residues 32 and 35 mutated to alanine (IκBαA32/A36), whose expression leads to a very efficient inhibition of NF-κB, and verified COX-2 levels under Rho signaling in MDCK cells (Karin et al., 2002). The induction of COX-2 by ectopic expression of both RhoAQL and Cdc42QL was drastically inhibited by IκBαA32/A36 (Figure 2B). The same result was obtained with two independent stable clones for RhoAQL (SP7.3 and SP7.29) and Cdc42QL (SP7.17 and SP7.18) (our unpublished data). As well, Rac1-dependent induction of COX-2 relies on the NF-κB pathway, because ectopic expression of dominant positive IκBα in SP7.7 cells inhibited COX-2 expression (Figure 2C). These results were also obtained with another clone, SP7.9 (our unpublished data). As expected, inhibition of Rho GTPases-induced COX-2 expression by IκBαA32/A36 was at the level of transcription because it abrogated COX2-Luc transcription when expressed in stable clones of each GTPase (Figure 2D). As a control of dominant positive IκBαA32/A36 activity, we verified that its expression led to inhibition of NF-κB activity and DNA-binding induced by Rho GTPases (Figure 2E; our unpublished data).
To further test whether NF-κB is involved in the induction of COX-2 by Rho GTPases, we next expressed the p65 subunit of NF-κB together with RhoAQL and Cdc42QL or control vector in MDCK cells. As seen in Figure 2F, transient coexpression of p65 with either RhoAQL or Cdc42QL in MDCK cells potentiated COX-2 expression. The same effect was observed when p65 was transiently transfected into two Rac1QL-expressing clones, SP7.7 and SP7.9, whereas overexpression of NF-κB alone in MDCK cells did not cause a significant elevation of COX-2 expression (Figure 2G; our unpublished data). Expression of p65 in RhoA, Rac1, or Cdc42QL stable clones led to an increase in cox-2 promoter activity by more than threefold compared with their respective controls (Figure 2H). Accordingly, coexpression of p65 increased NF-κB transcriptional activity induced by all three GTPases (Figure 2I). Thus, NF-κB mediates the induction of COX-2 by oncogenic RhoA, Rac1, and Cdc42 at the transcriptional level.
Induction of COX-2 by RhoGTPases Is Not via Stat3
Activation of Stat3 by members of the family of RhoGTPases, such as RhoA and Rac has been described previously (Simon et al., 2000; Aznar et al., 2001; Faruqi et al., 2001). Furthermore, Stat3 is necessary for RhoA-induced anchorage independent growth (Aznar et al., 2001). Because the cox-2 promoter contains putative Stat-binding elements, we sought to verify whether Stat3 might act downstream of Rho GTPases to induce COX-2 expression.
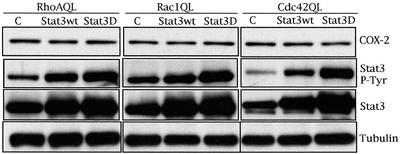
To that end, we expressed wild-type Stat3 (wt) or a dominant negative Stat3 with a mutated transactivation domain (Stat3D), in RhoAQL-, Rac1QL-, and Cdc42QL-expressing clones SP7.29, SP7.9, and SP7.17 (Figure 3). RhoA QL, Rac1 QL, and Cdc42QL efficiently induced tyrosine-705 phosphorylation of Stat3 in MDCK cells; however, no change in the level of COX-2 was observed upon transfection of either Stat3wt or Stat3D compared with vector control transfected cells. Thus, although the COX-2 promoter contains Statresponsive elements, and Stat3 is activated by Rho GTPases in MDCK epithelial cells, there is no functional relationship between Stat3 and COX-2 under Rho GTPases signaling.
Figure 3.
Stat3 transcription factor is not involved in the expression of COX-2 under Rho GTPases signaling. Activation of Stat3 and Stat3 expression were determined using an anti-Stat3PY-694 and anti-Stat3 antibody respectively. Equal loading was verified with anti-tubulin. Results shown are representative of five independent experiments.
ROCK, but Not PKN, Is Necessary for RhoA-induced COX-2 Expression via NF-κB
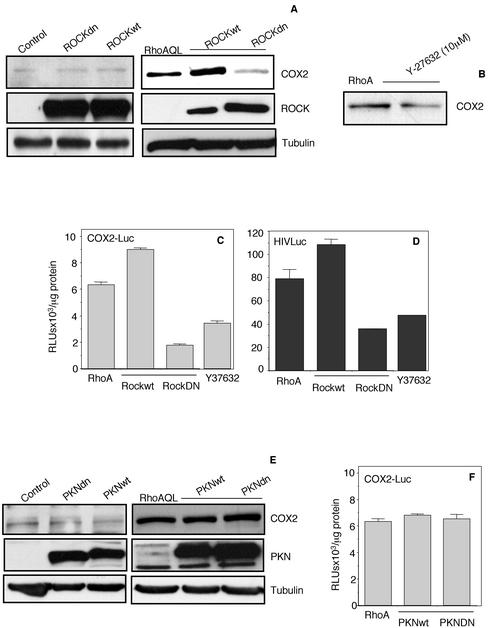
RhoA signaling is dependent on a large number of effector proteins that physically interact with RhoA and transmit the signal within the cell. Among the most studied of these are the families of ROCK and PKN (Van Aelst and D'Souza-Schorey, 1997). These families of effectors mediate most of the cytoskeletal changes induced by RhoA and have been implicated in some of the developmental processes that lead to growth and metastasis of human tumors by RhoA. Thus, we next studied the possible role of ROCK and PKN in RhoA-induced expression of COX-2. To that end, we expressed either ROCKwt or dominant negative ROCKdn in RhoAQL-expressing cells or MDCK parental cells, and measured the protein level of COX-2. Although expression of ROCKwt synergized with RhoA to induce expression of COX-2, ROCKdn greatly impaired COX-2 expression (Figure 4A). This effect was specific to RhoA signaling because overexpression of ROCKwt or ROCKdn in parental MDCK cells had no effect over COX-2 expression (Figure 4A). No effect of ROCK on COX-2 expression was observed in MDCK parental cells (Figure 4A). Inhibition of COX-2 expression was also observed when RhoAQL-expressing cells were treated with Y-27632, a specific inhibitor of ROCK kinases (Figure 4B).
