Abstract
Neuropeptide Y regulates numerous physiological processes via at least five different Y receptors, but the specific roles of each receptor are still unclear. We previously demonstrated that Y2 receptor knockout results in a lean phenotype, increased cancellous bone volume, and an increase in plasma pancreatic polypeptide (PP), a ligand for Y4 receptors. PP-overexpressing mice are also known to have a lean phenotype. Deletion of the Y4 receptor also produced a lean phenotype and increased plasma PP levels. We therefore hypothesized that part of the Y2 phenotype results from increased PP action on Y4 receptors and tested this in PP transgenic Y4−/− and Y2−/− Y4−/− double knockout mice. Bone mass was not altered in Y4 knockout mice. Surprisingly, despite significant hyperphagia, Y2−/− Y4−/− mice retained a markedly lean phenotype, with reduced body weight, white adipose tissue mass, leptinemia, and insulinemia. Furthermore, bone volume was also increased threefold in Y2−/− Y4−/− mice, and this was associated with enhanced osteoblastic activity. These changes were more pronounced than those observed in Y2−/− mice, suggesting synergy between Y2 and Y4 receptor pathways. The lack of bone changes in PP transgenic mice suggests that PP alone is not responsible for the bone mass increases but might play a major role in the lean phenotype. However, a synergistic interaction between Y2 and Y4 pathways seems to regulate bone volume and adiposity and could have important implications for possible interventions in obesity and for anabolic treatment of osteoporotic bone loss.
Neuropeptide Y (NPY) is an important regulator of numerous physiological processes, such as food intake (9, 43), energy homeostasis (22, 42), and growth and reproduction (2, 10). These processes are mediated at least partially within the hypothalamus, via five different Y receptors (Y1, Y2, Y4, Y5, and y6). All of these receptors are expressed in the hypothalamus, the brain stem, and peripheral tissues (12). Despite having highly divergent primary structures, different Y receptors have very similar pharmacological profiles, with few agonists and antagonists that are selective for specific Y receptors and active in vivo being presently available. Consequently, the specific roles of individual receptors and the mechanisms that mediate the diverse physiological effects of NPY are not yet clearly understood.
Recent studies with germ line and conditional Y receptor knockout mouse models have begun to shed some light on the functions of different Y receptors. We have shown that both hypothalamus-specific and germ line Y2 receptor knockout resulted in a lean phenotype in male mice (reduced body weight and white adipose tissue [WAT] mass) (35) and a twofold increase in cancellous bone volume associated with accelerated bone formation (3). Consistent with the general inhibitory functions of NPY, it appears from these data that Y2 receptors directly or indirectly mediate the tonic stimulation of adipocyte activity while also mediating tonic inhibition of osteoblastic bone-forming activity. The increased bone volume in hypothalamic Y2−/− mice was not associated with changes in the concentrations in plasma of hormones that are known to regulate bone physiology, notably leptin, thyroid hormones, insulin-like growth factor 1 (IGF-1), corticosterone, or testosterone (3). The concentration of pancreatic polypeptide (PP) in plasma was elevated threefold in these mice (35), however, which could contribute to the regulation of energy homeostasis and bone mass alone or in addition to Y2 deletion. PP is released from F cells in the islets of Langerhans in response to stimuli such as food ingestion, hypoglycemia, or neuroglucopenia, predominantly via a vagal mechanism (16, 39). Y4, a receptor for PP, has been found mainly in peripheral tissues such as pancreas, intestine, colon, heart, and liver (5, 23). However, high levels of Y4 receptors are also found in the brain stem, including the nucleus of the solitary tract, the dorsal vagal motor nucleus, and the area postrema, which is considered to be accessible to circulating factors from the periphery (21, 31). In addition to its direct action on the digestive system, it has also been shown that circulating PP enhances digestive events such as gastric secretion, motility, and emptying, through activation of a vagal mechanism via the central Y4 receptor population (25, 27). Furthermore, PP released postprandially has also been shown to function as a satiety signal, presumably again acting on specific Y4 receptors in the brain stem (19). Our previous data indicate that Y2 receptors, specifically hypothalamic Y2 receptors, are involved in the regulation of plasma levels of PP (35), perhaps by Y2-induced inhibition of vagal efferent output (8). Further evidence that Y2 receptor knockout may induce its effects on energy homeostasis via increased plasma PP levels comes from the observation that PP-overexpressing mice have a lean phenotype similar to that of germ line Y2 receptor knockout mice (45). In addition, germ line deletion of the Y4 receptor also produced a lean phenotype accompanied by increased plasma PP concentrations (37).
Whereas Y4 receptors in the brain stem can be accessed by their high-affinity ligand PP, other central Y4 receptors which are not accessible to circulating PP are likely to be activated by the lower-affinity ligand NPY if its concentration reaches sufficiently high levels. Such high hypothalamic NPY levels are found, for example, in animal models of obesity such as the leptin deficient ob/ob mouse, which interestingly also exhibits increased bone mass (11). Moreover, elevated NPY levels are also present in Y2−/− mice. Thus, it is possible that the elevation in bone mass seen in these mice may occur as a result of regulation via hypothalamic NPY, possibly through central Y4 receptors.
In order to delineate the different contributions of the Y2 and Y4 receptors to the regulation of energy homeostasis and bone formation and to determine the potential involvement of PP or central NPY in these effects, we generated Y2−/− Y4−/− double knockout mice and compared the effects on adiposity and bone mass with reference to control, Y2−/−, and Y4−/− mice. In addition, we investigated bone parameters in PP transgenic mice.
MATERIALS AND METHODS
The research described here was conducted in compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and with the New South Wales Animal Research Act, with the approval of the Garvan/St. Vincent's Animal Experimentation Ethics Committee.
Generation of germ line Y2−/−, Y4−/−, and Y2−/− Y4−/− double knockout mice and PP transgenic mice.
Germ line deletion of Y2 and Y4 receptor genes was achieved as previously described (35, 37) by crossing conditional-knockout floxed mice (Y2lox/lox or Y4lox/lox) with oocyte-specific Cre recombinase-expressing C57/BL6 mice (40). This results in the removal of the entire coding region of the Y2 or Y4 gene. Y2−/− Y4−/− double knockout mice were obtained by crossing Y2−/− and Y4−/− mice, and the lack of the genes was confirmed by Southern blot analysis. Noninduced floxed Y2lox/lox animals were used as controls. All mice generated were maintained on a mixed C57BL/6-129/SvJ background. PP transgenic and age-matched nontransgenic mice were generated as previously described (45).
Measurement of food intake and body weight.
