Abstract
Legionella pneumophila requires the Dot/Icm protein translocation system to replicate within host cells as a critical component of Legionnaire's pneumonia. None of the known individual substrates of the translocator have been shown to be essential for intracellular replication. We demonstrate here that mutants lacking the Dot/Icm substrate SdhA were severely impaired for intracellular growth within mouse bone marrow macrophages, with the defect absolute in triple mutants lacking sdhA and its two paralogs. The defect caused by the absence of the sdhA family was less severe during growth within Dictyostelium discoideum amoebae, indicating that the requirement for SdhA shows cell-type specificity. Macrophages harboring the L. pneumophila sdhA mutant showed increased nuclear degradation, mitochondrial disruption, membrane permeability, and caspase activation, indicating a role for SdhA in preventing host cell death. Defective intracellular growth of the sdhA− mutant could be partially suppressed by the action of caspase inhibitors, but caspase-independent cell death pathways eventually aborted replication of the mutant.
Keywords: apoptosis, Dot/Icm, sdhA
Legionella pneumophila is a fastidious Gram-negative bacterium that grows within alveolar macrophages during Legionnaire's disease (1). Pneumonic diseases caused by this microorganism are initiated after exposure of human hosts to aerosols of contaminated water sources that harbor L. pneumophila growing within amoebae (2). Intracellular replication in the lung seems to mimic the relationship of the microorganism with amoeba, its preferred environmental host, fostering the idea that growth within host macrophages is a fortuitous event (3). There is substantial evidence indicating that certain L. pneumophila proteins seem more critical for growth within some types of host cells, however, so bacterial proteins may exist that preferentially target macrophages (4, 5).
Intracellular growth of L. pneumophila follows a distinctive pathway that allows the bacterium to maintain contact with the host cell secretory system, promoting formation of a Legionella-containing vacuole (LCV). Shortly after uptake, the bacterium is found in a membrane-bound compartment that bypasses routing into the endocytic pathway and associates with endoplasmic reticulum-derived vesicles (6–11). The morphology of the LCV in both mammalian cells and amoebae is similar, further supporting the idea that there is an evolutionarily conserved pathway for promoting intracellular growth (12).
The ability to form the LCV requires the bacterial Dot/Icm protein translocation machine (13, 14). In the absence of the Dot/Icm system, the bacteria are targeted into the endocytic path and fail to replicate intracellularly (7). A large number of proteins have been identified that are translocated across the LCV membrane by the Dot/Icm system, including several that seem to manipulate vesicle traffic between the endoplasmic reticulum and the Golgi apparatus (15–17). Mutations eliminating production of many of these substrates have been isolated, but they have little or no effect on intracellular growth, probably because of functional redundancy (17).
The translocated substrates of the Dot/Icm system stimulate host cell death by multiple pathways (18–20). At a high multiplicity of infection (moi), target macrophages undergo rapid cell death, whereas at low moi, a fraction of host cells undergo apoptotic death (19, 21, 22). Although the killing of target cells may play some role in the pathogenesis of disease within the animal, host cell death is distinctly antagonistic to intracellular replication (23), and it is known that the bacterium limits host cell death by the induction of macrophage anti-apoptotic proteins (24). L. pneumophila, however, also uses other strategies to interfere with host cell death, as there seem to be Dot/Icm-dependent signals that antagonize host cell death (25).
In this article, we demonstrate that the SdhA protein is a Dot/Icm-translocated substrate that is critical for growth within macrophages. In its absence, L. pneumophila causes large-scale killing of target macrophages.
Results
Mutations in sdhA Are Defective for Intracellular Replication.
A mutant hunt was designed to determine whether there exist L. pneumophila translocated substrates required for maximal intracellular growth. To this end, a strategy was performed that selects against strains having insertion mutations in dot/icm genes, by using techniques similar to those previously described (Materials and Methods). One of two such mutants identified in this screen that displayed large defects in intracellular growth had an insertion in sdhA. The SdhA protein is a paralog of two other predicted proteins encoded by L. pneumophila philadelphia 1, sidH and sdhB (Fig. 1A and refs. 16 and 26).
