Abstract
Inflammatory mediators play a criticial role in ulcerative colitis immune and inflammatory processes. The aim of the study was to investigate the effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-α, NF-κBp65, IL-6) in TNBS-induced colitis in rats. Colitis in rats was induced by colonic administration with 2,4,6-trinitrobenzene sulfonic acid (TNBS, 150 mg/kg). EGB in doses of (50, 100, 200 mg/kg) was administered for 4 weeks to protect colitis. The results showed that EGB could significantly ameliorate macroscopic and histological damage, evidently elevate the activities of SOD and reduce the contents of MDA, inhibit the protein and mRNA expressions of TNF-α, NF-κBp65, and IL-6 in the colon tissues of experimental colitis in a dose-dependent manner compared with the model group. We concluded that the probable mechanisms of EGB ameliorated inflammatory injury in TNBS-induced colitis in rats by its modulation of inflammatory mediators and antioxidation
INTRODUCTION
Ulcerative colitis (UC) and Crohn's disease (CD) are the two major categories of inflammatory bowel disease (IBD). Although the etiology and pathophysiology still remains unclear, immune dysfunction plays a crucial role in the development of UC [1]. Inflammatory mediators, including reactive oxygen species (ROS) and cytokines, contribute to the inflammatory cascade in modulating the immune system of IBD [2–4]. Proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6(IL-6) in the colonic mucosa significantly increased and antiinflammatory cytokines such as interleukin-4(IL-4) and interleukin-10(IL-10) significantly decreased in UC [5]. Of the various kinds of inflammatory mediators, TNF-α, which is induced, synthesized and secreted from macrophages, lymphocytes, and polymorphonuclear neutrophils, is regarded as the most prominent “first-line” cytokines [6, 7]. TNF-α stimulate and induce the production of other inflammatory mediators such as ROS, and it also activate oxidative stress-responsive genes which amplify and prolong inflammation [8]. Moreover, TNF-α has overlapping and synergetic activities to induce the production of nuclear factor-κB (NF-κB) and other cytokines [9].
Growing evidence demonstrated the significance of oxidative stress both in the clinical and experimental studies of UC. The increase of ROS and the impairment of antioxidant defense mechanisms were postulated to be causative factors in inflammatory diseases [10]. Excessive production of ROS in mucosal cells induced inflammatory and immune responses which could directly or indirectly cause damage of intestinal epithelial cells, subsequently influence mucosal integrity or initiate an inflammatory signaling cascade and lead to severe impairment in experimental colitis [11, 12]
Furthermore, oxidative stress and TNF-α could activate and induce NF-κB. NF-κB existed in the cytoplasm in an inactive form by virtue of its association with IκBs. Once activated, NF-κB translocated to the nucleus from the cytoplasm, then activated the consensus sequence related gene, including TNF-α, IL-6, IL-2, IL-8, ICAM-1, and so forth, involved in immune and inflammatory responses [13, 14]. On the other hand, elevated TNF-α might be a positive-feedback signal that triggered NF-κB reactivation. Eventually, the inflammatory reaction of the UC was amplified and perpetuated by the pathogenic cascade.
Therapy of UC is difficult on account of the complex etiology of disease. As a naturally remitting and recurring disease, the patients with IBD run a higher risk to develop colorectal cancer than the average population. Although therapeutic drugs such as 5-aminosalicylic acid (5-ASA), sulfasulfapyridine (SASP), and glucocorticoids could inhibit the inflammatory mediators through different mechanisms which engaged in the down-regulation of the immune and inflammatory responses of IBD [15, 16], their adverse reactions during prolonged treatment and high relapse rate limited their use. It is important that effective drugs with fewer adverse reactions should be developed to prevent UC from initiating and relapsing.
Ginkgo biloba extract (EGB), a natural antioxidant, is an extract from green leaves of the Ginkgo biloba tree. The main ingredients of EGB contain 24% ginkgo-flavone glycosides and 6% terpenoids. It is well known for its cheap prices and negligible side effects. EGB has various biological activities and different pharmacologic effects, including antioxidation, antiinflammatory and modulation of immune response. For its few side effects, EGB is extensively used in the therapy of central neural system disorders, acute pancreatitis, myocardial, and intestine ischemia/reperfusion injury which are associated with inflammatory mediators [17–20].
The mechanism that EGB affords protection against ulcerative colitis remains obscure. This study was designed to determine the probable mechanisms of EGB in ameliorating inflammatory injury in TNBS-induced colitis in rats, and to investigate its effects on the production of inflammatory mediators involved in the immune and inflammatory responses, including Superoxide dismutase (SOD), malondialdehyde (MDA), TNF-α, NF-κBp65 and IL-6. The effects of EGB on colonic inflammation and macroscopic and histological damage were evaluated as well.
MATERIALS AND METHODS
Animals
Purebred male Wistar rats (180 ± 20 g) were purchased from the Experimental Animal Center of Hubei Province (Wuhan, China). The rats were allowed to adapt to our laboratory environment for one week before beginning the experiment. They were housed in standard cages with free access to tap water and maintained in a room under standard conditions of feeding and temperature with a 12 h : 12 h light-dark cycle. This animal study was approved by the Ethical and Research Committee of the Wuhan University Medical School.
