Abstract
Secretory proteins are transported from the endoplasmic reticulum (ER) to the Golgi complex in vesicles coated with coat protein complex II (COPII). The incorporation of certain transport molecules (cargo) into the COPII vesicles is thought to be mediated by cargo receptors. Here we show that Emp47p, a type-I membrane protein, is specifically required for the transport of an integral membrane protein, Emp46p, from the ER. Exit of Emp46p from the ER was saturable and dependent on the expression level of Emp47p. Emp46p binding to Emp47p occurs in the ER through the coiled-coil region in the luminal domains of both Emp47p and Emp46p, and dissociation occurs in the Golgi. Further, this coiled-coil region is also required for Emp47p to form an oligomeric complex of itself in the ER, which is essential for exit of Emp47p from the ER. Our results suggest that Emp47p is a receptor protein for Emp46p that allows for the selective transport of this protein, and this event involves receptor oligomerization.
INTRODUCTION
In eukaryotic cells, intracellular protein transport between the organelles of the secretory pathway is mediated by vesicular carriers that are released from a donor organelle and fuse with an appropriate acceptor organelle (Palade, 1975). The starting point of the secretory route is the ER. Transport of newly synthesized secretory proteins from the ER are sorted from ER-resident proteins and packaged into COPII-coated vesicles (Schekman and Orci, 1996). The formation of the COPII coat on the ER membranes occurs by sequential binding of the small GTPase Sar1p (Nakano and Muramatsu, 1989), the Sec23/24p complex, and the Sec13/31p complex (Barlowe et al., 1994), which drives vesicle budding. Budding from the ER involves activation of the Sar1p GTPase by the ER resident protein Sec12p, the Sar1p-specific guanine-nucleotide exchange factor (Nakano et al., 1988; Barlowe and Schekman, 1993). The molecular events that trigger the activation of Sar1p to the GTP-bound form to initiate budding are presently unknown. Soon after formation, COPII vesicles shed their coat and then fuse with the Golgi compartment. The incorporation of cargo into the COPII vesicle is believed to be selective and perhaps nonselective to some extent. Certain integral membrane cargos are concentrated into ER-derived vesicles through direct interactions with COPII subunits (Springer and Schekman, 1998; Shimoni et al., 2000). Evidence suggests that several soluble secretory proteins require specific transmembrane cargo receptors to mediate the interaction of the cargo proteins with the COPII coats, resulting in selective packaging (Kuehn et al., 1998; Appenzeller et al., 1999; Belden and Barlowe, 2001). The mechanism by which cargo association occurs and the receptor's mode of action upon exit from the ER are less clear.
The Emp47p and its close homologue Emp46p, type-I membrane proteins that cycle between the ER and the Golgi, have been proposed as cargo receptors at the ER exit site (Schroder et al., 1995; Sato and Nakano, 2002). Their luminal domains share homology with leguminous lectins, whereas the C-terminal cytoplasmic regions contain both COPII and COPI-binding sites. Thus, Emp47p and Emp46p may be required for selective packaging of specific glycoproteins into ER-derived vesicles. Indeed, the disruption of both EMP47 and EMP46 leads to a marked defect in the secretion of a subset of glycoproteins (Sato and Nakano, 2002). However, specific cargo proteins for Emp47p and Emp46p have not been identified thus far. In mammalian cells, the mannose-binding lectin ERGIC-53 appears to be the homolog of Emp46/47p and has been proposed as a soluble cargo receptor at the ER exit site. A chemical cross-link approach recently resulted in the coisolation of ERGIC-53 with a soluble cathepsin-Z–related glycoprotein (Appenzeller et al., 1999).
In this report, we demonstrate that Emp47p associates with Emp46p in the ER and acts in selecting Emp46p into COPII vesicles. The Emp46p-Emp47p complex persists in the ER and COPII vesicles but dissociates when it reaches the Golgi. Furthermore, our data indicate that it is the oligomerization state that confers on Emp46/47p the competence for the exit from the ER. Therefore, our findings may have important implications for recruitment of a variety of other secretory cargo into COPII vesicles.
MATERIALS AND METHODS
Yeast Strains and Plasmids
Saccharomyces cerevisiae strains used in this study were KSY062 (MATα sec12-4 ura3 leu2 trp1 his3 emp46::3HA-EMP46) and KSY008 (MATα ura3 lys2 adehis3 leu22 trp1 emp47::LEU2 emp46::HIS3; Sato and Nakano, 2002). To construct pKSY155, pKSY160, pKSY166, and pKSY177 carrying the GFP-myc-EMP47-Δ383, GFP-myc-EMP47-Δ333, GFP-myc-EMP47-Δ281, and myc-EMP47-Δ281-333 deletion mutants, respectively, under control of the EMP47 promoter, these fragments were generated by PCR mutagenesis and subcloned into pRS314 (Sikorski and Hieter, 1989). The fragment encoding HA-EMP46-Δ279-321 was generated by PCR mutagenesis and subcloned into pRS316 (Sikorski and Hieter, 1989) to yield the plasmid pKSY187. The plasmids pKSY105, pKSY126, and pKSY113, encoding myc-EMP47, GFP-EMP46, and HA-EMP46, respectively, were described previously (Sato and Nakano, 2002). The overexpression of HA-Emp46p was from the plasmid pKSY114 (2 μm; Sato and Nakano, 2002). For the overexpression plasmid of myc-Emp47p, the EcoRI fragment of pKSY105 was inserted into the corresponding site of the pYO324 (2 μm) to yield the plasmid pKSY169.
Antibodies
The anti-Sec61p and anti-Sec22p antibodies were gifts from R. Schekman. Monoclonal antimyc and anti-HA antibodies were obtained from Berkeley Antibody, and the anti-Pho8p antibody was from Molecular Probes (Eugene, OR). The anti-Sed5p antibody was generated against Ni-NTA purified Sed5p-his6 lacking the C-terminal transmembrane domains (amino acids 1–316).