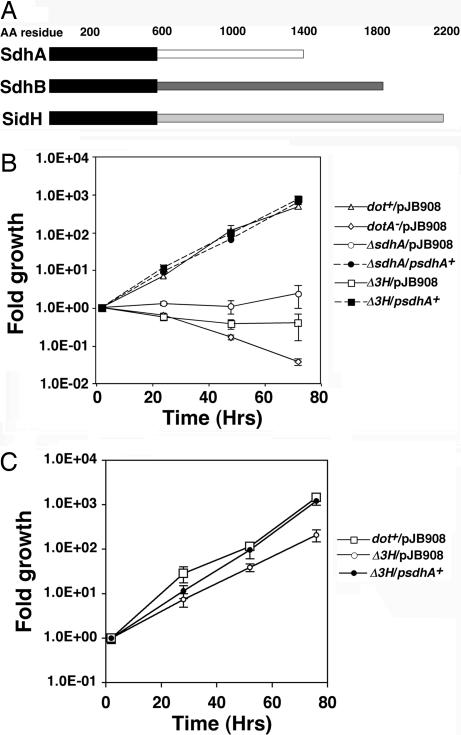
Fig. 1.
The absence of SdhA results in defective L. pneumophila growth in primary macrophages. (A) Amino acid sequence similarity at the amino termini of sdhA paralogs. Thick black bars, region of similarity; pale bars, regions of low similarity. (B) A triple mutant lacking sdhA family members shows a profound defect in intracellular growth. Primary bone marrow-derived macrophages from A/J mice were challenged at moi = 0.05 with the indicated strains and growth over 72 h was followed. (C) Defective intracellular growth of L. pneumophila ΔsdhA in D. discoideum. Strains used are described in Table 1. Δ3H, L. pneumophila ΔsdhAΔsidHΔsdhB. The plasmid pJB908 is a control vector, whereas psdhA+ contains an intact sdhA gene.
To determine the magnitude of the sdhA− defect, in frame deletion mutations in this gene and its paralogs were constructed, and the resulting strains were analyzed for intracellular growth in mouse bone marrow-derived macrophages (Fig. 1B; Materials and Methods). The ΔsdhA mutant showed severely defective intracellular growth, whereas complementation with a plasmid-encoded copy of sdhA gave bacterial yields indistinguishable from WT (Fig. 1B). Any residual intracellular replication observed in the single sdhA mutant was eliminated by a deletion of all three paralogs of sdhA (Fig. 1B). Interestingly, the growth defect of the triple mutant could be fully rescued by the presence of only sdhA on a multicopy plasmid (Fig. 1B). Single mutations affecting sidH and sdhB had no detectable effects on intracellular growth of L. pneumophila, although when mutations within these genes were individually combined with the sdhA mutation they did increase the severity of the sdhA defect (Fig. 6, which is published as supporting information on the PNAS web site).
The severity of the growth defects of the sdhA− mutant varied among the different host cells that can support L. pneumophila replication, with the most severe defects observed in primary macrophages. Growth of the mutants in phorbol ester transformed U937 cells was less defective than in mouse macrophages (data not shown), and similar results were obtained with the amoebal species Dictyostelium discoideum (Fig. 1C and Fig. 7, which is published as supporting information on the PNAS web site).
SdhA Is a Translocated Substrate of the Dot/Icm System.
The amino acid sequence similarity of SdhA to SidH indicated that SdhA is likely to be a translocated substrate of Dot/Icm. Immunofluorescence staining of macrophages incubated with L. pneumophila overproducing sdhA on a multicopy plasmid showed extensive staining of SdhA about the LCV (Fig. 2A and B; psdhA+). No such staining was observed after incubation with strains lacking either the three sdhA paralogs (Δ3H) or the Dot/Icm translocator (dotA−; Fig. 2 A and B). By 5 h after infection, ≈60% of the vacuoles harboring L. pneumophila/psdhA+ stained positively for SdhA, whereas <1% of the vacuoles harboring the dotA− strain showed such staining (Fig. 2B). Similarly, macrophages infected with the mutant lacking all three paralogs (3ΔH) showed no evidence of staining, but the lack of translocation could be rescued by the presence of plasmid-encoded sdhA (Fig. 2 A and B).
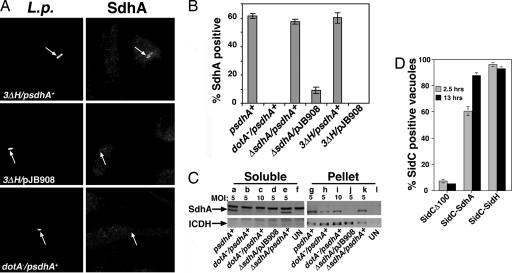
Fig. 2.
