Abstract
As a noninvasive method, exhaled breath condensate (EBC) has gained importance to improve monitoring of lung diseases and to detect biomarkers. The aim of the study was to investigate, whether erythropoietin (EPO) is detectable in EBC. EBC was collected from 22 consecutive patients as well as from healthy individuals. Using a multiplex fluorescent bead immunoassay, we detected EPO in EBC, as well as tumour necrosis factor-α (TNF-α) in 13 out of 22 patients simultaneously (EPO 0.21 ± 0.03 in U/mL and TNF-α 34.6 ± 4.2 in pg/mL, mean ± SEM). No significant differences for EPO levels or correlation between EPO and TNF-α were found but TNF-α was significantly higher in patients with chronic obstructive pulmonary disease (COPD) than in non-COPD (obstructive sleep apnoea, OSA, and lung healthy patients). This is the first report of detection of EPO in EBC. Due to the small study size more data is needed to clarify the role of EPO in EBC.
INTRODUCTION
Exhaled breath condensate (EBC) has gained increasing importance in noninvasive monitoring of airway inflammation [1–4]. This technique allows longitudinal sampling of biomarkers and may be repeated frequently with no difficulties even in patients with limitations to more invasive methods (ie, severe dyspnoea due to hypoxia) [5, 6]. Dyspnoea is the most frequent symptom in pulmonary diseases and is often associated with hypoxemia. Basically, low tissue oxygenation leads to stabilization of transcription factor hypoxia-inducible factor (HIF)-1α, and consequently production and secretion of erythropoietin (EPO) and the expression of EPO receptor (EPOR).
EPO is a 30.4 kd glycoprotein, which has a span of 165 amino acids. It has glycosylated chains, which are necessary for the biological function. Additional organs, apart from the kidney, liver, and uterus, have been found to secrete EPO, including peripheral vascular smooth muscle cells, endothelial cells, and insulin-producing cells [7]. Several proinflammatory cytokines, including tumour necrosis factor (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6), also can lead to increased expression of EPO and EPOR [8, 9]. On the other hand, EPO directly prevents cellular inflammation by inhibiting several proinflammatory cytokines, such as IL-6, TNF-α, and monocyte chemoattractant protein-1 (MIP) [8, 9]. Furthermore, EPO might provide vascular protection by preserving endothelial cell integrity and preventing apoptosis and cellular inflammation by inhibiting cellular phosphatidylserine (PS) membrane exposure and the subsequent targeting of cells for phagocytosis by macrophages [10]. Exposure of PS is believed to contribute significantly to a variety of diseases, such as ischemic stroke, dementia, Alzheimer disease, spinal cord injury, and myocardial infarction [10, 11]. Recently it has been shown that the EPO/EPOR system might also play a role in hypoxia-induced pulmonary hypertension [12].
Previously published data showed that the erythropoietic response did not correlate with the severity of hypoxia, hypoxemia, or erythrocytosis [13]. It is still unclear whether EPO plays a notable role either in patients with permanent hypoxemia (eg, patients with chronic obstructive pulmonary disease, COPD) or transient hypoxemia (eg, patients with obstructive sleep apnoea, OSA). There is increasing evidence that anaemia in chronic inflammatory diseases such as COPD might be caused by EPO resistance. But not in all of the anaemic patients with COPD elevated EPO levels were found [14]. There is a lack of data concerning prevalence of anaemia in COPD too [15]. Thus it appears that measuring of EPO in EBC, as a noninvasive technique, gains importance in the future.
The main purpose of our study was to investigate whether EPO is generally detectable in EBC, as EPO could be a versatile marker for various pathologic conditions, as well as for detection of exogenous supply of EPO in competitive sports or for further treatment strategies in advanced COPD.
METHODS
Subjects
Over a period of 6 weeks, 22 consecutive unselected in- and outpatients of the Section of Pulmonary Medicine, University of Ulm, Germany, were enrolled into the study. All patients were recruited from the lung function lab while routine tests. A detailed overview on patient characteristics is given in Table 1. From each patient, EBC was collected always in the morning and additional lung function measurements including blood gas analyses were done. All patients breathed in normal air at standard atmospheric pressure. Written informed consent was obtained from all. The study was approved by the Local Ethics Committee.
Table 1.
