Abstract
The proto-oncogene Sno has been shown to be a negative regulator of transforming growth factor beta (TGF-β) signaling in vitro, using overexpression and artificial reporter systems. To examine Sno function in vivo, we made two targeted deletions at the Sno locus: a 5′ deletion, with reduced Sno protein (hypomorph), and an exon 1 deletion removing half the protein coding sequence, in which Sno protein is undetectable in homozygotes (null). Homozygous Sno hypomorph and null mutant mice are viable without gross developmental defects. We found that Sno mRNA is constitutively expressed in normal thymocytes and splenic T cells, with increased expression 1 h following T-cell receptor ligation. Although thymocyte and splenic T-cell populations appeared normal in mutant mice, T-cell proliferation in response to activating stimuli was defective in both mutant strains. This defect could be reversed by incubation with either anti-TGF-β antibodies or exogenous interleukin-2 (IL-2). Together, these findings suggest that Sno-dependent suppression of TGF-β signaling is required for upregulation of growth factor production and normal T-cell proliferation following receptor ligation. Indeed, both IL-2 and IL-4 levels are reduced in response to anti-CD3ɛ stimulation of mutant T cells, and transfected Sno activated an IL-2 reporter system in non-T cells. Mutant mouse embryo fibroblasts also exhibited a reduced cell proliferation rate that could be reversed by administration of anti-TGF-β. Our data provide strong evidence that Sno is a significant negative regulator of antiproliferative TGF-β signaling in both T cells and other cell types in vivo.
Sno and Ski are the two most closely related members of the Ski/Sno proto-oncogene family. A third family member, Dach, is very distantly related and plays a role in central nervous system, eye, and skeletal-muscle formation (9, 24, 26). The Sno and Ski proteins localize to the nucleus (59) and appear to function as transcriptional activators or repressors (18, 43, 62). Loss of either Sno or Ski enhances tumor development in mice (52, 53), a phenotype also seen in the absence of E2F-1, a downstream mediator of transforming growth factor β (TGF-β) activity, and with other defects in TGF-β activity or signaling (20, 67, 72). Both Sno and Ski appear to function through interaction with other proteins that bind GTCTAGAC (11, 43), a consensus binding element for Smads, which are effector molecules for TGF-β signaling (29, 65). Indeed, both Sno and Ski can pair with Smad2, Smad3, and Smad4, repressing Smad trans-activation of target genes in vitro (1, 36, 56-58, 71). The complex regulatory interaction between Sno and the TGF-β pathway appears to involve both positive and negative feedback. Initially, TGF-β signaling induces degradation of Sno via Smad-dependent recruitment of the ubiquitin-dependent proteosome and the anaphase-promoting complex (4, 55). TGF-β later induces Sno expression, which then is thought to repress expression of TGF-β-responsive genes in a negative feedback loop (56). Sno- and Ski-mediated inhibition of TGF-β-induced gene expression appears to be effected by histone deacetylase recruitment (44, 52). However, Sno and Ski can also be shown to interact with other transcription factors, including TafII110 (11), N-CoR/SMRT (nuclear hormone receptor corepressor), Sin3A, and Rb (44, 52, 63).
Human Sno and Ski share a 106-amino-acid amino-terminal domain with 82% identity at the amino acid level. They appear to interact with each other through a 55% identical carboxyl-terminal region that includes two predicted α-helical coiled coils (25, 41). Sno and Ski are differentially expressed in some mature tissues and respond differently to several signals, suggesting that they produce nonredundant effects in vivo. It has been shown that Sno (but not Ski) is induced with immediate-early kinetics upon serum stimulation of quiescent mouse 10T1/2 fibroblasts (46). Sno is also selectively induced upon the onset of muscle cell differentiation, peaking prior to MyoD and myogenin induction (40). Although Sno and Ski are both cell cycle regulated in cycling myeloid cells in culture, with mRNA levels peaking in the early to mid-G1 phase of the cell cycle (45), these genes are differentially expressed in the cells of lymphoid lineages (45). It has been shown that Sno is selectively expressed in T lymphocytes but not in B lymphocytes, whereas Ski is expressed in both cell types (45). In this report, we show that Sno is expressed in the earliest stages of thymocyte development and in the spleen as soon as it is formed. Sno is also present in unstimulated primary mouse splenocytes in the adult and is upregulated within 1 h of T-cell receptor stimulation. Because of its restriction to cells of the T-lymphocyte lineage and its suspected relationship to TGF-β signaling, we hypothesized that Sno might be important in preventing suppressive TGF-β-mediated antiproliferative activity in the initial steps of T-cell activation during a productive immune response.