Dot/Icm-dependent translocation of SdhA into macrophages. (A) Dot/Icm-dependent export of SdhA. Bone marrow-derived macrophages were challenged for 3 h with the indicated L. pneumophila strains, fixed, and stained with rabbit anti-L. pneumophila (Left) and rat anti-SdhA (Right). (B) Dot/Icm-dependent export of SdhA. Quantitation of SdhA-positive LCVs was performed at 5 h postinfection, by visual inspection of 100 macrophages harboring bacteria. Data are means ± SE on triplicate coverslips. (C) Dot/Icm-dependent export of SdhA after contact with host cells. Macrophages were incubated with plasmid-harboring L. pneumophila strains at the noted moi for 1 h. Proteins were extracted with saponin (Materials and Methods), and saponin soluble (Soluble) and insoluble pellet (Pellet) fractions were separated by centrifugation and analyzed by immunoprobing with anti-SdhA or anti-ICDH as a nontranslocated protein control. Strains are described in Table 1. UN, uninfected macrophages. (D) SdhA and its paralog SidH have carboxyl-terminal translocation signals. The carboxyl termini of SdhA or SidH were translationally fused to a derivative of SidC lacking its carboxyl-terminal translocation signal, and L. pneumophila strains harboring these derivatives were incubated with bone marrow-derived macrophages for the noted times.
To obtain a second measure of SdhA export into macrophages by L. pneumophila, a previously described saponin extraction experimental protocol was performed (27). Western blot analysis with anti-SdhA of the different fractions showed that a large proportion of the total pool of SdhA was present in the saponin soluble (translocated) fraction of U937 cells incubated with L. pneumophila, but steady state amounts of SdhA were smaller in the dotA− strain than in WT strain backgrounds (data not shown). To overcome the issue of lowered SdhA levels in the dotA− strain, the multiplicity of infection was adjusted accordingly. When using a strain having an intact Dot/Icm system, export of SdhA into macrophages could clearly be detected at moi = 5 (Fig. 2C), whereas no export of the cytoplasmic protein isocitrate dehydrogenase (ICDH) was observed (Fig. 2C). In contrast, when the dotA− strain was incubated at moi = 10 with macrophages, there was no evidence for export of SdhA (Fig. 2C). Therefore, by both immunofluorescence analysis and cell fractionation studies, SdhA is a translocated substrate of the Dot/Icm apparatus.
It was next determined whether SdhA has a carboxyl-terminal translocation signal, as observed for other Dot/Icm substrates, by constructing hybrids between SdhA and the translocated substrate SidC (28). For this purpose, a gene fusion was constructed that encodes a chimera having the carboxyl-terminal 100 aa of SidC replaced by the carboxyl-terminal 200 aa of SdhA. Previous studies demonstrated that translocation of the Dot/Icm substrate SidC requires its carboxyl-terminal 100 aa, but that this region could be replaced by other translocated Dot/Icm substrates to restore transfer (Fig. 2D and ref. 27). When the 3′ region of sdhA replaced the 3′ region of sidC, there was robust translocation of the SidC-SdhA hybrid into bone marrow-derived macrophages (Fig. 2D). Similar results were obtained with the SidC-SidH hybrid (Fig. 2D), consistent with previous observations (26). Thus, SdhA has a carboxyl-terminal translocation signal.
The Absence of SdhA Is Associated with Death of Host Cells.
Mutations that interfere with the function of the Dot/Icm system result in improper trafficking of the L. pneumophila-containing vacuole, which can be demonstrated by the recruitment of the late endosomal markers LAMP-1 (19). We determined whether loss of SdhA resulted in a similar defect in replication vacuole biogenesis. Surprisingly, vacuoles containing the sdhA− mutant were devoid of LAMP-1, whereas there was heavy recruitment of the protein around the L. pneumophila dotA− compartment (Fig. 3A and ref. 7). Microscopic observation of bone marrow macrophages harboring the sdhA mutant, however, revealed an unexpected phenotype. There was a striking decline in the number of macrophages infected with the sdhA− mutant compared with macrophages incubated with WT (Fig. 3 B and C). We next determined whether cell loss was caused by macrophage death. A large fraction of macrophages harboring the L. pneumophila sdhA− mutant showed nuclear DNA degradation, based on TUNEL (Fig. 3 D–F). Therefore, whereas loss of the Dot/Icm system results in improper trafficking of the compartment containing the bacteria and little host cell death, macrophages harboring an L. pneumophila sdhA− mutant show normal trafficking of the compartment, but suffer increased cell death.