Patient characteristics of the study group. FEV1 = forced expiratory flow in 1 second, FEV1/VC = Tiffeneau index, pO2 = partial pressure of oxygen, pCO2 = partial pressure of carbon dioxide.
| Age (mean); range (years) | 65.9; 43–83 |
| Male/female | 15/7 |
| COPD, n | 7 |
| OSA, n | 7 |
| Lung healthy subjects*, n | 8 |
| FEV1 (mean ±95% Cl), in L | 2.2 ± 0.41 |
| FEV1/VC (mean ±95% Cl), in % pred | 72.4 ± 5.81 |
| pO2 (mean ±95% Cl), in mmHg | 68.5 ± 3.25 |
| pCO2 (mean ±95% Cl), in mmHg | 34.7 ± 1.67 |
*patients with arterial hypertension (n = 2), aortic valve stenosis (n = 1), atrial fibrillation (n = 2), and stable coronary heart disease (n = 3).
Exhaled breath condensate collection
EBC was collected according to the ATS/ERS Task Force [16]. We used a condenser, which permitted noninvasive collection of the nongaseous components of the expiratory air (EcoScreen; Jaeger, Germany). The subjects breathed through a mouthpiece and a two-way nonrebreathing valve, which also served as a saliva trap. They were instructed to breathe tidally, wearing a nose clip, for 15 minutes. If the subjects salivated, they were instructed to swallow. The obtained condensate (approximately 2-3 mL in each patient) was transferred to Eppendorf tubes and immediately stored at −80°C.
Cytometric bead array
A multiplex chemiluminescent immunoassay system (Immulite, DPC Biermann, Germany) was used to detect EPO and TNF-α. The measurement was done according to the manufacturer's protocol. The use of cytometric bead arrays has been validated for the use of EBC previously [4]. The detection limit was 0.24 mIU/mL for EPO and 1.7 pg/mL for TNF-α.
Lung function measurement
To measure lung function, a bodyplethysmograph was used (MasterScreen Body; Jaeger GmbH, Germany) and for blood gas analysis, a radiometer was used (ABL555, Radiometer A/S, Denmark). Lung function (spirometric parameters: forced expiratory volume in 1 second, FEV1, and inspiratory vital capacity, VC) was measured according to European Respiratory Society (ERS) recommendations [17].
Statistical analysis
Data were expressed as mean values and standard errors of mean (SEM). To compare EPO- and TNF-α levels between subgroups of patients, the Mann-Whitney U test was utilized. A value of P < .05 was considered statistically significant. Statistical analysis was performed with the Statistica software package (StatSoft Inc Tulsa, USA). Graphs were compiled using the GraphPad Prism Software (GraphPad Software, Inc, San Diego, USA).
RESULTS
EPO was detected in 15 and TNF-α in 13 out of 22 patients. In 13 cases, EPO and TNF-α could be detected simultaneously (EPO 0.21 ± 0.03 U/mL, TNF-α 34.6 ± 4.2 in pg/mL; mean ± SEM), see Figure 1.
Figure 1.
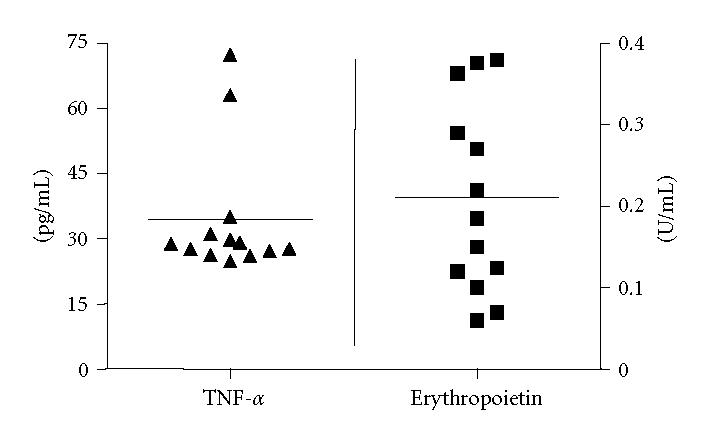
TNF-α- and EPO levels in exhaled breath condensate (EBC) from 13 nonhypoxic subjects in which both markers could be detected simultaneously, horizontal line = mean.
There were no significant differences in EPO levels between the groups (COPD, OSA, and lung healthy patients, pooled as non-COPD), as displayed in Figure 2. Additionally, hypoxia and the smoking status did not influence EPO levels in EBC. In 2 subjects in which EPO was detectable, TNF-α was not traceable.
