Abstract
Although adenoviral vectors provide prolonged gene expression in the brain by comparison to peripheral organs, expression is eliminated by a severe inflammatory infiltration (i.e., activated macrophages/microglia and T-lymphocytes) after peripheral infection with adenovirus. Here, we demonstrate that high-capacity adenoviral (HC-Ad) vectors succeed in maintaining long-term transgene expression in the brain, even in the presence of an active peripheral immunization with adenovirus that completely eliminates expression from first-generation vectors within 60 days. Importantly, even 60 days after the peripheral infection, brains injected with first-generation vectors exhibited evidence of a chronic infiltration of CD8+ cells, macrophage/microglial activation, and up-regulation of brain MHC-I expression. No inflammation was observed in the brains injected with the HC-Ad vector. Thus, these results demonstrate that HC-Ad vectors will allow safe, stable, and long-term transgene expression in the brain, even in the presence of peripheral infection with adenovirus. This markedly improves the prospects for the use of adenoviral vectors for long-term gene therapy of neurological disorders.
In peripheral tissues, adenovirus-induced inflammation resolves spontaneously within a few weeks (1–3). In the brain, the long-term outcome of inflammation remains unclear. In the light of ongoing clinical trials using adenoviral vectors for the treatment of brain disease, this issue is of great clinical relevance. We have shown recently that chronic brain inflammation can result from successful, short-term, tumor gene therapy using adenoviral vectors, even if the vector is only injected once (4). Vector systems useful for gene therapy of chronic neurological disorders should allow long-term transduction of brain cells in the complete absence of undesirable long-term side-effects. Particularly, chronic transgene expression and long-term vector persistence must evade any anti-vector immune responses generated through natural infection, vector re-administration, or substantial leak of vector from the brain to the periphery.
Recombinant adenovirus vectors are powerful tools for transduction of CNS glial cells and neurones (5). In the absence of prior immune priming to adenovirus, first-generation adenoviral vectors injected into the brain parenchyma can sustain prolonged transgene expression for months, relative to peripheral organs such as the liver, where transgene expression is generally undetectable after 14 days (1–3, 5–7). Although local inflammation is elicited in the brain in response to adenoviral vector injection, this is minimal and subsides within 1 month without clearing adenoviral-mediated transgene expression (8–10). Persistent expression in the brain is thought to be attributable to the failure to stimulate an effective anti-adenoviral T cell response (9–11). Activation of anti-viral T cells by peripheral immunization with adenovirus leads to massive T lymphocyte infiltration of the brain parenchyma, macrophage/microglial activation, and loss of vector-mediated transgene expression (12). Hence, the immunological instability of first-generation adenoviral vectors restricts their utility for long-term neurological gene therapy applications.
New generation, high-capacity adenoviral (HC-Ad) vectors that are deleted of all viral genes have been shown to mediate prolonged transgene expression compared with first-generation vectors after administration to the liver and muscle of unprimed animals (13–18). Similarly, herpes simplex virus 1 amplicon vectors, which like HC-Ads are devoid of all herpes simplex virus 1 coding sequences apart from those needed for vector DNA replication and packaging, have also been shown to allow long-term transgene transfer to the brain (19). However, the stability of any of these vectors in the presence of peripheral immune priming remains to be established. The question of whether gene expression from a viral vector is long-term and stable even after peripheral immune challenge, through natural infection or vector re-administration, is of considerable practical significance. We now demonstrate that, unlike first-generation vectors, HC-Ads can achieve long-term transduction of the rat brain, which is sustained even after the generation of an anti-adenoviral immune response. Our data are important not only for neurological gene therapy applications, but also for the treatment of other diseases that will require very long-term transgene expression.
Materials and Methods
Adenoviral Vectors.