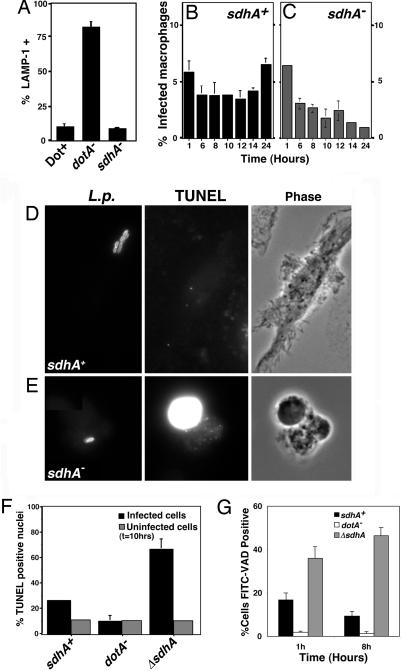
Fig. 3.
The absence of SdhA causes death of infected macrophages. (A) Trafficking of L pneumophila sdhA::Tn10 in macrophages. Bone marrow macrophages were incubated with noted strains, and percent colocalization with the late endosomal marker LAMP-1 was determined by immunofluorescence. Strains used were as follows: LP02 (Dot+); LP03 (dotA−); and RL1010 (sdhA−). (B and C) Loss of macrophages infected by strain lacking SdhA protein. Bone marrow-derived macrophages were incubated with strains for denoted lengths of time, and the number of infected macrophages was determined. (D–F) Bone marrow macrophages incubated with L. pneumophila sdhA::Tn10 are TUNEL-reactive. Macrophages were incubated with L. pneumophila strains for 10 h, stained for bacteria and TUNEL reaction, and observed microscopically. Infected cells, macrophages harboring bacteria. Uninfected cells, macrophages that have no observable associated bacteria. (G) Increased caspase activation in macrophages harboring L. pneumophila ΔsdhA. Macrophages were incubated for 1 or 8 h with L. pneumophila strains, and cells were stained with FITC-VAD-FMK.
The activation of caspases is associated with a number of pathways leading to cell death (29). As there is evidence for Dot/Icm-dependent caspase activation (20), macrophages incubated with bacteria were probed with FITC-VAD-FMK, a fluorescent pan-caspase activation marker. Association of cells with L. pneumophila sdhA− resulted in an increase in the number of macrophages reacting with the probe relative to that observed in macrophages incubated with WT bacteria (Fig. 3G), indicating that the presence of SdhA interferes with caspase activation in infected cells.
The Macrophage Response to L. pneumophila sdhA− Results in Mitochondrial Disruption.
As intrinsic signaling leading to cell death can result from dysfunction of mitochondria (29), we probed for morphological changes in the macrophage mitochondria by immunofluorescence staining of cytochrome c. One hour after contact of a dotA− strain at moi = 1, thin tubular structures were observed that were similar to those in uninfected cells (Fig. 4B and E). In cells infected with a strain having an intact Dot/Icm translocator, most cells showed normal cytochrome c staining (Fig. 4 A and E). In contrast, a 1-h incubation of macrophages with a sdhA− strain at moi = 1 resulted in drastic changes in cytochrome c staining. Intact macrophages harboring L. pneumophila sdhA− showed diffuse cytochrome c staining interspersed with punctate structures (Fig. 4C), whereas large-scale loss of cytochrome c staining was observed in cells that had grossly aberrant morphology (Fig. 4D). As soon as 5 min after incubation of macrophages with the sdhA− strain, there was clear evidence that mitochondrial morphology had been disrupted (Fig. 4E). The presence of sdhA+ on a multicopy plasmid allowed rescue of this defect (Fig. 4E).
Fig. 4.
Contact with L. pneumophila sdhA− results in disruption of macrophage mitochondria. Bone marrow-derived macrophages were incubated with L. pneumophila strains for 1 h, fixed, and stained with antibody directed against cytochrome c (green) and L. pneumophila (red). (A–D) Immunofluorescence analysis (Left) and phase contrast images (Right) of macrophages harboring L. pneumophila strains. Mitochondria are judged to be disrupted if their morphologies are similar to that seen in C (punctate staining) or D (lack of staining). (E) Rescue of mitochondrial disruption by the presence of plasmid-encoded SdhA. Macrophages incubated with L. pneumophila strains at moi = 1.0 were fixed at noted time points and assayed for mitochondrial disruption.
Macrophage Death Caused by L. pneumophila sdhA− Occurs via Multiple Mechanisms.
