Abstract
Forty Wistar rats were divided into 5 groups, including the control group, the acute pancreatitis group (AP group, induced by intraperitoneal injections of caerulein), and the AP group treated with baicalin, the AP group treated with LPS, and the AP group treated with LPS and baicalin. Pathological damage of pancreatic tissue was scored with hematoxylin and eosin (HE) staining. The mRNA expression of TNF-α was measured with semiquantitative RT-PCR, and activation of NF-κB was detected with flow cytometry assay. It was shown in the results that the expression of TNF-α mRNA, activation of NF-κB, and pathological score of AP group were all obviously higher than those of control group (P < .01). In AP group treated with LPS, further rise of these values were observed (P < .01). In the AP group treated with baicalin, activation of NF-κB decreased (P < .05), and expression of TNF-α mRNA also obviously decreased (P < .01), while pancreatic pathological damage was alleviated at the same time (P < .01); similar results were observed in AP group treated with LPS and baicalin (P < .01), which indicated that baicalin might be applied to inhibit NF-κB activating and TNF-α expressing so as to treat AP.
INTRODUCTION
Acute pancreatitis (AP) is characteristic of serious pathogenetic condition, with high mortality, and there is still no breakthrough in treatment. Common mortal causes of AP include systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), and so forth, which cannot be sufficiently explained with the traditional pancreatic autodigestion theory. It has been proved by a series of experiments in recent years that several inflammatory mediators (such as IL-1, IL-6, and TNF-α, etc) are related to the local and systemic tissue damages [1], and activation of these mediators are regulated by nuclear factor-κB (NF-κB) [2]. NF-κB is a kind of DNA binding protein, taking part in various kinds of inflammatory and immune responses by adjusting gene expression of cytokine and adhesion molecule [3, 4]. Research shows that NF-κB expression increased in AP, and it played an important role in initiation and development of AP [5]. Therefore, the subject how to restrain the generation of NF-κB and inflammatory mediators becomes a new focus of AP treatment.
In clinical AP treatment, some traditional Chinese medicinal prescriptions, such as Qing-Yi-Tang and Chai-Hu-Tang, and so forth, have been proved to be effective [6, 7]. According to chemical analysis, baicalin (5,6,7-trihydroxyflavone-7-O-D-glucuronic acid. C21H18O11) is an effective component in these prescriptions [8]. Baicalin is a kind of flavonoid compound extracted from dried root of Scutellaria baicalensis Georgi, a kind of Origanum. It features various effects such as heat-clearing and detoxicating, antiinflammation, antiallergic, and so forth [9]. In this paper, the influence of baicalin on NF-κB and the inflammatory mediator, for example, TNF-α, are observed in order to clarify the effects of baicalin in treating AP and its possible mechanism.
MATERIALS AND METHODS
Reagents
The rabbit monoclonal antibody against NF-κB p65 was obtained from Santa Cruz Biotechnology Inc (Santa Cruz, Calif, USA). Fluorescein isothiocyanate (FITC)-labeled anti-rabbit IgG antibody was purchased from Jackson Immuno Research Laboratory (Jackson Immuno Research, Westgrove, Pa, USA). Caerulein, propidium iodide (PI), triton X100, TRIzol, and lipopolysaccharide (LPS) were from Sigma Co (Sigma, St Louis, Mo, USA). DL-2000 DNA marker, RNAase, and reverse transcriptive-polymerase chain reaction (RT-PCR) kit were purchased from Promega Co (Promega, Madison, Wis, USA). Baicalin (purity > 90%) was obtained from Tongtai Pharmaceuticals Co (Tongtai, Sichuan, China).
Animals handling
Male Wistar rats (250 ± 20 g) were provided by the Animal Research Center of the First Clinical College of Harbin Medical University (Harbin, China). The experiments were carried out on 40 rats, housed individually in wired bottomed cages, at a room temperature of 18–22°C using a 12-hour light-dark cycle. All animals fasted for 12 hours before manipulation with free access to water. They were treated in accordance with the protocols approved by the local Animal Use and Care Committee and executed according to the National Animal Welfare Law.