RAd35 is a first-generation recombinant vector based on adenovirus type 5. The construction of RAd35 has been described earlier (20). In brief, an expression cassette containing the lacZ gene under the control of the hCMV promoter was inserted to replace the E1 region, thus rendering the virus replication-defective. RAdHPRT was constructed as described by Southgate et al. (21). Both E1a-deleted first-generation adenoviral vectors were propagated on E1a-expressing 293 cells, purified by double cesium chloride gradient centrifugation followed by dialysis and finally titered for infectious units by end point dilution on 293 cells, as described (22). The concentration of physical particles was determined for each preparation by optical absorbance at 260 and 280 nm as described by Mittereder et al. (23). The ratio of particles:infectious units (usually below 100:1 and providing a measure of the overall quality of the vector preparation) was 13:1 for RAd35 and 10:1 for RAdHPRT. The propagation and purification of the HC-Ad vector AdGS46 were performed as described for AdSTK109 by Schiedner et al. (15), using for production AdLC8cluc as helper virus (24) and a different Cre-expressing 293 cell line (G.S. and S.K., unpublished work). AdGS46 is a HC-Ad vector carrying an hCMV-lacZ expression cassette that was inserted into the pSTK120 backbone (15) between the HPRT and the C346 stuffer DNA. AdGS46 was titered by infecting HeLa cells with a known concentration of a standard virus in parallel with the AdGS46 preparation to be titered. Infected cells were slot-blotted, denatured, and hybridized to a DNA fragment corresponding to the left terminal 400 bp of the adenoviral genome. AdGS46 titer was calculated by comparing the intensities of the standard and AdGS46 vector bands. The particle:infectious units (i.u.) ratio for AdGS46 was 20:1, and helper virus contamination was 0.08%. Virus preparations were screened for replication competent adenovirus contamination by serial amplification on HeLa cells as described by Dion et al. (25) and Schiedner et al. (15). Replication competent adenovirus contamination was below 1:109 i.u. for all virus preparations. Adenoviruses were diluted in sterile saline solution for injection.
Psoralen-Inactivation of RAdHPRT.
The protocol used to inactivate RAdHPRT was modified from the work of Cotten et al. (26). A volume of 1.5 μl of 8-methoxypsoralen (Sigma), diluted to 33 mg/ml in DMSO, was added to 300 μl of purified RAdHPRT in a single well of a 24-well tissue culture plate. The plate was incubated for 20 min at room temperature to allow full penetration of the virion by the psoralen, before irradiating for 2 h with a long wave UV lamp placed 10 cm above the plate containing the virus. The irradiated vector was kept on ice throughout the procedure, and the plate was repositioned every 15 min to avoid shadows. Unincorporated psoralen was removed by gel filtration through a NAP5 column (Amersham Pharmacia), equilibrated with 10 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 135 mM NaCl, and 10% glycerol. No plaques were detected on 293 cells after titration of inactivated virus by end point dilution (22), indicating that the inactivation had been efficient and that there were less than 102 infectious units/ml of psoralen-RAdHPRT. The yield and particle concentration of inactivated virus was measured by optical absorbance at 260 and 280 nm, as described by Mittereder et al. (23). Non-inactivated RAdHPRT was also passed through a NAP-5 column and subsequently re-titered for infectious units and physical particles before injecting intradermally. The ratio of physical particles to infectious units after gel filtration and re-titration of RAdHPRT was 30:1. Comparable numbers of particles of psoralen-inactivated and active RAdHPRT were used for injection, based on OD260 and OD280 measurements.
Animals and Surgical Procedures.
Adult Sprague–Dawley rats of 250 g body weight (Charles River Breeding Laboratories) were anesthetized with halothane and were placed in a stereotaxic apparatus that was modified for use with inhalational anesthetic. Animals were injected in the left striatum (0.6 mm forward and 3.4 mm lateral from bregma, and 5.0 mm vertical from the dura) with 1.3 × 107 infectious units of either RAd35 or AdGS46 by using a 10-ml Hamilton syringe with a removable 26-gauge needle. Virus was administered in a volume of 2 μl, and each injection was performed over a period of 3 min, with the needle being left in place for a further 5 min before withdrawal. Anesthesia was maintained throughout surgery with 1% halothane in 67% medical O2 and 33% medical NO2. Sixty days after the striatal injection, rats were anesthetized with halothane and were injected intradermally in the back with either sterile saline solution, 5 × 108 infectious units of RAdHPRT, or the equivalent number of particles of psoralen-inactivated RAdHPRT. All intradermal injections were performed in a volume of 100 μl. Twenty-one or sixty days after the intradermal injection, rats were injected intraperitoneally with pentobarbitone and were transcardially perfused-fixed with heparinized saline and 4% paraformaldehyde in PBS. Brains were removed, were postfixed in 4% paraformaldehyde for 5 h, and were stored in PBS.