Chemical Cross-linking
For identification of Emp46p binding proteins, 75 mg of microsomes was incubated on ice with 0.5 mM DSP (Pierce, Rockford, IL) for 30 min in B88 (20 mM HEPES-KOH, pH 6.8, 150 mM KOAc, 5 mM MgOAc, and 250 mM sorbitol). The reaction was quenched by adding 50 mM Tris-Cl, pH 7.4, followed by 15-min incubation. Microsomes were lysed in 30 ml of 1% Triton X-100 in B88. After 1 h at 4°C, the detergent extract was centrifuged and supernatant was incubated with protein G-Sepharose bearing no IgGs at 4°C for 30 min. The flow through was collected, reapplied to the anti-HA immobilized protein G-Sepharose prepared as described (Ungermann et al., 1998), and incubated overnight at 4°C. The resins were washed, and bound proteins were eluted with 0.1 M glycine/HCl, 0.2% Triton X-100, precipitated with TCA, and washed with ice-cold acetone. Samples were analyzed by SDS-PAGE and Coomassie blue staining. Protein bands were excised from the gel and rinsed, and the protein samples were digested with trypsin in the gel matrix. Extracted peptide mixtures were analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF). Proteins were identified by comparing their tryptic peptide mass maps to the S. cerevisiae sequence database.
Coimmunoprecipitation
For coimmunoprecipitations, membranes were detergent solubilized in B88 containing 0.05% n-dodecyl maltoside. The detergent extract was placed on ice for 1 h, and the insoluble material was removed by centrifugation. The supernatant was applied to the protein G–immobilized antimyc or anti-HA antibody or to the protein A-immobilized anti-GFP antibody and incubated overnight at 4°C. The beads were washed five times in the same buffer and then were then eluted with the elution buffer (0.1 M glycine/HCl, pH 2.5, and 0.05% n-dodecyl maltoside). Samples were subjected to TCA precipitation, followed by SDS-PAGE and immunoblotting.
Gel Filtration Analyses
Gel filtrations were performed at 4°C on a G3000SWXL column (Tosoh, Tokyo, Japan) equilibrated in B88 containing 0.05% n-dodecyl maltoside and run at the flow rate of 0.5 ml/min. Detergent extracts of membranes were loaded onto the column, 0.2-ml fractions of were collected, and aliquots from the fractions were analyzed by immunoblotting. The column was calibrated with gel filtration standard proteins from Amersham Pharmacia Biotech (Piscataway, NJ).
Miscellaneous
In vitro vesicle budding assays, prebudding formation assays, subcellular fractionation, and confocal laser microscopy observation were performed as described (Sato and Nakano, 2002).
RESULTS
Emp46p Forms a Complex with Emp47p in the ER
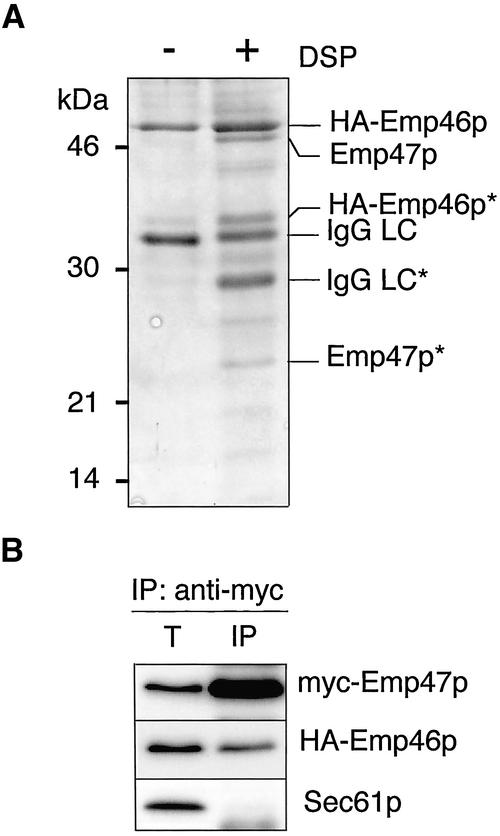
To identify proteins that interact with Emp46p in the ER, Emp46p was tagged by the hemagglutinin (HA) epitope at the N-terminus and expressed in the sec12-4 mutant cells, which have a temperature-sensitive defect in the COPII vesicle formation from the ER. Microsomes were prepared from the cells incubated at the restrictive temperature to accumulate HA-Emp46p in the ER and were subjected to cross-linking with the cleavable and membrane-permeable reagent dithiobis(succinimidylpropionate) (DSP). The microsomes incubated with or without DSP were solubilized with Triton X-100, and the extracts were incubated with the immobilized anti-HA antibody. Retained proteins were eluted from the column and analyzed by SDS-PAGE. The Coomassie-stained protein bands (Figure 1A) were identified by peptide mapping by MALDI-TOF mass spectrometry combined with sequence database searching. As a result, in addition to HA-Emp46p, Emp47p was specifically eluted from the anti-HA column. We further examined the interaction of Emp46p and Emp47p in microsomes by coimmunoprecipitation analysis. For these experiments, we used microsomes prepared from emp46Δemp47Δ cells that carried plasmids encoding HA-Emp46p and myc-Emp47p. Microsomes were solubilized in the presence of n-dodecyl maltoside and subjected to native immunoprecipitation with the antimyc antibody. HA-Emp46p was successfully coimmunopurified when myc-Emp47p was immunoprecipitated with the antimyc antibody, whereas a control ER protein, Sec61p, was not (Figure 1B). We conclude that Emp46p forms a complex with Emp47p in the ER.
Figure 1.