Experimental design
These rats were randomly divided into six groups of 12 each, normal group (N group), model group (M group), 5-aminosalicylic acid(5-ASA group), low-dose EGB group (LD group), middle-dose EGB group (MD group) and high-dose EGB group (HD group). Rat model of colitis was induced with 2,4,6-trinitrobenzene sulfonic acid (TNBS, Sigma Co, China) enema by using a technique modified from that of Morris et al [21]. Briefly, rats were fasted for 24 hours with access to water ad libitum and observed to ensure health before induction of colitis. The rats were lightly anesthetized with ether. A flexible plastic rubber catheter with an outside diameter of 2 mm was inserted 8 cm into the colon via the anus. TNBS (150 mg/kg) dissolved in 50% (vol/vol) ethanol (to break the intestinal barrier) was injected into the colon. Injection with 50% ethanol instead of TNBS served as a negative control in N group. All other rats received TNBS enema on day 0. EGB was mixed with saline to a 0.01 g/mL suspension. The drug-treated groups received 5-ASA (Guoyi Pharmaceutical Ltd, China) in a dose of 100 mg/kg and EGB (Guizhou Xinbang Pharmaceutical Company, China) in doses of 50, 100 and 200 mg/kg by stomach, respectively (once a day, from the 24 hours after colitis was established to the end of experiment). In the normal and model groups of rats, saline was given instead of 5-ASA and EGB, respectively. At the end of a four-week period, rats were killed using ether anesthesia and colonic biopsies were taken for macroscopic scoring, histopathological examination and biochemical studies.
Assessment of colon macroscopic and histological damage
Two pathologists who were unaware of the treatment conditions recorded the macroscopic and histological damage. The tissue of colon 8 cm proximal to anus was excised, opened longitudinally, and washed in saline buffer. The criteria of the macroscopic score used a previously validated scoring system from 0 to 4 that depends on the number and size of ulcers as well as the presence or absence of adhesions [21, 22].
Colon tissue was fixed in 4% paraformaldehyde, dehydrated, and paraffin embedded, processed, and sectioned in 4 μm thick sections, and stained with haematoxylin and eosin. The assessment criteria of the histological score was according to previous literature [21, 22]: (1) the infiltration of acute inflammatory cells: 0 = no, 1 = mild increasing, 2 = severe increasing; (2) the infiltration of chronic inflammatory cells: 0 = no, 1 = mild increasing, 2 = severe increasing; (3) the deposition of fibrotin protein: 0 = negative, 1 = positive; (4) the submucosa edema: 0 = no, 1 = patchy-like, 2 = fusion-like; (5) the epithelium necrosis: 0 = no, 1 = limiting, 2 = widening; (6) the epithelium ulcer: 0 = negative, 1 = positive. The ulceration, inflammation, lesion and fibrosis were scored and put together as a result ranging between the minimum of 0 and maximum of 10.
Determination of colonic SOD and MDA levels
The colon tissue was rinsed and weighed, then put into tubes with 9 volumes of 9 g/L normal saline. Then the tissue samples were homogenized for 10 minutes. After centrifugation at 4000 r/min for 10 minutes at 4 °C, the MDA contents and SOD activities in the supernatant were measured by the assay kit (Nanjing Jincheng Corp, China) according to its provider's instructions.
Immunohistochemistry
Sections of colon tissues were deparaffinized in xylene and hydrated in a series of graded alcohol. After dewaxing and rehydration, the antigen retrieval was done by microwave for 15 minutes. Sections were immersed in 3% hydrogen peroxide in methanol for 20 minutes at room temperature to abolish endogeneous peroxidase activities and then they were blocked with normal goat serum at 37°C for 15 minutes. Slides were incubated with polyclonal antibody of TNF-α (diluted to 1 : 100, Santa Cruz Biotechnology) at 37°C for 60 minutes. After PBS washing, the slides were incubated with a biotinylated horse peroxidase-conjugated secondary antibody and 0.1% DAB substrate, using the standard streptavidin-biotin-based method. Incubation with PBS instead of the primary antibody served as a negative control. The positive cells were observed and evaluated by two independent observers. A cytoplasmic brown granule was marked as a positive expression of TNF-α. The results were evaluated semiquantitatively according to the percentage of positive cells in ten randomly selected fields under high-power microscope (400-fold magnification) for each sample.
Western blotting
Nuclear protein was extracted as described by Huang et al [23] with some modification. Briefly, fresh colon tissue was pounded to pieces in liquid nitrogen, then minced and homogenized in 400 μL of hypotonic lysis buffer (10 mmol/L HEPES pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L DTT, 1 mmol/L PMSF, 1 μg/mL aprotinin, 1 μg/mL pepstatin and 1 μg/mL leupeptin). The samples were separated by centrifugation (0 °C, 2000 r/min, 15 seconds) and the supernatant was incubated in ice water for 5 minutes, and then spun by centrifugation (0 °C, 5000 r/min, 10 seconds) again. The cytoplasmic proteins were removed and the pellet nuclei were resuspended in 100 μL buffer (20 mmol/L HEPES pH 7.9, 420 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 1 mmol/L PMSF, 1 μg/mL aprotinin, 1 μg/mL pepstatin and 1 μg/mL leupeptin). The deposits were incubated in ice water for 20 minutes. Finally, the samples were separated by centrifugation (0 °C, 12 000 r/min, 15 minutes), the supernatants were collected as nuclear extracts in aliquots and stored at −80°C for western blotting of NF-κBp65. Protein concentrations were determined by Bradford assay.