Figure 4.
ROCK, but not PKN, is involved in RhoA-mediated COX-2 expression and NF-κB transcriptional activity. (A) ROCKwt and ROCKdn synergize and inhibit, respectively, RhoAQL-induced COX-2 expression (right) without any effect over COX-2 expression in parental MDKC-pcDNAIIIb cells (left). ROCKwt and ROCKdn (2.0 μg each) were cotransfected with pcDNAIIIb or RhoAQL in MDCK cells and whole cell extracts were collected 48 h posttransfection and subjected to Western blot analysis. Expression of ROCKwt and ROCKdn were verified with anti-myc antibody. (B) Y-27632 inhibits COX-2 expression induced by RhoAQL. MDCK-RhoAQL cells were treated with Y-27632 (10 μM) for 24 h, and whole cell extracts were then obtained and used to verify COX-2 expression. (C and D) ROCK is necessary for both RhoA-induced transcription of the cox2 proximal promoter region and NF-κB transcriptional activation. COX2-Luc or HIV-luc (0.5 μg) was cotransfected with ROCKwt or ROCKdn in MDCK cells along with empty vector or RhoAQL. Twenty-four hours posttransfection, luciferase activity was measured. As well, RhoAQL transfected cells were treated with Y-27632 (10 μM) for 24 h and luciferase activity was measured at this time. (E) PKN is not necessary for RhoAQL-induced COX-2 expression in MDCK cells. PKNwt or PKNdn (2.0 μg) were transfected into MDCK cells along with pcDNAIIIb or RhoAQL vectors, and 48 h posttransfection whole cell extracts were obtained. Expression of FLAG-PKNwt and FLAG-PKNdn was verified using an anti-FLAG antibody. (F) PKN has no effect overtranscription of the cox2 promoter under RhoA signaling. MDCK-RhoAQL cells were transfected either with empty vector or PKNwt and PKNdn along with the COX2-luc reporter vector. Twenty-four hours posttransfection, luciferase activity was measured as described in MATERIALS AND METHODS. Results shown in all parts are representative of three independent experiments.
As shown above, expression of COX-2 induced by RhoA is at the transcriptional level via the NF-κB pathway. Thus, we next verified whether ROCK was involved in this process. The COX2-Luc reporter vector was transfected in MDCK-RhoAQL cells together with control vector, ROCKwt, or ROCKdn and transcription of the COX-2 promoter was measured. As well, MDCK-RhoAQL cells transfected with the COX2-Luc reporter were treated with Y-27632 (10 μM) for 24 h. Although expression of ROCKwt resulted in a moderate increase in transcription of the COX-2 proximal promoter region, expression ROCKdn or treatment with Y-27632 inhibited such transcription (Figure 4C). The same type of experiment was carried out with the NF-κB–responsive reporter vector HIV-luc, to verify whether ROCK had any effect over NF-κB activity. Surprisingly, expression of ROCKwt or ROCKdn, or treatment with Y-27632 had the same effect over NF-κB activity than COX-2 promoter transcription.
The same experiments as described above were carried out with respect to PKN. However, expression of PKNwt of PKNdn in RhoAQL-transformed cells had no effect over COX-2 expression (Figure 4E). As well, no changes in the transcription of the COX-2 promoter were observed between MDCK cells transfected with RhoAQL or cotransfected with RhoAQL and PKNwt or PKNdn (Figure 4F). Expression of FLAG-tagged PKN and myc-tagged ROCK constructs were verified using anti-FLAG and anti-myc antibodies, respectively (Figure 4, A and E).
Thus, ROCK, but not PKN, affects RhoA-mediated COX-2 expression at the transcriptional level via modulation of the activity of NF-κB. To our knowledge, this is the first evidence for a relationship between NF-κB and ROCK kinases.
Nonsteroidal NSAIDs Inhibit Both Proliferation and Tumor Growth of RhoAQL Epithelial Cells
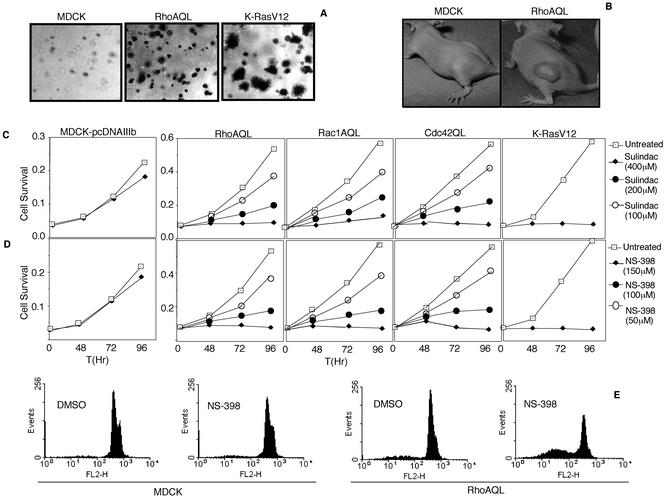
Given the implication of COX-2 and Rho GTPases overexpression in tumorigenesis and metastasis of human tumors, the above-mentioned results suggest that COX-2 might play a role in RhoA-mediated tumorigenesis. First, we analyzed the tumorigenic potential of stable MDCK-RhoAQL transfectants. Four different RhoAQL expressing clones were plated on soft agar to determine their capacity to grow under anchorage-independent conditions compared with MDCK-pcDNAIIIb (negative control) and MDCK-K-RasV12 cells (positive control). Although MDCK-pcDNAIIIb cells do not grow under these conditions, all MDCK-RhoAQL clones displayed anchorage-independent growth. Both the growth rate and size of RhoAQL clones in all cases was lower and smaller, respectively, compared with MDCK-K-RasV12 clones. Representative pictures of RhoAQL and K-RasV12 clones are shown in Figure 5A. Accordingly, four mice were injected with 2 × 106 MDCK-RhoAQL (SP7.3 and SP7.29) cells and tumor growth was monitored weekly up to 90 d after injection. As controls, MDCK-pcDNAIIIb and MDCK-KrasV12 cells were injected in the same conditions. Although control MDCK-pcDNAIIIb cells did not induce any detectable tumor, K-RasV12 cells developed tumors in 100% of injected mice approximately 10 to 15 d after injection. Both MDCK-RhoAQL clones (SP7.3 and SP7.29) induced tumor growth in three of four animals (75%). However, both the tumor volume and growth rate of RhoAQL clones were significantly lower than that displayed by K-RasV12 cells because RhoAQL tumors reached a size similar to K-RasV12 tumors ∼30 d after injection, with tumor volumes ranging 1 cm3 after 2 mo of inoculation. Representative pictures of MDCK-RhoAQL induced tumors are shown in Figure 5B.