Groups of 13 to 32 animals per genotype originating from four different breeding pairs were group housed and fed with standard chow. Body weight was monitored at the same time each week from 4 weeks of age onwards. Food intake was measured over 7 days at 12 weeks of age.
Tissue collection and analysis.
All analyses were carried out with male mice, which were injected with the fluorescent tetracycline compounds calcein and demeclocycline (Sigma Chemical Co., St. Louis, Mo.), each at 20 mg/kg, at 10 and 3 days prior to collection, respectively. At 15 to 17 weeks of age, control, Y2−/−, Y4−/−, and Y2−/− Y4−/− knockout mice were killed by cervical dislocation at between 10.00 to 14.00 h for collection of trunk blood. Plasma was separated and immediately frozen. The brain was removed and immediately frozen on dry ice, and interscapular brown adipose tissue (BAT) and WAT deposits (right inguinal, right epididymal, right retroperitoneal, and mesenteric) were collected and weighed. The small intestine was removed, its length was measured, and then it was flushed with isotonic saline, blotted, and weighed. Both femora were excised and fixed in 4% paraformaldehyde (Merck, Darmstadt, Germany) at 4°C for 16 h.
Plasma insulin and leptin levels were measured by radioimmunoassay (Linco Research, St. Louis, Mo.). Plasma PP was radioimmunoassayed by using guinea pig anti-rat PP serum, 2nd Antibody Precipitating Systems (Linco Research), and 125I-rPP (Auspep, Parkville, Australia). Plasma corticosterone, testosterone, and free T4 were measured by radioimmunoassay (ICN Biomedicals, Costa Mesa, Calif.); plasma IGF-1 concentrations were radioimmunoassayed with a kit from Bioclone Australia (Marrickville, Australia); and plasma glucose was determined by colorimetry (Trace Scientific, Melbourne, Australia, and Sigma Diagnostics, St. Louis, Mo.).
The right femora were bisected transversely at the midpoint of the shaft. Distal halves were embedded undecalcified in methyl-methacrylate (APS Chemicals, Sydney, Australia), and 5-μm sagittal sections were analyzed (Bioquant; R&M Biometrics Inc, Nashville, Tenn.) as described previously (3). Cortical parameters were measured at the bisection point, using the proximal portion of the bone. Cortical area was calculated by subtraction of the medullary area from the total bone area, and cortical thickness was calculated by radius difference, assuming circularity. Sections were stained for mineralized bone (28), and the cancellous bone volume, trabecular thickness, and number were calculated (30). Osteoblast parameters (osteoblast surface, osteoblast number, and osteoid surface) were estimated by using von Kossa- and toluidine blue-stained sections, with mineralizing surface, mineral apposition rate, and bone formation rate estimated following fluorescence microscopy (Leica, Heerbrugg, Switzerland) of unstained sections (29). Osteoclast surface and osteoclast number were estimated from sections stained for tartrate-resistant acid phosphatase activity as described previously (17).
In situ hybridization.
Coronal slices (20 μm) of frozen brains were cut and thaw mounted on charged slides. For in situ hybridization, DNA oligonucleotides complementary to mouse NPY (5′-GAGGGT CAGTCCACACAGCCCCATTCGCTTGTTACCTAGCAT-3′), proopiomelanocortin (POMC) (5′-TGGCTGCTCTCCAGGCACCAGCTCCACACATCTATGGAGG-3′), cocaine- and amphetamine-regulated transcript (CART) (5′-TCCTTCTC GTGGGACGCATCATCCACGGCAGAGTAGATGTCCAGG-3′), agouti-related protein (AgRP) (5′-AGCTTGCGGCAGTAGCAAAAGGCATTGAAGAAGCGGCAGTAGCAC-3′), corticotropin-releasing hormone (CRH) (5′-CCGATAATCTCCATCAGTTTCCTGTTGCTGTGAGCTTGCTGAGCT-3′), thyrotropin-releasing hormone (TRH) (5′-AACCTTACTCCTCCAGAGGTTCCCTGACCCAGGCTTCCAGTTGTG-3′), and gonadotropin-releasing hormone (GnRH) (5′-CAAACACACAGTCAGCAGTAGAATGCCGGCCATCAGTTTGAGGATC-3′) mRNAs were labeled with [35S]thio-dATP (Amersham, Buckinghamshire, United Kingdom) by using terminal deoxynucleotidyltransferase (Roche, Mannheim, Germany). Matching sections from the same portion of the hypothalamus (approximately −1.8 mm [for POMC and CART] to −1.9 mm [for NPY and AgRP] from Bregma for arcuate neurons and approximately −0.8 mm from Bregma for the paraventricular nucleus) of knockout and control mice were assayed together, as described previously (14, 35). Hybridizations with the respective sense oligonucleotides and in the presence of an excess of unlabeled antisense oligonucleotide were included as controls in some experiments.
For evaluation of in situ hybridization, digitized images of the areas of interest were acquired from photoemulsion-dipped and superficially counterstained brain slices at a magnification of ×200 with a digital camera (ProgRes 3008; Zeiss, Heidelberg, Germany) mounted onto a Zeiss Axiophot microscope, as previously described (36). Silver grain density was evaluated by an experimentally blinded observer by outlining single neurons and measuring the total neuronal area and the area covered by silver grains (black grains in bright-field image), using NIH Image software (version 1.61; available free from the Research Services Branch, National Institute of Mental Health [http://rsbweb.nih.gov/nih-image/index.html]). The percentages of silver grain area compared to total area calculated for single neurons were averaged. Data are given as percentage of control silver grain density averaged from at least four sections (80 neurons) per peptide per animal.
Statistical analyses.
Results were assessed by factorial analysis of variance. When there was a significant overall effect of Y2, Y4, or Y2 and Y4 deficiency, or interaction effects, Fisher's or Contrasts posthoc test was performed to identify differences, using StatView version 4.5 (Abacus Concepts Inc., Berkeley, Calif.). For all statistical analyses, a P value of <0.05 was accepted as being statistically significant.
RESULTS
Increased food intake and reduced adiposity in Y2−/− Y4−/− double knockout mice.