Emp46p forms a complex with Emp47p in the ER. (A) KSY062 (sec12-4, HA-EMP46) cells were incubated at 32°C for 120 min before microsome preparation, and microsomes (75 mg) were incubated in the presence (+) or absence (-) of DSP. HA-Emp46p was immunoprecipitated from the Triton X-100–solubilized membrane with the anti-HA antibody. Samples were separated by SDS-PAGE under a reducing condition and visualized by Coomassie staining. Specific protein bands were excised and trypsin-digested, and the resulting peptide mixtures were analyzed by MALDI mass spectrometry. Identified proteins are indicated on the right. IgG-LC, IgG light chain; asterisks, partial proteolytic breakdown products of indicated proteins. (B) Microsomes from emp46Δemp47Δ cells expressing myc-Emp47p and HA-Emp46p were solubilized with 0.05% n-dodecyl maltoside. The resulting extract was cleared by centrifugation and immunoprecipitated (IP) with the antimyc antibody followed by immunoblotting with the antimyc or anti-HA antibody. Total lane (T) represents 5% of the starting microsomal extract.
Emp47p Is Required for the ER Exit of Emp46p.
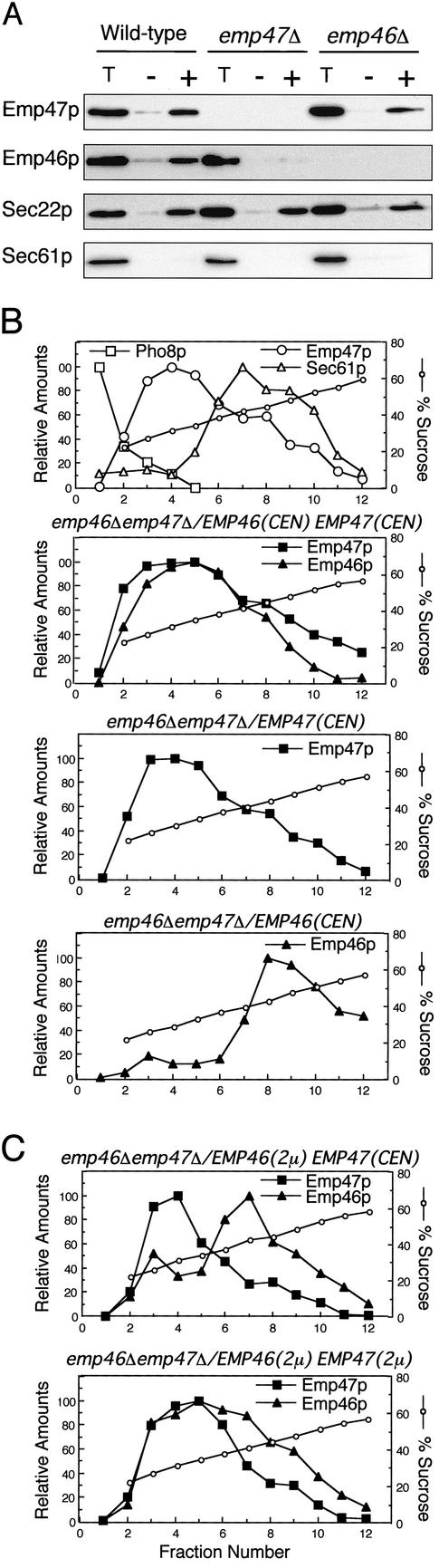
To examine whether the complex formation between Emp46p and Emp47p has a role in the ER exit of these proteins, we performed the reconstituted in vitro budding reactions (Figure 2A). In both wild-type and Emp46p-lacking microsomes, ER-derived COPII vesicles incorporated Emp46p and Emp47p at a level comparable to another characterized cargo protein, Sec22p, but not the resident ER protein, Sec61p. Strikingly, no incorporation of Emp46p into COPII vesicles was detected in the microsomes lacking Emp47p, whereas the control Sec22p was unaffected. Thus, in the absence of Emp47p, ER-derived vesicles normally formed but eliminated Emp46p, suggesting that Emp47p is required for the ER exit of Emp46p.
Figure 2.
Emp47p is required for the ER exit of Emp46p. (A) In vitro budding reactions with microsomes prepared from emp46Δemp47Δ cells expressing both myc-Emp47p and HA-Emp46p (Wild-type), or either HA-Emp46p (emp47Δ) or myc-Emp47p (emp46Δ). Ten percent of the total reaction (T) and budded COPII vesicles isolated after incubation with (+) or without (-) COPII proteins were analyzed by SDS-PAGE followed by immunoblotting with the anti-HA or antimyc antibody. Sec61p as a negative control and Sec22p as a positive control were detected with polyclonal antibodies. (B) Subcellular distribution of Emp46p in the presence or absence of Emp47p. Whole cell lysates from indicated strains were separated on 20–60% sucrose density gradient. Relative levels of Pho8p (vacuole marker), myc-Emp47p (Golgi marker), HA-Emp46p, and Sec61p (ER marker) in each fraction were quantified by densitometry of immunoblots. (C) Expression level of Emp47p influences the subcellular distribution of Emp46p.
As an independent method to evaluate Emp46p exit from the ER in emp47Δ cells, we performed cell fractionation experiments to determine where Emp46p accumulates in the cell (Figure 2B). Previous sucrose gradient fractionation of membrane organelles from wild-type cells documented the Golgi localization pattern for both Emp46p and Emp47p (Schroder et al., 1995; Sato and Nakano, 2002). As shown in Figure 2B, the Golgi localization pattern of Emp47p was not affected in the absence of Emp46p. In contrast, Emp47p deletion caused a strong shift of residual Emp46p to the ER membrane fractions. These results indicate that Emp46p fails to exit from the ER and accumulate there in the absence of Emp47p, which is entirely consistent with the in vitro budding results.