Tissue lysates were prepared by sonicating tissues in lysis buffer (50 mmol/L Tris-HCl buffer, pH 8.0, 150 mmol/LNaCl, 1 mmol/L EDTA, 1%NP-40, 1%SDS, 0.02% Sodium Azide containing 1 g/mL aprotinin, 1 g/mL leupeptin, and 0.2 mmol/L PMSF) and centrifuged at 12 000 r/min for 10 minutes at 4°C, the supernatants were collected and stored at −80°C for Western blotting of TNF-α and IL-6. Protein concentrations were determined by Bradford assay. Samples containing equal amounts of protein (40 μg/lane) were separated on a denaturing 12% polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked in 5% Blotto in TBS +0.1% Tween 20, and incubated with anti-TNF-α, anti-NF-κBp65, anti-IL-6, and anti-β-actin rabbit polyclonal antibodies (diluted to 1 : 500, 1 : 500, 1 : 500, and 1 : 1000, resp, Santa Cruz Biotechnology). The membranes were then treated with horseradish peroxidase-conjugated secondary antibodies (diluted to 1 : 2000, Santa Cruz Biotechnology). Detection of antibody binding was done using the Western blotting Luminol Reagent kit (Santa Cruz Biotechnology) using the appropriate secondary antibody supplied with the kit. The membranes were exposed to Kodak X-ray film and developed accordingly. The bands were quantified by the average ratios of integral optic density (IOD) of TNF-α/β-actin, NF-κBp65/-β-actin and IL-6/β-actin, respectively.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from a fresh colon sample with Trizol reagent (Invitrogen Life Technologies Co Ltd, USA.) following the manufacturer's protocol. An aliquot of total RNA was reverse transcribed and amplified using MMLV and Taq DNA polymerase (Promega, Southampton, UK), respectively. The conditions of the PCR amplification were 3 minutes at 94°C for one cycle, 30 seconds at 94°C, 30 seconds at 50°C and 45 seconds at 72°C for 35 cycles, 7 minutes at 72°C for one cycle. The sequences of primers of NF-κBp65 (493 bp) were: sense, 5′-AAGATCAATGGCTACACGGG-3′, antisense, 5′-CCTCAATGTCTTCTTTCTGC-3′; the primer for TNF-α (357 bp) sense, 5′-GCCAATGGCATGGATCTCAAAG-3′, antisense, 5′-CAGAGCAATGACTCCAAAGT-3′; the primer for IL-6(557 bp) sense, 5′-TCTCTCCGCAAGAGACTTCC-3′, antisense, 5′-TCTTGGTCCTTAGCCACTCC-3′; the primer for GAPDH (308 bp) sense, 5′-TCCCTCAAGATTGTCAGCAA-3′, antisense, 5′-AGATCCACAACGGATACATT-3′. Each PCR products were electrophoresed in 2% agarose gel and stained with ethidium bromide. The intensities of the specific bands were analyzed to determine whether the differences were statistically significant.
Statistical analysis
The results were presented as mean ± standard deviation. Statistical analysis was performed with SPSS 11.5 statistical software. One way analysis of variance and Student Newman Keuls Test were used for data analysis. Differences were considered significant if P < .05.
RESULTS
Macroscopic presentation and histological evaluation
As shown in Table 1, macroscopic presentation of the colon after TNBS treatment revealed colonic mucosal hyperaemia, oedema, erosion, and some small punctate ulceration.
Table 1.
Effects of EGB on the macroscopic and histological damage scores and the levels of SOD and MDA of colon tissues in rats. The results were expressed as means ± SD (n = 12).
| Group | Macroscopic score | Histological score | SOD (U/mg) | MDA (nmol/mg) |
| N | 0.00 ± 0.00 | 0.33 ± 0.49 | 41.51 ± 8.28 | 2.51 ± 0.44 |
| M | 3.67 ± 0.49## | 7.17 ± 1.26## | 22.04 ± 3.26## | 6.62 ± 0.80## |
| 5-ASA | 2.33 ± 0.49**## | 4.50 ± 1.00** | 27.10 ± 2.87**## | 4.77 ± 0.60**## |
| LD | 3.33 ± 0.49## | 6.42 ± 1.4## | 26.63 ± 3.68*## | 5.87 ± 0.96*## |
| MD | 1.67 ± 0.65**## | 3.75 ± 0.87**## | 31.58 ± 2.98**## | 4.49 ± 0.79**## |
| HD | 1.25 ± 0.45**## | 2.25 ± 0.87**## | 39.33 ± 3.67** | 3.70 ± 0.79**## |
* denotes that P < .05 versus the model group.
** denotes that P < .01 versus the model group.
# denotes that P < .05 versus the normal group.
## denotes that P < .01 versus the normal group.
Conglutination was obvious later. No changes could be observed in N group. Treatment with 5-ASA reduced the intensity of the macroscopic score. Treatment with Ginkgo biloba extract significantly reduced the severity of gross lesion score in a dose dependent manner. On the other hand, EGB in the small dose used had no significant effect while the higher doses used had a significant effect on the intensity of inflammatory response.
No histological damage was seen in N group. Rats with TNBS-induced colitis showed a number of neutrophils, macrophages, lymphocytes and eosinophil infiltration in mucosa and submucosa. Ulceration and mucosal damage were obviously seen. Fibroblasts and granuloma-like structures were also seen. Treatment with 5-ASA significantly attenuated the extent and severity of the histological signs. Administration with EGB at different dosage (50, 100, and 200 mg/kg) could inhibit the extents of inflammation, prevent the mucosa injury, minimize the ulceration area, and alleviate the colitis compared with that in model control animals. The inhibition effect was most obvious with EGB at the dose of 200 mg/kg (Table 1, Figure 1).
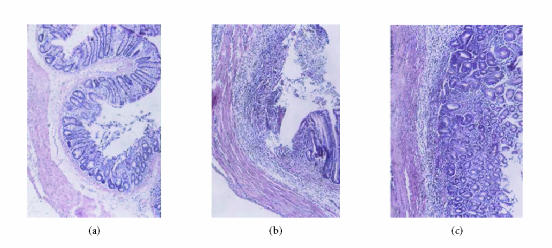
Figure 1.