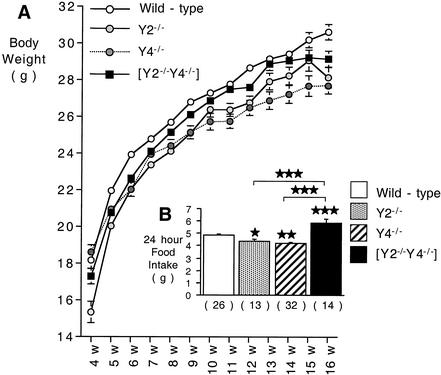
In order to examine the effect on energy balance of Y4 receptor activity alone or in the context of Y2 deletion, Y4−/− and Y2−/− Y4−/− mice were compared to control and Y2−/− mice. The body weights of male Y2−/− and Y4−/− animals were significantly reduced compared with those of control animals (Fig. 1A). In Y2−/− Y4−/− double knockout mice there was also a significant decrease in body weight compared with control mice, although the difference was less pronounced than in Y2−/− and Y4−/− animals (Fig. 1A). The 24-h food intake of Y2−/− Y4−/− double knockout mice was significantly increased compared to those of control, Y2−/−, and Y4−/− mice (Fig. 1B).
FIG. 1.
Body weight (A) and food intake (B) of male Y2−/−, Y4−/−, and Y2−/− Y4−/− double knockout mice compared to those of controls. Data are means ± standard errors of the means per group. The number of mice is indicated in parentheses. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (versus control or the comparison indicated by horizontal bars).
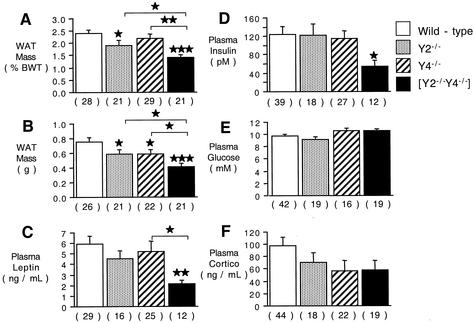
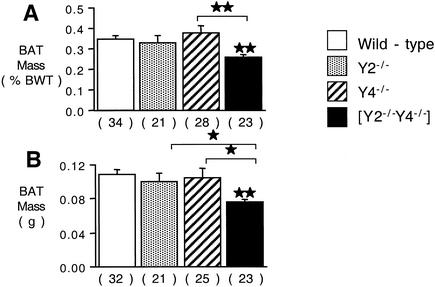
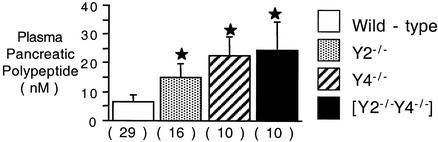
WAT mass, either as a percentage of body weight or as an absolute weight, was slightly but significantly decreased in male Y2−/− and Y4−/− mice compared with control mice (Fig. 2A and B), with a greater reduction in WAT in Y2−/− Y4−/− double knockout mice compared to that in control mice (Fig. 2A and B). The decreased adiposity of Y2−/− Y4−/− double knockout mice was associated with significant reductions in the plasma leptin (Fig. 2C) and insulin (Fig. 2D) concentrations compared with those in control mice, changes that did not reach significance in either Y2−/− or Y4−/− mice. Plasma glucose and corticosterone concentrations were not significantly different among the four groups of mice studied, although there was a nonsignificant trend to decreased corticosterone levels in the knockout mice (Fig. 2E and F). Since Y2−/− Y4−/− double knockout mice had significantly greater food intake than wild-type mice yet were lighter and leaner than controls, we investigated BAT for evidence of increased metabolic rate. Whereas Y2 or Y4 receptor knockout in isolation had no significant effect on BAT weight (Fig. 3), Y2−/− Y4−/− double knockout mice showed a marked reduction in BAT weight, both relative (Fig. 3A) and absolute (Fig. 3B). These changes in BAT in Y2−/− Y4−/− mice were not associated with alterations in the concentrations of free T4 in plasma, which were 21.8 ± 3.0 pM in Y2−/− Y4−/− mice versus 20.0 ± 2.1 pM in wild-type mice (not significant; n = 10 mice per group). Similarly, there was no significant change in plasma IGF-1 concentrations (214 ± 30 mg/dl in Y2−/− Y4−/− mice [n = 9] versus 304 ± 29 mg/dl in wild-type mice [n = 19] [P = 0.1]) and no significant change in plasma testosterone levels (21.7 ± 9.3 nM in Y2−/− Y4−/− mice [n = 19] versus 14.2 ± 3.9 nM in wild-type mice [n = 17] [P = 0.37]). Neither the relative weight (as a percentage of body weight) nor the absolute weight of the small intestine was significantly altered by double deletion of Y2 and Y4 receptors (data not shown). There was no difference in the length of the small intestine between Y2−/− Y4−/− and wild-type mice (data not shown). Plasma PP levels, however, were significantly elevated over control values in Y2−/−, Y4−/−, and Y2−/− Y4−/− double knockout mice (Fig. 4).
FIG. 2.
Adiposity and plasma hormone and metabolite concentrations in male Y2−/−, Y4−/−, and Y2−/− Y4−/− double knockout mice compared to those in controls. (A and B) Masses of the right inguinal, right epididymal, right retroperitoneal, and mesenteric WAT depots, expressed as a percentage of body weight (BWT) (A) or as absolute WAT weight (B). (C to F) Concentrations of leptin (C), insulin (D), glucose (E), and corticosterone (cortico) (F) in plasma. Data are means ± standard errors of the means for the number of mice indicated in parentheses. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus control or the comparison indicated by horizontal bars).
FIG. 3.
Mass of BAT, expressed as a percentage of body weight (BWT) (A) or as absolute weight (B). Data are means ± standard errors of the means for the number of mice indicated in parentheses. *, P < 0.05; **, P < 0.01 (versus wild-type control or the comparison indicated by horizontal bars).
FIG. 4.
Plasma PP concentrations in Y2−/−, Y4−/−, and Y2−/− Y4−/− double knockout mice compared to those in controls. Data are means ± standard errors of the means for the number of mice indicated in parentheses. *, P < 0.05 versus control mice.
Synergistic increase in cancellous bone volume and bone formation in Y2−/− Y4−/− double knockout mice.