Immunohistochemistry.
Coronal sections 50 μm thick were cut through the striatum by using a vibratome. Free-floating immunohistochemistry was performed to detect transgene or inflammatory and immune cell markers. Endogenous peroxidase was inactivated with 0.3% hydrogen peroxide, and sections were blocked with 10% horse serum (Life Technologies, Paisley, Scotland) before incubating overnight with primary antibody diluted in PBS containing 1% horse serum and 0.5% Triton X-100. The primary antibodies and the dilutions at which they were used were anti-β-galactosidase (1:1,000; Promega), anti-MHC class I (OX-18, 1: 200; Serotec), anti-CD8 (cytotoxic T lymphocytes and NK cells, 1:500; Serotec), ED1 (activated macrophages/microglial cells, 1:1,000; Serotec), and anti-myelin basic protein (1:2,000; Dako). All primary antibodies were mouse monoclonal anti-rat, except for anti-myelin basic protein, which was rabbit polyclonal anti-human. Secondary antibodies were biotinated rabbit anti-mouse or biotinated swine anti-rabbit (Dako), diluted 1:200 in 0.5% Triton X-100 with 1% horse serum, and were detected by using the Vectastain Elite ABC horseradish peroxidase method (Vector Laboratories). After developing with diaminobenzidine and glucose oxidase, sections were mounted on gelatinized glass slides and were dehydrated through graded ethanol solutions before coverslipping.
Quantification.
Quantitative analysis to determine the area occupied by cells immunoreactive with antibodies against β-galactosidase and immune markers within single 50-μm brain sections was performed by using a Leica Quantimet 600 Image Analysis System controlled by qwin software (Leica Microsystems, Cambridge, U.K.) using a Leica RMDB microscope. Brain sections containing the needle track (and thus displaying the highest levels of immunoreactivity) were used for the quantitative analysis. Student's t test was used to determine the degree of statistical significance between values from different experimental groups.
Anti-Adenovirus Neutralizing Antibody Assay.
Neutralizing antibody titers were measured in serum samples from animals killed 21 days after the intradermal injection of virus. Serum samples were heat-inactivated at 56°C for 30 min and were serially diluted 2-fold in MEM containing 2% FCS. The range of dilutions was 1:2–1:4,096. Each serum dilution (100 μl) was incubated with 1 × 106 i.u. of RAd35 (in 10 μl) for 90 min at 37°C. Volumes of 50 μl of each dilution were then added to wells of a 96-well plate containing 4 × 104 293 cells per well and were incubated at 37°C for 60 min. A further 50 μl of medium containing 10% FCS was added to each well, and cells were incubated at 37°C for 20 h before fixing with 4% paraformaldehyde in PBS, and staining with 5-bromo-4-chloro-indolyl-β-d-galactoside (X-gal). The neutralizing antibody titer for each animal is given as the reciprocal of the highest dilution of serum at which 50% of RAd35-mediated transduction was inhibited.
Results
Long-Term Transgene Expression and Inflammation in the Brain, After Intracranial Injection of Adenovirus Vector Followed by Intradermal Injection of Adenovirus Vector.
The HC-Ad vector AdGS46, or the first-generation vector RAd35, were stereotaxically injected into the striatum of Sprague–Dawley rats. Both adenoviral vectors expressed β-galactosidase from an hCMV promoter. Sixty days after the striatal injection, animals were immunized against adenoviral vectors by injecting RAdHPRT intradermally. RAdHPRT is a first-generation adenovirus encoding the hypoxanthine-guanine phosphoribosyltransferase gene under the transcriptional control of the hCMV promoter. This vector shares with RAd35 both the vector backbone and the viral capsid whereas with the HC-Ad vector AdGS46 it shares only the viral capsid. Control groups of animals were injected intradermally with psoralen-inactivated RAdHPRT or saline. Our prediction was that eliciting an anti-first generation vector genome immune response would lead to inflammation in brains injected with RAd35, but not AdGS46, whereas generation of an anti-capsid immune response would lead to brain inflammation in all animals. Thus, we did not examine the role of the transgene in these experiments, but focused on those components of adenoviral vectors that are constant among vectors of the same class: i.e., the vector genome and the viral vector capsid. Importantly, our strategy is supported by data from Byrnes et al. (12), who have previously shown that peripheral immune stimulation generates an anti-genome, but not an anti-β-galactosidase response. Our data further support their claims.