The haematoxylin and eosin staining of colon tissues: (a) no damage in N group (H&E, ×100); (b) the changes of histology with M group, the colonic mucosa showed necrotic destruction of epithelium, hemorrhage, edema, inflammatory cellular infiltration and ulceration at mucous and submucous layers (H&E, ×100); (c) the changes of histology with HD EGB (200 mg/kg) group (H&E, ×100).
Determination of colonic SOD, MDA
Compared with the N group, the activities of SOD notably decreased and the contents of MDA significantly increased in the M group (P < .01). Administration with 5-ASA and EGB (LD, MD, and HD) could elevate the activities of SOD and reduce the contents of MDA in a dose-dependent manner. The change was most significant in the HD group (P < .05 or P < .01, Table 1).
Immunohistochemistry
The expressions of TNF-α in the M group significantly increased compared with the N group. The positive cells of TNF-α predominantly located within the mucosa and mucosa lamina propria with brown-yellow cytoplasm. Administration of 5-ASA resulted in a significant reduction of colon TNF-α levels. Compared with the M group, expressions of TNF-α in EGB group weakened significantly in a dose-dependent manner, and the expression in HD group was the lowest (P < .01, Figures 2 and 3).
Figure 2.

Immunohistochemical staining for TNF-α: (a) expression of TNF-α in the N group (brown staining, SP ×400), (b) expression of TNF-α in the M group (brown staining, SP ×400), (c) expression of TNF-α in the HD EGB group (brown staining, SP ×400).
Figure 3.
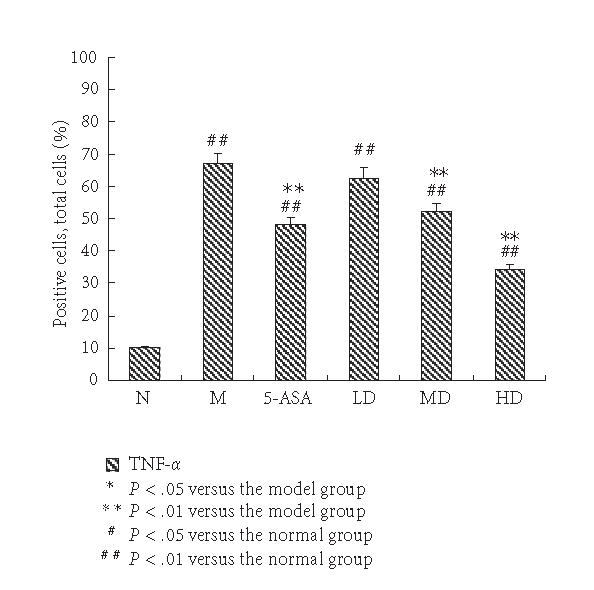
Expression of TNF-α immunohistochemical staining sections in each group. The percentage of positive cells in ten randomly selected fields under high-power microscope (400-fold magnification) for each sample. The expression of TNF-α in the N group was weak; it was elevated significantly in the M group. EGB treatment suppressed the expression of TNF-α in a dose-dependent manner to some degree, but still higher than normal group. The results were expressed as means ± SD.
Western blotting
The Western blotting results showed that the expressions of TNF-α, NF-κBp65 and IL-6 in the M group were significantly increased compared with N group (P < .01). EGB treatment decreased the expressions of TNF-α, NF-κBp65 and IL-6 in a dose-dependent manner to some degree, 5-aminosalicylic acid reduced the expression, too. EGB in a dose of 200 mg/kg was the most effective in reducing TNF-α, NF-κBp65, and IL-6 levels. (P < .01, Figures 4 and 5). These experiments were performed six times with similar results.
Figure 4.
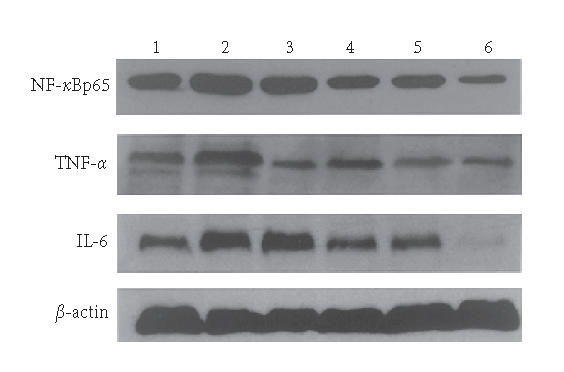
Western blotting showed levels of TNF-α, NF-κBp65 and IL-6 in colon tissue of rats. Lane 1: the N group, Lane 2: the M group, Lane 3: the 5-ASA group, Lane 4: the LD group, Lane 5: the MD group, and Lane 6: the HD group.
Figure 5.
