Abstract
The restoration of planar cell polarity is an essential but poorly understood step toward physiological recovery during sensory-organ regeneration. Investigating this issue in the lateral line of the zebrafish, we found that hair cells regenerate in pairs along a single axis established by the restricted localization and oriented division of their progenitors. By analyzing mutants lacking the planar-polarity determinant Vangl2, we ascertained that the uniaxial production of hair cells and the subsequent orientation of their hair bundles are controlled by distinct pathways, whose combination underlies the establishment of hair-cell orientation during development and regeneration. This mechanism may represent a general principle governing the long-term maintenance of planar cell polarity in remodeling epithelia.
Keywords: auditory system, balance, hearing, lateral line, vestibular system
Epithelial cells are polarized along the apicobasal axis and often also perpendicularly to this axis, within the plane of the epithelium. The latter type of cellular organization, termed planar cell polarity, has been studied extensively during development in Drosophila (1, 2). Because the epithelia of adult fruit flies harden and, consequently, cannot undergo repair after mechanical damage or cell death, they are not amenable to studies of the postembryonic recovery of planar cell polarity. In vertebrates, the orientation of the hair bundle that projects from the apical surface of a sensory hair cell represents a striking example of planar cell polarity (3, 4). Because the hair bundle's axis of morphological polarization defines the cell's axis of responsiveness to mechanical stimuli, the senses of hearing and equilibrium rely on the coordinated orientation of hair cells across the sensory epithelium (5, 6).
The loss of cochlear hair cells in mammals is irreversible. However, other vertebrates can regenerate hair cells (7–9). In birds, for example, regenerated hair cells appear normal and regain their proper orientation (10–12). The mechanisms that underlie the establishment of hair-cell orientation during development remain poorly understood, however, and those active during hair-cell regeneration are unknown. Direct and continuous observation of hair-cell development and regeneration has not yet been achieved, for live imaging of the ear's mechanosensory epithelium is hampered by the inaccessibility of the cochlea within the skull. Furthermore, the long periods needed by some animals to regenerate hair cells pose severe limitations to analyzing the process continuously. Attempts to monitor cellular behavior in neuromasts after hair-cell ablation in salamanders suffered from the absence of cellular and molecular markers (13), which prevented the unambiguous identification of cellular types and the analysis of the recovery of epithelial architecture upon regeneration. In this study, we used the lateral-line system of the zebrafish to circumvent these limitations and to acquire time-lapse sequences of hair-cell formation and regeneration. The results from transgenic wild-type and mutant animals suggest how the oriented cell division of identified hair-cell progenitors and the subsequent activity of Vangl2 control the establishment of hair-cell orientation during development and its recovery during regeneration.
Results
Hair Bundles Are Produced in Pairs Along a Single Axis.
During development, neuromasts acquire two equally populated groups of hair cells whose hair bundles are oriented along a single axis but in opposite directions (14). For example, the first neuromast of the posterior lateral line, conventionally named L1, has an axis of planar cell polarity that is oriented parallel to the animal's anteroposterior body axis: each hair bundle directs its kinocilium toward either the head or the tail (Fig. 1A). To learn whether this polarization reappears during the postembryonic repair of neuromasts, we took advantage of the robust regenerative capacity of hair cells in the lateral line (15, 16). Exposing zebrafish larvae to neomycin leads to hair-cell apoptosis (Fig. 1 B and C), extrusion from the epithelium, and clearance by circulating cells (Movie 1, which is published as supporting information on the PNAS web site). Neuromasts become devoid of hair cells within 1 h of treatment, whereas supporting cells are not affected (Fig. 1 D and E). Throughout regeneration, most organs display equal numbers of hair cells of each orientation along their original axis of planar cell polarity both in parallel (Fig. 1F) and in perpendicular neuromasts (14; data not shown). Innervation and the presence of neural crest-derived glia are dispensable for this process, for neurogenin1 mutant zebrafish regenerate hair cells as in wild-type control specimens (data not shown). Because the total absence of hair cells, innervation, or glia does not affect this process, we conclude that supporting cells or perhaps the extracellular matrix contains sufficient information to instruct hair-cell orientation during neuromast repair.