To characterize Sno function in lymphoid cells and other cells in vivo, we used homologous recombination gene targeting in mice to make two deletion mutations. A 5′ deletion, removing 1.7 kb of regulatory sequences at the 5′ end of the Sno gene, leaves coding sequences intact. This mutation decreased Sno mRNA and protein expression to low basal levels but appeared to leave activation-induced increases intact. A second construct deleted exon 1, removing the coding sequences for the first half of the protein and effectively eliminating Sno expression in homozygous mice. Mice homozygous for either deletion are viable and show no deviant phenotype on gross inspection in either a C57BL/6, 129/Sv, or mixed 129/Sv-C57BL/6 genetic background. However, both mutations caused a reproducible defect in T-cell activation, accompanied by signs of increased sensitivity to TGF-β. The mutant defect is rescued by the addition of exogenous interleukin-2 (IL-2) to the cultures. This suggests a direct relationship between Sno activity and the production of proproliferative cytokines. Indeed, we found that Sno-null splenocytes express reduced levels of IL-2 as well as IL-4 and that Sno overexpression can trans-activate the IL-2 promoter in transfection assays. These results suggest that Sno functions early in the T-cell activation pathway(s) to inhibit tonic TGF-β modulation of the pathway in response to strong activating signals and that early response of the Sno gene to T-cell receptor triggering is required for optimal IL-2 production and subsequent T-cell proliferation.
MATERIALS AND METHODS
RNA isolation and RT-PCR.
RNA was isolated as described previously (10) and reverse transcribed using random hexamer primers. Reverse transcription (RT)-PCR is performed with specific primers and [32P]dCTP in the reaction. Pilot studies were used to determine the conditions for linear incorporation of 32P with the amount of input RNA (27 cycles with 0.7 μg of total RNA for Sno and 0.05 μg for GAPDH [glyceraldehyde-3-phosphate dehydrogenase] [Table 1 ]) (17, 49). One-fifth of the [32P]dCTP-labeled reaction mixture was loaded on a 5% acrylamide gel, electrophoresed, exposed to a phosphor screen, and scanned for image analysis. The band identities were confirmed by the band sizes and by Southern blot hybridization of cold RT-PCR products to radiolabeled Sno probes (not shown). Real-time PCR was performed, using SYBR Green dye and primer concentration conditions that we optimized in pilot experiments, in a Prism 7700 sequence detector (Applied Biosystems, Inc., Foster City, Calif.). Oligonucleotide primer sequences and template amounts are presented in Table 1. Real-time PCR for IL-2 and IL-4 was performed using predeveloped TaqMan primers (Applied Biosystems, Inc.).
TABLE 1.
Oligonucleotide primer sequences and conditions
| Gene | Oligonucleotide
|
Annealing temp (°C) | Size (nt) | Amt of template (μg in 50 μl) | 5′- to-3′ sequence | |
|---|---|---|---|---|---|---|
| 5′ | 3′ | |||||
| SnoN, SnoN2 | oM094 | 62 | 427, 289 | 0.7 | CTGCTGCGTCCCAGTCTA | |
| oM065 | TGAACTGCTCAGCATCTCCACCTCCAT | |||||
| Ski | oM099 | 62 | 283 | 0.7 | GTTTTGGGTCTTATGGAAGCTGGGGGCTC | |
| oM100 | GCTGCCTGCATCCAGTGCCTCGACTGCCGCCTCATGT | |||||
| IL-2a | oM245 | 60 | 413 | 0.0125 | TTCAAGCTCCACTTCAAGCTCTACAGCGGAAG | |
| oM246 | GACAGAAGGCTATCCATCTCCTCAGAAAGTCC | |||||
| IL-2Rαa | oM257 | 60 | 700 | 0.0125 | ATGGAGCCACGCTTGCTGATGTTG | |
| oM258 | CCATTGTGAGCACAAATGTCTCCG | |||||
| IL-4a | oM247 | 60 | 357 | 0.025 | CCAGCTAGTTGTCATCCTGCTCTTCTTTCTCG | |
| oM248 | CAGTGATGTGGACTTGGACTCATTCATGGTGC | |||||
| GAPDHa | oM205 | 62 | 983 | 0.05 | TGAAGGTCGGTGTGAACGGATTTGGC | |
| oM206 | CATGTAGGCCATGAGGTCCACCAC | |||||
| L7 | oM092 | 62 | 210 | 0.7 | GGAGCTCATCTATGAGAAGGC | |
| oM093 | AAGACGAAGGAGCTGCAGAAC | |||||
| JunB | oM277 | 60 | 134 | 0.6 | GTTCCTGGCTCTTAGTTTGCCG | |
| oM278 | CTGGAGTCCGTGAATTGGATTG | |||||
Sequences originally from Clontech.
Splenocyte stimulation.
Splenocytes were isolated from unprimed C57BL/6 mice (Fig. 1) or B6;129/Sv mice of the different genotypes and plated on 10-cm-diameter dishes precoated with 100 μg of anti-CD3ɛ (αCD3) antibody/ml (catalog no. 145-2C11; BD Biosciences Pharmingen, San Diego, Calif.). Adherent cells were harvested after 1 to 48 h for RNA isolation. Both adherent and nonadherent cells were collected for immunophenotyping; some splenocytes were harvested prior to plating (zero hour).