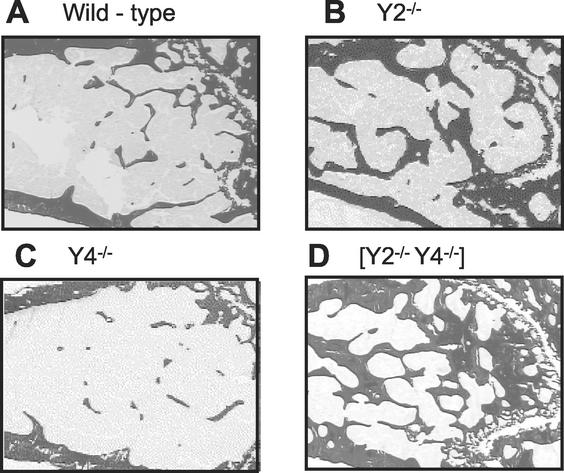
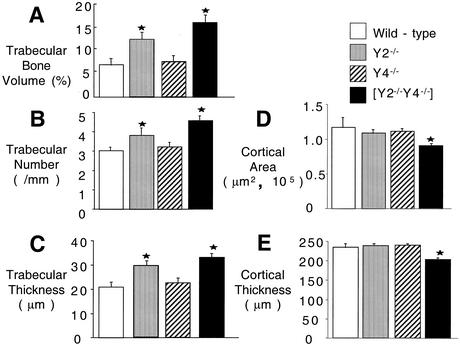
As Y2 receptor deletion had previously been shown to have a profound effect on bone volume and osteoblast activity, these parameters were compared in Y2−/−, Y4−/−, and Y2−/− Y4−/− mice. The cancellous bone volume was increased over control values in Y2−/− but not in Y4−/− mice (Fig. 5 and 6A to C). Y2−/− Y4−/− double knockout mice exhibited a profound increase in bone volume, exceeding the increase induced by Y2 receptor deficiency alone (Fig. 5 and 6). Cancellous bone volume, trabecular number, and trabecular thickness were significantly increased over control values in Y2−/− but not Y4−/− mice. Moreover, Y2−/− Y4−/− double knockout mice showed even greater increases over control mice in these parameters. The increased bone volume of Y2−/− Y4−/− double knockout mice was associated with a significant increase in bone turnover, with osteoblast, osteoclast, and osteoid surfaces elevated as much as 100% compared to those of control, Y2−/−, or Y4−/− mice (Table 1). However, osteoblast and osteoclast numbers were not different from those of controls in the double knockout mice, unlike the case for Y2−/− mice, in which osteoclast number was decreased, and Y4−/− mice, in which osteoblast number was decreased (Table 1). The osteoblastic bone formation rate was significantly increased over control values in Y2−/− Y4−/− double knockout mice, driven by an increase in mineral apposition rate, with no change in mineralizing surface (Table 2).
FIG. 5.
Sagittal micrographs of distal femoral metaphyses of Y2−/− Y4−/− double knockout mice (D) compared to those of control (A), Y2−/− (B), or Y4−/− (C) male mice. The micrographs show darkly stained bone tissue and are representative of the respective groups. Bar, 1 mm.
FIG. 6.
Effect of Y2−/− Y4−/− double knockout on bone morphology compared with no knockout, Y2−/− knockout, or Y4−/− knockout in male mice. Trabecular bone volume (A), trabecular number (B), trabecular thickness (C), cortical area (D), and cortical thickness (E) were determined. Data are means ± standard deviations for 6 to 14 mice per group. *, P < 0.05 versus control mice.
TABLE 1.
Histomorphometric analysis of bone cells in distal femoral metaphysis in Y receptor knockout micea
| Mice | Osteoblast surface (% OS) | Osteoblast no. (/mm of OS) | Osteoid surface (% BS) | Osteoclast surface (% BS) | Osteoclast no. (/mm) |
|---|---|---|---|---|---|
| Control | 10.4 ± 4.1 | 9.5 ± 2.5 | 12.7 ± 5.8 | 8.0 ± 2.3 | 4.2 ± 1.2 |
| Y2−/− | 7.9 ± 2.7 | 7.3 ± 2.7 | 11.1 ± 4.4 | 6.4 ± 1.9 | 2.9 ± 0.6* |
| Y4−/− | 10.1 ± 3.7 | 5.0 ± 1.5* | 12.0 ± 4.6 | 9.4 ± 2.9 | 4.5 ± 1.7 |
| Y2−/− Y4−/− | 18.6 ± 5.9* | 10.8 ± 3.4 | 23.2 ± 8.1* | 14.1 ± 5.8* | 4.1 ± 1.1 |
Data are means ± standard deviations for 6 to 14 mice per group. *, P < 0.05 versus control. OS, osteoid surface; BS, bone surface.
TABLE 2.
Osteoblastic bone formation in Y2−/− Y4−/− double knockout micea
| Mice | Mineralizing surface (%, BS) | Mineral apposition rate (μm/day) | Bone, formation rate (μm2/μm/day) |
|---|---|---|---|
| Control | 38.4 ± 12.7 | 1.1 ± 0.2 | 0.41 ± 0.1 |
| Y2−/− Y4−/− | 39.6 ± 7.2 | 1.4 ± 0.2* | 0.58 ± 0.2* |
Data are means ± standard deviations for 9 to 14 mice per group. *, P < 0.05 versus control. BS, bone surface.
Whereas the Y2−/− Y4−/− double knockout increased the volume and rate of formation of cancellous bone, these mice showed significant reductions in the area and thickness of cortical bone compared to control mice (Fig. 6D and E). This change was associated with a reduction in the periosteal diameter of the cortical shaft, with no change in the endosteal diameter (data not shown). There was no effect of Y2 or Y4 single gene deletion on cortical area or cortical thickness (Fig. 6D and E).
PP overexpression does not alter bone volume or bone turnover.
In order to investigate the effect of chronically elevated plasma PP levels on bone homeostasis in animals with a complete set of Y receptors, bone tissue from male PP transgenic mice and age-matched control mice on the same genetic background was investigated (45). Neither cancellous bone volume nor cortical area was different from control values in PP transgenic mice (data not shown). Bone turnover was also similar to control values, with no differences in osteoblast and osteoclast surface and number and osteoid surface values (data not shown). These data suggest that an elevated PP level alone is not capable of changing bone mass.
Altered expression of neuropeptides in the hypothalamus of Y2−/− Y4−/− double knockout mice.