Figure 5.
Sulindac and NS-398 inhibit proliferation and induce apoptosis of RhoAQL-, Rac1QL-, Cdc42QL-, and KrasV12-expressing epithelial cells. (A) RhoAQL and KrasV12 promote anchorage independent growth in MDCK cells. (B) RhoAQL promotes tumor growth in vivo. MDCK or MDCK-RhoAQL cells (2 × 106) were inoculated subcutaneously in athymic mice, and tumor volume was monitored at 2-d intervals. Pictures were taken 60 d upon inoculation. (C and D) Indicated concentrations of sulindac or NS-398 inhibit proliferation of MDCK cells transformed with RhoAQL, Rac1QL, Cdc42QL, and MDCK-RasV12 cells with no significant effect on MDCK parental cells. (E) NS-398 induces apoptosis of MDCK-RhoAQL but not MDCK parental cells. NS-398 (150 μM) was added to MDCK-pcDNAIIIb or MDCK-RhoAQL, and cells were treated for 96 h. At this point, cells were collected by trypsin treatment and were stained with propidium iodide for fluorescence-activated cell sorting analysis (see MATERIALS AND METHODS).
We next verified whether RhoAQL clones were susceptible to growth inhibition upon treatment with two NSAIDs, sulindac and NS-398. MDCK, MDCK-K-RasV12, and MDCK-RhoAQL (SP7.29) cells were treated with increasing amounts of Sulindac or NS-398, and cell viability was measured at 24-h intervals up to 4 d (Figure 5, C and D). A slight, nonsignificant decrease in cell growth was observed on MDCK-pcDNAIIIb cells upon treatment with both NSAIDs. However, in keeping with previous publications (Taylor et al., 2000), K-RasV12-expressing cells were significantly more sensitive to sulindac and NS-398. RhoAQL-expressing cells were also highly sensitive to sulindac and NS-398 treatment as early as 24 h upon treatment. Inhibition of proliferation was observed at concentrations of 50 μM for NS-398 and 100 μM for sulindac, with strong inhibition at 100 and 200 μM, respectively (Figure 5, C and D). However, maximal inhibition was achieved at concentrations of 150 and 400 μM for NS-398 and sulindac, respectively. Inhibition of cell proliferation with both NSAIDS was also observed upon treatment of MDCK-Rac1QL and MDCK-Cdc42QL cells at the same concentrations as those used for RhoAQL.
The growth inhibitory action of both drugs over RhoAQL-expressing cells versus control MDCK cells was due to strong induction of apoptosis rather than a cell cycle arrest as determined by flow cytometry. MDCK and RhoAQL cells were treated with sulindac (400 μM) and NS-398 (150 μM) for 120 h and analysis of propidium iodide incorporation by flow cytometry was carried out at 24-h intervals. Figure 5E shows a representative histogram at 96 h of NS-398 treatment of MDCK and MDCK-RhoAQL cells. Although control MDCK cells exhibited a residual 13.79% of apoptosis at 96 h of treatment with NS-398, >45% of RhoAQL cells were undergoing apoptosis. Although a higher toxicity was observed with sulindac in MDCK control cells, a strong induction of apoptosis was observed in RhoAQL expressing clones upon sulindac treatment (our unpublished data).
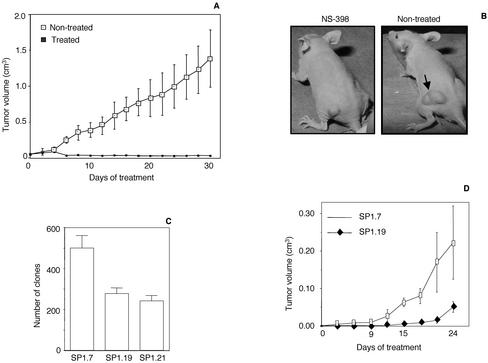
In addition, the capacity of NS-398 to inhibit RhoAQL-induced tumor growth in vivo was studied. To this end, 12 mice were injected with MDCK-RhoAQL (SP7.29) cells and when tumors had reached a mean volume of 0.05–0.1 cm3 (approximately 1 mo after inoculation), mice were treated intraperitoneally with either NS-398 (3 mg/kg, 3 times a week during 4 wk) or vehicle, and tumor growth was compared between both populations. As observed in Figure 6A, a strong tumor growth inhibition was obtained in NS-398–treated mice that was statistically significant after 1 wk of treatment (p < 0.05). A slight decrease in tumor volume was observed at this time, yet tumor size was maintained all throughout the time of treatment after this first week. A representative picture of a treated and a nontreated mouse is depicted in Figure 6B. Therefore, NS-398 is a very efficient antitumoral agent against tumors that arise as a consequence of RhoAQL overexpression.