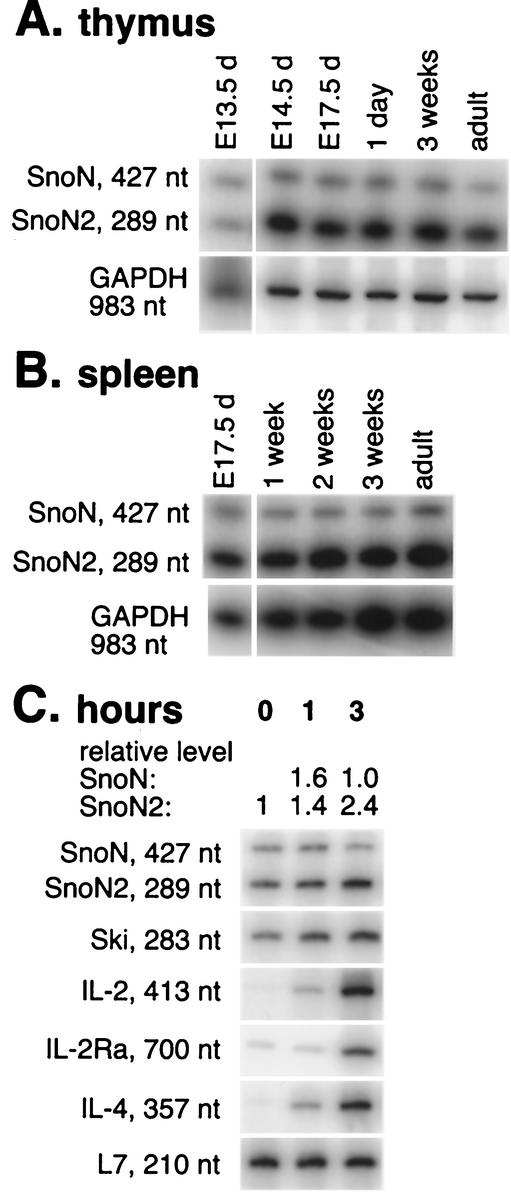
FIG. 1.
Sno is expressed in embryonic thymus and spleen from the earliest stages. (A and B) Thymus tissue (A) or whole spleens (B) were isolated from outbred Swiss-Webster embryos or mice at the times indicated (d, dpc), and then total RNA was purified and RT-PCR was performed with specific primers as described in Materials and Methods. RT-PCR with GAPDH or L7 primers showed that reverse-transcribed-RNA was present in all samples in comparable amounts. The images shown are composites of phosphorimager files; the relationship between the signal and image intensity is linear. Sno and Ski mRNAs are upregulated within 1 h after in vitro stimulation of primary splenocytes. (C) Splenocytes freshly isolated from wild-type mice were plated on plate-bound αCD3 antibody. Total RNA was isolated after 0, 1, or 3 h of culture and subjected to RT-PCR using primers as described in Materials and Methods. The calculated levels of SnoN and SnoN2 mRNAs relative to zero time (set at 1), and independently verified using real-time RT-PCR, are shown above the figure.
Targeting vector and gene targeting.
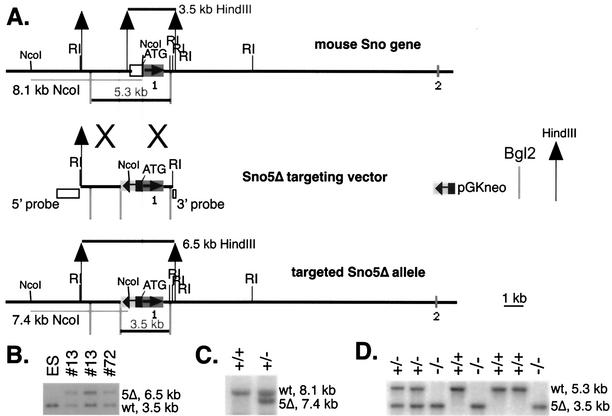
A 20-kb 129/Sv strain mouse Sno genomic DNA clone in lambda FIX II (Stratagene) phage was used to generate the targeting vectors. A pGK-neo gene expression cassette (50) was substituted for 1.7 kb of Sno promoter immediately upstream of the NcoI site at the ATG translation initiation codon (Fig. 2A). The similarity of the sequence of this region in the mouse Sno gene to the chicken genomic Sno sequence (21) led us to expect that this deletion would remove promoter elements controlling Sno expression. The Sno5Δ construct also deleted the 500-nucleotide (nt) 5′ untranslated region and the context for the initiation of translation. Fill-in of the NcoI 5′ overhang to make a blunt end disrupted the NcoI recognition site but regenerated the ATG codon (boldface); the Kozak consensus context was altered from GTGGCCATGG to GAATTCATGG. Since the coding region remained intact in the 5Δ mutant, we generated another allele deleting all of exon 1 (designated ex1), thus deleting all of the biological activities so far described for Sno (Fig. 2A). A lacZ gene with a nuclear localization signal was substituted for the exon 1 coding sequences that were deleted. The targeting constructs (Fig. 2A and 3A) were electroporated into R1 ES cells (42) and selected in 200 μg of G418/ml on irradiated G418-resistant mouse embryo fibroblast (MEF) feeders isolated from our TgN(pGKneo)066Spw transgenic mice. ES clones exhibiting homologous integration of the targeting vector (n = 2 for 5Δ and n = 4 out of the 12 clones isolated for ex1) were thawed, expanded briefly, injected into C57BL/6 blastocysts, and implanted into pseudopregnant recipient mice, which were allowed to deliver litters (7). We obtained one or more germ line-transmitting male chimeras for each line. One line of Sno5Δ was chosen for further analysis. Two lines of Snoex1 were studied and behaved identically; the results reported here were from one of these lines. Animal use complied fully with federal guidelines, with prior approval of the animal protocol by the Institutional Animal Care and Use Committee.