Fig. 1.
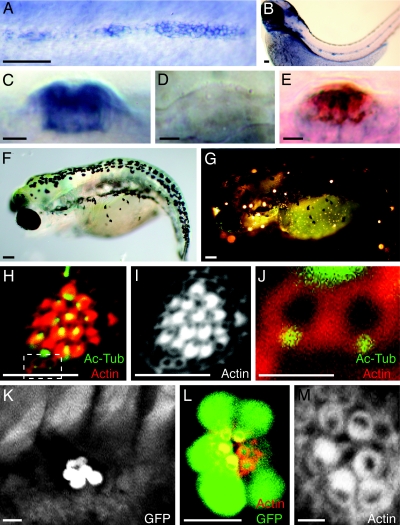
Characterization of hair-cell death and ET4 transgenic zebrafish. (A) In a confocal image of a neuromast in a normal 3-day-old larva, stereocilia are labeled with rhodamine-phalloidin to reveal the polarization of hair bundles toward the animal's head or tail. (B) In a control larva, only one peripheral cell displays TUNEL staining (green) indicative of apoptosis. To-Pro-3 labeling (red) marks all nuclei. (C) Neomycin treatment increases the number of apoptotic cells at the center of the neuromast. (D) In an untreated animal, immunolabeling with the hair cell-specific marker HCS1 (green) identifies hair cells, and claudin b labeling (blue) designates supporting cells. (E) A neomycin-treated larva lacks hair cells. (F) In a regenerating L1 neuromast, actin staining shows that hair bundles orient along the neuromast's original, anteroposterior axis of planar cell polarity. (G) GFP-positive hair cells (green) in the ear of a 3-day-old ET4 transgenic animal are also positive for the specific hair-cell marker HCS1 (red). Claudin b immunoreactivity (blue) marks supporting cells. (H) In a lateral view of a neuromast from an ET4 transgenic animal labeled for claudin b (blue) and HCS1 (red), GFP-positive hair cells (green) are also marked by HCS1. (I) Labeling stereocilia with rhodamine-phalloidin (red) confirms that planar cell polarity is normal in an ET4 larva, whose hair cells express GFP (green). (I Inset) Only the actin staining is shown. (J) Incubation of an ET4 larva in FM4–64 (red) reveals a single, weakly GFP-positive cell (asterisk) that does not incorporate this vital dye. (K–M) Labeling of an ET4 larva with HCS1 (red) shows a gradient of intensity for this specific hair-cell marker and for GFP (green) toward the periphery of the neuromast. A single weakly GFP-positive cell at the lower edge of the neuromast (arrowheads) is negative for HCS1. In these and all subsequent figures, the animal's anterior is oriented to the left and its dorsum is situated to the top. (Scale bars: 10 μm.)
Pairs of Hair Cells Develop from a Common Progenitor.
To characterize the pattern of hair-cell production, we used the transgenic zebrafish line ET4 that expresses green fluorescent protein (GFP) in hair cells of the inner ear and lateral line (Fig. 1 G and H) (17). A careful analysis of this transgenic line revealed that ET4 animals are normal: The onset of hair-cell development, death after neomycin exposure, and regeneration follow a pattern and time course identical to that of nontransgenic wild-type control animals (data not shown). Furthermore, hair cells polarize normally in ET4 neuromasts (Fig. 1I).