AP was induced by 4 intraperitoneal injections of caerulein (20 μg/kg body weight) with 1-hour interval, at the beginning, and consecutively the first, the second, and the third hours of the experiment. For control rats, only saline solution was given. For the LPS-treated rats, the solution of LPS (2 mg/kg body weight) was given intraperitoneally 30 minutes after the last caerulein injection. To treat the rats with baicalin, the solution of baicalin (50 mg/kg body weight) was given intraperitoneally 1, 12, 24, and 36 hours after the final caerulein injection.
The experimental animals were randomly divided into to 5 groups including the control group, the AP group, the AP group treated with baicalin, the AP group treated with LPS, and the AP group treated with baicalin and LPS.
Forty eight hours after the final caerulein injection, rats were anaesthetized with pentobarbital sodium (40 mg/kg body weight). Then, laparotomy was performed, and pancreas was rapidly removed for further analyses.
Scoring pathological damage of pancreatic tissue
The pancreas specimen was fixed with 20% formaldehyde, imbedded with paraffin, and then stained with hematoxylin and eosin (HE) for tissue morphological observation. Pathological damage of pancreatic tissue was evaluated semiquantitatively with modified Grewal method [10] by exclusive personnel with the double-blind method under a light microscope (Olympus, Japan).
TNF-α mRNA expression by RT-PCR
Total RNA was extracted from pancreatic tissues by using TRIzol reagent and was reversely transcribed into single-strand cDNA, which was used as template for subsequent polymerase chain reaction (PCR). The sequences of rat-specific primers for TNF-α (415 bp) were as follows: upstream primer, 5′GCCAATGGCATGGATCTCCAAAG3′ (P1); downstream primer, 5′CAGAGCAATGACTCCAAAGT3′ (P2). Housekeeping gene β-actin (348 bp) was used as a control, upstream primer: 5′CATCACCATTGGCAATGAGCG3′ (β1) and downstream primer: 5′′CTAGAAGCATTTGCGGTCGGAC3′ (β2). Conditions for amplification were as follows: preheating at 94°C for 2 minutes; denaturing at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extending at 68°C for 30 seconds, for 30 cycles using a thermal cycler (PerkinElmer, Mass, USA). Following these steps, a final extension was carried out at 72°C for 10 minutes. The PCR product was separated by gel electrophoresis on 1.0% agarose containing ethidium bromide and analyzed under ultraviolet light. The bands were quantitated by densitometry.
NF-κB activation in pancreatic tissue by flow cytometry
The surfactant processing method was applied to prepare nucleus suspension. Fresh pancreatic tissue was cut into pieces of 1.0 mm3 and was centrifuged at 200 xg for 5 minutes. After 10 ml detergent solution containing 1% triton X100 added and stored in a refrigerator at 4°C for 18 hours, nucleus suspension could be obtained by being filtered through a 50 μm nylon mesh. Based on methods of Foulds [11] and Deptala et al[12], 1 μL RNAase was added into 50 μL nucleus suspension and water-bathed at 37°C for 30 minutes.Then 40 μL NF-κB p65 monoclonal antibody was added and cultured at room temperature for 20 minutes; 1 μL FITC-labeled IgG was added and cultured at room temperature for 20 minutes; 20 μL PI was added and stored in a dark room for 30 minutes; then analyzed using a FACScan flow cytometer (BD Biosciences, USA).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Comparison between groups was performed with analysis of variance (ANOVA) and Student-Newman-Keuls test (q test). Simple linear correlation analysis was applied to investigate the relation between two parameters. Statistical significance was assumed at a value of P < .05.