To investigate whether additional factors might be required for the Emp46p-Emp47p interaction, subcellular localizations of overexpressed Emp46p and Emp47p were examined (Figure 2C). Overexpression of Emp46p from a multicopy plasmid with endogenous Emp47p leads to ER accumulation of Emp46p. To examine whether this was because the limited pool of Emp47p in the ER was titrated out, subcellular localization of Emp46p was examined in the cells overproducing both Emp46p and Emp47p. We found that the overproduced Emp46p was efficiently targeted to the Golgi when Emp47p was overexpressed as well. These results suggest that no additional factor is necessary as a bridge between Emp46p and Emp47p in the ER.
Emp46p Binds Emp47p through the Coiled-Coil Region of Emp47p in the ER and Dissociates in the Golgi
We next sought to identify a region in Emp47p required for Emp46p binding in the ER. Emp47p has a single transmembrane domain near its C termini, and a 200-residue segment in the N-terminal luminal domain that shares homology with leguminous lectins. These two regions were connected by a predicted coiled-coil segment (Figure 3A). For convenience, we divided the Emp47p into three segments, designated the lectin domain (Δ281), the coiled-coil region (Δ333 and Δ281–333), and the transmembrane domain with the cytoplasmic tail (Δ383). Microsomes were prepared from cells expressing HA-Emp46p and one each of the myc-tagged deletion mutants and applied to native immunoprecipitation with the anti-HA antibody followed by the antimyc detections. As shown in Figure 3B, the only deletion proteins found to fail to associate with Emp46p were the ones lacking the coiled-coil region (Δ281 and Δ281–333). These results suggest that the molecular escort is associated with Emp47p through the coiled-coil region in the luminal domain. We further examined whether the coiled-coil domain of Emp46p is also required for the Emp46/47p complex formation. Microsomes expressing both the coiled-coil deleted HA-Emp46p (Emp46p-Δ279–321) and myc-Emp47p were subjected to native immunoprecipitation with the antimyc antibody as above (Figure 3C). The result clearly showed that the Emp46p without coiled-coil region fails to associate with Emp47p, indicating that the complex formation between Emp47p and Emp46p is mediated by the coiled-coil region.
Figure 3.
Emp46p is associated with Emp47p through the coiled-coil region in its luminal domain, which dissociates in the Golgi. (A) Diagram of constructs encoding Emp47p and Emp46p derivatives. Emp47p derivatives are N-terminally tagged with myc and GFP (Δ383, Δ333, and Δ281) or 2× myc (Δ281–333). Emp46p-Δ281–333 is tagged by HA. (B) Immunoblots of anti-HA native immunoprecipitations from solubilized microsomes expressing HA-Emp46p with Emp47p-Δ383, -Δ333, -Δ281, and -Δ281–333. Total lanes (T) represent 5% of immunoprecipitation (IP) lanes. (C) Solubilized microsomes expressing myc-Emp47p and HA-Emp46p-Δ281–333 were subjected to native immunoprecipitation with antimyc antibody as above. (D) ER and Golgi fractions from sucrose density gradient in Figure 2B (ER, fraction 8; Golgi, fraction 4) and microsome-derived COPII vesicles were solubilized, and Emp47p-Emp46p complexes were detected by native immunoprecipitation as in Figure 1B. ER-Golgi marker, Sed5p, is shown as a negative control.
Our results indicating Emp46p and Emp47p as interacting proteins suggest that Emp47p may be a transport receptor for Emp46p targeting. If this were the case, their association might be reversible so that disassembly occurs at some point after export from the ER. To test this possibility, their coimmunoprecipitation was examined in different intracellular compartments. ER and Golgi membranes were prepared from the cells expressing myc-Emp47p and HA-Emp46p by sucrose gradient fractionation. COPII vesicles were also prepared by GTP-driven in vitro budding reactions with this strain. Membranes were solubilized with a detergent and myc-tagged Emp47p was immunoprecipitated. HA-Emp46p associated with these native immune complexes was then examined by immunoblotting (Figure 3D). Strikingly, the Emp46p-Emp47p complex was detected in the ER and COPII vesicles, but not at all in the Golgi fraction. These results demonstrate that the interaction of Emp47p and Emp46p is transient; cargo binding occurs in the ER and release in the Golgi.
Emp47p Forms an Oligomeric Complex in the ER, whereas Emp46p Alone Is Present as a Monomer
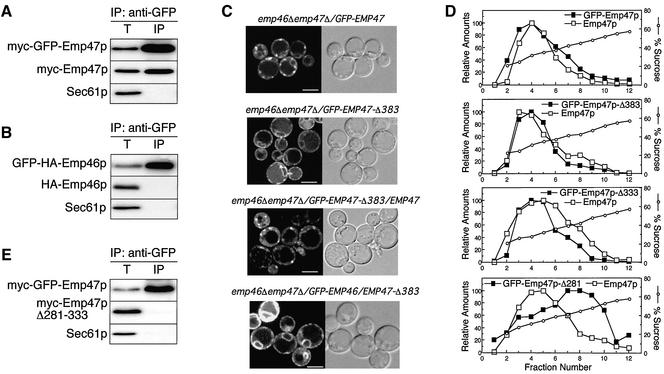
We have shown before that the C-terminal region of Emp46p and Emp47p contain signals required for COPII binding (Sato and Nakano, 2002). The question is raised then why Emp46p requires Emp47p for exit from the ER, whereas Emp47p alone is efficiently exported out from the ER. We speculated that the presence of COPII-binding motifs is not enough for incorporation into COPII vesicles and that some structural difference exists between Emp46p and Emp47p in the ER. To test this idea, we constructed emp46Δemp47Δ cells expressing differently tagged Emp47p (myc-GFP-Emp47p and myc-Emp47p) or Emp46p (GFP-HA-Emp46p and HA-Emp46p) to analyze the oligomeric state of each protein in the ER. myc-GFP-Emp47p complemented the growth defect of the emp47Δ strain in the presence of Ca2+ (Sato and Nakano, 2002) and showed exactly the same distribution to myc-Emp47p on sucrose gradients (unpublished data). Microsomes from these strains were solubilized with a detergent and then a portion of GFP-tagged protein was immunoprecipitated with the anti-GFP antibody. The amounts of coprecipitated myc-Emp46/47p were determined by immunoblotting. The results shown in Figure 4A demonstrate that myc-GFP-Emp47p was coimmunoprecipitated with myc-Emp47p, suggesting that Emp47p exists as a homooligomer in the ER at least two Emp47p molecules in a complex. In contrast, no Emp46p homooligomers were detected at all in the microsomes (Figure 4B). Our findings showed that Emp47p alone forms more than a dimer in the ER, whereas Emp46p alone is present as a monomer.