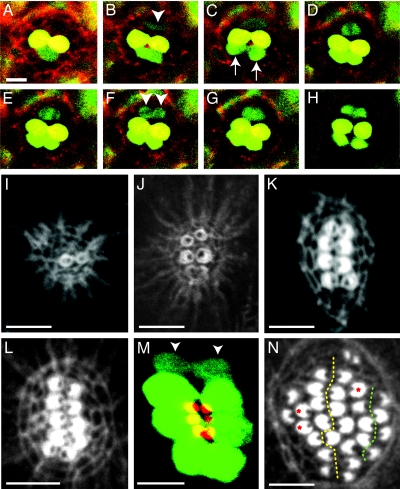
Examination of ET4 animals occasionally reveals low levels of GFP expression in solitary cells that are negative for markers diagnostic of the hair-cell phenotype and that do not incorporate the fluorophore FM4–64, a vital marker for mature hair cells (Fig. 1 J–M). Live imaging during development and regeneration revealed that these cells divide in a plane perpendicular to the neuromast's axis of planar cell polarity, producing two hair cells in each of 14 instances (Fig. 2A–H and Movies 2 and 3, which are published as supporting information on the PNAS web site). Because these solitary, GFP-positive cells do not replenish themselves, they represent transient hair-cell progenitors that are produced by putative stem cells. These mitotic cells were observed regularly at the dorsal and ventral poles of developing or regenerating neuromasts such as L1, whose axis of planar cell polarity is oriented anteroposteriorally. In a neuromast whose axis of polarization is instead directed dorsoventrally (14), the positions of the progenitors were also rotated by 90°, that is, at the anterior and posterior extremes of the organ. The progenitors were encountered rarely in quiescent, nonproliferating neuromasts (data not shown), which may explain why they heretofore have escaped identification. In none of the 14 live-imaging recordings did we observe hair cells developing singly or independently of a mitosis. These results demonstrate that regenerating lateral-line hair cells are born in pairs from a localized progenitor that divides in a plane perpendicular to the neuromast's axis of planar polarity. The process of hair-cell regeneration therefore recapitulates that of development.
Fig. 2.
Time-course analysis of hair-cell production. (A–H) In a 12-h-long series of confocal images begun 2 days after fertilization, labeling of an ET4 larva with Texas red-ceramide (red) reveals two GFP-positive mature hair cells (yellow). At the lower edge of the neuromast, a pair of immature hair cells (green; arrows in C), whose proximity initially makes them appear confluent, separate over the course of 3 h (B and C) and become yellow as they mature (D–H). A hair-cell precursor meanwhile develops at the upper edge of the neuromast (arrowhead in B). Over the next hour, this precursor increases its green fluorescence and commences mitosis; the absence of GFP identifies the chromosomes congregated in the metaphase plate (D) and segregated in anaphase (E). The daughter cells (arrowheads in F) eventually separate to form two hair cells (H). This time-lapse series and the supporting movies indicate that hair cells develop in pairs along a single axis in neuromasts. (I) Only 6 h after ablation of hair cells in a wild-type larva, actin staining delineates the first, immature pair of hair bundles. (J) By 10 h after treatment, a neuromast has developed three mature pairs of hair bundles whose opposing polarities are apparent. (K) A neuromast at 16 h after treatment shows the orientation of four pairs of hair bundles. (L) In another animal at the same stage of recovery, five pairs of hair bundles demarcate a line of symmetry dividing the neuromast into two halves along the dorsoventral midline. All of the hair bundles on each side of the line of symmetry have the same orientation. (M) A composite of two confocal images of a regenerating ET4 animal, in which the apical surfaces of the hair cells are pseudocolored red and the cell bodies green, shows that the line of symmetry defined by the hair bundles corresponds to the arrangement of cell bodies. Two immature hair cells have not yet developed hair bundles (arrowheads). (N) By 40 h after treatment, the original line of symmetry is still evident (dotted yellow line), but a second line also has appeared on one side of the neuromast (dotted green line). Three unpaired hair cells are marked with red asterisks. (Scale bars: 10 μm.)
Sibling Hair Cells Develop Opposite Polarities.
Neuromasts normally contain equal numbers of hair cells of each polarity, suggesting that the process of hair-bundle orientation is tightly regulated. One possible explanation of this fidelity is that the hair bundles of sibling cells consistently assume opposite orientations. To test this hypothesis, we analyzed the orientation of hair bundles in sibling hair cells during the 24 h after drug exposure. The sequential regeneration of hair cells along a single axis creates a line of mirror symmetry in neuromasts (Fig. 2 I–L). All hair cells lying anterior to this line are polarized posteriorly, whereas the posterior cells adopt an anterior orientation. We conclude that the orientation of a hair bundle can be predicted from the position of any given hair cell with respect to the line of symmetry. Together with the observation that hair cells develop in pairs, these results suggest that the position of a hair cell with respect to its sibling determines the polarization of its hair bundle.