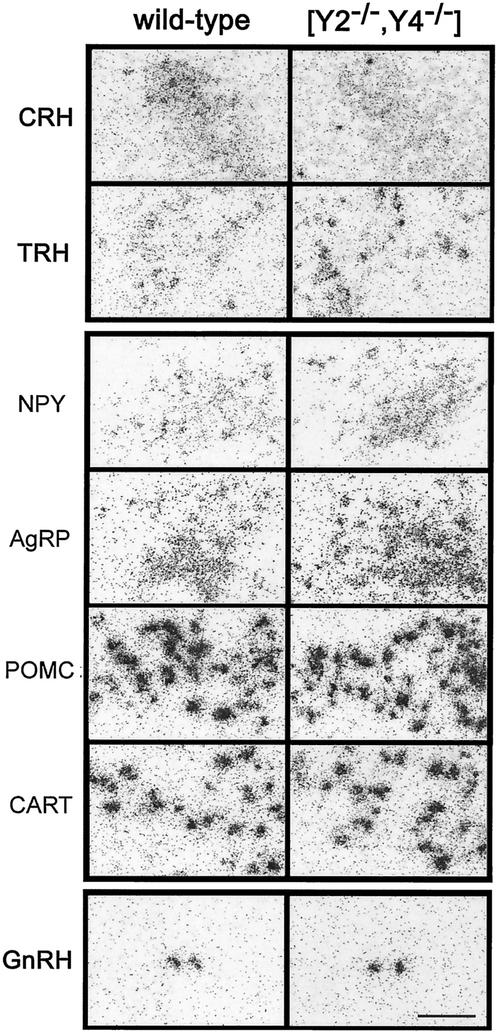
As both Y2 and Y4 receptors are expressed in hypothalamic nuclei that are important in the regulation of energy homeostasis and autonomic output, we investigated the expression of several important neurotransmitters in these areas in the different models. In accordance with previous data, Y2 deficiency tended to increase NPY and AgRP mRNA levels, while POMC and CART mRNA levels in the arcuate nucleus were down-regulated compared to those in control mice (35). Y4 deletion did not significantly affect the expression of these neuropeptides in the arcuate nucleus (Fig. 7, Table 3). Interestingly, Y2−/− Y4−/− animals displayed an even greater increase in NPY mRNA levels than Y2−/− mice, with significant increases in AgRP mRNA and significant decreases in CART mRNA also observed in the arcuate nucleus compared to control animals (Fig. 7; Table 3). The decreased POMC mRNA levels seen in Y2−/− mice were normalized by the double knockout (Fig. 7; Table 3). In the paraventricular nucleus, Y2 receptor knockout resulted in strong decreases in the expression of both CRH and TRH mRNAs, while Y4−/− mice displayed increased TRH and unchanged CRH mRNA levels. In Y2−/− Y4−/− animals the decreased CRH mRNA levels seen in Y2−/− mice were unaffected by the additional deletion of the Y4 receptor. However, the strong reduction in TRH mRNA in Y2−/− mice was fully reversed, with TRH actually significantly increased in the Y2−/− Y4−/− animals over control animals (Fig. 7; Table 3), suggesting that different NPY pathways regulate these two endocrine parameters. In addition to pure hypothalamic regulation of energy homeostasis and bone formation, we also investigated functional changes in the gonadotropic axis, which also plays a role in bone homeostasis. The increased GnRH levels observed in scattered forebrain neurons of Y4−/− mice were also observed in the Y2−/− Y4−/− double knockout mice but not in Y2−/− mice, further confirming that Y4 but not Y2 receptors are involved in the regulation of the gonadotropic axis (Fig. 7; Table 3) (37).
FIG. 7.
Effect of Y2−/− Y4−/− double knockout on CRH and TRH mRNA levels in neurons of the paraventricular nucleus (upper panels); on NPY, AgRP, POMC, and CART mRNA levels in the arcuate nucleus (middle panels); and on GnRH mRNA levels in forebrain neurons (lower panels) in male mice. High-power bright-field photomicrographs of dipped sections obtained from control and Y2−/− Y4−/− double knockout mice after in situ hybridization for the respective mRNA are shown. Bar, 100 μm. The paraventricular nucleus is depicted at about −0.8 mm and the arcuate nucleus is depicted at about −1.8 to −1.9 mm from Bregma as low-magnification film autoradiographs for TRH and CART.
TABLE 3.
Expression levels of neuropeptide mRNAs in hypothalamic and forebrain nuclei of Y receptor knockout mice
| mRNA | Expressiona in the following mice:
|
|||
|---|---|---|---|---|
| Control | Y2−/− | Y4−/− | Y2−/− Y4−/− | |
| Paraventricular nucleus | ||||
| CRH | 100 ± 3.5 (5) | 66 ± 9.4 (10)*** | 103 ± 5.1 (4) | 69 ± 2.9 (5)*** |
| TRH | 100 ± 2.6 (6) | 58 ± 5.5 (10)*** | 113 ± 6.9 (4) | 117 ± 5.9 (5)* |
| Arcuate nucleus | ||||
| NPY | 100 ± 4.2 (10) | 115 ± 7.5 (10)* | 100 ± 2.4 (10) | 131 ± 10 (5)** |
| AgRP | 100 ± 3.3 (9) | 126 ± 5.7 (10)** | 99 ± 6.7 (10) | 120 ± 1.8 (10)** |
| POMC | 100 ± 2.7 (10) | 88 ± 3.5 (10)* | 99 ± 2.2 (9) | 100 ± 4.5 (5) |
| CART | 100 ± 2.7 (10) | 79 ± 4.4 (10)** | 103 ± 2.4 (9) | 88 ± 6.7 (5)* |
| Forebrain neurons (GnRH) | 100 ± 6.6 (5) | 104 ± 0.9 (5) | 123 ± 8.5 (5)** | 119 ± 3.8 (5)* |
Data represent mean labeling intensity of neurons, given as percentage of the control value, ± standard error of the mean for the number of mice shown in parentheses. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus control).
DISCUSSION
This study shows that simultaneous deletion of Y2 and Y4 receptors results in striking reductions in adiposity, leptinemia, and insulinemia and a marked threefold increase in cancellous bone volume. These findings demonstrate synergy between Y2 and Y4 receptors in the regulation of adiposity, since individual deletion of Y2 or Y4 receptors resulted in only slight reductions in adiposity, with no significant effects on leptinemia or insulinemia. The double knockout thus reveals a level of complexity in the regulation of Y receptor-mediated signals that was not made apparent in previous single knockout models. Synergy was also evident in the bone mass increase, as Y2 but not Y4 receptor single gene knockout resulted in increased bone mass, whereas double knockout of Y2 and Y4 receptors enhanced this phenotype by a further 90%. The initial hypothesis that elevated PP levels can act via Y4 receptors to promote decreased adiposity and increased bone mass did not hold, as the phenotypes seen in Y2 knockout mice were not prevented by simultaneous deletion of Y4 receptors. Moreover, PP overexpression in transgenic animals did not increase bone mass. This is in keeping with the observations that leptin-deficient ob/ob mice have a high bone mass (11) but low circulating levels of PP (18). However, the possibility that the elevated plasma PP levels in these knockout models (increased severalfold in all mutants) may influence energy homeostasis (45) and insulin effects (15) by activating other Y receptors with lower but still significant affinity for PP must be considered. The Y2−/− Y4−/− double knockout mice will provide a useful model in the investigation of these pathways.