As incubation with L. pneumophila sdhA− strains resulted in increased caspase activation, we tested whether inhibition of caspase activity with the pan-caspase inhibitor Z-VAD-FMK could rescue intracellular growth in bone marrow macrophages. The presence of the pan-caspase inhibitor resulted in increased intracellular growth of the ΔsdhA strain over a 72-h period compared with growth in untreated macrophages (Fig. 5A). There was also enhanced growth of the WT strain during the first 48 h. of incubation, indicating that the presence of intact SdhA is not sufficient to interfere completely with caspase-dependent cell death pathways.
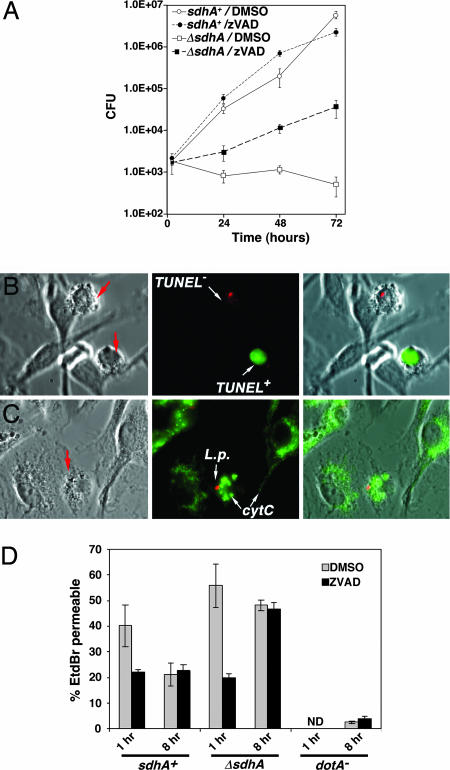
Fig. 5.
Inhibition of caspase activity does not fully rescue the intracellular growth defect of a L. pneumophila ΔsdhA strain. (A) Enhanced intracellular replication of a L. pneumophila ΔsdhA mutant. Macrophages were incubated in the presence of Z-VAD-FMK throughout the incubation period. (B) Cells with aberrant morphologies in the presence of Z-VAD-FMK have multiple TUNEL phenotypes. Arrows point to cells with aberrant morphologies. (C) zVAD-FMK is unable to protect against mitochondrial disruption by 8 h postinfection. Displayed are macrophages showing punctate cytochrome c staining in the presence of z-VAD-FMK. (D) Protection by caspase inhibitor is transient. Noted strains were assayed for ethidium bromide (EtdBr) permeability at 1 h and 8 h postinfection in the presence or absence of Z-VAD-FMK, after incubation with L. pneumophila at moi = 1.0.
The presence of the caspase inhibitor, however, did not allow complete rescue of the sdhA− defect during growth in macrophages (Fig. 5A). This result presumably was not due to decay of the inhibitor, as fresh Z-VAD-FMK was added to the cultures every 24 h (Materials and Methods). To determine whether this observation indicated there was a second defect in sdhA− strains unrelated to causing cell death, the survival of infected host cells in the presence of the caspase inhibitor was analyzed. At 8 h postinfection in the presence of the caspase inhibitor, most of the infected cells showed no nuclear DNA degradation (Fig. 8, which is published as supporting information on the PNAS web site), but there were infected cells showing clear signs of aberrant morphologies (Fig. 5B, arrows) as well as evidence of punctate cytochrome c staining (Fig. 5C, arrows; and Fig. 8). Furthermore, based on an ethidium bromide permeability assay system for detection of dead macrophages, the caspase inhibitor seemed to protect from cell death caused by the ΔsdhA strain at early time points, but not at later times (8 h postinfection, Fig. 5D). Similar results were obtained with cytochrome c staining, in which early protection from disruption of cytochrome c localization was transient (Fig. 8). Death depended on contact with Dot/Icm+ bacteria, as uninfected macrophages in Z-VAD-FMK treated cultures as well as macrophages in contact with the dotA− strain showed little signs of death (Fig. 5D). Therefore, although caspase inhibition allowed enhanced replication of the sdhA− mutant within macrophages, the macrophages eventually succumbed, consistent with the model that defective intracellular growth of the sdhA− mutant is due to triggering of host cell death.