Figure 6.
NS-398 inhibits tumor growth of RhoAQL-transformed MDCK cells. (A) NS-398 inhibits tumor growth promoted by oncogenic RhoA. Cells (2 × 106) were inoculated subcutaneously in nu/nu mice and when tumors had reached a mean volume of 0.05–0.1 cm3, mice were treated intraperitoneally with NS-398 (3 mg/kg body weight) at 3-d intervals. Statistical significance was achieved at day 8 of treatment and was maintained throughout the rest of the treatment (p < 0.05). (B) Representative pictures of NS398-treated and vehicle-treated mice inoculated with MDCK-RhoAQL cells at 2 wk of treatment. (C) Inhibition of Cdc42 in HT29 cells results in a 50% reduction of anchorage-independent growth in soft agar. Clones were stained with crystal violet and were quantified 1 mo after seeding of cells. (D) Tumor growth of HT29-Cdc42N17 (SP1.19) in syngeneic mice is delayed with respect to empty vector transfected cells HT29 cells (SP1.7). Cells (2 × 106; SP1.7 and SP1.19) were inoculated subcutaneously in immunosuppressed mice and tumor volume was monitored at 2-d intervals. All experiments shown were performed three independent times with similar results.
Overexpression of members of the family of Rho GTPases in diverse human tumors has been described. Moreover, in several tumoral models, Rho GTPases have been found to be essential either for tumor growth or metastasis. Thus, we next evaluated whether inhibition of Rho GTPases in human colorectal carcinoma-derived cell line HT29, which overexpress both Cdc42 and RhoA, would have any effect over their capacity to promote tumor growth in vivo. As mentioned above, we could not obtain viable stable RhoAN19-HT29 clones; however, we established two HT29-Cdc42N17 clones (SP1.19 and SP1.21), which have completely lost expression of COX-2 (Figure 1F). Both HT29-Cdc42N17 stable cell lines showed an approximate 50% reduction with respect to vector transfected HT29 cells in their capability to grow under anchorage independent conditions in soft agar (Figure 6C). Furthermore, both SP1.19 and SP1.21 clones were injected each in four nude mice and tumor growth was monitored at 3-d intervals compared with control vector transfected HT29 cells (SP1.7). Tumor growth of HT29-Cdc42N17 cells (clones SP1.19) was significantly delayed compared with that of parental HT29 cells (SP1.7), with statistical significance (p <0.05) (Figure 6D). Thus, Cdc42 is an important signaling component that contributes to tumor growth of HT29 human colorectal carcinoma cells.
NF-κB Activity Is Affected by Both Sulindac and NS-398 Treatment in RhoAQL-expressing Cells and Is Necessary for Cell Proliferation
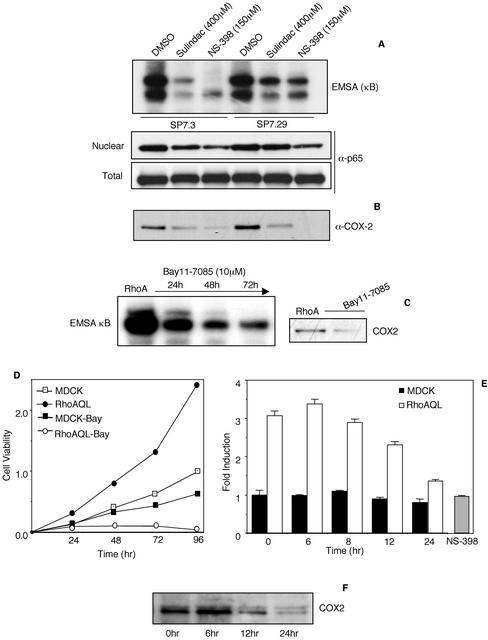
Several works have shown that different NSAIDs elicit their antiinflammatory and antitumoral effects by COX-2–independent mechanisms (Tegeder et al., 2001). In fact, sulindac and NS-398 have been shown to affect NF-κB activity as well as other proteins in different cell types that would account for some of their specific effects (Yamamoto et al., 1999; Shao et al., 2000; Mack et al., 2001). This is of particular interest in our system where both proteins, COX-2 and NF-κB, are directly connected. Thus we sought to determine the effect of sulindac and NS-398 treatment on NF-κB activity under RhoAQL signaling. To that end, two RhoAQL expressing clones, SP7.3 and SP7.29, were treated with sulindac (400 μM) or NS-398 (150 μM) for 72 h, and DNA-binding of NF-κB studied using a κB consensus element. As shown in Figure 7A, NF-κB DNA-binding was significantly impaired upon treatment with both drugs. Accordingly, the level of nuclear p65 subunit was verified to be lower in treated versus untreated cells. Whole cell lysates were obtained under the same conditions and the total level of cellular p65 was determined to remain constant (Figure 7A). Thus, inhibition of NF-κB by sulindac and NS-398 is not due to reduced NF-κB synthesis but rather to a specific inhibition of its nuclear migration and DNA-binding activity.
Figure 7.