It is of particular interest that double knockout of Y2 and Y4 receptors resulted in such profound decreases in adiposity, leptinemia, and insulinemia, because no single Y receptor deletion alone has resulted in such an obvious lean phenotype on an already lean background. As action of NPY in the hypothalamus causes increases in body weight, fat mass (42), leptinemia (4), and insulinemia (1, 38, 46), it was expected that knockout of the Y receptors for NPY would induce opposite effects. However, this has not been the case with germ line knockout of individual Y receptors, with reports describing modest decreases in such parameters in Y2 and Y4 receptor knockout mice (35, 37), no change in these parameters in young Y5 receptor knockout mice (24), or counterintuitive increases in these parameters consistent with positive energy balance in Y1 receptor knockout mice (20, 32). The latter observations lent support to the hypothesis that if Y receptors do mediate the NPY-induced obesity syndrome, then there must be considerable redundancy and/or compensation among the receptor types or by other regulators of energy balance that would obscure the consequences of single Y receptor deletion. Our present data clearly reveal a less flexible system than was initially postulated. The synergy between Y2 and Y4 receptors in the regulation of adiposity demonstrates a lack of compensation for the roles of these receptors by other regulators of energy balance when both Y2 and Y4 receptors are missing. The lack of compensation by other mechanisms is also particularly evident in the increased cancellous bone volume of Y2−/− Y4−/− mice compared to mice with either Y2−/− or Y4−/− single deletion.
Interestingly, food intake was significantly increased in Y2−/− Y4−/− mice over control, Y2−/−, and Y4−/− values. A mechanism for this strong increase in food intake may involve a lack of feedback inhibition from postprandially released factors such as PYY(3-36) and PP, each of which have been shown to reduce food intake in humans and rodents by acting on central Y2 and possibly Y4 receptors (6). Failure of these inhibitory pathways due to the lack of both Y2 and Y4 receptors might leave unchecked the orexigenic actions of NPY, the expression of which was increased in the hypothalamus of Y2−/− Y4−/− mice. NPY action in the hypothalamus through Y receptors other than Y2 or Y4 may thereby also contribute to the large increase in food consumption in the double knockout animals.
Importantly, however, this increase in food consumption was accompanied by decreased adiposity in Y2−/− Y4−/− mice, indicating a distinct mechanism that decreased fuel efficiency in the double knockout animals. This mechanism does not appear to involve increased physical activity, since the activity of double knockout mice in a plus maze was not significantly different from that of controls (data not shown). Although the length and weight of the small intestine was not different in Y2−/− Y4−/− and wild-type mice, the possibility that decreased intestinal absorption of nutrients contributed to the lean phenotype despite hyperphagia in the double knockout mice cannot be excluded. BAT weight was significantly decreased in Y2−/− Y4−/− mice, suggesting increased thermogenic activity and combustion of the fat deposits within this tissue. These data collectively suggest that increased metabolic rate, possibly via enhanced thermogenesis in BAT, contributed to the decreased fuel efficiency of double knockout mice.
Increases in metabolic rate in small mammals can be brought on by increases in sympathetic tonus and/or increases in T3 concentration within the BAT. Indeed, thyroid hormones and sympathetic neurotransmitters act in a coordinate fashion to increase facultative thermogenesis (33, 41). Y2−/− Y4−/− double knockout mice did not show any change in concentrations in plasma of free T4, a marker of thyrometabolic status established by the hypothalamo-pituitary-thyrotropic axis (34). However, this does not exclude the possibility that there may have been increased production of metabolically active T3 within BAT by local activity of type II iodothyronine 5′ deiodinase (41). Indeed, TRH expression was significantly increased in the paraventricular nucleus of the hypothalamus in Y2−/− Y4−/− mice.
The mRNA expression of hypothalamic neuropeptides that are important in the regulation of energy homeostasis is strongly altered in Y2−/− knockout mice (35); however, with the exception of slightly elevated TRH mRNA levels, these are unaffected in Y4−/− mice (37). In Y2−/− Y4−/− mice, the expression of the orexigenic NPY and AgRP peptides increased and the expression of the anorexic CART peptide decreased, following the trends observed in the Y2−/− mice. Thus, the orexigenic stimulus associated with this set of changes in NPY, AgRP, and CART expression levels is consistent with the increase in food intake in Y2−/− Y4−/− double knockout mutant mice. These mice, however, unlike Y2−/− mice with intact Y4-mediated satiety signaling pathways, lack both feedback inhibition by PYY(3-36) and PP, which may also contribute to their hyperphagia, as discussed above. The strongly reduced expression of CRH mRNA in the paraventricular nucleus of Y2−/− was not influenced by Y4 receptor deletion, suggesting a purely Y2-mediated inhibitory effect on this pathway. On the other hand, POMC and TRH mRNA expression patterns in the double knockout mice exhibited changes opposite to the Y2−/− patterns, with Y4 receptor deletion on the Y2−/− background restoring POMC levels to normal. More interestingly, not only did Y4 knockout abolish a Y2−/−-associated reduction in TRH mRNA levels, Y4 deletion actually increased the TRH mRNA signal in the double knockout mice to levels significantly greater than those in control mice. NPY is known to inhibit the hypothalamo-pituitary-thyrotropic axis by inhibition of TRH expression in the paraventricular nucleus (13), but the Y receptor type responsible for this effect is not known. This observed pattern of TRH expression in our knockout models suggests that the elevated NPY expression observed in Y2−/− mice inhibited TRH expression via Y4 receptors, thereby implicating Y4 receptors in the regulation of the thyrotropic axis. This observation also indicates that elevated NPY levels in the Y2−/− mice have the potential to act on other Y receptors and thereby indirectly modulate additional pathways such as the central regulation of bone mass.
Coordinate deletion of the Y2 and Y4 receptors resulted in a profound increase in cancellous bone volume associated with marked increases in trabecular thickness and number, with these anabolic changes enhanced compared to the Y2 knockout mouse phenotype. This increase in bone volume occurred as a result of elevated bone formation, with an increased mineral apposition rate consistent with more active osteoblasts. We previously demonstrated that selective deletion of hypothalamic Y2 receptors alone released tonic inhibition of bone formation by a mechanism independent of classic hormonal mediators of bone homeostasis. Based on the present study, plasma PP, which is strongly elevated in Y2−/− mice, can now be ruled out as the sole causative agent in the Y2−/− bone phenotype, as PP overexpression is not by itself capable of stimulating bone formation. Moreover, as in Y2−/− mice, Y2−/− Y4−/− double knockout did not produce significant changes in plasma corticosterone, free T4, IGF-1, or testosterone concentrations that would be expected to explain the increased bone mass phenotype of these mice. However it must be noted that the trend to reduced plasma IGF-1 levels in Y2−/− Y4−/− mice and the pattern of change in bone morphology in these animals is consistent with the observed effects in bone of reduced activity of the growth hormone-IGF-1 axis (7, 26).