Discussion
A number of studies have demonstrated that the L. pneumophila Dot/Icm system is responsible for causing death to host cells (30). The involvement of Dot/Icm in promoting cell death, however, raises a serious problem for the microorganism, as both the Dot/Icm translocator and survival of host cells are required for intracellular growth. We show here that SdhA provides one solution to this problem. The protein was translocated after uptake into host cells (Fig. 2) and was continually expressed throughout the intracellular growth cycle (data not shown), arguing that it is involved in protecting against stress conditions leading to host cell death throughout intracellular replication. Caspase inhibition only partially reversed the intracellular growth defect resulting from loss of SdhA (Fig. 5A), presumably because there is more than one pathway leading to cell death antagonized by SdhA.
A number of viral pathogens express proteins that antagonize apoptotic pathways (31). Many of these proteins show sequence similarity to cellular proteins that are known to interfere with apoptosis. Although no proteins encoded by intracellular bacteria that interfere with host cell death have been identified, there is ample evidence that such proteins must exist. For instance, cultured cells harboring replicating Chlamydia and Bartonella species are highly resistant to apoptosis-inducing agents (32–35). In the case of Chlamydia, resistance to apoptosis seems to involve degradation of a variety of proapoptotic BH3-only domain proteins as well as sequestration of the proapoptotic Bid protein. Death inhibition by SdhA, may not be limited to targeting apoptosis, however, based on the diverse morphology of dead cells and the results with the caspase inhibitor (Fig. 5 B and C and Fig. 8). It is also formally possible that SdhA does not act directly on host cell death pathways, but rather its absence results in hypertranslocation of toxic Dot/Icm substrates. Mutations in sdhA, however, do not cause global increases in translocation, as the translocation efficiencies of the Dot/Icm substrates LidA and SidC were found to be unaffected by the absence of SdhA (Fig. 9, which is published as supporting information on the PNAS web site). This observation does not eliminate the possibility that a specific subset of toxic substrates is hypertranslocated in the sdhA− strains.
The work presented here is consistent with previous results arguing that active caspases 1 and 3, which are inhibited by Z-VAD-FMK, interfere with intracellular growth of L. pneumophila (36). It has been shown that after L. pneumophila contact with macrophages at high moi, there is rapid host cell death that is blocked either by caspase 1 inhibition or in macrophages derived from caspase-1−/− mice (21). Also consistent with a negative role for these caspases are results indicating that intracellular growth requires inhibition of an apoptotic pathway initiated by caspase 3 (37). There is no evidence from our work, however, that activation of caspase 3 is a requirement for L. pneumophila intracellular growth, as suggested by previous work (37), as inhibition of this protease resulted in efficient intracellular growth (Fig. 5A).
Despite the fact that >40 translocated substrates of the Dot/Icm system have been identified, no other substrate is known to play as significant a role in intracellular growth as does SdhA (38). Even so, not all host cells are equally restrictive of the sdhA− mutant. The growth rates of the SdhA family triple mutant (3ΔH) within D. discoideum amoebae (Fig. 1C) or within phorbol ester transformed U937 cells (data not shown) were not as attenuated as in primary macrophages. This effect may be explained by the presence of other bacterial or host cell factors that work more efficiently in some cell types than in others to suppress host cell death. For instance, we have recently demonstrated an alternate pathway in which L. pneumophila can activate NFκB-dependent anti-apoptotic signaling to suppress host cell death (24). In addition, other work has indicated that there are probably L. pneumophila translocated substrates that interfere with cell death (25), so it is possible that multiple substrates act with a similar purpose. Finally, some of the death pathways induced by strains lacking SdhA may not exist in host cells that support growth of a sdhA− mutant.
It is not clear what bacterial proteins are causing death to macrophages. It has been shown that there is flagellin-dependent death of macrophages that occurs via caspase 1 activation (19, 21, 22), although at low mois this pathway does not seem to be a significant cause of death in macrophages from the A/J mouse strain (21). We have found that strains that lack both sdhA and flaA, the structural gene for flagellin, are extremely defective for intracellular growth, indicating that flagellin cannot be the cause of death in sdhA− mutants (data not shown). Other proteins that require the Dot/Icm system for translocation, or components of the translocation apparatus itself, may cause damage to the target cell. This damage may occur either as the primary function of these macromolecules or as side-products of other activities. For those bacterial products that function primarily to kill host cells, SdhA may act as a timing factor that prevents premature termination of the intracellular replication cycle. In the case of bacterial products that are toxic for host cells, but are important for intracellular growth of the microorganism, it may be that the large-scale changes in the host cell resulting from these L. pneumophila proteins could have the unintended consequence of causing death. Identification of the nature of these products, as well as understanding the mechanisms that allow SdhA to protect the host cell from damage, will likely give insight into how the bacterium promotes intracellular replication.