Sulindac and NS-398 inhibit both NF-κB DNA-binding and COX-2 expression induced by RhoAQL. (A) Sulindac and NS-398 inhibit NF-κB DNA-binding without affecting p65 expression. MDCK-RhoQL cells (clones SP7.3 and SP7.29) were treated with NS-398 and sulindac at the indicated concentrations for 72 h, and nuclear and whole cell extracts were obtained. EMSA analysis was carried out using a κB consensus sequence, and p65 expression was verified using an anti-p65 antibody. (B) Sulindac and NS-398 treatment inhibit COX-2 expression induced by RhoAQL. Same whole cell extracts as in A were used to verify COX-2 expression with an anti-COX-2 antibody. (C) MDCK-RhoAQL cells (SP7.3) were treated with Bay11-7083 (10 μM) for 72 h and nuclear and whole cell extracts were obtained. EMSA analysis and COX-2 expression were performed as in A and B. (D) Bay11-7083 inhibits MDCK-RhoAQL cell proliferation with a minor effect over empty vector transfected MDCK cells. MDCK-pcDNAIIIb and MDCK-RhoAQL cells were plated on 24-well dishes and were treated with Bay11-7083 (10 μM) at the indicated time points. Cell viability was determined by the crystal violet method. (E) Bay11-7083 does not affect the enzymatic activity of COX-2 (PGE2 production) in MDCK cells transformed with RhoAQL. MDKC-pcDNAIIIb and MDCK-RhoAQL (SP7.3) were plated in 24-well dishes and were treated with Bay11-7083 (10 μM) for the indicated period of time (0, 6, 8, and 24 h). Supernatant was collected and PGE2 concentration was measured as described in MATERIALS AND METHODS. As a positive control, NS-398 (100 μM) was added for 8 h and PGE2 synthesis was measured as described above. Ordinate indicate fold induction of PGE2 relative to control cells. (F) Bay11-7083 inhibits RhoAQL-induced COX-2 expression in MDCK cells at 12 h of treatment. The same experiment as in E was carried in parallel and whole cell extracts were obtained and subjected to Western blot analysis by using an anti-COX-2 antibody. All experiments were performed three times with similar results.
Expression of COX-2 by RhoAQL is dependent on NF-κB. Therefore, we hypothesized that inhibition of NF-κB upon sulindac and NS-398 treatment would lead to a reduction in the level of expressed COX-2. Whole cell extracts were obtained from SP7.3 and SP7.29 cells treated during 24 h with 400 μM sulindac or 150 μM NS-398, and COX-2 expression was determined by Western immunoblotting (Figure 7B). Interestingly, the level of COX-2 was significantly reduced in sulindac-treated cells and complete loss of expression was observed upon NS-398 treatment. This effect was not due to a general loss of expression of cellular proteins because the level of endogenous p65 was unaffected upon treatment with both drugs. A similar effect was observed with 50 μM NS-398, although the effect was not as drastic (our unpublished data). Thus, both sulindac and NS-398 affect both COX-2 and NF-κB activities.
Last, we verified whether treatment of MDCK-pcDNAIIB and MDCK-RhoAQL cells with a specific inhibitor of NF-κB, termed Bay11-7083, would confirm these results. As shown in Figure 7C, treatment of RhoAQL-expressing cells with Bay11-7083 resulted in inhibition of both COX-2 expression and NF-κB DNA-binding. We next verified if exposure of MDCK-RhoAQL cells to Bay11-7083 would lead to inhibition of cell proliferation. MDCK-pcDNAIIIb and MDCK-RhoAQL cells were treated with Bay11-7083 (10 μM) during 96 h, and cell viability was measured at 24-h intervals (Figure 7D). Although treatment of MDCK cells with Bay11-7083 produced an inhibition of cell proliferation, this inhibition was drastically increased in MDCK-RhoAQL cells. Thus, inhibition of NF-κB with Bay11-708 drastically interferes with cell proliferation driven by oncogenic RhoA, in keeping with the results shown above on a similar effect induced by the COX-2 inhibitors sulindac and NS-398.
To discriminate the effects on NF-κB from those of COX-2, we next analyzed the possible inhibitory effect of Bay11-7083 directly on COX-2 activity at early stages of treatment (Figure 7E). For this, we measured synthesis of PGE2 in both MDCK control cells (transfected with the empty vector) and in RhoAQL transformed cells. Bay11-7083 had no significant effect on COX-2 activity up to 8 h of treatment, whereas a partial inhibition of PGE2 production could be observed at 12 h of treatment. This effect was due to inhibition of COX-2 expression rather than a direct inhibition over its enzymatic activity (Figure 7F). As well, as a positive control we treated MDCK-RhoAQL cells with NS-398 (100 μM) for 8 h in the same experiment and verified that there was a much more efficient inhibition of COX-2 enzymatic activity. Thus, NF-κB activity is necessary for cell proliferation induced by RhoA.
DISCUSSION
An emerging interest in Rho GTPases signaling as plausible targets for the development of antitumoral strategies is a consequence of the overwhelming evidence that relates the overexpression of either the GTPase itself or the deregulation of some downstream signaling component in a high number of human cancers (Aznar and Lacal, 2001, 2003; Sahai and Marshall, 2002). Many works have delineated the downstream effectors to RhoA, Rac1, and Cdc42 that contribute to the tumoral phenotype. On the other hand, there is a significant lack of knowledge about the physiological target genes whose expression is modulated under persistent Rho signaling contributing to tumor development and metastasis. In this sense, cyclin D1, c-Myc, p21 (Cip1), and p27 (Kip1) are among the few known targets that might enable aberrant growth stimulated by Rho GTPases (Pruitt and Der, 2001).
In this work, we identify COX-2 as a target gene that is transcriptionally regulated by Rho GTPases in several cell lines of murine, canine, and human origin. Activation of COX-2 by Rho GTPases may be cell specific, as many other Rho-dependent signaling pathways (reviewed in Aznar and Lacal, 2001b). Thus, overexpression of COX-2 in human colon cancer HT29 cells is completely dependent on Cdc42, because stable expression of dominant negative Cdc42N17 led to complete loss of COX-2 expression. However, although transient expression of oncogenic RhoAQL or Rac1QL in HT29 results in an increase in COX-2 expression, inhibition of endogenous Rac1 or RhoA through expression of their dominant negative inhibitory mutants did not affect COX-2 expression in the cell line. However, DLD1 cells that express low levels of Rho GTPases and completely lack endogenous COX-2 expression, express COX-2 upon RhoA, but not Rac1 and Cdc42 overexpression. These results suggest that Rho GTPases might be involved in the expression of COX-2 in a cell type- and tumor-specific manner.