The spatial organization of hair-cell apices mirrors that of the hair cells themselves, indicating that the orientation of the mitoses that generate them directly translates into that of the hair bundles (Fig. 2M). The preferential localization and oriented division of transient hair-cell progenitors produce regional differences in the growth rate of neuromasts, with the consequent elongation of the epithelium along the line of symmetry (Fig. 2 I–M). Continuous expansion along this axis eventually reaches a limit, which is probably imposed by the size of the neuromast. Large neuromasts experience this limit later than small ones, explaining why long lines of symmetry develop in large neuromasts during regeneration but rarely in small neuromasts during development (Fig. 1A). After 24 h, however, the primary line of symmetry becomes less recognizable. The macula of nonproliferating neuromasts loses its oval shape, and many hair bundles become located in rows parallel to the primary line of symmetry (Fig. 2N). This observation suggests either that later-born hair cells are produced by progenitors located ectopically or that hair cells relocate within the neuromast after regeneration ceases. The observation that most hair bundles continue to face one another, forming several smaller lines of symmetry, lends support to the former possibility.
The Orientation of Hair Bundles Is Disrupted in trilobite Mutants.
To gain insight into the molecular bases of hair-cell orientation during development and regeneration, we analyzed trilobite mutant zebrafish lacking the protein Vangl2 (18), a determinant of planar cell polarity that controls the orientation of hair bundles in the mammalian inner ear (19). We first ascertained that the vangl2 gene is expressed in the migrating lateral-line primordium (Fig. 3A) and in the supporting cells and hair cells of mature neuromasts (Fig. 3 B–E). Next, we found that trilobite mutants develop both major branches of the primary lateral line (Fig. 3 F and G) but display a pronounced defect in the planar polarity of their hair cells (Fig. 3 H–J).
Fig. 3.
Random orientation of hair bundles in neuromasts of trilobite mutant zebrafish. (A) In situ hybridization shows that vangl2 is expressed by the migrating primordium of the posterior lateral line in a 2-day-old wild-type embryo. (B) Expression is apparent in mature neuromasts at 3 days of age. (C) In a lateral view of a mature neuromast, the labeling indicates that the vangl2 transcript is expressed strongly by most neuromast cells. (D) A trilobitevu7 deletion mutant lacks vangl2 labeling. (E) A two-color in situ hybridization for vangl2 (blue) and the hair-cell marker parvalbumin 3a (red) indicates that vangl2 is expressed by both hair cells and supporting cells. (F) A brightfield micrograph displays the shortened, curled body of a trilobitem209 mutant larva at 5 days of age. (G) Fluorescence microscopy after exposure to 4-Di-2-Asp reveals that the trilobite larva possesses functional neuromasts along both the anterior and the posterior branches of its lateral-line system. (H–J) A neuromast of a 5-day-old trilobitem209 larva labeled for acetylated α-tubulin (green) and actin (red) shows that hair bundles assume random orientations. Two young hair cells occur at the lower edge of the neuromast (dashed box). (I) Staining of actin alone accentuates the defect in planar cell polarity. (J) A high-magnification view of the same neuromast reveals the mislocalization of basal bodies in two, probably sibling hair cells that have not yet formed mature hair bundles. (K) A low-magnification view of the trunk of an ET4;trilobite larva demonstrates that hair-cell pairs are aligned along a single axis in a neuromast. (L) A high-magnification view of a regenerating neuromast in an ET4;trilobite animal expressing GFP (green) and stained for actin (red) shows the alignment of hair cells along the neuromast's line of symmetry. (M) In a higher-magnification view of this neuromast, actin staining demonstrates that, although the hair bundles respect the line of symmetry, they are misoriented with respect to one another. (Scale bars: A, B, F, and G, 100 μm; C–E, H, I, K, and L, 10 μm; J and M, 2 μm.)