Materials and Methods
Bacterial Strains and Cell Culture Conditions.
Bacterial strains are shown in Table 1, which is published as supporting information on the PNAS web site, and strains were grown as described (39). A/J mice were used as source of primary bone marrow-derived macrophages (7), and intracellular growth was determined by using standard procedures (16). D. discoideum was cultured and incubated with L. pneumophila as described (7).
Plasmid and Strain Constructions.
Plasmids were introduced into L. pneumophila by either electroporation (39) or natural transformation (40, 41). Isolation of random miniTn10(kanR) insertions in L. pneumophila chromosome were made by using the suicide transposition vector pJK211 (42). All insertions were transferred into unmutagenized backgrounds by natural transformation (41). Deletion mutations in selected genes on the L. pneumophila chromosome and complementation plasmids were constructed by using standard strategies (42).
Isolation of sdhA Insertion Mutants.
To identify intracellular growth defective L. pneumophila mutants that had lesions outside the dot/icm loci, a pool of L. pneumophila KanR miniTn10 insertions (42) was isolated in a strain that expresses GFP under Ptac control (43). The pool was then incubated with phorbol ester transformed U937 cells in media containing 100 nM wortmannin, which interferes with uptake of L. pneumophila dot/icm mutants (44). After a 1-h incubation, 50 μg/ml of gentamicin was added for 1 h. Eleven hours later, the infected U937 cells were lifted in trypsin/EDTA, washed, and subjected to FACS, pooling cells showing the lowest levels of GFP fluorescence. The infected cells from this fraction were lysed, incubated on CYE plates for 3 days, and individual purified colonies were assayed for growth within U937 cells after 72 h incubation (42).
LAMP-1 Targeting and Mitochondrial Morphology Assays.
Targeting of bacteria to a LAMP-1 containing compartment was determined by fluorescence microscopy (7, 10). To determine the morphology of mitochondria, coverslips were probed with mouse anti-cytochrome c (Invitrogen, Carlsbad, CA) (45). Mitochondria were judged to have normal morphology if the cytochrome c staining was largely rodlike or tubular, whereas aberrant morphology was judged to be either the absence of cytochrome c staining or the appearance of punctate structures for 100 infected cells per coverslip.
Cell Death Assays and Inhibition of Caspase Activity.
Staining for nuclear DNA degradation was performed by using TUNEL (Roche Applied Science, Indianapolis, IN). The assay for general caspase activation was performed by using FITC-VAD-FMK (Promega, Madison, WI). For inhibition of caspase activity, Z-VAD-FMK was added at a concentration of 20 μM 1 h before incubation, by using DMSO as the control. Media containing the caspase inhibitor was replenished every 24 h with fresh doses of inhibitor.
Translocation of SdhA After Contact with Macrophages.
Rabbit and rat antisera were raised against His-SdhA protein to assay for translocation (7, 42). Assay of SidC translocation was performed similarly, by using rabbit anti-SidC and rat anti-L. pneumophila (27). Dot/Icm-dependent translocation in phorbol ester-transformed U937 cells was assayed by using a published detergent extraction procedure, by using saponin in place of digitonin (46).
Supplementary Material
Acknowledgments
We thank Tamara O'Connor, Molly Bergman, Matthias Machner, Vicki Auerbuch, Matt Heidtman, and Alex Ensminger for reviewing the manuscript. This work was supported by the Howard Hughes Medical Institute, by Training Grant 5 T32 AI07422 (to R.K.L.), and by a postdoctoral fellowship from the National Institutes of Health (to N.V.). R.R.I. is a Howard Hughes Medical Institute Investigator.