FIG. 2.
(A) Sno5Δ gene targeting construct [B6;129-TgH(Skir5Δ)1Spw]. The 6.1-kb EcoRI (RI) fragment that contained exon 1 from the Sno gene was used to generate the targeting vector. An internal fragment extending from the NcoI site at the ATG translation start site to a BstEII site 1.7 kb upstream was replaced with the pGKneo cassette. This mutation is called 5Δ due to the removal of sequence 5′ of the coding region. The deletion removes a HindIII site, and the resulting larger HindIII fragment (6.5 kb) hybridizes with a probe outside the targeting construct. With the wild-type gene, the probe hybridizes with a 3.5-kb HindIII band. The symbols for the pGKneo cassette, Bgl2 site, and HindIII site are defined to the right of the figure. (B) Autoradiogram of Southern blot of 10 μg of DNA from ES cells and two targeted clones digested with HindIII, electrophoresed on a 1.2% agarose gel, alkaline blotted to Hybond N+ (Amersham Biosciences, Piscataway, N.J.), and probed with the radiolabeled 3′ probe shown in panel A. Clone 13 appears in duplicate at two concentrations. wt, wild type. (C) The NcoI site at the ATG codon is destroyed, but another is introduced in pGKneo that is closer to the probe, making a 7.4-kb band with the 5′ probe; the wild-type band is 8.1 kb. The autoradiogram shows a Southern blot of an NcoI digest from a wild type (+/+) and an Sno5Δ+/− (+/−) heterozygote probed with the 5′ probe shown in panel A. (D) The pGKneo insert introduces a BglII site so that an exon 1 probe hybridizes with a 3.5- instead of a 5.3-kb band when digested with BglII. The autoradiogram shows a Southern blot of a Bgl2 digest from a weanling Sno5Δ+/− heterozygote intercross litter probed with exon 1; three mice are homozygous mutant at the Sno locus.
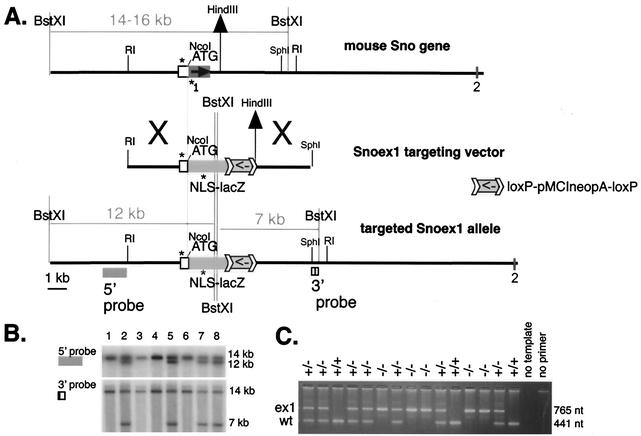
FIG. 3.
(A) Snoex1 targeting construct [B6;129-TgH(Skirex1)2Spw]. The 6.1-kb EcoRI fragment described in the legend to Fig. 2 was used for the 5′ end, subcloning the first 4.4 kb up to the NcoI site into ApaI-NcoI-digested pSKTNLSLacZ (60, 61), generously provided by Shahragim Tajbakhsh and Margaret Buckingham. pMMneoflox8 (23), generously provided by Tim Bender and Klaus Rajewsky, was cut with XbaI and ligated to BamHI adaptor linkers (catalog no. 1108 and 1133; New England Biolabs) and then cut with HindIII and ligated into the BamHI-digested lacZ construct (described above), together with a 4.1-kb BamHI/HindIII fragment purified from a downstream SphI genomic subclone. The symbol used for the loxP-flanked pMCIneo cassette is shown at the right. The targeting vector introduces two nearby BstXI sites that cleave a 14- to 16-kb band that hybridizes with both 5′ and 3′ flanking probes to give an apparent 12- or 7-kb band, respectively. This mutation is called ex1 to indicate the deletion of exon 1 coding sequences. The PCR primers (asterisks) are oM263 (AATGTGAGCGAGTAACAACCCG) in the lacZ segment of the targeting vector, oM264 (CCAACCAAACTCAACCATTACACC) in the Sno promoter, and oM265 (TTCACAGGAGGACTGCCATCATCC) in wild-type Sno. (B) Autoradiogram of Southern blot of 10 μg of DNA from eight ES clones digested with BstXI and probed sequentially with the 5′ and 3′ probes (Fig. 2A). Four of the eight clones shown are targeted; the overall targeting frequency was 12 of 200. (C) PCR assay of weanling heterozygote (+/−) intercross mice; five homozygous mutant (−/−) animals are in this group. wt, wild type.
Immunoprecipitation of Sno protein.
To examine Sno protein, 13.5-day postcoitum (dpc) embryos were eviscerated and homogenized in 10 ml of RIPA buffer per g of tissue (56) with fresh protease inhibitor cocktail (Complete; Roche Applied Science, Indianapolis, Ind.). Eviscerated embryos were used because LacZ staining of embryos (not shown) revealed them to have the highest expression of Sno protein. Precleared lysates were immunoprecipitated overnight with anti-Sno polyclonal antibody (H317; Santa Cruz Biotechnology, Santa Cruz, Calif.) or preimmune serum, electrophoresed, Western blotted using a mixed anti-Sno monoclonal antibody probe generously provided by Shunsuke Ishii (52) and goat anti-mouse horseradish peroxidase-coupled secondary antibody (Sigma, St. Louis, Mo.), and developed by Western Lightning chemiluminescent detection (New England Nuclear, Boston, Mass.).