Because it has been reported that the orientation of cell divisions during zebrafish gastrulation depends on Vangl2 function (20), the loss of Vangl2 might disrupt hair-cell polarization by perturbing the division of transient hair-cell progenitors. Our observations of trilobite mutants showed, however, that these mitoses are normal (Movie 4, which is published as supporting information on the PNAS web site). The analysis of hair-cell regeneration in trilobite mutants revealed that sibling hair cells are arrayed along the usual single axis in neuromasts (Fig. 3 K–M). The defects in hair-bundle orientation caused by Vangl2 deficiency thus are not a consequence of the misoriented divisions of hair-cell progenitors.
During the processes of hair-bundle orientation, a basal body localizes apically in maturing hair cells, forming the precursor of a single kinocilium that extends from one edge of the cellular apex. The peripheral movement of the kinocilium signals the onset of hair-cell planar polarization and antecedes the final step of hair-bundle morphogenesis, the graded stereociliary elongation that underlies hair-bundle orientation. The peripheral movement of the kinocilium is impaired in Vangl2 mutant mice (19). By labeling neuromasts with an antibody to acetylated α-tubulin, we found that the hair cells of trilobite mutant larvae display randomly oriented basal bodies and kinocilia (Fig. 3 H–M).
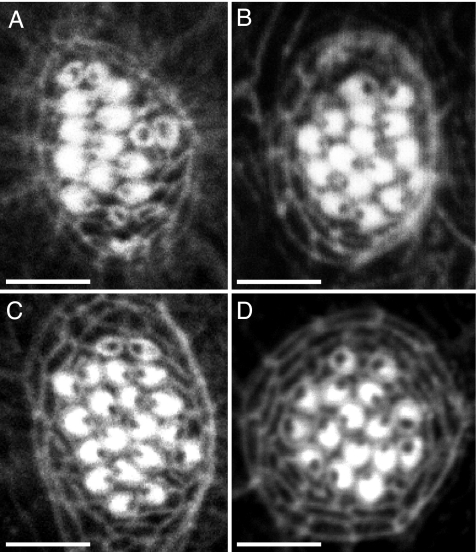
Because the hair-cell abnormality associated with the trilobite mutation might represent a secondary effect of the strong mispatterning of the body owing to defective cell migration during gastrulation, we analyzed other mutations that affect gastrulation in zebrafish. Gastrulation defects are associated with the silverblick/wnt11 and pipetail/wnt5a mutations (21, 22). Analyzing these mutations singly or in combination, we observed no defect in hair-bundle orientation in neuromasts (Fig. 4A and B and data not shown). A stronger body-patterning defect owing to failed cell movements during gastrulation occurs in the knypek mutation (23). Although knypek mutant animals resemble trilobite larvae, they develop normally oriented hair bundles during hair-cell development and regeneration in neuromasts (Fig. 4C and data not shown). We conclude that the planar polarity defects associated with the trilobite mutation (Fig. 4D) are due to a specific requirement for Vangl2 in neuromasts in the proper localization of basal bodies.
Fig. 4.
Characterization of hair-cell regeneration in several zebrafish mutant lines. (A–C) Gastrulation defects in zebrafish are associated with the silverblick/wnt11, pipetail/wnt5a, and knypek mutations (21–23). Labeling of neuromasts in 6-day-old larvae with rhodamine-phalloidin reveals that the hair bundles are properly orientated in pipetail (A), silberblick (B), and knypek mutant animals (C). (D) A neuromast of a trilobite mutant animal is included for comparison. The planar polarity defects of hair cells in trilobite mutants are associated with the loss of Vangl2 function specifically in neuromasts, rather than a consequence of gastrulation defects. (Scale bars: 10 μm.)
Discussion
The establishment of planar cell polarity relies on a highly conserved genetic program that defines the differentiation of two orthogonal axes in epithelial cells. Although the activity of the planar polarity pathway during embryonic development has been extensively analyzed, its function during the postembryonic maintenance of tissue architecture is not known. We report here a mechanism that controls the recovery of planar cell polarity during the regeneration of epithelial mechanoreceptors. By monitoring continuously the production of hair cells, we revealed a simple process that establishes planar cell polarity during hair-cell development and regeneration.