Figure 4.
Emp47p alone forms oligomers through its coiled-coil region in the ER, whereas Emp46p alone is present as a monomer. (A and B) Microsomes from emp46Δemp47Δ cells expressing differently tagged Emp47p (a, myc-GFP-Emp47p and myc-Emp47p) and Emp46p (b, GFP-HA-Emp46p and HA-Emp46p) were solubilized and applied to native immunoprecipitation as in Figure 1B, followed by immunoblotting with antimyc (a) or anti-HA (b). (C) emp46Δemp47Δ cells expressing indicated proteins were visualized by confocal laser microscopy. Bars, 5 μm. (D) Subcellular localization of Emp47p deletion constructs shown in Figure 3A coexpressed with wild-type Emp47p. (E) Anti-myc immunoblots of anti-GFP native immunoprecipitation from solubilized microsomes expressing myc-GFP-Emp47p and myc-Emp47p-Δ281–333 as in A and B. Total (T) represents 5% of the immunoprecipitation lane.
The state of Emp47p homooligomerization was further examined. If Emp47p exists as a homooligomer in the ER, the prediction is that wild-type Emp47p and Emp47p without the cytoplasmic export signals would be traveling to the Golgi together. As can be seen in Figure 4C, GFP-Emp47p alone was localized to punctate structures, representing the yeast Golgi. In contrast, when the mutant that lacks the C-terminal COPII-binding sites and the transmembrane region, GFP-Emp47p-Δ383, was expressed alone in emp46Δemp47Δ cells, the Golgi fluorescence was virtually gone and a continuous pattern typical of the yeast ER emerged. This indicates the incapability of ER exit of this soluble-form mutant. However, when GFP-Emp47p-Δ383 was coexpressed with wild-type Emp47p in emp46Δemp47Δ cells, GFP-Emp47p-Δ383 was now converted to punctate Golgi structures. This shift appears to correlate with the association of GFP-Emp47p-Δ383 with wild-type Emp47p, again supporting homooligomerization of Emp47p upon exit from the ER. These results further indicate that the essential domain for the formation of Emp47p homooligomer is in the luminal domain of Emp47p. We further examined the effect of C-terminal deletion of Emp47p on Emp46p localization. When GFP-Emp46p was expressed with GFP-Emp47p-Δ383, ER accumulation of GFP-Emp46p was observed (Figure 4C). This result confirms that the ER export of Emp46p is dependent on Emp47p.
We addressed the mechanism by which Emp47p forms a homooligomer by more precise identification of the domain of Emp47p required for oligomer formation. We tested a series of deletion mutants used in Figure 3A, for intracellular localization in the presence of wild-type Emp47p as in Figure 2B. We found that the C-terminal deletion up to the coiled-coil region had an obvious effect, which appeared to shift the mutant Emp47p toward ER membrane fractions (Figure 4D). When the internal deletion mutant of the coiled-coil region from full-length Emp47p (myc-Emp47p-Δ281–333) was coexpressed with GFP-tagged wild-type Emp47p and subjected to native immunoprecipitation as in Figure 4A, no association was observed between these proteins in the microsomes (Figure 4E). This implies that the coiled-coil region of Emp47p is responsible for the oligomer formation as well as Emp46p binding. Emp46p has 66% amino acid similarity to Emp47p, and indeed the alignment of Emp46p protein with Emp47p demonstrates the probable coiled-coil domain at similar locations in Emp46p and Emp47p (Sato and Nakano, 2002). It should be noted, however, that Emp46p has deletions within this region, which may contribute to homooligomerization. ERGIC-53, a mammalian homologue of Emp47p, forms disulfide-bond–linked homodimers and homohexamers in the ER (Lahtinen et al., 1992). This is not likely the case for Emp47p, because the coiled-coil region contains no cysteines and Emp47p migrated as its monomer size in a nonreduced gel (unpublished data).
Emp47p Oligomerization Acts as a Signal for Its Incorporation into COPII Vesicles but Is Not Required for COPII Binding
We next tested the influence of the myc-Emp47p-Δ281–333 mutation on the incorporation of this protein into COPII vesicles. As shown in Figure 5A, in the reconstituted in vitro budding reaction with microsomes expressing both myc-GFP-Emp47p and myc-Emp47p-Δ281–333, the packaging of myc-Emp47p-Δ281–333 into ER-derived COPII vesicles was undetectable, whereas myc-GFP-Emp47p budding was at a level comparable to Sec22p. This result suggests that the presence of COPII-binding motifs in Emp47p per se is not sufficient for this cargo to be sorted into COPII vesicles but the oligomerization is also required. Presumably, the failure of Emp46p alone to exit the ER is reasoned by its monomeric state in the ER. We also tested if myc-Emp47p-Δ281–333 affects the packaging of Emp46p into COPII vesicles using microsomes derived from emp46Δemp47Δ cells expressing HA-Emp46p and myc-Emp47p-Δ281–333. As expected, the budding efficiency of HA-Emp46p was very low along with myc-Emp47p-Δ281–333, again confirming that the coiled-coil region is sufficient for Emp46p binding (Figure 5A).