Animals were killed 21 and 60 days after the intradermal injections (n = 3 per group for each time point; also see Tables 2 and 3, published as supplementary material on the PNAS web site, www.pnas.org), and the brains were analyzed by immunohistochemistry. At the 21-day time point (Fig. 1), we detected widespread CD8+ immunostaining and intense microglial activation (as evidenced by both ED1 and MHC class I immunoreactivity) throughout the ipsilateral striatum in all three animals that had received RAd35 in the brain and RAdHPRT in the periphery (Fig. 1, row a). The perivascular localization of many ED1 positive cells suggested monocyte infiltration of the brain. In contrast, in the three animals that had received AdGS46 in the brain and RAdHPRT in the periphery (Fig. 1, row c), less than 10 CD8 immunoreactive cells per section were detected. Activated macrophages/microglia and MHC class I immunoreactive cells in these animals were correspondingly few and were exclusively restricted to the scar remaining at the site of the needle injection. Similarly, all animals that had received psoralen-inactivated RAdHPRT in the periphery (Fig. 1, rows b and d) showed minimal evidence of inflammation, suggesting that activated T cells specific for viral gene products, but not those specific for capsid proteins, are important mediators of the inflammatory response to first-generation vectors in the brain.
Figure 1.
Transgene expression [β-galactosidase (β-gal)] and inflammatory responses (CD8, MHC I, ED1) in the brain, 21 days after intradermal injection of adenovirus. Rows a–d show brains from animals injected intrastriatally with 1.3 × 107 i.u. of RAd35 (rows a and b) or AdGS46 (rows c and d) at day 0, and intradermally with 5 × 108 i.u. of RAdHPRT (rows a and c), or the equivalent number of particles of psoralen-inactivated RAdHPRT (ps-RAdHPRT, rows b and d) at day 60. Bold font indicates the vector used for the striatal injection and normal font indicates the vector used for the dermal injection. Perfused-fixed brains from animals killed 21 days after the intradermal injection (i.e., day 81 post-brain injection) were cut into serial 50-μm sections and were stained for β-galactosidase (shown at low magnification in the first column with boxed area at high magnification in the second column), CD8+ cells (third column), MHC class I (fourth column), and activated microglial cells and macrophages (ED1 immunoreactivity; fifth column). Red arrowheads indicate the needle track. [Bar = 1 mm (in row d of the first column; scale for entire first column) and 200 μm (in row d of the fifth column; scale for the second through fifth columns).]
The extent of β-galactosidase immunoreactivity was comparable in all animals injected in the brain with the HC-Ad vector AdGS46, regardless of whether they had received RAdHPRT or psoralen-inactivated RAdHPRT in the periphery (Fig. 1, rows c and d). In contrast, in all animals that had received RAd35 in the brain and RAdHPRT in the periphery (Fig. 1, row a), levels of β-galactosidase staining were markedly lower than those that had been injected with psoralen-inactivated RAdHPRT in the periphery (Fig. 1, row b). Distribution of β-galactosidase, CD8, ED1, and MHC class I immunoreactivity in animals that had received peripheral injections of saline were comparable to those that had received psoralen-inactivated RAdHPRT (not shown).
To confirm that the peripheral injection of vectors had led to the priming of an anti-adenoviral immune response, neutralizing antibody titers were measured in the serum from animals killed 21 days after the intradermal injection (Table 1). All animals injected intradermally with RAdHPRT had high levels of circulating neutralizing antibodies; animals injected with psoralen-inactivated RAdHPRT had low levels, or undetectable levels of neutralizing antibodies, and no neutralizing antibodies could be detected in any of the animals injected intradermally with saline.