Figure 2.
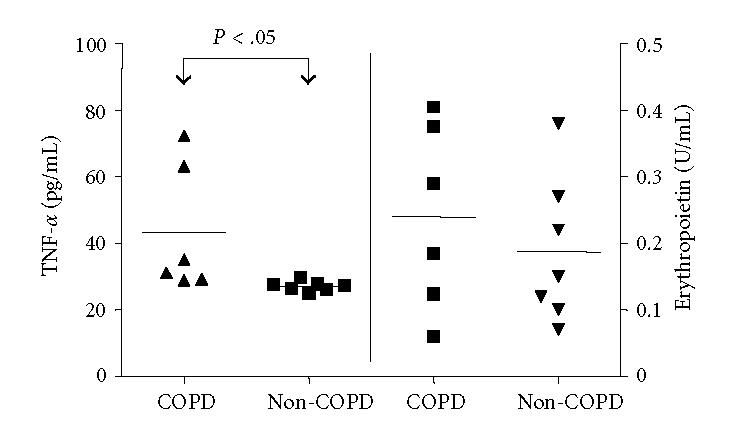
TNF-α- and EPO levels in EBC. In COPD, TNF-α levels were significantly higher compared to OSA and lung healthy patients (pooled as non-COPD), calculated using Mann Whitney U Test, P < .05 denotes significance, horizontal line = mean.
In COPD, TNF-α levels were significantly higher than in OSA and lung healthy patients (pooled as non-COPD, P < .05), see Figure 2.
Lung function measurement showed normal lung functions in the majority of individuals, as displayed in Table 1, especially no severe hypoxia or hypercapnia was noted.
DISCUSSION
The use of EBC to monitor inflammation in airways is well documented, and might be expanded to other fields of diagnosis [18–21]. In our study, EBC from 22 consecutive patients was collected and EPO was detected in 15/22 (68.1%) patients.
In patients with COPD, we noted a trend towards higher EPO levels than those with OSA or in lung healthy patients (pooled as non-COPD), however this was statistically not significant. These were comparable to normal reference values of EPO in serum (differences were not significant).
Higher EPO levels in COPD might be expected due to the nature of disease leading to chronic hypoxia. However, it is unclear if EPO or TNF-α can be expected in EBC in all individuals at all times. Furthermore, no reliable data exist concerning the right point in time to measure biomarkers in EBC.
TNF-α levels in COPD patients were significantly higher than in OSA patients or lung healthy individuals (pooled as non-COPD). No significant correlation of EPO levels with TNF-α was found. Our results concerning TNF-α were higher but still in the range of data described previously [4, 21, 22].
EPO could not be detected in 7 subjects, whereas TNF-α was not measured in 2 cases in which EPO was found. Even though the sampling of EBC was strictly performed according to the ATS/ERS Task Force Report on EBC, the inconstant detection of EPO, respectively, TNF-α might be explained by unresolved and previously reported methodological problems like unknown dilution of each biomarker in EBC [16]. The small size of our study can be the cause for these differences. On the other hand, temporary hypoxia in OSA patients seems not to be an adequate stimulus leading to increased levels of EPO.
Reviewing the literature, some attempts have been made to assess the dilution of substances in EBC samples, which represent more than 99.9% of condensate volumes. Neither the measurement of exhaled volume, exhaled ions, urea, protein concentration nor the conductance of lyophilised samples and external dilution markers (for internal or external standards) could resolve this problem completely [23–27].
To improve the method, combined efforts of all persons involved in collecting and handling of EBC samples are needed. A standardization of the method to collect EBC was recently accomplished and published by the European Respiratory Society (ERS) [16]. Furthermore, more sensitive analysis methods should be developed and validated, preferably with repeated measurements of biomarkers. Thus, many questions for interpretation of EBC data regarding the uncertain source of condensate solutes and the variable dilution of respiratory droplets from condensed water vapour have to be answered.
In severe COPD, anaemia is present up to 23% and higher EPO levels are expectable as disease progresses [15]. Based on these data, new treatment strategies could be developed regarding the role of EPO in advanced COPD. It is still unclear whether higher EPO levels or EPO resistance determines anaemia in these patients. EBC as a noninvasive and easy-to-use diagnostic method could help to monitor disease state by determination of characteristic biomarkers as a diagnostic tool or to control the treatment success of patients.