Neuronal mechanisms of bone regulation may contribute to the bone phenotype of Y2−/− Y4−/− mice, as recently described (44). Since leptin inhibits bone formation (11) via central activation of sympathetic nervous output to bone tissue (44), the reduced leptinemia of Y2−/− Y4−/− double knockout mice may contribute to their increased rate of bone formation by abolishing hypothalamic NPY signaling through these Y receptors, thus reducing sympathetic nervous activity and thereby releasing β-adrenergic inhibition of osteoblasts (44). Importantly, Y2 and Y4 receptors are localized not only in the hypothalamus but also in areas of the brain stem where they have modulatory effects on sympathetic outflow (31). As the selective deletion of hypothalamic Y2 receptors alone is sufficient to significantly increase bone formation, double knockout of these receptors might further reduce sympathetic outflow by direct effects within the brain stem, thereby increasing bone formation to an even greater extent.
In conclusion, these results demonstrate that Y2 and Y4 receptors act in a synergistic manner in the regulation of adiposity and bone volume. Deletion of both receptors in Y2−/− Y4−/− double knockout mice results in greater reductions in adiposity and greater increases in cancellous bone volume than in mice with deficiency of either the Y2 or Y4 receptor alone. Of particular importance in the Y2−/− Y4−/− bone phenotype is the increase in trabecular number, which represents a degree of anabolic activity not achieved with any current osteoporosis therapies. These findings have important implications for intervention in obesity and for effective anabolic treatment of even severe osteoporotic bone loss.
Acknowledgments
Amanda Sainsbury and Paul A. Baldock contributed equally to this work.
We thank Julie Ferguson for invaluable veterinary advice, and we thank the staff of the Garvan Institute Biological Testing Facility. We are grateful to John Eisman for critical review of the manuscript.
This research was supported by the National Health and Medical Research Council of Australia (NHMRC) Centre Block Grant, a Human Frontier Science Program grant (RG0045/2000-B), an NHMRC/Diabetes Australia Career Development Award (188 827) to A. Sainsbury, and an Auslands-Stipendium of the University of Innsbruck to C. Schwarzer.
REFERENCES
- 1.Abe, M., M. Saito, and T. Shimazu. 1989. Neuropeptide Y and norepinephrine injected into the paraventricular nucleus of the hypothalamus activate endocrine pancreas. Biomed. Res. 10:431-436. [Google Scholar]
- 2.Aubert, M. L., D. D. Pierroz, N. M. Gruaz, V. d'Alleves, B. A. Vuagnat, F. P. Pralong, W. F. Blum, and P. C. Sizonenko. 1998. Metabolic control of sexual function and growth: role of neuropeptide Y and leptin. Mol. Cell. Endocrinol. 140:107-113. [DOI] [PubMed] [Google Scholar]
- 3.Baldock, P. A., A. Sainsbury, M. Couzens, R. F. Enriquez, G. P. Ghomas, E. M. Gardiner, and H. Herzog. 2002. Hypothalamic Y2 receptors regulate bone formation. J. Clin. Investig. 109:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baran, K., E. Preston, D. Wilks, G. J. Cooney, E. W. Kraegen, and A. Sainsbury. 2002. Chronic central melanocortin-4 receptor antagonism and central neuropeptide-Y infusion in rats produce increased adiposity by divergent pathways. Diabetes 51:152-158. [DOI] [PubMed] [Google Scholar]
- 5.Bard, J. A., M. W. Walker, T. A. Branchek, and R. L. Weinshank. 1995. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J. Biol. Chem. 270:26762-26765. [DOI] [PubMed] [Google Scholar]
- 6.Batterham, R. L., M. A. Cowley, C. J. Small, H. Herzog, M. A. Cohen, C. L. Dakin, A. M. Wren, A. E. Brynes, M. J. Low, M. A. Ghatei, R. D. Cone, and S. R. Bloom. 2002. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 418:650-654. [DOI] [PubMed] [Google Scholar]
- 7.Bikle, D., S. Majumdar, A. Laib, L. Powell-Braxton, C. Rosen, W. Beamer, E. Nauman, C. Leary, B. Halloran, and D. D. Bikle. 2001. The skeletal structure of insulin-like growth factor I-deficient mice. J. Bone Mineral Res. 16:2320-2329. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. H., R. L. Stephens, Jr., and R. C. Rogers. 1997. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol. Motil. 9:109-116. [DOI] [PubMed] [Google Scholar]
- 9.Clark, J. T., P. S. Kalra, W. R. Crowley, and S. P. Kalra. 1984. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115:427-429. [DOI] [PubMed] [Google Scholar]
- 10.Clark, J. T., P. S. Kalra, and S. P. Kalra. 1985. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology 117:2435-2442. [DOI] [PubMed] [Google Scholar]
- 11.Ducy, P., M. Amling, S. Takeda, M. Priemel, A. F. Schilling, F. T. Beil, J. Shen, C. Vinson, J. M. Rueger, and G. Karsenty. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197-207. [DOI] [PubMed] [Google Scholar]
- 12.Dumont, Y., D. Jacques, P. Bouchard, and R. Quirion. 1998. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J. Comp. Neurol. 402:372-384. [PubMed] [Google Scholar]
- 13.Fekete, C., J. Kelly, E. Mihaly, S. Sarkar, W. M. Rand, G. Legradi, C. H. Emerson, and R. M. Lechan. 2001. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology 142:2606-2613. [DOI] [PubMed] [Google Scholar]
- 14.Franklin, K. B., and G. Paxinos. 1997. The mouse brain in stereotaxic coordinates. Academic Press, San Diego, Calif.