RESULTS
Influence of baicalin on the pathological damage of pancreatic tissue
In the control group, morphology of the pancreas was normal, the lobule structure existed, and mesenchyma was clear, with a pathological score of 0.375 ± 0.04. In the AP group, pancreatic congestion and edema were observed, and the pancreatic cells were basically in normal shapes. Lobular mesenchymal rarefaction, edema, and inflammatory cell infiltration occurred, which were the main manifestations of acute edematous pancreatitis (mild AP) and with a pathological score of 3.5 ± 0.75. When compared with the control group, the difference was significant (P < .01). In the AP group treated with baicalin, the degree of mesenchymal edema and inflammatory cell infiltration was alleviated as compared with the AP group (P < .05).
In the AP group treated with LPS, hemorrhagic and necrotic spots were found sporadic in pancreatic tissue, with cell membrane dissolved and nucleus disappeared. Acinus and lobule structure were not clear. Around necrotic focus, numerous leukocyte and monocyte infiltration could be observed, which were main manifestations of acute necrotizing pancreatitis (severe AP), with a pathological score of 7.0±0.75, and pathological changes were remarkably heavier than that of the AP group (P < .01); while in the AP group treated with baicalin and LPS, the lobule structure was destroyed, with acinus arranging in disorder. There were a lot of inflammatory cell infiltrations, which were still manifestations of acute necrotizing pancreatitis (with a pathological score of 5.75 ± 1.25), but pathological changes were obviously alleviated (compared with the AP group treated with LPS, P < .05) (see Figure 1).
Figure 1.
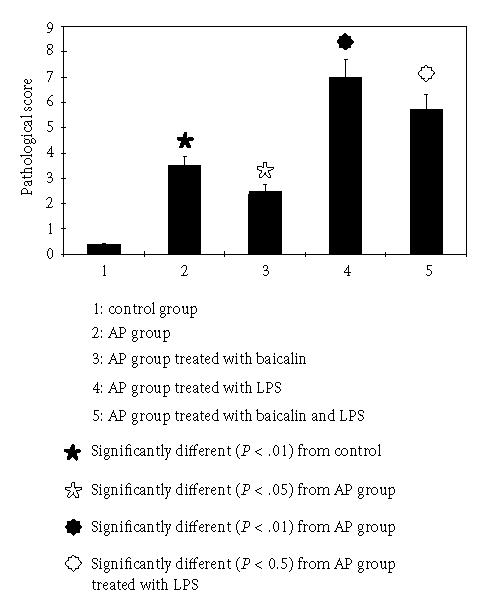
Pathological score of pancreatic tissue.
Effects of baicalin on TNF-α mRNA of pancreatic tissue
Semiquantitative analysis was carried out for mRNA expression of target gene of pancreatic tissue with the optical density ratio of the target gene/β-actin. TNF-α mRNA was expressed at a low level (0.72±0.10) in the control group; in the AP group, the TNF-α mRNA level increased to 1.94 ± 0.23; while in the AP group treated with baicalin, the level was 1.59 ± 0.16, which is obviously lower than that of the AP group (P < .01). In the AP group treated with LPS, it reached 3.79 ± 0.40, and when treated with baicalin, the level decreased to 3.35 ± 0.35, which makes significant difference when compared with the group treated with LPS (P < .05) (see Figure 2).
Figure 2.
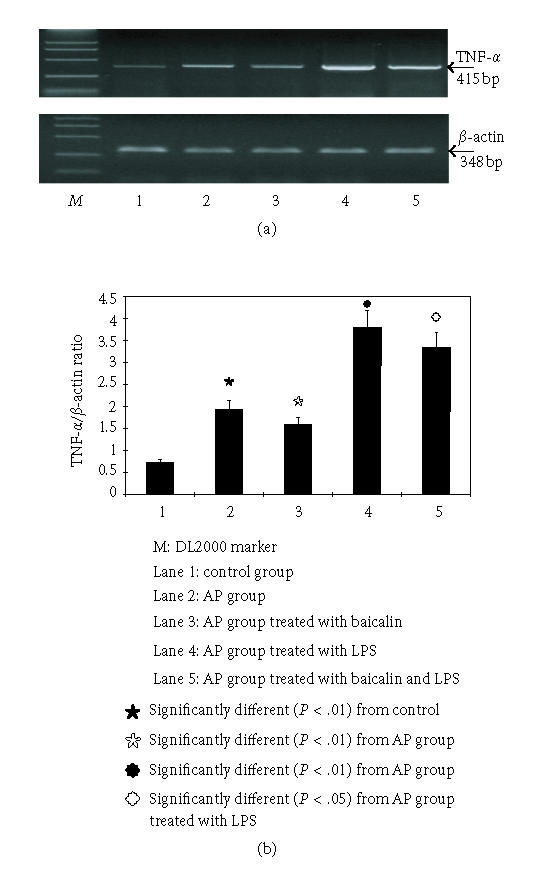
The mRNA expressions of TNF-α assessed with semiquantitive RT-PCR.