Figure 5.
Emp47p oligomerization acts as a signal for its incorporation into COPII vesicles but is not required for COPII binding. (A) COPII vesicle budding from emp46Δemp47Δ cells expressing Emp47p-Δ281–333 with GFP-Emp47p (left) or HA-Emp46p (right) microsomes as in Figure 2A. (B) Microsomes prepared from cells expressing myc-Emp47p and HA-Emp46p (left) or myc-Emp47p-Δ281–333 and HA-Emp46p (right) were incubated with GST-Sar1p and the purified Sec23/24p complex in the presence of GMP-PNP or GDP-βS. Subsequently, digitonin-soluble prebudding complexes were recovered on glutathione beads. Emp46p, Emp47p, Sec22p, and Sec61p in the prebudding complex were detected by immunoblotting. Total (T) represents 0.5% of the total solubilized membranes.
We next examined whether the coiled-coil deletion of Emp47p and Emp46p alone failed to be incorporated into COPII vesicles because they could not bind to COPII subunits or whether they bound but were not packaged into COPII vesicles. These were investigated by testing whether those monomers were recovered in detergent-soluble prebudding complexes (Figure 5B). The prebudding complexes are detected when microsomes are incubated with a subset of COPII components, GST-Sar1p, and the Sec23/24p complex in the presence of GMP-PNP (Kuehn et al., 1998). GMP-PNP-bound GST-Sar1p stabilizes the assembled prebudding complex for isolation on glutathione-Sepharose in the presence of detergent. Microsomes were prepared from cells expressing Emp46p and Emp47p or Emp46p and Emp47p-Δ281–333 and were incubated in the presence of GST-Sar1p with GMP-PNP and the Sec23/24p complex. Emp46p and Emp47p from wild-type microsomes were efficiently recovered in the prebudding complex, whereas the ER resident protein Sec61p was not. Sec22p was also found in the prebudding complex, which was shown as a positive control (Kuehn et al., 1998; Sato and Nakano, 2002).
We next asked whether the Emp46/47p monomers were able to be incorporated into the prebudding complex. Surprisingly, when microsomes expressing both HA-Emp46p and myc-Emp47p-Δ281–333 were tested, both proteins were present in the prebudding complex. These results indicate that the oligomerization of Emp46/47p is not required for efficient binding of COPII subunits. The deletion of the coiled-coil region of the Emp47p may disturb the protein stability. It should be noted, however, that the Emp47p-Δ281–333 expressed indistinguishable amounts of protein from the wild-type Emp47p (our unpublished observation). Moreover, the result indicating that the coiled-coil deleted Emp47p retains partial function to form prebudding complexes argues against the possibility that the transport defect of this protein is caused by its misfolding and interactions with the ER quality control machinery. Taken together, the results above suggest that the packaging of Emp46/47p into the COPII vesicles is due to a combined action of COPII binding and oligomer formation of Emp46/47p.
Emp47p Forms a Large Oligomeric Complex
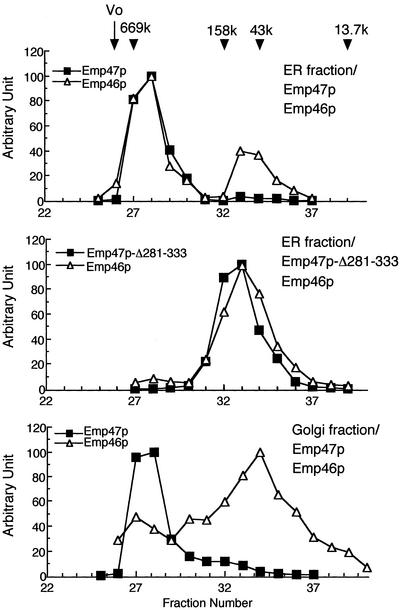
The results described above strongly suggest that Emp47p is found in complexes with Emp46p or with Emp47p itself in the ER. To extend our analysis and to determine the size of Emp47p-containing complex, we performed gel filtration separation of the detergent solubilized ER and the Golgi isolated by sucrose gradient, which would be a more direct test of Emp47p oligomerization. Significant proportions of Emp47p and Emp46p in the ER from wild-type cells were found to coelute at the molecular mass of ∼600 kDa (Figure 6). When extracts were prepared from the ER fraction expressing both myc-Emp47p-Δ281–333 and HA-Emp46p, these proteins were eluted at lower molecular mass positions, most consistent with their monomer sizes. Thus, it seems unlikely that the high-molecular-mass complexes comprising Emp47p and Emp46p are monomeric forms of each molecule surrounded by detergent micelles. The figure 600 kDa corresponds to 10–12 mers if only Emp47p and/or Emp46p are present. In agreement with the coimmunoprecipitation results in Figure 3D, ∼70% of Emp46p were eluted in fractions corresponding to its monomer size when the Golgi fractions were analyzed, representing the cargo dissociation from the receptor in the Golgi. However, no apparent molecular shift was detected for the Emp47p containing complexes between the ER and the Golgi. We cannot rule out that this ∼600-kDa complex may be associated with additional proteins in the Golgi, and they may contribute to the dissociation of Emp47p-Emp46p protein complexes. Because efficient transport of the soluble truncated Emp47p is observed without its cytoplasmic COPII-binding domain when wild-type Emp47p is present (Figure 4, C and D), the oligomerization to ∼600 kDa might not be the minimal requirement for Emp47p to exit from the ER.
Figure 6.