Table 1.
Neutralizing serum antibody titers from animals killed 21 days after the intradermal injection of virus
| Striatal injection (day 0): | RAd35 | RAd35 | RAd35 | AdGS46 | AdGS46 | AdGS46 | Saline | Saline | |
|---|---|---|---|---|---|---|---|---|---|
| Dermal injection (day 60): | RAdHPRT | Ps-RAdHPRT | Saline | RAdHPRT | Ps-RAdHPRT | Saline | RAdHPRT | Saline | |
| Neutralizing | Animal 1 | 256 | 8 | <2 | 32 | 4 | <2 | 256 | <2 |
| antibody | Animal 2 | 64 | 8 | <2 | 64 | <2 | <2 | 32 | <2 |
| titre | Animal 3 | 64 | <2 | <2 | 256 | <2 | <2 | 32 | <2 |
Animals with titers of <2 had less than 50% inhibition of RAd35 transduction in the in vitro assay at 1:2 serum dilution.
Sixty days after the intradermal injection (Fig. 2), we were unable to detect β-galactosidase positive cells in the brains of any of the three animals injected intrastriatally with RAd35 and intradermally with RAdHPRT (Fig. 2, row a). Importantly, numerous CD8+-, ED1+-, and MHC I-positive cells were still present in these brains, indicating that the anti-adenoviral immune priming had caused a chronic inflammatory response in the brain. The distribution of the inflammatory markers correlated with the pattern of β-galactosidase immunoreactivity in the control animals. In contrast, many β-galactosidase-positive cells, but few CD8+-, ED1+-, or MHC I-positive cells were detected in the brains of animals injected intrastriatally with RAd35 and intradermally with psoralen-inactivated RAdHPRT (Fig. 2, row b). Similarly, all animals injected in the brain with AdGS46 exhibited high levels of β-galactosidase immunoreactivity with no evidence of inflammation at this time point (Fig. 2, rows c and d).
Figure 2.
Transgene expression [β-galactosidase (β-gal)] and inflammatory responses (CD8, MHC I, ED1) in the brain, 60 days after intradermal injection of adenovirus. Rows a–d show brains from animals injected intrastriatally with 1.3 × 107 i.u. of RAd35 (rows a and b) or AdGS46 (rows c and d) at day 0, and intradermally with 5 × 108 i.u. of RAdHPRT (rows a and c), or the equivalent number of particles of psoralen-inactivated RAdHPRT (ps-RAdHPRT, rows b and d) at day 60 (i.e., day 120 postbrain injection). Scale bars and anatomical and immunohistochemical markers shown as for Fig. 1.
A quantitative analysis of the levels of transgene expression and inflammation in animals injected intracranially with RAd35 (Fig. 3) highlighted the high levels of inflammation and the loss of transgene expression in animals injected peripherally with RAdHPRT. Levels of CD8 and MHC I immunoreactivity at 21 days post-intradermal injection and ED1 and MHC I immunoreactivity at 60 days post-intradermal injection were statistically significantly higher in animals injected intradermally with RAdHPRT than in animals injected with psoralen-inactivated RAdHPRT. Correspondingly, β-galactosidase expression at 60 days postintradermal injection was completely absent in animals injected intradermally with RAdHPRT.
Figure 3.
Quantification of the area of 50-μm brain sections occupied by β-galactosidase, ED1, CD8, and MHC I immunoreactivity. Error bars show the SEM value from the three animals in each experimental group. At 60 days post-intradermal injection, there was no β-galactosidase immunoreactivity remaining in any of the animals injected intracranially with RAd35 and peripherally with RAdHPRT.
Because viral infection has been implicated in the activation of autoreactive demyelinating T cell inflammation in the brain (27), we assessed the integrity of brain myelin in each of our animals. No differences in myelin basic protein immunoreactivity were detected between the ipsilateral or contralateral sides of the brain in any of the animals, indicating that a primary demyelination had not occurred (Fig. 4). Accordingly, the distribution of CD8+ immunoreactive cells in the brains displaying strong inflammatory infiltration was not localized to the white matter.