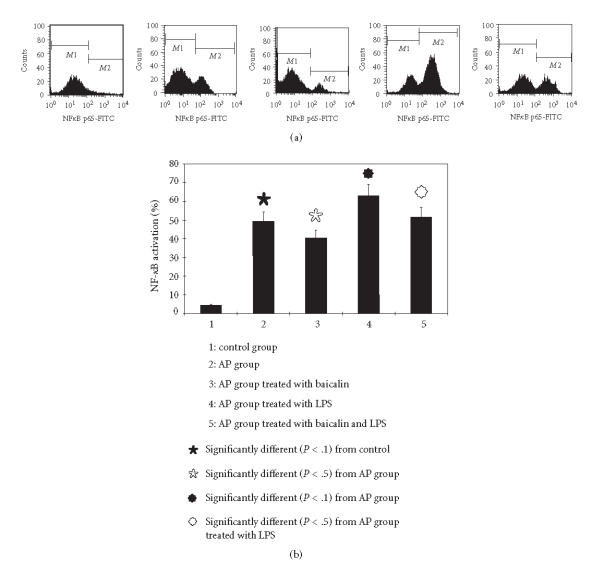
Effects of baicalin on activation of NF-κB
In the control group, activation of NF-κB in normal pancreatic nucleus was in a low level (< 5%), and in the AP group, it increased obviously to 49.38% ± 5.9%. After treatment with baicalin, it decreased to 40.46% ± 7.6% (versus AP group, P < .05). In the AP group treated with LPS, the activation level of NF-κB reached 62.8%±8.38%, and when treated with baicalin, it also decreased obviously to 51.58% ± 6.05 (versus AP group treated with LPS, P < .05) (see Figure 3).
Figure 3.
The NF-κB activation detected with flow cytometry assay.
DISCUSSION
At present, pathogenesis of AP is still unclear, which leads to lack of proper treatment in AP therapeutic strategy. The traditional hypotheses such as pancreatic autodigestion theory or microcirculatory disturbance theory cannot satisfactorily explain the mechanism that AP develops SIRS, MODS, and even death. However, the inflammatory mediator theory proposed recently provides a new clue for AP research [13]. It is explained in this theory that in the early stage of AP, the abnormal-activated pancreatin damages adjacent pancreatic cells and leads to local infiltration of inflammatory cells. Activated inflammatory cells such as macrophage, neutrophil, and pancreatic acinar cells produce and release proinflammatory cytokines [14]. The rising degree of inflammatory cytokines is closely associated with the severity of AP, and it is these inflammatory cytokines that result in SIRS and MODS.
It is shown in the study that among various inflammatory mediators related with AP, TNF-α plays a key role and is an important index that indicates the severity of AP [15]. Therefore pathological damage of AP may be alleviated by restraining TNF-α expression. This assumption has been confirmed in research of some scholars. For example, Grewal et al [10] reported that blocking TNF-α with anti-TNF-α polyclonal antibody could significantly decrease the severity of AP in rats. Fijen et al [16] had succeeded in reducing proinflammatory cytokines such as TNF-α, IL-1, and IL-8 in blood with the depressor obtained by activating mitogen-activated protein kinase (MAPK) with p38 cytomin (mitogen), and alleviated inflammatory response caused by endotoxin, which eventually increased the survival rate of patients.