Emp47p forms a large oligomeric complex. ER and Golgi fractions were prepared from emp46Δemp47Δ cells expressing indicated proteins by sucrose density gradient as in Figure 2B (ER, fraction 8; Golgi, fraction 4), and solubilized with 0.05% n-dodecyl maltoside. Extracts were applied to a G3000SWXL gel filtration column. Eluates were subjected to SDS-PAGE followed by immunoblotting using antimyc and anti-HA antibodies. Molecular size standards used are thyroglobulin (669 kDa), aldolase (158 kDa), ovalbumin (43 kDa), and ribonuclease A (13.7 kDa).
DISCUSSION
Emp47p and Emp46p were previously shown to be selectively recruited into COPII vesicles and were required for the export of a subset of glycoproteins. In this study, we show that Emp47p physically interacts with Emp46p in the ER. To identify the region of the Emp47p responsible for this association, we constructed a series of deletion mutants of Emp47p and found that the coiled-coil region in the luminal domain of this protein is critical for association with Emp46p. Furthermore, the Emp46p-Emp47p association is reversible such that complex dissociates when it reaches the Golgi. Based on these findings, Emp47p has typical characteristics as a receptor protein for the ER-Golgi transport. Additional studies will be required to elucidate the mechanism of complex dissociation in the Golgi. It is not clear whether Emp47p is a receptor specialized only for Emp46p, because our cross-link experiment showed several bands other than Emp46/47p that we could not identify. Emp47p has homologues in all higher eukaryotes, and its mammalian homologue, ERGIC-53, has been shown to be a receptor for soluble glycoprotein cargo acting in ER-to-Golgi transport (Appenzeller et al., 1999). It remains to be determined if the lectin-like domains of Emp46/47p bind glycoproteins and indeed act as glycoprotein cargo receptors as well.
Another saturable transport receptor that has been characterized in yeast, Erv29p, shows a limited secretion of several soluble cargo molecules when inactivated (Belden and Barlowe, 2001). Integral membrane cargo proteins have also been thought to depend on saturable receptors for export from the ER. Candidates for this function include Emp24p and Erv14p, which have been postulated to be required for selection of specific GPI anchored and integral membrane cargo, respectively, into COPII vesicles (Muniz et al., 2000; Powers and Barlowe, 2002). Our present study on Emp46/47p provides another piece of evidence to prove that the integral membrane secretory protein depends on an adaptor/receptor protein for export from the ER.
We have demonstrated before that both the cytoplasmic regions of Emp46p and Emp47p have the ability to bind COPII proteins (Sato and Nakano, 2002). The decreased rate, but not completely, of ER exit of Emp46p with mutations in this region was observed. This raised the possibility that the multiple signals may be required in Emp46p and Emp47p for efficient export. In the present work, we have demonstrated that Emp46p associates with Emp47p in the ER and this Emp46/47p hetero-oligomer is competent for export. This finding readily explains why the disruption of COPII-binding motif cannot completely block the ER export. The Emp46p with disrupted COPII-binding motif still can exit the ER through association with Emp47p but at reduced efficiency. The COPII binding to each subunit of the Emp46/47p complex is likely to be required for maximal transport efficiency. We also have shown before that the overexpression of Emp46p suppresses the temperature and calcium sensitivities of the emp47Δ cells, whereas Emp47p is required for the ER export of Emp46p. The explanation for this is that the ER-to-Golgi flow of Emp46p is achieved by the overexpression presumably by bulk flow export. In support of this idea, a small amount of Golgi localization of Emp46p is observed when Emp46p alone is overexpressed (unpublished data), which might be enough to suppress the phenotypes associated with EMP47 deletion.
The formation of a homooligomeric cargo complex upon exit from the ER is also reported for the yeast plasma membrane H+-ATPase Pma1p (Bagnat et al., 2001; Lee et al., 2002). In comparison with these examples, Emp47p oligomerization seems to has a different role upon ER exit, because the ER export of Pma1p is not dependent on its oligomeric state. Recent studies on Erv41p-Erv46p complex, which cycles between the ER and Golgi, have identified multiple ER export signals contained within the C-terminal cytoplasmic tails of both Erv41p and Erv46p. Sequence information contained in these tails of both the Erv41p and the Erv46p is required in its proper context for efficient packaging of the Erv41p-Erv46p complex into COPII vesicles (Otte and Barlowe, 2002). Requirement of the export signals on separate subunits of the complex for the ER export is similar to Emp46/47p transport.
Is cargo oligomerization a general mechanism of protein sorting into COPII vesicles or specific for the Emp46/47p? It is well known that a number of proteins need to homo- or heterooligomerize in order to be exported out from the ER in mammalian cells, such as immune receptors (Bonifacino et al., 1990), ATP-dependent K+ channels (Zerangue et al., 1999), γ-aminobutyric acid GABAB receptors (Margeta-Mitrovic et al., 2000; Pagano et al., 2001), glutamate NMDA receptors (Perez-Otano et al., 2001), and G-protein–coupled receptors (Bouvier, 2001). However, in those membrane protein complexes whose trafficking is known to be assembly dependent, at least one of the subunits contains an ER retrieval signal that is shielded on subunit assembly, allowing the assembled protein complex to traffic to the plasma membrane. The unassembled subunits recycle between the ER and the Golgi until the complexes are properly formed. Therefore, the oligomerization of those proteins are required for the plasma membrane targeting of fully assembled complexes, but not essential for each subunit to exit the ER. In fact, the disruption of those ER retrieval signals allows each unassembled subunit to localize to the plasma membrane. In comparison with those documented examples of assembly-dependent ER export, Emp46/47p seems to act through a different mechanism. Here, Emp46/47p oligomerization itself is required for the export competence of Emp46/47p complexes. Interestingly, the oligomeric association of other type-I transmembrane cargos has also been reported for p24 family members, which also depends on the coiled-coil region of their luminal domain (Marzioch et al., 1999; Ciufo and Boyd, 2000). Moreover, the vesicular stomatitis virus (VSV) G protein trimerizes in the ER and only trimers are transported to the Golgi (Doms et al., 1987). Although the precise role of cargo oligomerization in the ER export remains to be established, these observations strongly support the presence of assembly dependent cargo selection at the ER exit sites.