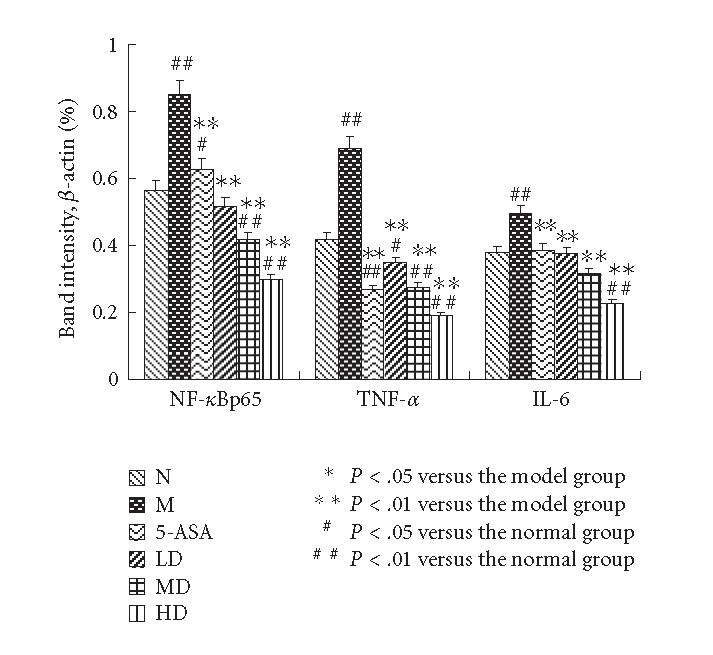
The Western blotting results of TNF-α, NF-κBp65, and IL-6 were measured by average ratios of TNF-α/β-actin, NF-κBp65/β-actin and IL-6/β-actin, respectively. The levels of TNF-α, NF-κBp65, and IL-6 of the M group showed a significantly high expression compared with normal group. EGB treatment decreased the increase in a dose-dependent manner. The results were expressed as means ± SD.
RT-PCR
The mRNA levels of TNF-α, NF-κBp65 and IL-6 of the M group showed a significantly high expression compared with N control (P < .01). TNF-α, NF-κBp65, and IL-6 mRNA expressions were inhibited dose dependently when animals were treated with EGB and 5-ASA. Maximum inhibition effect was observed with EGB at a concentration of 200 mg/kg. These results are in accord with Western blotting analysis of protein expression (P < .01, Figures 6 and 7). Data is representative of colons from at least six rats.
Figure 6.
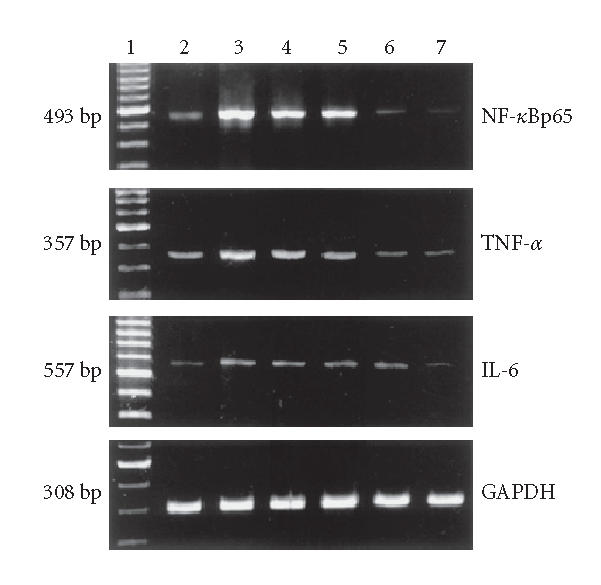
TNF-α, NF-κBp65, IL-6, and GAPDH mRNA expressions in colon tissues. Lane 1: 100 bp marker, Lane 2: the N group, Lane 3: the M group, Lane 4: the 5-ASA group, Lane 5: the LD group, Lane 6: the MD group, and Lane 7: the HD group.
Figure 7.
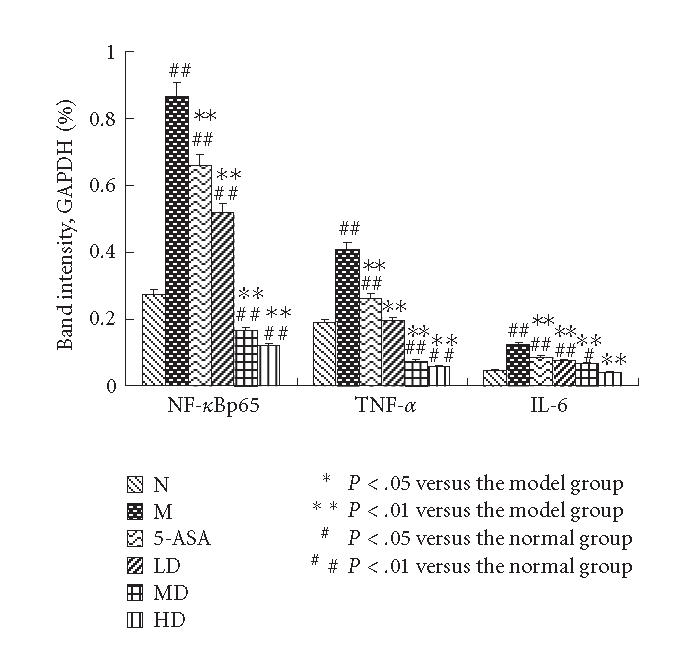
The mRNA expressions of TNF-α, NF-κBp65, and IL-6 were measured by average ratios of TNF-α/GAPDH, NF-κBp65/GAPDH, and IL-6/GAPDH, respectively. RT-PCR analysis showed increased TNF-α, NF-κBp65, and IL-6 mRNA levels in colon tissues of the M group; EGB could reduce the mRNA expressions dose dependently, which were consistent with the Western blot analysis. The results were expressed as means ± SD.
DISCUSSION
The present study demonstrated clearly that EGB in a dose-dependent manner affected the release of inflammatory mediators which resulted in a remarkable improvement of inflammation injury in TNBS-induced colitis in rats. EGB had a basic character, antioxidant properties and antiinflammatory functions, and these effects most probably contributed to its therapy of the ulcerative colitis. Compared with the model group, treatment with EGB for 4 weeks significantly reduced colon macroscopic and histological damage in a dose-dependent manner.