Some works had previously suggested that Rho GTPases might trigger transcription of the cox-2 promoter, with reporter assays and chemical inhibitor experiments (Slice et al., 1999, 2000; Hahn et al., 2002). Slice et al. (2000) have shown that RhoA and Rac1 but not Cdc42 induce transcriptional activation of a reporter vector that contains the cox-2 promoter region spanning from -963 to +50 in NIH3T3 fibroblasts. Herein, we provide evidence that either wild-type or constitutively active forms of Cdc42 stimulate expression of endogenous COX-2 via the NF-κB pathway to a similar extent to that found for RhoA and Rac1. This discrepancy might be due to differences in the selected promoter region used for the reporter vector, because it lacked all putative κB elements present in the endogenous cox-2 promoter. Interestingly, in the same work it was determined that the elements located in the cox-2 promoter between -80 and -40 were critical for Rac- and Rho-induced transcription of the reporter vector. In addition, a CRE/ATF element was shown to be essential for transcriptional stimulation of the reporter by Rac1, but not for RhoA. In our work, we have observed that inhibition of NF-κB leads to complete loss of COX-2 expression. Although, this does not exclude the possibility that other cis-acting elements might be relevant for the physiological expression of COX-2 under Rho signaling. Interestingly, although Stat3 is involved in Rho-mediated anchorage independent growth, and the cox-2 promoter contains at least two STAT putative binding elements, we have not observed any effect of Stat3 signaling over Rho GTPases-induced expression of COX-2.
In terms of the molecular mechanism involved, we have identified ROCK as one of the RhoA effector proteins involved in the expression of COX-2. The role of ROCK in Rho-mediated tumorigenesis has been extensively studied. However, besides their profound effects over cell cytoarchitecture and motility, both of which have been related to its capability to promote tumor invasion, little is known as to whether this family of kinases regulates transcriptional pathways that promote tumor growth. Herein, we demonstrate that ROCK is necessary for RhoA-induced expression of COX-2 at the transcriptional level and that ROCK modulates the transcriptional activity of NF-κB.
In keeping with the possible functional relationship between Rho GTPases and COX-2, there are many similarities between the expression profiles of both proteins in human tumors. For instance, both proteins are up-regulated and necessary for aberrant epidermal growth factor receptor signaling and tumor growth induced by the receptor. The same holds true for tumor growth induced by oncogenic Ras, activation of c-Myc by growth factors, or signaling from the PI3K/Akt pathway (Taylor et al., 2000; Sheng et al., 2001a,b; Murga et al., 2002; Pai et al., 2002). Furthermore, overexpression of COX-2 and members of the family of Rho GTPases has been detected in same human tumors such as breast, colon, pancreas, and head and neck squamous carcinomas.
The relationship between the NF-κB/COX-2 pathway and Rho proteins in neoplastic transformation might provide an additional way to treat tumors where Rho proteins are implicated. Several inhibitors of Rho signaling are available that exhibit antitumoral and antimetastatic activities (Aznar and Lacal, 2001b, 2003). In identifying the ROCK/NF-κB/COX-2 pathway as a physiological Rho target, new antitumoral approaches can be made with respect to tumors where Rho GTPases are an issue. MDCK cells transformed with RhoA, which are tumorigenic as determined by anchorage-independent growth and in vivo tumor growth studies, are susceptible to efficient inhibition of proliferation by sulindac and NS-398. Thus, NSAIDs inhibit cell proliferation, induce apoptosis, and prevent tumor growth of cells transformed with Rho proteins with little effects over parental untransformed cells. The differences between MDCK and MDCK-RhoAQL cells upon NSAIDs treatment are probably due to the differential activities of signaling pathways, including COX-2 and NF-κB. Indeed, the inhibitory effects of both NSAIDs over Rho-transformants cannot be solely related to COX-2 inhibition, because both drugs have been reported to affect several other pathways (Tegeder et al., 2001). These pathways regulated by Rho would presumably set the different behavior of the parental cells versus RhoA-transformed cells upon drug exposure. Of particular interest is the inhibitory action of sulindac and NS-398 over NF-κB activation. More importantly, COX-2 expression is completely inhibited upon 24 h of NS-398 treatment of the cells. Thus, given that the half-life of COX-2 ranges from 3.5 to 8 h, the antitumoral effect of both NSAIDs over RhoAQL transformed cells might take place via inhibition of the preexisting COX-2 at early stages of treatment and to sustained NF-κB inhibition during early and late stages of the treatment. This idea is further strengthened by the fact that BAY11-7083, an inhibitor of NF-κB, drastically blocks proliferation of RhoAQL transformed cells with no direct effect on COX-2 enzymatic activity upon early stages of treatment. In addition, these observations suggest that treatment of tumors induced by Rho GTPases with conventional NSAIDs might be equally valid with respect to treatment with yet to be synthesized specific COX-2 inhibitors.
Last, we have provided evidence that inhibition of endogenously overexpressed Cdc42 in HT29 cells leads to a significant delay of tumor growth in vivo, further potentiating the knowledge of an important role of Rho GTPases in human cancer. Thus, overall, these results suggest that inhibition of Rho GTPases signaling via COX-2, NF-κB, or ROCK may constitute a plausible strategy to inhibit tumor growth and open a new alley for the development of a novel antitumoral strategy against human tumors where Rho GTPases play an important role.
Acknowledgments
This work was supported by grants SAF2001-2042 and SAF2002-2437 from Ministerio de Ciencia y Tecnología. S.A.B. is a fellow from Fondo deInvestigación Sanitaria (Instituto deSalud Carlos III).
References
- Aznar, S., and Lacal, J.C. (2001a). Rho signals to cell growth and apoptosis. Cancer Lett. 165, 1-10. [DOI] [PubMed] [Google Scholar]
- Aznar, S., and Lacal, J.C. (2001b). Searching new targets for anticancer drug design: the families of Ras and RhoGTPases and their effectors. Prog. Nucleic Acid Res. Mol. Biol. 67, 193-234. [DOI] [PubMed] [Google Scholar]
- Aznar, S., and Lacal, J.C. (2003). Rho GTPases in human carcinogenesis: a tale of excess. Rev. Oncol. (in press).