Throughout an animal's life, neuromasts maintain approximately half their complement of hair cells in each orientation. How does this occur? In the lateral-line system of the zebrafish, hair-cell death induces the mitotic proliferation of supporting cells (24). Although it had been suggested that the recovery of the lost sensory cells depends on this mitotic activity, it remained possible that supporting cells transdifferentiate into hair cells during neuromast repair. We find this unlikely in the present experimental circumstances for two reasons. First, each neuromast contains <30 supporting cells; if these were to transdifferentiate into hair cells without intervening mitoses, neuromasts would lose all supporting cells during the two-day period between hair-cell ablation and complete anatomical repair. We found instead that the number of supporting cells remains essentially constant during regeneration (data not shown). Second, the orderly sequence in which hair cells reappear after neomycin treatment does not reflect the widespread hair-cell regeneration that would be expected if supporting cells were to transdifferentiate into hair cells. Instead, new hair cells always are born in pairs along two rows orthogonal to the neuromast's axis of planar polarity.
The expression of GFP in ET4 larvae provides one of the earliest known molecular markers for a hair-cell fate. By continuously monitoring regeneration in ET4 transgenic animals, we were able to identify progenitors of hair cells localized at the neuromast's poles. Although the hair-cell progenitors resident in sensory epithelia have been sought for >20 years, we know of no prior demonstration of such cells. The delineation of a hair-cell progenitor in neuromasts permits the analysis of hair-cell development from its very outset, suggests the existence of a stem-cell population, and pinpoints its location within the neuromast.
We used trilobite mutant zebrafish to gain insight into the cellular bases of hair-bundle polarization during hair-cell development and regeneration. Although the migration of the lateral-line primordium and the deposition of individual neuromasts are not affected, this mutation causes a prominent defect in the orientation of hair bundles. By analyzing other mutations that affect gastrulation in zebrafish, we confirmed that the misorientation of hair bundles is not a consequence of the defective cell migration during gastrulation of trilobite larvae. The defects in planar polarization of hair cells therefore reflect specifically the loss of Vangl2 in neuromasts. By continuously monitoring the behavior of hair-cell progenitors, we showed that the randomized polarization of hair cells in trilobite mutants is unlikely to be a consequence of defects in the oriented division of the progenitors.
Other aspects of hair cells are preserved in trilobite mutants, including their shape and apicobasal polarity as revealed by the formation of hair bundles. A central role of cilia in the specification of planar cell polarity emerges from studies of Bardet–Biedl Syndrome (BBS) proteins, whose loss of function produces defective basal bodies (25) and causes polarity defects in the murine cochlea (26). Interestingly, a dominant genetic interaction between Bbs6 and Vangl2 produces rotated hair bundles in mice, and knockdown of bbs4 expression enhances the gastrulation defects in trilobite mutant zebrafish. A constituent of basal bodies and ciliary axonemes (26, but see ref. 27), Vangl2 may directly govern the behavior of basal bodies, in turn instructing the localization of kinocilia and the orientation of hair bundles. Consistent with this logic, we found that the hair cells of trilobite mutant larvae display randomly oriented basal bodies and kinocilia.
Our results indicate that the recovery of planar cell polarity during neuromast repair is achieved by the combination of two processes. First, the oriented division of progenitors consistently produces pairs of hair cells along a single axis. This step occurs independently of Vangl2. Second, the Vangl2-dependent localization of basal bodies or kinocilia adjacent to each other ensures that sibling hair cells invariably adopt opposite orientations. The combined activity of these two steps underlies the fidelity of the mechanism that governs hair-cell planar polarization during development and regeneration. Because the orientation of hair bundles ultimately determines the sensitivity of hair cells to directional mechanical stimuli, cell-replacement therapies for the treatment of human deafness would benefit from the ability to control the orientation of hair cells within the sensory epithelium. Our findings should facilitate progress toward this goal and provide an experimental system with which to explore the mechanisms that govern the long-term maintenance of tissue architecture in remodeling epithelia.