T-cell proliferation assay for function of unprimed T cells.
Spleen cells from littermates of each genotype were seeded into microwells. The following stimulation conditions were employed: 10 ng of 145-2C11 (αCD3)/well plate-bound in 96-well plates, 50 ng of phorbol myristate acetate (PMA)/ml with 1 μg of ionomycin/ml, and 20 U of recombinant human IL-2/ml. Recombinant human TGF-β1 (catalog no. 240-B; R& D Systems, Minneapolis, Minn.) was used at 100 pM. Anti-TGF-β antibody (MAB1835, clone 1D11 anti-TGF-β1, -β2, -β3; R&D Systems) was used at 2 μg/ml. Cells were incubated for 66 h at 37°C with a stimulator in the medium or plate bound and then for an additional 6 h with [3H]thymidine and were then harvested onto glass fiber filters (Tomtec harvester and Wallac 1450 MicroBeta scintillation counter; Perkin-Elmer, Boston, Mass.) and counted in scintillation fluid to determine the [3H]thymidine incorporation.
Transient transfections and luciferase assays.
10T1/2 cells (3 × 105 per 60-mm-diameter plate) were transfected with 1 μg of reporter with or without 2 μg of Sno expression construct in a total DNA mass of 3 μg using lipofectAMINE (Gibco-Invitrogen, Grand Island, N.Y.). Transfections and assays were done in triplicate. Cells were harvested 48 h after transfection in groups of six or fewer to minimize variations in activity (8). The washed cell monolayers were scraped into reporter lysis buffer (Promega, Madison, Wis.) for harvest. The lysates were assayed for protein concentration (6) (Bio-Rad [Hercules, Calif.] protein assay), and 50 μg of protein from each sample was measured into tubes in duplicate for luciferase assays in a Pharmingen luminometer with Promega dual-injection reagents. An internal transfection control, such as pSVβgal, was not included with each experimental sample, since it had been demonstrated that Sno activates the simian virus 40 and several other enhancers and distorts the control (K. Jessen and S. Pearson-White, unpublished data), as does Ski (32). pSVβgal transfection controls were done in parallel and stained for LacZ activity (Roche Applied Science) to compare the transfection efficiencies of MEFs of different genotypes.
Cell proliferation assay.
MEFs were isolated by trypsinizing and mechanically dissociating 13.5-dpc embryo carcasses as described previously (2). Multiple separate cell preparations were tested for each genotype. MEFs were plated at equal densities in quadruplicate in 96-well plates at the appropriate concentrations of TGF-β; cultured for 24 h, with the final 3 h in [3H]thymidine; and then harvested onto glass fiber filters and counted as described above to determine the [3H]thymidine incorporation. Anti-TGF-β antibody (MAB1835; R&D Systems) was used at 2 μg/ml.
Statistics.
Statistical significance was calculated using the paired one-tailed Student's t test. The probabilities given are the calculated likelihood that the two sets of values are from the same population. All experiments were performed completely at least three times; representative data from one typical experiment are shown.
RESULTS
Sno mRNA expression in the immune system.
Two isoforms of Sno mRNA have been described (46, 47). SnoN is the product of the full-length mRNA. The SnoN2 isoform (also called sno-dE3) is generated by use of an internal alternative splice donor site within exon 3 (46, 47). The SnoN2 transcript is 138 nt shorter than SnoN, predicting the translation of a protein product missing a 46-amino-acid segment (46, 47). Mouse Sno expression contrasts with that of the human gene, which produces SNON2 as a minor percentage of total SNO mRNA in the tissues examined (46). Mouse SnoN and SnoN2 mRNAs are expressed throughout thymocyte and splenocyte development, appearing first in thymic progenitor cells at 13.5 dpc (Fig. 1A and B), as determined by semiquantitative 32P RT-PCR. The ratio between SnoN and SnoN2 is 1:4 in spleen and in thymus tissue at most ages tested (Fig. 1A and B) but is closer to 1:1 in embryonic 13.5-dpc thymic cells. These expression levels and ratios were confirmed using isoform-specific primers in real-time PCRs (data not shown). It has been shown that the SnoN/SnoN2 ratio is also 1:4 in many nonhematopoietic tissues (e.g., in liver, kidney, and skeletal muscle) but is consistently 1:1 in the brain (46). The functional significance of the two isoforms is unknown, but tissue-specific differences in their relative expression levels suggest important functional differences between them.
Sno mRNA is induced upon T-cell receptor stimulation of wild-type splenocytes.