- 15.Gettys, T. W., R. Garcia, K. Savage, D. C. Whitcomb, S. Kanayama, and I. L. Taylor. 1991. Insulin-sparing effects of pancreatic polypeptide in congenitally obese rodents. Pancreas 6:46-53. [DOI] [PubMed] [Google Scholar]
- 16.Havel, P. J., J. O. Akpan, D. L. Curry, J. S. Stern, R. L. Gingerich, and B. Ahren. 1993. Autonomic control of pancreatic polypeptide and glucagon secretion during neuroglucopenia and hypoglycemia in mice. Am. J. Physiol. 265:R246-R254. [DOI] [PubMed]
- 17.Hayman, A. R., P. Macary, P. J. Lehner, and T. M. Cox. 2001. Tartrate-resistant acid phosphatase (Acp 5): identification in diverse human tissues and dendritic cells. J. Histochem. Cytochem. 49:675-684. [DOI] [PubMed] [Google Scholar]
- 18.Jia, B. Q., and I. L. Taylor. 1984. Failure of pancreatic polypeptide release in congenitally obese mice. Gastroenterology 87:338-343. [PubMed] [Google Scholar]
- 19.Katsuura, G., A. Asakawa, and A. Inui. 2002. Roles of pancreatic polypeptide in regulation of food intake. Peptides 23:323-329. [DOI] [PubMed] [Google Scholar]
- 20.Kushi, A., H. Sasai, H. Koizumi, N. Takeda, M. Yokoyama, and M. Nakamura. 1998. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc. Natl. Acad. Sci. USA 95:15659-15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen, P. J., and P. Kristensen. 1997. The neuropeptide Y (Y4) receptor is highly expressed in neurones of the rat dorsal vagal complex. Brain Res. Mol. Brain Res. 48:1-6. [DOI] [PubMed] [Google Scholar]
- 22.Leibowitz, S. F. 1991. Brain neuropeptide Y: an integrator of endocrine, metabolic and behavioral processes. Brain Res. Bull. 27:333-337. [DOI] [PubMed] [Google Scholar]
- 23.Lundell, I., A. G. Blomqvist, M. M. Berglund, D. A. Schober, D. Johnson, M. A. Statnick, R. A. Gadski, D. R. Gehlert, and D. Larhammar. 1995. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J. Biol. Chem. 270:29123-29128. [DOI] [PubMed] [Google Scholar]
- 24.Marsh, D. J., G. Hollopeter, K. E. Kafer, and R. D. Palmiter. 1998. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat. Med. 4:718-721. [DOI] [PubMed] [Google Scholar]
- 25.McTigue, D. M., G. E. Hermann, and R. C. Rogers. 1997. Effect of pancreatic polypeptide on rat dorsal vagal complex neurons. J. Physiol. 499:475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberbauer, A. M., J. Cruickshank, A. Thomas, A. Stumbaugh, K. D. Evans, J. D. Murray, and A. R. Egan. 2001. Effects of pre and antenatal elevated and chronic oMT1a-oGH transgene expression on adipose deposition and linear bone growth in mice. Growth Dev. Aging 65:3-13. [PubMed] [Google Scholar]
- 27.Okumura, T., T. N. Pappas, and I. L. Taylor. 1994. Intracisternal injection of pancreatic polypeptide stimulates gastric emptying in rats. Neurosci. Lett. 178:167-170. [DOI] [PubMed] [Google Scholar]
- 28.Page, K. 1977. Bone and preparation of bone sections, p. 223-248. In J. D. Bancroft and A. Stevens (ed.), Theory and practice of histological techniques. Churchill Livingston, London, United Kingdom.
- 29.Parfitt, A. M., M. K. Drezner, F. H. Glorieux, J. A. Kanis, H. Malluche, P. J. Meunier, S. M. Ott, and R. R. Recker. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Mineral Res. 2:595-610. [DOI] [PubMed] [Google Scholar]
- 30.Parfitt, A. M., C. H. Mathews, A. R. Villanueva, M. Kleerekoper, B. Frame, and D. S. Rao. 1983. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J. Clin. Investig. 72:1396-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker, R. M., and H. Herzog. 1999. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 11:1431-1448. [DOI] [PubMed] [Google Scholar]
- 32.Pedrazzini, T., J. Seydoux, P. Kunstner, J. F. Aubert, E. Grouzmann, F. Beermann, and H. R. Brunner. 1998. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat. Med. 4:722-726. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro, M. O., S. D. Carvalho, J. J. Schultz, G. Chiellini, T. S. Scanlan, A. C. Bianco, and G. A. Brent. 2001. Thyroid hormone-sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J. Clin. Investig. 108:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins, J., and J. E. Rall. 1967. The iodine-containing hormones, p. 383-490. In C. H. Gray, and A. L. Bacharach (ed.), Hormones in blood, 2nd ed. Academic Press, London, United Kingdom.
- 35.Sainsbury, A., C. Schwarzer, M. Couzens, S. Fitissov, S. Furtinger, A. Jenkins, H. M. Cox, G. Sperk, T. Hokfelt, and H. Herzog. 2002. Important role of hypothalamic Y2 receptors in bodyweight regulation revealed in conditional knockout mice. Proc. Natl. Acad. Sci. USA 99:8938-8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sainsbury, A., C. Schwarzer, M. Couzens, and H. Herzog. 2002. Y2 receptor deletion attenuates the type 2 diabetic syndrome of ob/ob mice. Diabetes 51:3420-3427. [DOI] [PubMed] [Google Scholar]
- 37.Sainsbury, A., C. Schwarzer, M. Couzens, A. Jenkins, S. R. Oakes, C. J. Ormandy, and H. Herzog. 2002. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 16:1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sainsbury, A., D. Wilks, and G. J. Cooney. 2000. Interaction between adrenal glucocorticoids and parasympathetic activation in mediating hyperinsulinemia during long-term central neuropeptide Y infusion in rats. Diabetologia 43:859-865. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz, T. W. 1983. Pancreatic polypeptide: a unique model for vagal control of endocrine systems. J. Auton. Nervous Syst. 9:99-111. [DOI] [PubMed] [Google Scholar]
- 40.Schwenk, F., U. Baron, and K. Rajewsky. 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23:5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva, J. E., and P. R. Larsen. 1983. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305:712-713. [DOI] [PubMed] [Google Scholar]
- 42.Stanley, B. G., S. E. Kyrkouli, S. Lampert, and S. F. Leibowitz. 1986. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides 7:1189-1192. [DOI] [PubMed] [Google Scholar]
- 43.Stanley, B. G., and S. F. Leibowitz. 1984. Neuropeptide Y: Stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 35:2635-2642. [DOI] [PubMed] [Google Scholar]
- 44.Takeda, S., F. Elefteriou, R. Levasseur, X. Liu, L. Zhao, K. L. Parker, D. Armstrong, P. Ducy, and G. Karsenty. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305-317. [DOI] [PubMed] [Google Scholar]
- 45.Ueno, N., A. Inui, M. Iwamoto, T. Kaga, A. Asakawa, M. Okita, M. Fujimiya, Y. Nakajima, Y. Ohmoto, M. Ohnaka, Y. Nakaya, J. I. Miyazaki, and M. Kasuga. 1999. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology 117:1427-1432. [DOI] [PubMed] [Google Scholar]
- 46.Zarjevski, N., I. Cusin, R. Vettor, F. Rohner-Jeanrenaud, and B. Jeanrenaud. 1993. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology 133:1753-1758. [DOI] [PubMed] [Google Scholar]