TNBS-induced colitis was widely adopted to observe the effects of drugs because of its similarity to human IBD and the availability of a quantitative scoring system. It was characterized by oxidative stress, mucosal infiltration by polymorphonuclear cells, at least in part, the activation of TNF-α and IL-6 and activation of the NF-κB pathway, led to inflammation cascade effects and tissue damage [7, 24].
Oxidative stress and its consequent lipid peroxidation could aggravate free radicals chain reactions, disrupt the integrity of intestinal mucosa barrier, and activate inflammatory mediators. It has been shown that colonic MDA contents increased and colonic SOD Levels decreased both in human and experimental animal studies [25, 26]. The levels of MDA were often used as an indication of oxidative damage and as a marker for free radicals-induced lipid peroxidation. SOD, a primary defense, could reduce the oxidative stress and the activation of inflammatory mediators. Segui et al [27] reported that administration of SOD significantly reduced lipid peroxidation, recruited leukocytes into the inflamed intestine and ameliorated colonic inflammatory in UC. Some previous data have showed that the levels of MDA were decreased and SOD were increased by antioxidant and antiinflammatory agents in UC [28]. EGB acting as free radical scavenger has been shown to have an SOD-like activity. EGB could counteract the function of ROS, directly scavenge superoxide anion, hydroxyl radicals, peroxyl radical species, and nitric oxide [18, 29]. EGB has been reported to enhance the activities of SOD to decrease lipidperoxidation in liver fibrosis induced by carbon tetrachloride (CCl4) [30]. In our study, compared with the N group, the Levels of SOD in colon tissue dwindled, while MDA contents improved remarkably in the M group. Therapy with EGB for 4 weeks resulted in a marked increase in SOD and decrease in MDA in colon tissue in a dose-dependent manner. Our results suggested that EGB successfully inhibited lipid peroxidation induced by TNBS. EGB provided protective effects in UC probably by the radical scavenging and antioxidant properties. This may be an important and underlying mechanism of EGB protection against UC.
As the most important cytokine in “inflammation cascade” of UC, TNF-α stimulated the synthesis of oxygen free radicals and IL-6, IL-1, NO and other inflammatory mediators, activated leucocytes, promoted inflammatory cells migration in the intercellular matrix, thus amplified the inflammatory response by activating a cascade of immune cells [7]. So TNF-α may represent a potential target for down-regulating the immune and inflammatory responses. Oxidative stress was a major factor-activated local inflammation during inflammatory process. Intestinal inflammation was considered a consequence of an imbalance between prooxidant and antioxidant mechanisms [31]. Gossart et al [32] confirmed that ROS was an early cause of injury and pretreatment with free radical scavengers resulted in a decreased expression of TNF-α in rat's lung inflammation. Considering our immunohistochemical observation, Western blotting analysis, and RT-PCR results, we found that the expression of TNF-α significantly increased in the TNBS model group and significantly reduced in the EGB group in a dose-dependent manner both at mRNA and protein levels. Our study was consistent with the results described by Mustafa et al [33], who found that EGB resulted in a significant reduction in colonic TNF-α levels of colitis rats. They have shown that EGB could suppress the synthesis, release, and biological activity of TNF-α in colitis at least in part by scavenging ROS. Besides a direct scavenging effect on active oxygen species, EGB could exert an antiinflammatory effect by suppressing the production of active oxygen and nitrogen species [34]. Our study demonstrated that EGB had marked antiinflammatory action in addition to its antioxidation properties.
Oxygen free radicals and TNF-α released during inflammation could activate NF-κB, a redox-sensitive transcription factor, and could activate the subsequent inflammatory cascade in TNBS-induced colitis in rats, and this process could be inhibited by EGB. The precise mechanism in which EGB decreased the levels of NF-κB has not been completely elucidated. Although the interaction between ROS on the NF-κB signaling pathway has not been completely defined, it has been suggested that NF-κB could be activated by oxidative stress and inhibited by antioxidants and radical scavengers such as vitamin E derivatives, N-acetyl-cysteine, pyrrolidine dithiocarbamate, and curcumin [35, 36]. All these results indicated that ROS was involved in NF-κB activation process. Schreck et al [37] indicated that ROS could serve as messengers that directly or indirectly caused the release of IκB from the p50-p65-IκB complex which had been implicated in the activation of NF-κB. Wei et al [38] found that EGB could inhibit H2O2-induced activation of NF-κB owing to its antioxidant properties by directly scavenging H2O2 and elevating intracellular GSH levels. TNF-α could trigger degradation of IκB, the inhibitor of NF-κB, then NF-κB especially p65 translocated to the nucleus to activate related gene. Chen et al [39] have showed that EGB could inhibit TNF-α-induced reactive oxygen species generation and NF-κB activation in human aortic endothelial cells.
The important discovery of this study was that antiinflammatory effect of EGB may be linked with down-regulation in NF-κBp65 activity in TNBS induced colitis in rats. In our study, Western blotting and RT-PCR results showed that increasing expression of NF-κBp65 in TNBS induced colitis and decreased significantly in groups with continuous 4-week treatment with EGB in a dose-dependent manner. So we concluded that EGB decreased the activation of NF-κBp65 by serving as antioxidants. On the other hand we found that EGB could suppress NF-κBp65 production in the colitis through inhibited TNF-α-induced NF-κBp65 activation in colonic mucosa. Then EGB could curb the inflammatory cascade effects of inflammatory mediators to protect UC.