Abbreviations
- LCV
Legionella-containing vacuole
- moi
multiplicity of infection
- ICDH
isocitrate dehydrogenase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Horwitz MA, Silverstein SC. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y. Appl Environ Microbiol. 2005;71:20–28. doi: 10.1128/AEM.71.1.20-28.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien M, Morozova I, Shi S, Sheng H, Chen J, Gomez SM, Asamani G, Hill K, Nuara J, Feder M, et al. Science. 2004;305:1966–1968. doi: 10.1126/science.1099776. [DOI] [PubMed] [Google Scholar]
- 4.Hales LM, Shuman HA. J Bacteriol. 1999;181:4879–4889. doi: 10.1128/jb.181.16.4879-4889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao LY, Harb OS, Kwaik YA. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz MA. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson MS, Isberg RR. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. J Cell Sci. 2001;114:4637–4650. doi: 10.1242/jcs.114.24.4637. [DOI] [PubMed] [Google Scholar]
- 9.Kagan JC, Roy CR. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 10.Derre I, Isberg RR. Infect Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagan JC, Stein MP, Pypaert M, Roy CR. J Exp Med. 2004;199:1201–1211. doi: 10.1084/jem.20031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao LY, Harb OS, Abu Kwaik Y. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal G, Purcell M, Shuman HA. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel JP, Andrews HL, Wong SK, Isberg RR. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 15.Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- 16.Luo ZQ, Isberg RR. Proc Natl Acad Sci USA. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shohdy N, Efe JA, Emr SD, Shuman HA. Proc Natl Acad Sci USA. 2005;102:4866–4871. doi: 10.1073/pnas.0501315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao LY, Abu Kwaik Y. Infect Immun. 1999;67:862–870. doi: 10.1128/iai.67.2.862-870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby JE, Vogel JP, Andrews HL, Isberg RR. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Zant A, Santic M, Molmeret M, Jones S, Helbig J, Abu Kwaik Y. Infect Immun. 2005;73:5339–5349. doi: 10.1128/IAI.73.9.5339-5349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derre I, Isberg RR. Infect Immun. 2004;72:6221–6229. doi: 10.1128/IAI.72.11.6221-6229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Losick VP, Isberg RR. J Exp Med. 2006;204:2177–2189. doi: 10.1084/jem.20060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Zant A, Jones S, Asare R, Suttles J, Price C, Graham J, Kwaik YA. Cell Microbiol. 2006 Aug 15; doi: 10.1111/j.1462–5822.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 26.Ninio S, Zuckman-Cholon DM, Cambronne ED, Roy CR. Mol Microbiol. 2005;55:912–926. doi: 10.1111/j.1365-2958.2004.04435.x. [DOI] [PubMed] [Google Scholar]
- 27.VanRheenen SM, Luo ZQ, O'Connor T, Isberg RR. Infect Immun. 2006;74:3597–3606. doi: 10.1128/IAI.02060-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. Proc Natl Acad Sci USA. 2005;102:826–831. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel RM. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 30.Molmeret M, Bitar DM, Han L, Kwaik YA. Microbes Infect. 2004;6:129–139. doi: 10.1016/j.micinf.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G. Biochim Biophys Acta. 2004;1659:178–189. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Verbeke P, Welter-Stahl L, Ying S, Hansen J, Hacker G, Darville T, Ojcius DM. PLoS Pathog. 2006;2:e45. doi: 10.1371/journal.ppat.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Y, Zhong Y, Greene W, Dong F, Zhong G. Infect Immun. 2004;72:5470–5474. doi: 10.1128/IAI.72.9.5470-5474.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, Ying S, Hacker G. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirby JE, Nekorchuk DM. Proc Natl Acad Sci USA. 2002;99:4656–4661. doi: 10.1073/pnas.072292699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, et al. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 37.Molmeret M, Zink SD, Han L, Abu-Zant A, Asari R, Bitar DM, Abu Kwaik Y. Cell Microbiol. 2004;6:33–48. doi: 10.1046/j.1462-5822.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 38.Segal G, Feldman M, Zusman T. FEMS Microbiol Rev. 2005;29:65–81. doi: 10.1016/j.femsre.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Berger KH, Merriam JJ, Isberg RR. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 40.Stone BJ, Kwaik YA. J Bacteriol. 1999;181:1395–1402. doi: 10.1128/jb.181.5.1395-1402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sexton JA, Vogel JP. J Bacteriol. 2004;186:3814–3825. doi: 10.1128/JB.186.12.3814-3825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conover GM, Derre I, Vogel JP, Isberg RR. Mol Microbiol. 2003;48:305–321. doi: 10.1046/j.1365-2958.2003.03400.x. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Solomon JM, Isberg RR. Cell Microbiol. 2005;7:431–442. doi: 10.1111/j.1462-5822.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 44.Khelef N, Shuman HA, Maxfield FR. Infect Immun. 2001;69:5157–5161. doi: 10.1128/IAI.69.8.5157-5161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrews HL, Vogel JP, Isberg RR. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derre I, Isberg RR. Infect Immun. 2005;73:2128–2134. doi: 10.1128/IAI.73.7.4370-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.