We had previously shown that serum stimulation of fibroblasts upregulates SnoN2 expression by 1 h, resulting in peak expression at 3 h (46). To examine whether Sno was inducible by antigen receptor triggering in T cells, splenocytes from unprimed mice were isolated and cultured for 0, 1, or 3 h with plate-bound antibody specific for the receptor-associated signaling molecule CD3ɛ, a strong proliferative stimulus for normal T cells. Increased expression of both SnoN and SnoN2 was detectable by 1 h after T-cell receptor stimulation (Fig. 1C). The level of increase in Sno expression was modest but was independently confirmed using isoform-specific primers in real-time PCR experiments (data not shown). SnoN2 mRNA remained the dominant isoform in stimulated T cells, increasing by twofold in splenocytes within 3 h. As expected, autocrine growth factor signaling pathways, represented by IL-2, IL-2 receptor α-chain (IL-2Rα), and IL-4 mRNAs, were induced with slightly delayed kinetics relative to Sno but were also detected by 3 h (Fig. 1C). Ski was also upregulated 2.5-fold upon T-cell receptor stimulation (Fig. 1C).
Gene targeting produces altered expression of Sno in vivo.
To examine Sno function in vivo, we performed gene targeting in mice. A pGK-neo gene expression cassette (50) was substituted for 1.7 kb of Sno promoter immediately upstream of the NcoI site at the ATG translation initiation codon (Fig. 2A) to make the 5Δ mutation. The coding region remains intact in this mutant, allowing a low basal level of expression of the entire Sno gene product. A second mutant allele (ex1), generated by deleting all of exon 1, removes that portion of the coding sequence responsible for all of the biological activities described to date for Sno (Fig. 3A). After electroporation into ES cells, selection, and screening, 2 out of 160 clones (5Δ) and 12 out of 200 clones (ex1) had undergone homologous recombination at one allele of the Sno locus (Fig. 2B and C and 3B) and were successfully passed through the mouse germ line.
When bred from heterozygotes, mice homozygous for either mutant Sno allele are viable at weaning (Fig. 2D and 3C) in the expected 1:2:1 ratio in mice carrying a mixed 129/Sv-C57BL/6 genetic background, as well as in mice backcrossed through several generations onto either 129/Sv or C57BL/6. No gross morphological defects have been noted in homozygotes carrying either mutation, and the mice are not subject to early mortality on a mixed genetic background or after backcrossing. Maintenance of the lines on a 129/Sv background was difficult, probably because of reduced fertility of this strain. However, both mutant alleles were successfully propagated by backcrossing them to C57BL/6. Heterozygote intercrosses were used to generate animals for testing but not to propagate the line. Mice used in these studies resulted from intercrosses using the 11th generation of backcrossing for Sno5Δ and the 3rd and 4th generations for Snoex1. Two independent lines initially established for the Snoex1 mutant gave identical results in phenotypic screening.
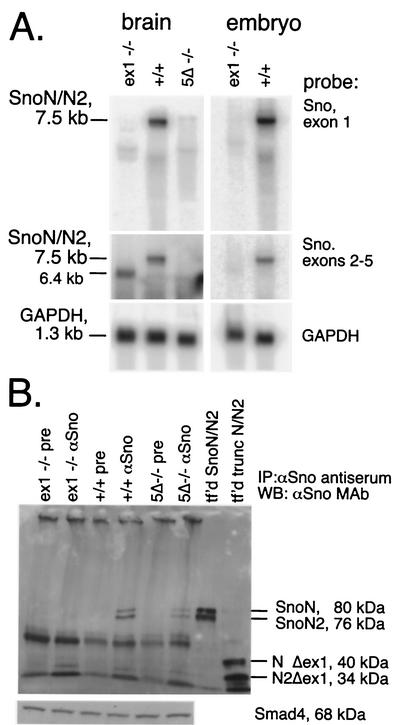
Expression of Sno is highest in embryonic mouse skin; after birth, Sno expression is highest in brain tissue. In the Snoex1−/− tissues, Sno poly(A)+ mRNA was reduced below the limit of detection with a probe specific for the exon 1 sequence that was deleted (Fig. 4A, top, exon 1 probe). Using this exon 1 probe, a minor amount of Sno mRNA with retarded mobility was detectable in the brains of mice homozygous for the Sno5Δ mutation, which retains this sequence (Fig. 4A, exon 1 probe). Rehybridization with a separate probe for exons 2 to 5 showed that although exon 1 was deleted from Snoex1−/− mice, downstream initiation produced a 6.4-kb 3′-end RNA species in the brain, but there was much less produced in embryos (Fig. 4A, panels with the probe from exons 2 to 5, lanes 1 and 4). This species is not detected in the Sno5Δ−/− tissue on Northern blots (Fig. 4A, panels with the probe from exons 2 to 5, lane 3) but is detectable using RT-PCR and primers spanning exons 3 to 5 (data not shown). The probes used do not cross-react with Ski. A faint background band (or doublet) at ∼4.8 kb is seen with these probes and comigrates with mouse 28S RNA in total-RNA Northern blots. Thus, we suspect that this faint band is due to the residual 28S RNA remaining after polyA+ purification and is not related to Sno or Ski. Rehybridization of the blot with a GAPDH probe showed that the lanes were comparably loaded with RNA (Fig. 4A). Similar results were observed with RNAs from other tissues in both lines (data not shown), although lower expression levels in tissues other than these make definitive demonstration of decreased or absent mRNA levels less convincing.
FIG. 4.