There is ample evidence that a complex network of inflammatory cytokines and chemokines has a prominent role in mediation and perpetuation of the processes of acute lung injury [28].
Detection of EPO in EBC might further be a useful tool in a variety of diseases, however especially in conditions with reduced tissue oxygenation, for example, COPD, pulmonary hypertension, various states of shock and myocardial infarction, but might develop into a useful noninvasive tool for detection of exogenous supply of EPO in competitive sports on the one hand, and for monitoring training effects in legal sports on the other hand, after data on EPO detection are bolstered by other groups and larger trials. In this context, noninvasive measurement of EPO in EBC (eg, in mechanically ventilated patients) might provide new insights into mechanisms of disease and might develop into a tool which allows to early counteract undesirable effects of tissue hypoxia apart from clinical shock as, for example, induction of apoptosis or inflammation. For example, EPO has been linked to cardiac ischemia and reperfusion injury and it has been shown that administration either before or during myocardial ischemia-reperfusion can protect against myocardial cell apoptosis and decrease infarct size, resulting in enhanced cardiac function and improved left ventricular contractility in a rabbit model of infarction [29].
Our results are further in contrast to recent publications that measured levels of biomarkers in EBC vary among each other, due to variable dilution of the ELF droplets with condensed water vapour (approximately up to 20 000-fold) as compared to serum levels [25, 30]. But up till now, no systematic examinations exist to resolve this problem completely. The detection of EPO in EBC is, even in that high amount, somewhat unexpected, following the current perception that molecular size limits the detection of volatile compounds. Review existing literature shows that this hypothesis has to be revised. More complex proteins (ie, keratins with 40–52 kd), with similar or larger molecular weight compared to EPO (30.4 kd), could be detected in EBC [31, 32]. But these findings are still not validated in larger populations.
To the best of our knowledge, this is the first description of detection of EPO in EBC in patients with COPD and OSA, however the clinical significance remains unclear.
ACKNOWLEDGMENT
We are indebted to Professor Dr E. Marion Schneider for helpful discussion and methodological help.
References
- 1.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124(4):1386–1392. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 2.Montuschi P, Kharitonov SA, Ciabattoni G, Barnes PJ. Exhaled leukotrienes and prostaglandins in COPD. Thorax. 2003;58(7):585–588. doi: 10.1136/thorax.58.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondino C, Ciabattoni G, Koch P, et al. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. Journal of Allergy and Clinical Immunology. 2004;114(4):761–767. doi: 10.1016/j.jaci.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Sack U, Scheibe R, Wōtzel M, et al. Multiplex analysis of cytokines in exhaled breath condensate. Cytometry Part A. 2006;69(3):169–172. doi: 10.1002/cyto.a.20231. [DOI] [PubMed] [Google Scholar]
- 5.Scheideler L, Manke H-G, Schwulera U, Inacker O, Hammerle H. Detection of nonvolatile macromolecules in breath: a possible diagnostic tool? American Review of Respiratory Disease. 1993;148(3):778–784. doi: 10.1164/ajrccm/148.3.778. [DOI] [PubMed] [Google Scholar]
- 6.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. Journal of Allergy and Clinical Immunology. 2002;110(1):28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 7.Chong ZZ, Kang J-Q, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. Journal of Hematotherapy and Stem Cell Research. 2002;11(6):863–871. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- 8.Chong ZZ, Kang J-Q, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. Journal of Cerebral Blood Flow and Metabolism. 2002;22(5):503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Restorative Neurology and Neuroscience. 2004;22(2):105–119. [PubMed] [Google Scholar]
- 10.Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restorative Neurology and Neuroscience. 2004;22(2):87–104. [PubMed] [Google Scholar]
- 11.Sakamaki K. Regulation of endothelial cell death and its role in angiogenesis and vascular regression. Current Neurovascular Research. 2004;1(4):305–315. doi: 10.2174/1567202043362072. [DOI] [PubMed] [Google Scholar]