To further elucidate the mechanism of EGB in UC, we observed the levels of IL-6, downstream pathway of NF-κBp65. IL-6 could stimulate neutrophil chemotaxis and relate to the presence of necrosis in the colon which led to tissue destruction. Our experiment indicated that the levels of IL-6 in rat TNBS colitis increased more distinctly than normal group and declined more obviously after treatment with EGB in response to TNF-α and NF-κB. Since the promoter regions of IL-6 had also been shown to contain consensus-binding motifs for NF-κB. We presumed that the reduced of IL-6 by EGB might be through modulation of NF-κB.
UC seriously affected the patient's quality of life in that no specific treatment was available and it had a high recurrence rate. With the development of immunology and molecular biology, anti-TNF-α antibodies and antisense oligonucleotides against NF-κB and anti-IL-6R mAb were effective tools in the treatment of colitis. But their price was expensive and the safety need to be further identified [24, 40, 41]. Thus, EGB, natural antioxidant, was safe and cheap, and it provided an interesting alternative approach for the treatment of UC.
Although the antioxidant properties and antiinflammatory effects of EGB could exert a beneficial effect in UC, it was probable that additional mechanisms were also involved. Cheng et al [42] have found that EGB efficiently blocked several cytokines, including TNF-α production. It was likely to be mediated through the down-regulation of JNK-AP-1 signaling path in vitro experiment. Future studies from our group will investigate the upstream pathways of TNF-α and NF-κBp65 in order to elucidate the active principles and underlying molecular mechanisms.
In conclusion, the present results demonstrated that EGB dose dependently exerted a beneficial effect in the TNBS-induced colitis in rats. As a possible mechanism EGB could scavenge oxidative-free radicals, down-regulate some of the inflammatory mediators involved in the intestinal immune and inflammatory responses, including TNF-α, NF-κBp65 and IL-6 resulting in the improvement of UC.
We suggest that EGB alone or in combination with other drugs is a promising agent for the treatment of ulcerative colitis or is used as a dietary supplement for prevention from the recurrence of UC. Further sufficient preclinical and clinical studies should be conducted to prove it.
ACKNOWLEDGMENTS
This study was supported by Grant from the Foundation of Department of Education of Hubei Province of China (no B200528003) and supported in part by National Natural Science Foundation of China (no 30470782). The first author is employed in Xianning University now.
References
- 1.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. American Journal of Gastroenterology. 1997;92(suppl 12):5S–11S. [PubMed] [Google Scholar]
- 2.Nieto N, Torres MI, Fernandez MI, et al. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Digestive Diseases and Sciences. 2000;45(9):1820–1827. doi: 10.1023/a:1005565708038. [DOI] [PubMed] [Google Scholar]
- 3.Wendland BE, Aghdassi E, Tam C, et al. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. American Journal of Clinical Nutrition. 2001;74(2):259–264. doi: 10.1093/ajcn/74.2.259. [DOI] [PubMed] [Google Scholar]
- 4.Murata Y, Ishiguro Y, Itoh J, Munakata A, Yoshida Y. The role of proinflammatory and immunoregulatory cytokines in the pathogenesis of ulcerative colitis. Journal of Gastroenterology. 1995;30(suppl 8):56–60. [PubMed] [Google Scholar]
- 5.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World Journal of Surgery. 1998;22(4):382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 6.Reinecker H-C, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-α, IL-6, and IL-1β by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clinical and Experimental Immunology. 1993;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neurath MF, Fuss I, Pasparakis M, et al. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. European Journal of Immunology. 1997;27(7):1743–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 8.Larrick JW, Wright SC. Cytotoxic mechanism of tumor necrosis factor-α. FASEB Journal. 1990;4(14):3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- 9.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 10.Han SN, Meydani SN. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. Journal of Infectious Diseases. 2000;182(suppl 1):S74–S80. doi: 10.1086/315915. [DOI] [PubMed] [Google Scholar]
- 11.Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants as novel therapy in a murine model of colitis. Journal of Nutritional Biochemistry. 2005;16(5):297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Kurutas EB, Cetinkaya A, Bulbuloglu E, Kantarceken B. Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediators of Inflammation. 2005;2005(6):390–394. doi: 10.1155/MI.2005.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldwin AS., Jr Series introduction: the transcription factor NF-κB and human disease. The Journal of Clinical Investigation. 2001;107(1):3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogler G, Brand K, Vogl D, et al. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115(2):357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 15.Joshi R, Kumar S, Unnikrishnan M, Mukherjee T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: mechanistic aspects and antioxidant activity. Free Radical Research. 2005;39(11):1163–1172. doi: 10.1080/10715760500177880. [DOI] [PubMed] [Google Scholar]
- 16.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of N NF-κB activity through induction of IkB synthesis. Science. 1995;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 17.Pehlivan M, Dalbeler Y, Hazinedaroglu S, et al. An assessment of the effect of Ginkgo biloba egb 761 on ischemia reperfusion injury of intestine. Hepato-Gastroenterology. 2002;49(43):201–204. [PubMed] [Google Scholar]