Sno mRNA and protein are expressed in wild-type but not mutant tissue. (A) Five micrograms of poly(A)+ RNA from brain or eviscerated 13.5-dpc embryo tissue of mice of the indicated genotypes was electrophoresed and subjected to Northern blotting. The blot was hybridized with a probe for exon 1 and then stripped and rehybridized with a probe for exons 2 to 5 and then with a GAPDH probe to show that the lanes were equally loaded with RNA. The probe used for each autoradiogram is shown to the right of each blot. (B) To examine Sno protein expression, lysates from wild-type or mutant eviscerated 13.5-dpc embryos were subjected to immunoprecipitation (IP) and Western blotting (WB). Total protein lysates (140 mg of tissue per lane) were immunoprecipitated with anti-Sno polyclonal antiserum (αSno) or preimmune serum (pre). The immunoprecipitated products were electrophoresed on a 7.5% polyacrylamide Tris-glycine gel, blotted to polyvinylidene difluoride membranes, and probed with a mixture of four anti-Sno monoclonal antibodies (αSno MAb) (52). X-Ray film exposed by the enhanced chemiluminescence signal was laser scanned to make the image. 293T cells were transfected separately with Sno expression vectors, immunoprecipitated with anti-FLAG-agarose beads, and pooled to provide positive controls (tf'd SnoN/N2 at 80 and 76 kDa; tf'd trunc N/N2 at 40 and 34 kDa). Aliquots of each mouse embryo lysate were electrophoresed, blotted, and probed with anti-Smad4 antibody (Santa Cruz) to show that equal amounts were loaded. Molecular mass markers (not shown) were used to measure the approximate molecular masses of the bands.
Despite the presence of RNA derived from downstream coding sequence in Snoex1−/− mice, no mature full-length protein is detectable in these mice on immunoblots (Fig. 4B). To control for the possibility that truncated mutant proteins are produced, we made Sno exon 1 deletion mutant cDNA constructs containing only cDNA from exons 2 to 6 for SnoN and SnoN2 and used them to transfect 293T cells (Fig. 4B, lane tf'd trunc N/N2). Transfected protein lysates were then electrophoresed in parallel with the lysates from embryo fibroblasts. Although a minor band of the appropriate size for truncated SnoN2 (Fig. 4B, tf'd N2Δex1; 34 kDa) was observed in the Snoex1−/−-immunoprecipitated lane, no truncated SnoN band is detected. The truncated SnoN2 band is also present in the wild-type lane at a similar level. It is therefore unlikely to be responsible for the phenotypes observed in Snoex1−/− cells. Since the Sno5Δ deletion leaves exon 1 coding sequence intact, we expected to see Sno protein in embryo lysates from Sno5Δ−/− mice but at decreased levels based on the small amounts of Sno mRNA detected by Northern blot analysis. As predicted, Sno protein was detected in mice homozygous for Sno5Δ at reduced levels. We measured ∼40% as much Sno5Δ protein as seen in wild-type mice, using densitometric measurement of the laser-scanned autoradiogram (Fig. 4B). The polyclonal antibodies used for the immunoprecipitation and the monoclonal antibody mixture used for the blot hybridization are both capable of binding to any remaining carboxyl-terminal peptide that the 6.4-kb Snoex1−/− mRNA might encode. Thus, the homozygous Snoex1 mutation appears to eliminate Sno expression, as expected, while the Sno5Δ mutation significantly decreases Sno expression. An anti-Smad4 probe detected comparable levels of Smad4 in mice of each genotype, suggesting no feedback alteration of Smad4 expression in either line (Fig. 4B).
Sno mutations produce no gross phenotypic changes in homozygous mice.
At 3 months of age, littermates of all three genotypes (Sno5Δ−/−, Snoex1−/−, and their Sno+/+ siblings) displayed no differences in the gross morphology of any organ system. Tissue sections from testis, salivary gland, skeletal muscles, heart, lung, and liver, as well as smears taken from bone marrow and blood, were also unremarkable. No significantly increased incidence of somatic tumors has been observed up to 2 years of age. Splenomegaly (enlarged spleen) was noted among a few Sno5Δ−/− and Sno5Δ+/− mice >6 months old in a mixed 129/Sv-C57BL/6 genetic background. However, weights and cell counts of spleens harvested from mice at younger ages (2 to 6 months) or mice with a higher percentage of C57BL/6 background have yielded no significant difference in the numbers of splenocytes from wild-type and mutant Snoex1−/− or Sno5Δ−/− spleens. Similarly, age- and sex-matched mutant mice and their sibling controls maintain similar numbers of thymocytes.
T-cell subpopulations from thymus and spleen were analyzed by flow cytometry. There were no significant differences between homozygous mutants of either line and their sibling controls in thymocyte or splenocyte expression of the CD4, CD8, CD25, or CD62L differentiation antigens (data not shown). Sno is not expressed in B cells, and as expected, no differences in the percentages of B220+ splenocytes were observed (data not shown). These results have remained consistent through 11 generations of backcrossing to C57BL/6 for the Sno5Δ mutation.
Sno5Δ−/− and Snoex1−/− splenocytes exhibit reduced T-cell activation in response to T-cell receptor stimulation.