- 12.Satoh K, Kagaya Y, Nakano M, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006;113(11):1442–1450. doi: 10.1161/CIRCULATIONAHA.105.583732. [DOI] [PubMed] [Google Scholar]
- 13.Tsantes AE, Papadhimitriou SI, Tassiopoulos ST, et al. Red cell macrocytosis in hypoxemic patients with chronic obstructive pulmonary disease. Respiratory Medicine. 2004;98(11):1117–1123. doi: 10.1016/j.rmed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 14.John M, Hoernig S, Doehner W, Okonko DD, Witt C, Anker SD. Anemia and inflammation in COPD. Chest. 2005;127(3):825–829. doi: 10.1378/chest.127.3.825. [DOI] [PubMed] [Google Scholar]
- 15.John M, Lange A, Hoernig S, Witt C, Anker SD. Prevalence of anemia in chronic obstructive pulmonary disease: comparison to other chronic diseases. International Journal of Cardiology. 2006;111(3):365–370. doi: 10.1016/j.ijcard.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 16.Horváth I, Hunt J, Barnes PJ, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official statement of the European respiratory society. The European Respiratory Journal. Supplement. 1993;16:5–40. [PubMed] [Google Scholar]
- 18.Gessner C, Hammerschmidt S, Kuhn H, et al. Exhaled breath condensate acidification in acute lung injury. Respiratory Medicine. 2003;97(11):1188–1194. doi: 10.1016/s0954-6111(03)00225-7. [DOI] [PubMed] [Google Scholar]
- 19.Gessner C, Hammerschmidt S, Kuhn H, Wirtz H. Expired diagnosis?—The potential of exhaled breath analysis. Pneumologie. 2004;58(4):230–237. doi: 10.1055/s-2004-818411. [DOI] [PubMed] [Google Scholar]
- 20.Gessner C, Kuhn H, Toepfer K, Hammerschmidt S, Schauer J, Wirtz H. Detection of p53 gene mutations in exhaled breath condensate of non-small cell lung cancer patients. Lung Cancer. 2004;43(2):215–222. doi: 10.1016/j.lungcan.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Gessner C, Scheibe R, Wōtzel M, et al. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respiratory Medicine. 2005;99(10):1229–1240. doi: 10.1016/j.rmed.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Garey KW, Neuhauser MM, Robbins RA, Danziger LH, Rubinstein I. Markers of inflammation in exhaled breath condensate of young healthy smokers. Chest. 2004;125(1):22–26. doi: 10.1378/chest.125.1.22. [DOI] [PubMed] [Google Scholar]
- 23.Kietzmann D, Kahl R, Muller M, Burchardi H, Kettler D. Hydrogen peroxide in expired breath condensate of patients with acute respiratory failure and with ARDS. Intensive Care Medicine. 1993;19(2):78–81. doi: 10.1007/BF01708366. [DOI] [PubMed] [Google Scholar]
- 24.Effros RM, Hoagland KW, Bosbous M, et al. Dilution of respiratory solutes in exhaled condensates. American Journal of Respiratory and Critical Care Medicine. 2002;165(5):663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 25.Effros RM, Biller J, Foss B, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. American Journal of Respiratory and Critical Care Medicine. 2003;168(12):1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 26.Dwyer TM. Cigarette smoke-induced airway inflammation as sampled by the expired breath condensate. American Journal of the Medical Sciences. 2003;326(4):174–178. doi: 10.1097/00000441-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Zacharasiewicz A, Wilson N, Lex C, et al. Repeatability of sodium and chloride in exhaled breath condensates. Pediatric Pulmonology. 2004;37(3):273–275. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]
- 28.Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2001;164(10 pt I):1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 29.Parsa CJ, Kim J, Riel RU, et al. Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. Journal of Biological Chemistry. 2004;279(20):20655–20662. doi: 10.1074/jbc.M314099200. [DOI] [PubMed] [Google Scholar]
- 30.Effros RM, Dunning MB, III, Biller J, Shaker R. The promise and perils of exhaled breath condensates. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2004;287(6):L1073–L1080. doi: 10.1152/ajplung.00069.2004. [DOI] [PubMed] [Google Scholar]
- 31.Gianazza E, Allegra L, Bucchioni E, et al. Increased keratin content detected by proteomic analysis of exhaled breath condensate from healthy persons who smoke. American Journal of Medicine. 2004;117(1):51–54. doi: 10.1016/j.amjmed.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Nissenson AR, Nimer SD, Wolcott DL. Recombinant human erythropoietin and renal anemia: molecular biology, clinical efficacy, and nervous system effects. Annals of Internal Medicine. 1991;114(5):402–416. doi: 10.7326/0003-4819-114-5-402. [DOI] [PubMed] [Google Scholar]