- 18.Maitra I, Marcocci L, Droy-Lefaix MT, Packer L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochemical Pharmacology. 1995;49(11):1649–1655. doi: 10.1016/0006-2952(95)00089-i. [DOI] [PubMed] [Google Scholar]
- 19.Kusmic C, Basta G, Lazzerini G, Vesentini N, Barsacchi R. The effect of Ginkgo biloba in isolated ischemic/reperfused rat heart: a link between vitamin E preservation and prostaglandin biosynthesis. Journal of Cardiovascular Pharmacology. 2004;44(3):356–362. doi: 10.1097/01.fjc.0000137164.99487.42. [DOI] [PubMed] [Google Scholar]
- 20.Zeybek N, Gorgulu S, Yagci G, et al. The effects of gingko biloba extract (EGb 761) on experimental acute pancreatitis. Journal of Surgical Research. 2003;115(2):286–293. doi: 10.1016/s0022-4804(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 21.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. [PubMed] [Google Scholar]
- 22.Mei Q, Yu J-P, Xu J-M, Wei W, Xiang L, Yue L. Melatonin reduces colon immunological injury in rats by regulating activity of macrophages. Acta Pharmacologica Sinica. 2002;23(10):882–886. [PubMed] [Google Scholar]
- 23.Huang Y, Johnson KR, Norris JS, Fan W. Nuclear factor-κB/IκB signaling pathway may contribute to the mediation of paclitaxel-induced apoptosis in solid tumors cells. Cancer Research. 2000;60(16):4426–4432. [PubMed] [Google Scholar]
- 24.Neurath MF, Pettersson S, Meyer zum Buschenfelde K-H, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates established experimental colitis in mice. Nature Medicine. 1996;2(9):998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 25.Girgin F, Karaoglu O, Erkus M, et al. Effects of trimetazidine on oxidant/antioxidant status in trinitrobenzenesulfonic acid-induced chronic colitis. Journal of Toxicology and Environmental Health - Part A. 2000;59(8):641–652. doi: 10.1080/009841000156637. [DOI] [PubMed] [Google Scholar]
- 26.Verspaget HW, Pena AS, Weterman IT, Lamers CBHW. Diminished neutrophil function in Crohn's disease and ulcerative colitis identified by decreased oxidative metabolism and low superoxide dismutase content. Gut. 1988;29(2):223–228. doi: 10.1136/gut.29.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segui J, Gil F, Gironella M, et al. Down-regulation of endothelial adhesion molecules and leukocyte adhesion by treatment with superoxide dismutase is beneficial in chronic immune experimental colitis. Inflammatory Bowel Diseases. 2005;11(10):872–882. doi: 10.1097/01.mib.0000183420.25186.7a. [DOI] [PubMed] [Google Scholar]
- 28.Kuralay F, Yildiz C, Ozutemiz O, et al. Effects of trimetazidine on acetic acid-induced colitis in female swiss rats. Journal of Toxicology and Environmental Health - Part A. 2003;66(2):169–179. doi: 10.1080/15287390306402. [DOI] [PubMed] [Google Scholar]
- 29.Sakarcan A, Sehirli O, Velioglu-Ovunc A, et al. Ginkgo biloba extract improves oxidative organ damage in a rat model of thermal trauma. Journal of Burn Care and Rehabilitation. 2005;26(6):515–524. doi: 10.1097/01.bcr.0000185115.17261.50. [DOI] [PubMed] [Google Scholar]
- 30.Liu S-Q, Yu J-P, Chen H-L, Luo H-S, Chen S-M, Yu H-G. Therapeutic effects and molecular mechanisms of Ginkgo biloba extract on liver fibrosis in rats. American Journal of Chinese Medicine. 2006;34(1):99–114. doi: 10.1142/S0192415X06003679. [DOI] [PubMed] [Google Scholar]
- 31.Cruz T, Galvez J, Ocete MA, Crespo ME, Sanchez de Medina L-HF, Zarzuelo A. Oral administration of rutoside can ameliorate inflammatory bowel disease in rats. Life Sciences. 1998;62(7):687–695. doi: 10.1016/s0024-3205(97)01164-8. [DOI] [PubMed] [Google Scholar]
- 32.Gossart S, Cambon C, Orfila C, Seguelas M-H. Reactive oxygen intermediates as regulators of TNF-α production in rat lung inflammation induced by silica. Journal of Immunology. 1996;156(4):1540–1548. [PubMed] [Google Scholar]
- 33.Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacological Research. 2006;53(4):324–330. doi: 10.1016/j.phrs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Ilieva I, Ohgami K, Shiratori K, et al. The effects of Ginkgo biloba extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Experimental Eye Research. 2004;79(2):181–187. doi: 10.1016/j.exer.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Karin M. Is NF-κB the sensor of oxidative stress? FASEB Journal. 1999;13(10):1137–1143. [PubMed] [Google Scholar]
- 36.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB Journal. 1996;10(7):709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 37.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO Journal. 1991;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Z, Peng Q, Lau BHS, Shah V. Ginkgo biloba inhibits hydrogen peroxide-induced activation of nuclear factor κ B in vascular endothelial cells. General Pharmacology. 1999;33(5):369–375. doi: 10.1016/s0306-3623(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 39.Chen J-W, Chen Y-H, Lin F-Y, Chen Y-L, Lin S-J. Ginkgo biloba extract inhibits tumor necrosis factor-α-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(9):1559–1566. doi: 10.1161/01.ATV.0000089012.73180.63. [DOI] [PubMed] [Google Scholar]
- 40.Videla S, Garcia-Lafuente A, Antolin M, et al. Antitumor necrosis factor therapy in rat chronic granulomatous colitis: critical dose-timing effects on outcome. Journal of Pharmacology and Experimental Therapeutics. 1998;287(3):854–859. [PubMed] [Google Scholar]
- 41.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. Journal of Immunology. 2000;164(9):4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 42.Cheng S-M, Yang S-P, Ho L-J, et al. Down-regulation of c-jun N-terminal kinase-activator protein-1 signaling pathway by Ginkgo biloba extract in human peripheral blood T cells. Biochemical Pharmacology. 2003;66(4):679–689. doi: 10.1016/s0006-2952(03)00388-5. [DOI] [PubMed] [Google Scholar]