Generation of TNF-α is adjusted by NF-κB as there are binding sites of NF-κB on the TNF-α promoter [17]. NF-κB is a protein with the gene-transcription regulation effect, which can participate in regulation of many inflammatory cytokines [18]. It is proved in research that activation of NF-κB can result in increased expression of cytokines such as TNF-α, monocyte chemoattractant protein-1 (MCP-1) and intercellular adhesion molecule-1 (ICAM-1), leading to immune and inflammatory responses in organisms [19]. It is proposed by Altavilla et al [20] that NF-κB activated by lipid peroxide could increase generation of TNF-α, while NF-κB and TNF-α decreased after using lipid peroxide inhibitors.
In our previous study, NF-κB activation, TNF-α mRNA expression, and pathological score were measured 12, 24, 36, and 72 hours after inducing AP to clarify the relationship among them. Results showed that in each time point, there was relatively close correlation among NF-κB activation, TNF-α mRNA, and pathological score [21]. In this experiment, relationship among them was studied again. After inducing AP, NF-κB was activated significantly (P < .01), while expression of TNF-α mRNA and the pathological score of pancreatic damage also increased obviously. Correlation analysis of NF-κB and TNF-α mRNA showed relatively high correlation between them (with a correlation coefficient of 0.7423, P < .05); there was also relatively close correlation between expression of TNF-α and pathological score, with a correlation coefficient of 0.7154 (P < .05). In order to further explore the regulating effect of NF-κB activation on TNF-α, LPS was applied as the stimulator to activate NF-κB in this experiment [22]. After AP was induced with caerulein, large dosage of LPS was injected intraperitoneally. The result showed that LPS strengthened NF-κB activation, and expression of TNF-α mRNA obviously increased simultaneously and resulted in more severe pathological damage, which suggests that in AP, activation of NF-κB may enhance expression of TNF-α mRNA and participate in aggravation of pancreatic damage. Hence, it can be inferred that inhibiting activation of NF-κB may reduce expression of proinflammatory genes, and alleviate inflammatory response and pancreatic tissue damage, which may have positive sense for AP therapy.
In traditional Chinese medicine, some important prescriptions have been successfully applied in AP treatment, in which one of the mechanisms is to suppress inflammatory response. Baicalin is an important active ingredient in these prescriptions [23]. It is shown in modern pharmacological studies that baicalin features multiple effects, such as broad-spectrum antibacterial, immune regulation, antitumor, antioxidation, and inducing apoptosis [24]. More research has focused on exploring antiinflammatory naturally occurring drugs. Effects of baicalin on inflammatory mediators have also been studied more than ever. In research of Lo et al [25] on lung injury caused by endotoxin, it was discovered that baicalin could decrease contents of IL-1β, TNF-α, and MCP-1 in serum, which was similar to the antiinflammation effect of dexameson. In research of Shen et al [26] on peripheral blood leucocytes of patients with inflammation, results showed that baicalin had antiinflammation effects via decreasing myeloperoxidase (MPO) and inhibiting leucocyte adhesion. It was proved in in vitro study that baicalin could inhibit T lymphocyte proliferation, decreasing expression of inflammatory cytokines such as IL-1, IL-6, TNF, and macrophage inflammatory protein (MIP) [27]. In our study, effects of baicalin on inflammatory mediator- TNF-α and its regulating factor NF-κB in pancreatic tissue of rats were studied. It was found that in the AP group, after treatment with baicalin, activation of NF-κB decreased (P < .05), and expression of TNF-α mRNA also significantly decreased (P < .01), while pancreatic pathological damage was alleviated at the same time (P < .05); similar results were observed in the AP group treated with LPS and baicalin. These results indicate that baicalin can inhibit NF-κB and the generation of inflammatory mediator in pancreatic tissue, and eventually results in alleviated pathological changes in AP. Therefore, baicalin has helpful effects in treating AP.