Figure 4.
Immunohistochemical detection of myelin antigens during chronic inflammation caused by peripheral immunization with adenovirus in animals injected with first generation vectors into the brain. No loss of myelin basic protein immunoreactivity in the ipsilateral subcortical white matter or striatum was detected at day 120 in animals injected intrastriatally with RAd35 at day 0, and intradermally at day 60 with RAdHPRT. Thus, the chronic inflammation is not accompanied by loss of myelin specific proteins. (Bar = 1 mm.)
Long-Term Transgene Expression and Inflammation in the Brain, After a Single Intracranial Injection of Adenovirus Vector.
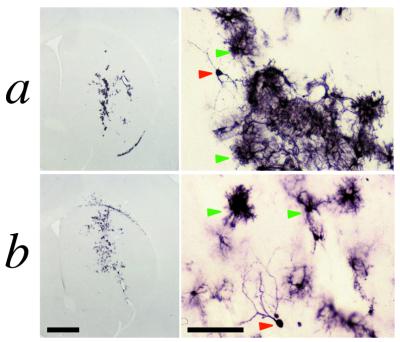
In contrast to the persistent inflammation observed in the brains of primed animals injected in the brain with RAd35, the duration of inflammation elicited by a single administration of either RAd35 or GS46 to the brains of naive animals was limited to less than 30 days. Animals were injected intrastriatally with 1.3 × 107 infectious units of RAd35 or AdGS46 and were killed at 3, 14, and 30 days and 6 months after the striatal injection, without peripheral re-exposure to adenovirus. Although significant inflammation was observed at 3 days after virus administration (i.e., infiltration of CD8+ cells and up-regulation of MHC class I and ED1 expression) (Fig. 5a), this was greatly diminished at 14 days postadministration (not shown). By 30 days postvector administration CD8, ED1, and MHC class I immunoreactivity was restricted to the scar surrounding the site of needle injection (Fig. 5b). The inflammatory responses to a single administration of either RAd35 or AdGS46 were comparable, and the anatomical distribution of β-galactosidase expression remained stable throughout the period analyzed. Importantly, all animals injected in the brain with RAd35 or GS46 displayed high levels of β-galactosidase immunoreactivity 6 months after virus administration (Fig. 6), indicating that both first-generation and HC-Ad vectors can sustain persistent expression in the absence of peripheral re-exposure to adenovirus. Morphological examination of stained cells indicated that predominantly glial cell types had been transduced and were stably expressing transgene.
Figure 5.
Immunohistochemical detection of transgene expression and inflammatory responses in the brain, 3 and 30 days after intrastriatal injection of a single dose of adenovirus. Rows a (3 days postinfection) and b (30 days postinfection) show the immunohistochemical detection of transgene expression and inflammatory markers, after a single administration of the first-generation vector RAd35, or the high-capacity vector AdGS46 to the brains of naïve animals (n = 3 per virus, per time point). Adenovirus-induced brain infiltration of CD8+ cells, ED1+ macrophages, and up-regulation of MHC class I expression subsided within 30 days whereas levels of β-galactosidase expression remained stable. Red arrowheads indicate the needle track. [Bar = 1 mm (in row b of the first column; scale for the low magnification images) and 150 μm (in row b of the fifth column; scale for the higher magnification images).]
Figure 6.
Expression of β-galactosidase seen 6 months after injection of RAd35 (row a) or AdGS46 (row b). Animals were injected with 1.3 × 107 infectious units of vector and were left to survive for 6 months without re-exposure to adenovirus. Brains were subsequently analyzed by immunohistochemistry for β-galactosidase immunoreactivity. Red arrowheads delineate transduced cells with neuronal morphology and green arrowheads delineate astroglial-like cells (the predominant cell-type transduced). [Bar = 1 mm (low magnification images) and 100 μm (high magnification images).]