Materials and Methods
Zebrafish Strains and Husbandry.
Zebrafish were maintained under standardized conditions (28). Naturally spawned eggs were collected, cleaned, and maintained in system water at 28.5°C at a density of 50 per 85-mm Petri dish. Embryos were raised in system water and staged (28). Wild-type fish were Tübingen Long Fin (TUL) and albino. To analyze the neurogenin1, pipetail, silverblick, knypek, and trilobite mutations, we used respectively the ngn1hi1059, ppt298, slbtx226, knyhi1934, and trirw75, trim209, and trivu7 alleles (18, 21–23, 29). Transgenic and mutant combinations were generated by the intercrossing of carrier lines.
Labeling Procedures and Imaging.
For immunohistochemistry, manually dechorionated embryos or hatched larvae were fixed overnight at 4°C in a solution of 4% paraformaldehyde in PBS solution containing 1% Tween-20. After fixation, samples were washed in the same solution without fixative and blocked at room temperature with 10% BSA. Primary- and secondary-antibody incubations were conducted overnight at 4°C in PBS solution with 0.2% Tween-20. Labeling with rhodamine-phalloidin (Invitrogen, Carlsbad, CA) was accomplished during the first wash after secondary-antibody incubation, either overnight at 4°C or for 4–8 h at room temperature. Primary antibodies were used at the following dilutions: rat anti-claudin b (14), 1/250; mouse anti-acetylated α-tubulin (clone 6-11B-1; Sigma, St. Louis, MO; ref. 30), 1/1,000; and mouse monoclonal HCS1 (31, 32), 1/100. Fluorescein-labeled donkey anti-mouse and -rabbit, Texas red-labeled donkey anti-mouse, and Cy5- and Texas red-labeled donkey anti-rabbit immunoglobin secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used at 1/200.
Apoptosis was detected with the Fluorescein in situ Cell Death Detection kit according to the protocol provided by the manufacturer (Roche).
Whole-mount in situ hybridizations with antisense digoxigenin- or fluorescein-labeled RNA probes were conducted with a minor modification: Older larvae treated with anti-digoxigenin and anti-fluorescein antibodies were washed for 4 days at room temperature in PBS solution with 0.2% Tween-20. Two-color, whole-mount in situ hybridizations were performed according to Jowett (33). Probes were generated by using the T7/Sp6 digoxigenin-labeling kit (Roche).
For vital labeling of hair cells, ET4 larvae were immersed in a 100 μM solution of FM4–64 (Invitrogen) for 10 min at room temperature in the dark. Treated larvae were washed briefly to remove excess fluorophore, anesthetized in a 610-μM solution of 3-aminobenzoic acid ethyl ester (Sigma), mounted in 3% methylcellulose on a glass slide, and aligned by using a hair loop.
For live imaging, dechorionated ET4 and tri;ET4 animals at 12 h after fertilization were incubated in the dark for 12 h at 28.5°C in Bodipy-Texas red-ceramide (Invitrogen) at a final concentration of 500 μg/ml in half-strength Danieau's solution, then rinsed and kept in system water until analysis. Each larva was mounted on a glass coverslip, covered with 1% low-melting-point agarose in anesthetic solution, and imaged with a Bio-Rad 2000 confocal system. Figures were assembled by using Adobe (San Jose, CA) Photoshop and Illustrator; movies were processed by using Metamorph software.
Ablation of Hair Cells.
Hair-cell ablation was performed by treating wild-type and mutant zebrafish larvae 5 to 7 days of age with neomycin sulfate (500 μM) for 1 h at room temperature and then leaving them to recover in fish water at 28.5°C.
Scoring of Hair-Cell Orientation.
Unless otherwise noted, we conducted analyses of hair-cell orientation on L1, the first neuromast of the posterior lateral line. Actin- and acetylated α-tubulin-labeled neuromasts were imaged by using a confocal microscope. Orientations of hair cells in neuromasts were determined by drawing a line connecting the kinocilum to the opposite edge of the hair bundle. The angle between this line and a reference line parallel to the animal's body axes defined the bundle's orientation.