Generally, proinflammatory cytokine, such as TNF-α, play an important role in pathogenesis of AP. However, generation of inflammatory mediator is regulated by the transcription factor NF-κB. Baicalin extracted from traditional Chinese medicine can treat AP via decreasing NF-κB and TNF-α.
ACKNOWLEDGMENT
This work was supported by the Provincial Natural Sciences Foundation of Heilongjiang, China (no. D0227).
References
- 1.Bhatia M. Inflammatory response on the pancreatic acinar cell injury. Scandinavian Journal of Surgery. 2005;94(2):97–102. doi: 10.1177/145749690509400203. [DOI] [PubMed] [Google Scholar]
- 2.Murr MM, Yang J, Fier A, et al. Regulation of Kupffer cell TNF gene expression during experimental acute pancreatitis: the role of p38-MAPK, ERK1/2, SAPK/JNK, and NF-κB. Journal of Gastrointestinal Surgery. 2003;7(1):20–25. doi: 10.1016/s1091-255x(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 3.Li J-H, Yu J-P, Yu H-G, et al. Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediators of Inflammation. 2005;2005(4):185–193. doi: 10.1155/MI.2005.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homaidan FR, Chakroun I, El-Sabban ME. Regulation of nuclear factor-κB in intestinal epithelial cells in a cell model of inflammation. Mediators of Inflammation. 2003;12(5):277–283. doi: 10.1080/09629350310001619681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-κB attenuates the severity of cerulein-induced acute pancreatitis. Journal of the American College of Surgeons. 2002;195(4):497–505. doi: 10.1016/s1072-7515(02)01222-x. [DOI] [PubMed] [Google Scholar]
- 6.Li Z-L, Wu C-T, Lu L-R, Zhu X-F, Xiong D-X. Traditional Chinese medicine “Qing Yi Tang” alleviates oxygen free radical injury in acute necrotizing pancreatitis. World Journal of Gastroenterology. 1998;4(4):357–359. doi: 10.3748/wjg.v4.i4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Q, Yuan L, Yang X-N, Tang W-F, Jiang J-M. Comparison of integrated Chinese and Western medicine with and without somatostatin supplement in the treatment of severe acute pancreatitis. World Journal of Gastroenterology. 2005;11(7):1073–1076. doi: 10.3748/wjg.v11.i7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu I. Current status of treatment of heapatobiliary disorders in Japan. Sho-saiko-to: Japanese herbal medicine for protection against hepatic fibrosis and carcinoma. Journal of Gastroenterology and Hepatology. 2000;15(suppl):D84–D90. doi: 10.1046/j.1440-1746.2000.02138.x. [DOI] [PubMed] [Google Scholar]
- 9.Zobayed SMA, Murch SJ, Rupasinghe HPV, De Boer JG, Glickman BW, Saxena PK. Optimized system for biomass production, chemical characterization and evaluation of chemo-preventive properties of Scutellaria baicalensis Georgi. Plant Science. 2004;167(3):439–446. [Google Scholar]
- 10.Grewal HP, Mohey el Din A, Gaber L, et al. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-α polyclonal antibody. American Journal of Surgery. 1994;167(1):214–219. doi: 10.1016/0002-9610(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 11.Foulds S. Novel flow cytometric method for quantifying nuclear binding of the transcription factor nuclear factor kappa B in unseparated human monocytes and polymorphonuclear cells. Cytometry. 1997;29(2):182–186. [PubMed] [Google Scholar]
- 12.Deptala A, Bedner E, Gorczyca W, Darzynkiewicz Z. Activation of nuclear factor Kappa B (NF-κB) assayed by laser scanning cytometry (LSC) Cytometry. 1998;33(3):376–382. doi: 10.1002/(sici)1097-0320(19981101)33:3<376::aid-cyto13>3.0.co;2-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felderbauer P, Müller C, Bulut K, et al. Pathophysiology and treatment of acute pancreatitis: new therapeutic targets - a ray of hope? Basic and Clinical Pharmacology and Toxicology. 2005;97(6):342–350. doi: 10.1111/j.1742-7843.2005.pto_274.x. [DOI] [PubMed] [Google Scholar]