Discussion
Realistic implementation of long-term gene delivery with first generation adenoviruses has been hampered by (i) their intrinsic inflammatory potential and (ii) short-term transgene expression, linked to their immunological instability in the presence of an anti-viral immune response. The intrinsic inflammatory potential in the brain is transitory: 30 days after administration of effective vector doses to the brain, all traces of acute inflammation have disappeared. Short-term expression is not a limitation of first-generation adenoviral vectors administered into the brain. We here show that they can express at stable levels for at least 6 months, and behavioral recoveries for up to 4 months have been described before (28). The immunological instability of adenoviral vectors has been the handicap most detrimental to their utility for neuroscience applications. During the lifetime of an individual previously subjected to gene transfer with an adenoviral vector, an immune challenge by natural infection with the same or a closely related viral serotype will be a likely occurrence. Thus, we demonstrate here that HC-Ad vectors are completely immune to an anti-adenoviral immune response.
Our data confirm that first-generation vectors are inefficient at sustaining high level, long-term transgene expression in the brain in the presence of a subsequent peripheral immune challenge with adenovirus. Consistent with the findings of Byrnes et al. (12), the activation of a peripheral anti-first-generation adenovirus vector immune response (i.e., a response against capsid proteins and viral gene products) caused brain inflammation and elimination of first-generation vector-mediated gene expression from the brain. Activation of a peripheral anti-adenovirus capsid immune response (a result of peripheral infection with psoralen-inactivated adenovirus, the genome of which cannot express any viral proteins) failed to cause inflammation or loss of first-generation vector-mediated transgene expression from the brain. Thus, it would appear that the principal targets of brain inflammation in animals intracranially injected with first-generation adenoviral vectors are proteins encoded by the adenoviral genome and expressed by persistently transduced cells. Interestingly, our data do not corroborate that immune responses to persistent first-generation vector in the brain cause demyelination, as reported earlier (12). Whether this is a result of the different techniques used to detect demyelination, rat strains, or identity of viral vectors, remains to be determined. Nevertheless, the association of T cell-mediated inflammation with autoimmune disorders of the brain renders unsafe any gene therapy strategy that carries the risk of eliciting such inflammation. Whether peripheral restimulation will simply eliminate transgene expression or stimulate an anti-CNS immune response remains to be determined. Interestingly, however, we have previously reported that, after successful inhibition of syngeneic glioma growth in the brain by using adenoviruses encoding herpes simplex virus 1-TK and peripheral administration of ganciclovir, a chronic inflammatory T-cell and macrophage/microglial response is generated accompanied by demyelination, which is secondary to cytotoxicity, but not autoimmune responses (4).
Importantly, we demonstrate that HC-Ad vectors deliver transgenes to the brain without risk of expressing any potential targets to CNS invading inflammatory cells, generated during an immune response resulting from subsequent peripheral infection with adenovirus or vector re-administration. Additionally, we report that such vectors can sustain stable and long-term transgene expression. These data confirm that the advantages of HC-Ads over first-generation vectors are attributable to the lack of viral coding sequences within the HC-Ad vector genome.
A further advantage of HC-Ad vectors is their high insert capacity, which allows the delivery of multiple expression cassettes or even genomic DNA (15, 18). This, together with the data of the present study, suggests that the use of HC-Ad vectors will markedly improve the prospects for long term, stable neurological gene therapy.
Supplementary Material
Acknowledgments
We acknowledge Frank Graham for the helper virus AdLC8cluc. We thank Tricia Maleniak, Emma Jones, and Peter Stanley for excellent technical assistance and Ros Poulton for expert secretarial help. We also thank Peter Wood for discussions regarding the peripheral immunizations, Ignacio Anegon for advice regarding the neutralizing antibody assay, and John Denton for his help with the quantitative analysis. P.R.L. is a fellow of The Lister Institute of Preventive Medicine. This work was supported by Grant 3087 from the Parkinson's Disease Society (United Kingdom) and European Union/Biomed Program Grants BMH4-CT98–3277 (to M.G.C. and P.R.L.) and QLK3-CT-1999-00364 (to M.G.C., P.R.L., and S.K.) and Grants 01KS9502 and 0311708 from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Germany (to S.K.).