Supplementary Material
Acknowledgments
We thank the following colleagues for the generous provision of reagents: J. Corwin (HCS1 antibody; University of Virginia, Charlottesville, VA), V. Korzh (ET4 zebrafish line; Institute of Molecular and Cell Biology, Singapore), L. Solnica-Krezel (tri line; vangl1 and vangl2 DNA clones; Vanderbilt University, Nashville, TN), N. Hopkins (ngn1 line; Massachusetts Institute of Technology, Cambridge, MA), S. Wilson (ppt and slb lines; University College London, London, U.K.), and the Zebrafish International Resource Center (tri and kny lines; University of Oregon, Eugene, OR). We thank A. Afolalu and P. Espitia for expert fish husbandry; M. Rauf for help with the analysis of neurogenin1 mutants; J. Jessen and L. Solnica-Krezel for advice; and J. Corwin, S. Desbordes, C. Desplan, S. Heller, A. Martínez-Arias, and the members of our research group for comments on the manuscript. This research was supported by National Institutes of Health Grant DC00241. H.L.-S. was supported by a Life Sciences Research Foundation Fellowship from Howard Hughes Medical Institute, of which A.J.H. is an Investigator.
Footnotes
The authors declare no conflict of interest.
References
- 1.Uemura T, Shimada Y. J Biochem (Tokyo) 2003;134:625–630. doi: 10.1093/jb/mvg186. [DOI] [PubMed] [Google Scholar]
- 2.Klein TJ, Mlodzik M. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 3.Lewis J, Davies A. J Neurobiol. 2002;53:190–201. doi: 10.1002/neu.10124. [DOI] [PubMed] [Google Scholar]
- 4.Barald KF, Kelley MW. Development (Cambridge, UK) 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- 5.Shotwell SL, Jacobs R, Hudspeth AJ. Ann NY Acad Sci. 1981;374:1–10. doi: 10.1111/j.1749-6632.1981.tb30854.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida N, Liberman MC. Hear Res. 1999;131:29–38. doi: 10.1016/s0378-5955(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 7.Ryals BM, Rubel EW. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 8.Corwin JT, Cotanche DA. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 9.Corwin JT, Cotanche DA. J Comp Neurol. 1989;288:529–537. doi: 10.1002/cne.902880402. [DOI] [PubMed] [Google Scholar]
- 10.Cotanche DA, Corwin JT. Hear Res. 1991;52:379–402. doi: 10.1016/0378-5955(91)90027-7. [DOI] [PubMed] [Google Scholar]
- 11.Duckert LG, Rubel EW. Hear Res. 1990;48:161–182. doi: 10.1016/0378-5955(90)90206-5. [DOI] [PubMed] [Google Scholar]
- 12.Komeda M, Raphael Y. Hear Res. 1996;102:81–89. doi: 10.1016/s0378-5955(96)00150-5. [DOI] [PubMed] [Google Scholar]
- 13.Balak KJ, Corwin JT, Jones JE. J Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Schier H, Starr CJ, Kappler JA, Kollmar R, Hudspeth AJ. Dev Cell. 2004;7:401–412. doi: 10.1016/j.devcel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández PP, Moreno V, Olivari FA, Allende ML. Hear Res. 2006;213:1–10. doi: 10.1016/j.heares.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Parinov S, Kondrichin I, Korzh V, Emelyanov A. Dev Dyn. 2004;231:449–459. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- 18.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Mo C, Fraser SE. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 21.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 22.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 23.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 24.Williams JA, Holder N. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 25.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 26.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 27.Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, et al. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 29.Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Proc Natl Acad Sci USA. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddon C, Lewis J. J Comp Neurol. 1996;365:113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Gale JE, Meyers JR, Corwin JT. J Assoc Res Otolaryngol. 2000;1:172–182. doi: 10.1007/s101620010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Schier H, Hudspeth AJ. Proc Natl Acad Sci USA. 2005;102:1496–1501. doi: 10.1073/pnas.0409361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jowett T. Methods. 2001;23:345–358. doi: 10.1006/meth.2000.1147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.