- 14.Norman JG, Fink G, Franz M, et al. Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Annals of Surgery. 1996;223(2):163–169. doi: 10.1097/00000658-199602000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laveda R, Martínez J, Muñoz C, et al. Different profile of cytokine synthesis according to the severity of acute pancreatitis. World Journal of Gastroenterology. 2005;11(34):5309–5313. doi: 10.3748/wjg.v11.i34.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fijen JW, Zijlstra JG, De Boer P, et al. Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clinical and Experimental Immunology. 2001;124(1):16–20. doi: 10.1046/j.1365-2249.2001.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udalova IA, Knight JC, Vidal V, Nedospasov SA, Kwiatkowski D. Complex NF-κB interactions at the distal tumor necrosis factor promoter region in human monocytes. Journal of Biological Chemistry. 1998;273(33):21178–21186. doi: 10.1074/jbc.273.33.21178. [DOI] [PubMed] [Google Scholar]
- 18.Christman JW, Lancaster LH, Blackwell TS. Nuclear factor κB: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Medicine. 1998;24(11):1131–1138. doi: 10.1007/s001340050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Algül H, Tando Y, Schneider G, Weidenbach H, Adler G, Schmid RM. Acute experimental pancreatitis and NF-κB/Rel activation. Pancreatology. 2002;2(6):503–509. doi: 10.1159/000066090. [DOI] [PubMed] [Google Scholar]
- 20.Altavilla D, Famulari C, Passaniti M, et al. Lipid peroxidation inhibition reduces NF-κB activation and attenuates cerulein-induced pancreatitis. Free Radical Research. 2003;37(4):425–435. doi: 10.1080/1071576031000070093. [DOI] [PubMed] [Google Scholar]
- 21.Xue D, Wang H, Zhang W. Observation of the trends of nuclear factor-κB, tumor necrosis factor-α and pathologic injury in rats with acute pancreatitis. China Journal of Modern Medicine. 2005;15(10):1512–1514. [Google Scholar]
- 22.Qiu Z, Kwon A-H, Tsuji K, Kamiyama Y, Okumura T, Hirao Y. Fibronectin prevents D-galactosamine/lipopolysaccharide-induced lethal hepatic failure in mice. Shock. 2006;25(1):80–87. doi: 10.1097/01.shk.0000185797.04589.5c. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Sun A, Liu R. Separation and purification of baicalin and wogonoside from the Chinese medicinal plant Scutellaria baicalensis Georgi by high-speed counter-current chromatography. Journal of Chromatography A. 2005;1066(1-2):243–247. doi: 10.1016/j.chroma.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 24.Ueda S, Nakamura H, Masutani H, et al. Baicalin induces apoptosis via mitochondrial pathway as prooxidant. Molecular Immunology. 2002;38(10):781–791. doi: 10.1016/s0161-5890(01)00115-8. [DOI] [PubMed] [Google Scholar]
- 25.Lo YC, Lin YL, Yu KL, et al. San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. Journal of Ethnopharmacology. 2005;101(1–3):68–74. doi: 10.1016/j.jep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Shen Y-C, Chiou W-F, Chou Y-C, Chen C-F. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. European Journal of Pharmacology. 2003;465(1-2):171–181. doi: 10.1016/s0014-2999(03)01378-5. [DOI] [PubMed] [Google Scholar]
- 27.Krakauer T, Li BQ, Young HA. The flavonoid baicalin inhibits superantigen-induced inflammatory cytokines and chemokines. FEBS Letters. 2001;500(1-2):52–55. doi: 10.1016/s0014-5793(01)02584-4. [DOI] [PubMed] [Google Scholar]