In conclusion, although the function of Emp46p and Emp47p proteins remains to be determined, we have demonstrated the Emp47p acts in sorting an integral membrane secretory cargo during export from the ER and this process includes cargo oligomerization. To confirm a direct role of cargo oligomerization in concert with activated Sar1p and Sec23/24p complex, it will be necessary to reconstitute these associations in purified proteoliposomes. Such a system may provide an explanation of the contribution of a cargo oligomerization to its recruitment.
Acknowledgments
We are grateful to Randy Schekman for providing strains and antibodies. This work was supported by PRESTO, Japan Science and Technology Corporation, by a Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan, by a research grant of the Human Frontier Science Program, and by a fund from the Bioarchitect Research Project of RIKEN.
References
- Appenzeller, C., Andersson, H., Kappeler, F., and Hauri, H.P. (1999). The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol 1, 330-334. [DOI] [PubMed] [Google Scholar]
- Bagnat, M., Chang, A., and Simons, K. (2001). Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell 12, 4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C. et al. (1994). COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895-907. [DOI] [PubMed] [Google Scholar]
- Barlowe, C., and Schekman, R. (1993). SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature 365, 347-349. [DOI] [PubMed] [Google Scholar]
- Belden, W.J., and Barlowe, C. (2001). Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science 294, 1528-1531. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., Cosson, P., and Klausner, R.D. (1990). Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell 63, 503-513. [DOI] [PubMed] [Google Scholar]
- Bouvier, M. (2001). Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2, 274-286. [DOI] [PubMed] [Google Scholar]
- Ciufo, L.F., and Boyd, A. (2000). Identification of a luminal sequence specifying the assembly of Emp24p into p24 complexes in the yeast secretory pathway. J. Biol. Chem. 275, 8382-8388. [DOI] [PubMed] [Google Scholar]
- Doms, R.W., Keller, D.S., Helenius, A., and Balch, W.E. (1987). Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J. Cell Biol. 105, 1957-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn, M.J., Herrmann, J.M., and Schekman, R. (1998). COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature 391, 187-190. [DOI] [PubMed] [Google Scholar]
- Lahtinen, U., Dahllof, B., and Saraste, J. (1992). Characterization of a 58 kDa cis-Golgi protein in pancreatic exocrine cells. J. Cell Sci. 103(Pt 2), 321-333. [DOI] [PubMed] [Google Scholar]
- Lee, M.C., Hamamoto, S., and Schekman, R. (2002). Ceramide biosynthesis is required for the formation of the oligomeric H+-ATPase Pma1p in the yeast endoplasmic reticulum. J. Biol. Chem. 277, 22395-22401. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic, M., Jan, Y.N., and Jan, L.Y. (2000). A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27, 97-106. [DOI] [PubMed] [Google Scholar]
- Marzioch, M., Henthorn, D.C., Herrmann, J.M., Wilson, R., Thomas, D.Y., Bergeron, J.J., Solari, R.C., and Rowley, A. (1999). Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Mol. Biol. Cell 10, 1923-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz, M., Nuoffer, C., Hauri, H.P., and Riezman, H. (2000). The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum-derived vesicles. J. Cell Biol. 148, 925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, A., Brada, D., and Schekman, R. (1988). A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J. Cell Biol. 107, 851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, A., and Muramatsu, M. (1989). A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J. Cell Biol. 109, 2677-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte, S., and Barlowe, C. (2002). The Erv41p-Erv46p complex: multiple export signals are required in trans for COPII-dependent transport from the ER. EMBO J. 21, 6095-6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, A. et al. (2001). C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J. Neurosci. 21, 1189-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade, G. (1975). Intracellular aspects of the process of protein synthesis. Science 189, 347-358. [DOI] [PubMed] [Google Scholar]
- Perez-Otano, I., Schulteis, C.T., Contractor, A., Lipton, S.A., Trimmer, J.S., Sucher, N.J., and Heinemann, S.F. (2001). Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J. Neurosci. 21, 1228-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, J., and Barlowe, C. (2002). Erv14p directs a transmembrane secretory protein into COPII-coated transport vesicles. Mol. Biol. Cell 13, 880-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., and Nakano, A. (2002). Emp47p and its close homolog Emp46p have a tyrosine-containing endoplasmic reticulum exit signal and function in glycoprotein secretion in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2518-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman, R., and Orci, L. (1996). Coat proteins and vesicle budding. Science 271, 1526-1533. [DOI] [PubMed] [Google Scholar]
- Schroder, S., Schimmoller, F., Singer-Kruger, B., and Riezman, H. (1995). The Golgi-localization of yeast Emp47p depends on its dilysine motif but is not affected by the ret1–1 mutation in alpha-COP. J. Cell Biol. 131, 895-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni, Y., Kurihara, T., Ravazzola, M., Amherdt, M., Orci, L., and Schekman, R. (2000). Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J. Cell Biol. 151, 973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, S., and Schekman, R. (1998). Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science 281, 698-700. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., Nichols, B.J., Pelham, H.R.B., and Wickner, W. (1998). A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated organelles, is disassembled and activated for docking and fusion. J. Cell Biol. 140, 119-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue, N., Schwappach, B., Jan, Y.N., and Jan, L.Y. (1999). A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22, 537-548. [DOI] [PubMed] [Google Scholar]