- Aznar, S., Valeron, P.F., del Rincon, S.V., Perez, L.F., Perona, R., and Lacal, J.C. (2001). Simultaneous tyrosine and serine phosphorylation of stat3 transcription factor is involved in rho a GTPase oncogenic transformation. Mol. Biol. Cell 12, 3282-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi, D., and Hall, A. (2000). Ras and Rho GTPases: a family reunion. Cell 103, 227-238. [DOI] [PubMed] [Google Scholar]
- Boerner, J.M., Danielsen, A.J., McManus, M.J., and Maihle, N.J. (2000). Activation of Rho is required for ligand-independent oncogenic signaling by a mutant EGF receptor. J. Biol. Chem. 10, 3691-3695. [DOI] [PubMed] [Google Scholar]
- Cao, Y., and Prescott, S.M. (2002). Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J. Cell. Physiol. 190, 279-286. [DOI] [PubMed] [Google Scholar]
- Crofford, L.J., Tan, B., McCarthy, C.J., and Hla, T. (1997). Involvement of nuclear factor kappa B in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum. 40, 226-236. [DOI] [PubMed] [Google Scholar]
- Chiariello, M., Marinissen, M.J., and Gutkind, S. (2001). Regulation of c-myc expression by PDGF through Rho GTPases. Nat. Cell Biol. 3, 580-586. [DOI] [PubMed] [Google Scholar]
- Danen, E.H., Sonneveld, P., Sonnenberg, A., and Ymamda, K.M. (2000). Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to fibronectin supports growth factorstimulated cell cycle progression. J. Cell Biol. 151, 1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Peso, L., Hernandez-Alcoceba, R., Embade, N., Carnero, A., Esteve, P., Paje, C., and Lacal, J.C. (1997). Rho proteins induce metastatic properties in vivo. Oncogene 15, 3047-3057. [DOI] [PubMed] [Google Scholar]
- Diaz-Cazorla, M., Perez-Sala, D., Ros, J., Jimenez, W., Fresno, M., and Lamas, S. (1999). Regulation of cyclooxygenase-2 expression in human mesangial cells-transcriptional inhibition by IL-13. Eur. J. Biochem. 260, 268-274. [DOI] [PubMed] [Google Scholar]
- Dubois, R.N. (2001). New paradigms for cancer prevention. Carcinogenesis 22, 691-692. [DOI] [PubMed] [Google Scholar]
- Embade, N., Valeron, P.F., Aznar, S., Lopez-Collazo, E., and Lacal, J.C. (2000). Apoptosis induced by Rac GTPase correlates with induction of FasL and ceramides production. Mol. Biol. Cell 11, 4347-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruqi, T.R., Gomez, D., Bustelo, X.R., Bar-Sagi, D., and Reich, N.C. (2001). Rac1 mediates Stat3 activation by autocrine IL-6. Proc. Natl. Acad. Sci. USA 98, 9014-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R.A., and Dubois, R.N. (2001). Colorectal cancer prevention and treatment by inhibition of cyclooxygenase 2. Nat. Rev. Cancer 1, 11-21. [DOI] [PubMed] [Google Scholar]
- Hahn, A., Barth, H., Kress, M., Mertens, P.R., and Goppelt-Struebe, M. (2002). Role of Rac and Cdc42 in lysophosphatidic acid-mediated cyclo-oxygenase-2 gene expression. Biochem. J. 362, 33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.K., Wang, H., Peskar, B.M., Levin, E., Itani, R.M., Sarfeh, I.J., and Tarnawski, A.S. (1999). Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat. Med. 5, 1418-1423. [DOI] [PubMed] [Google Scholar]
- Karin, M., Cao, Y., Greten, F.R., and Li, Z.W. (2002). NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2, 301-310. [DOI] [PubMed] [Google Scholar]
- Lim, J.W., Kim, H., and Kim, K.H. (2001). Nuclear factor κB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab. Investig. 81, 349-360. [DOI] [PubMed] [Google Scholar]
- Liu, C.H., Chang, S-H., Narko, K., Trifan, O.C., Wu, M-T., Smith, E., Haudenschild, C., Lane, T.F., and Hla, T. (2001). Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J. Biol. Chem. 276, 18563-18569. [DOI] [PubMed] [Google Scholar]
- Mack Strong, V.E., Mackrell, P.J., Concannon, E.M., Mestre, J.R., Smyth, G.P., Schaefer, P.A., Stapleton, P.P., and Daly, J.M. (2001). NS-398 treatment after trauma modifies NF-κB activation and improves survival. J. Surg. Res. 98, 40-46. [DOI] [PubMed] [Google Scholar]
- Muller, J.M., Metzger, E., Greschik, H., Bosserhoff, A.K., Mercep, L., Buettner, R., and Schule, R. (2002). The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 21, 736-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, S.M., Okan, E., and Jones, P. (2000). Regulation of urokinase receptor transcription by Ras- and Rho-family GTPases. Biochem. Biophys. Res. Commun. 270, 892-898. [DOI] [PubMed] [Google Scholar]
- Murga, C., Zohar, M., Teramoto, H., and Gutkind, J.S. (2002). Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-κB. Oncogene 21, 207-216. [DOI] [PubMed] [Google Scholar]
- Nur-E-Kamal, M.S.A., Kamal, J.M., Qureshi, M.M., and Maruta, H. (1999). The CDC42-specific inhibitor derived from ACK-1 blocks v-Ha-Ras-induced transformation. Oncogene 18, 7787-7793. [DOI] [PubMed] [Google Scholar]
- Pai, R., Sorenghan, B., Szabo, I.L., Pavelka, M., Baatar, D., and Tarnawski, A.S. (2002). Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 8, 289-293. [DOI] [PubMed] [Google Scholar]