Abbreviations
- HC-Ad vector
high-capacity adenovirus vector
- i.u.
infectious units
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120474397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120474397
References
- 1.Kaplan J M, Armentano D, Sparer T E, Wynn S G, Peterson P A, Wadsworth S C, Couture K K, Pennington S E, St. George J A, Gooding L R, Smith A E. Hum Gene Ther. 1997;8:45–56. doi: 10.1089/hum.1997.8.1-45. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Su Q, Wilson J M. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Jooss K U, Su Q, Ertl H C, Wilson J M. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 4.Dewey R A, Morrissey G, Cowsill C M, Stone D, Bolognani F, Dodd N J F, Southgate T D, Klatzmann D, Lassmann H, Castro M G, Löwenstein P R. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 5.Le Gal La Salle G, Robert J J, Berrard S, Ridoux V, Stratford-Perricaudet L D, Perricaudet M, Mallet J. Science. 1993;59:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 6.Davidson B L, Allen E D, Kozarsky K F, Wilson J M, Roessler B J. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- 7.Akli S, Caillaud C, Vigne E, Stratford-Perricaudet L D, Poenaru L, Perricaudet M, Kahn A, Peschanski M. Nat Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- 8.Byrnes A P, Rusby J E, Wood M J, Charlton H M. Neuroscience. 1995;66:1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes A P, Wood M J, Charlton H M. Gene Ther. 1996;3:644–651. [PubMed] [Google Scholar]
- 10.Kajiwara K, Byrnes A P, Charlton H M, Wood M J, Wood K J. Hum Gene Ther. 1997;8:253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- 11.Wood M J, Charlton H M, Wood K J, Kajiwara K, Byrnes A P. Trends Neurosci. 1996;19:497–501. doi: 10.1016/S0166-2236(96)10060-6. [DOI] [PubMed] [Google Scholar]
- 12.Byrnes A P, MacLaren R E, Charlton H M. J Neurosci. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morsy M A, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks R J, Graham F L, et al. Proc Natl Acad Sci USA. 1998;95:965–976. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiedner G, Morral N, Parks R J, Wu Y, Koopmans S C, Langston C, Graham F L, Beaudet A L, Kochanek S. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 16.Morral N, Parks R J, Zhou H, Langston C, Schiedner G, Quinones J, Graham F L, Kochanek S, Beaudet A L. Hum Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 17.Chen H H, Mack L M, Choi S Y, Ontell M, Kochanek S, Clemens P R. Hum Gene Ther. 1999;10:365–373. doi: 10.1089/10430349950018814. [DOI] [PubMed] [Google Scholar]
- 18.Kochanek S. Hum Gene Ther. 1999;10:2451–2459. doi: 10.1089/10430349950016807. [DOI] [PubMed] [Google Scholar]
- 19.Costantini L C, Jacoby D R, Wang S, Fraefel C, Breakefield X O, Isacson O. Hum Gene Ther. 1999;10:2481–2494. doi: 10.1089/10430349950016825. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson G W, Akrigg A. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southgate T D, Bain D, Fairbanks L D, Morelli A E, Larregina A T, Simmonds H A, Castro M G, Löwenstein P R. Metab Brain Dis. 1999;14:207–222. doi: 10.1023/a:1020728924026. [DOI] [PubMed] [Google Scholar]
- 22.Löwenstein P R, Sherring A F, Bain D, Castro M G, Wilkinson G W G. In: Protocols for Gene Transfer in Neuroscience. Löwenstein P R, Enquist L W, editors. New York: Wiley; 1996. pp. 93–114. [Google Scholar]
- 23.Mittereder N, March K L, Trapnell B C. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks R J, Chen L, Anton M, Sankar U, Rudnicki M A, Graham F L. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dion L D, Fang J, Garver R I., Jr J Virol Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- 26.Cotten M, Saltik M, Kursa M, Wagner E, Maass G, Birnstiel M L. Virology. 1994;205:254–261. doi: 10.1006/viro.1994.1641. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Z S, Granucci F, Yeh L, Schaffer P A, Cantor H. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 28.Geddes B J, Harding T C, Lightman S L, Uney J B. Nat Med. 1997;3:1402–1404. doi: 10.1038/nm1297-1402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.