- Perona, R., Esteve, P., Jimenez, B., Ballestero, R.P., Ramon y Cajal, S., and Lacal, J.C. (1993). Tumorigenic activity of rho genes from Aplysia californica. Oncogene 8, 1285-1292. [PubMed] [Google Scholar]
- Pruitt, K., and Der, C.J. (2001). Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 17, 1-10. [DOI] [PubMed] [Google Scholar]
- Psichari, E., Balmain, A., Plows, D., Zoumpourlis, V., and Pintzas, A. (2002). High activity of serum response factor in the mesenchymal transition of epithelial tumor cells is regulated by RhoA signaling. J. Biol. Chem. 277, 29490-29495. [DOI] [PubMed] [Google Scholar]
- Qiu, R.G., Chen, J., Kirn, D., McCormick, F., and Symons, M. (1995b). An essential role for Rac in Ras transformation. Nature 37, 457-459. [DOI] [PubMed] [Google Scholar]
- Qiu, R.G., Chen, J., McCormick, F., and Symons, M. (1995a). A role for Rho in Ras transformation. Proc. Natl. Acad. Sci. USA 92, 11781-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J. (2001). Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471-477. [DOI] [PubMed] [Google Scholar]
- Sachdev, P., Jiang, X.Y., Li, W., Miki, T., Nur-E-Kamal, M.S., Wang, L.H. (2001). Differential requirement for Rho family GTPases in an oncogenic insulin-like growth factor-I receptor-induced cell transformation. J. Biol. Chem. 276, 26461-26471. [DOI] [PubMed] [Google Scholar]
- Sahai, E. and Marshall, C.J. (2002) Rho-GTPases and cancer. Rev. Cancer 2, 133-142. [DOI] [PubMed] [Google Scholar]
- Schmedtje, J.F., Ji, Y.S., Lui, W.L., DuBois, R.N., and Runge, M.S. (1997). Hypoxia induces cyclooxygenase-2 via NF-κB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 272, 601-608. [DOI] [PubMed] [Google Scholar]
- Schmitz, A.A.P., Govek, E-E., Bottner, B., and Van Aelst, L. (2002). Rho GTPases: signaling, migration, and invasion. Exp. Cell Res. 261, 1-12. [DOI] [PubMed] [Google Scholar]
- Shao, J., Sheng, H., Inoue, H., Morrow, J.D., and DuBois, R.N. (2000). Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J. Biol. Chem. 275, 33951-33956. [DOI] [PubMed] [Google Scholar]
- Sheng, H., Shao, J., and Dubois, R.N. (2001a). K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves activation of the protein kinase B1. Cancer Res. 61, 2670-2675. [PubMed] [Google Scholar]
- Sheng, H., Shao, J., Washington, M.K., and DuBois, R.N. (2001b). Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 276, 18075-18081. [DOI] [PubMed] [Google Scholar]
- Simon, A.R., Vikis, H.G., Stewart, S., Fanburg, B.L., Cochran, B.H., and Guan, K.L. (2000). Regulation of STAT3 by direct binding to the Rac1 GTPase. Science 290, 144-147. [DOI] [PubMed] [Google Scholar]
- Slice, L.W., Bui, L., Mak, C., and Walsh, J.H. (2000). Differential regulation of COX-2 transcription by Ras- and Rho-family of GTPases. Biochem. Biophys. Res. Commun. 276, 406-410. [DOI] [PubMed] [Google Scholar]
- Slice, L.W., Walsh, J.H., and Rozengurt, E. (1999). Gα 13 stimulates Rho-dependent activation of the cyclooxygenase-2 promoter. J. Biol. Chem. 274, 27562-27566. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos, A., Gineitis, D., Copeland, J., and Treisman, R. (1999). Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98, 159-169. [DOI] [PubMed] [Google Scholar]
- Taylor, M.T., Lawson, K.R., Ignatenko, N.A., Marek, S.E., Stringer, D.E., Skovan, B.A., and Gerner, E.W. (2000). Sulindac sulfone inhibits K-ras-dependent cyclooxygenase-2 expression in human colon cancer cells. Cancer Res. 60, 6607-6610. [PubMed] [Google Scholar]
- Tegeder, I., Pfeilschifter, and Geisslinger, G. (2001). Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 15, 2057-2072. [DOI] [PubMed] [Google Scholar]
- Tsujii, M., Kawano, S., Tsuji, S., Sawaoka, H., Hori, M., and Dubois, R.N. (1998). Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93, 705-716. [DOI] [PubMed] [Google Scholar]
- Van Aelst, L., and D'Souza-Schorey, C. (1997). Rho GTPases and signaling network. Genes Dev. 11, 2295-2322. [DOI] [PubMed] [Google Scholar]
- van Leeuwen, F.N., van der Kammen, R.A., Habets, G.G., and Collard, J.G. (1995). Oncogenic activity of Tiam1 and Rac1 in NIH3T3 cells. Oncogene 11, 2215-2221. [PubMed] [Google Scholar]
- Welsh, C.F., Roovers, K., Villanueva, J., Liu, Y., Schwartz, M.A., and Assoian, R.K. (2001). Timing of Cyclin D1 expression within G1 phase is controlled by Rho. Nat. Cell Biol. 3, 950-957. [DOI] [PubMed] [Google Scholar]
- Whitehead, I.P., Lambert, Q.T., Glaven, J.A., Abe, K., Rossman, K.L., Mahon, G.M., Trzaskos, J.M., Kay, R., Campbell, S., and Der, C.J. (1999). Dependence of Dbl and Dbs transformation on MEK and NF-κB activation. Mol. Cell. Biol. 19, 7759-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, Y., Yin, M.-J., Lin, K.-M., and Gaynor, R. (1999). Sulindac inhibits activation of the NF-κB pathway. J. Biol. Chem. 274, 27307-27314. [DOI] [PubMed] [Google Scholar]