The T-cell receptor complex consists of an antigen-specific heterodimer, structurally related to immunoglobulin molecules, and an associated multimeric signaling complex designated CD3. Plate-bound αCD3 antibody is a potent T-cell receptor stimulatory signal. Sno5Δ−/− and Snoex1−/− splenocytes have a T-cell proliferation response to αCD3 stimulation that is 15 to 60% of wild type (Fig. 5A; 26 and 36% in the example shown). Heterozygote values were somewhat variable, ranging from fully wild type to intermediate between wild type and homozygous mutant in many experimental replicates (data not shown). Analysis on days 2 to 5 of culture showed that the peak proliferative response was on day 3 for both wild-type and mutant cultures; day 3 data are shown. Mutant splenocytes also showed similar decreases in proliferative response after stimulation by the bacterial superantigen staphylococcal enterotoxin B and by mitomycin C-treated allogeneic spleen cells (data not shown), demonstrating that the proliferative defect seen in these mice is not restricted by the type of external stimulus applied through the T-cell receptor or its signaling partners.
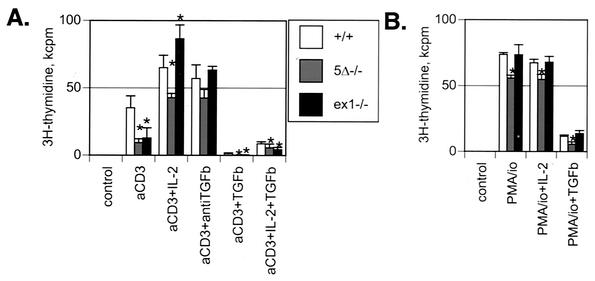
FIG. 5.
T-cell proliferation assays examining functions of unprimed T cells show that Sno5Δ−/− and Snoex1−/− mutant T cells have a T-cell activation defect that is largely compensated for by addition of excess IL-2 or incubation with anti-TGF-β antibody. (A) Spleen cells from littermates of each genotype were seeded at a density of 500 × 103 responder cells per microwell in 96-well plates. The cells were incubated for 66 h at 37°C and then for a final 6 h with 1 μCi of [3H]thymidine and then harvested onto glass fiber filters to determine the [3H]thymidine incorporation. The numbers presented are kilocounts of [3H]thymidine per minute (background samples without stimulator were subtracted) in the average of triplicate wells from a representative experiment. The error bars indicate the calculated standard deviations. For T-cell receptor stimulation of splenocytes, 10 ng of 145-2C11 αCD3 allogeneic major histocompatibility complex anti-T-cell receptor (T-cell receptor) monoclonal antibody was preincubated in each well as indicated (aCD3). Additional antibody or cytokines were added as indicated (TGFb, TGF-β). Control wells had no αCD3 stimulator or other additions to the media and had very low proliferation. The genotypes were wild type (Sno+/+), Sno5Δ−/−, and Snoex1−/−. The asterisks indicate results that were statistically significantly (P < 0.05) different from the wild type. (B) Splenocytes were stimulated with PMA-ionomycin (PMA/io), with added cytokines as indicated. [3H]thymidine incorporation was measured on day 4 after plating. The genotypes were as in panel A. Representative experiments of 5 to 12 repetitions are shown in both panels.
Despite the defective proliferation seen in response to triggering through the T-cell receptor, T cells from Snoex1−/− mice could proliferate well in culture after stimulation with the combination of PMA and ionomycin. PMA and calcium ionophores together bypass the early steps in T-cell activation that require the T-cell receptor complex by direct activation of downstream intracellular signaling cascades (48). Interestingly, differences were noted in the ability of PMA-ionomycin to induce proliferation of cells carrying the two Sno mutations: Sno5Δ−/− cells proliferated only 75% as well as wild-type cells in response to PMA-ionomycin, whereas Snoex1−/− cells proliferated as well as the wild type under these conditions (Fig. 5B). This may reflect differences in compensation by other genes regulating cell growth that are invoked in Sno-null cells but not in the hypomorph.
Exogenous IL-2 also reverses the Sno mutant defect.
T cells require the support of growth factors in order to proliferate after stimulation through the T-cell receptor complex. IL-2 is the major proproliferative cytokine produced to support clonal expansion of antigen-activated T cells. It is primarily, if not exclusively, produced by T cells in vivo and functions in an autocrine, as well as a paracrine, fashion. Addition of IL-2 to cultures of Snoex1−/− cells effectively reversed the proliferation defect in response to αCD3 stimulation (Fig. 5A). Although IL-2 completely restored the deficit in proliferative response in T cells from Snoex1−/− mice, it only partially restored proliferation of Sno5Δ−/− cells (66% of wild type). This difference was observed consistently in all the repetitions of this experiment and mirrors the incomplete recovery of proliferative capacity seen with PMA-ionomycin stimulation.
The Sno mutant defect may be due to reduced antagonism of TGF-β signaling.
TGF-β is known to inhibit T-cell activation through suppression of IL-2 production (5, 22, 31). Since Sno has been shown to suppress the response to signaling through TGF-β receptors in other cell types (55, 56, 58), we tested whether the Sno mutant defect in T-cell proliferation could be reversed by addition of panspecific anti-TGF-β antibody. Blocking endogenous TGF-β signaling with anti-TGF-β antibody completely rescued the proliferation of the Snoex1−/− mutant T cells in response to αCD3 stimulation. Although rescue of Sno5Δ−/− T cells by anti-TGF-β antibody consistently appeared to be incomplete (averaging 75% of Snoex1 recovery), it was not statistically significantly different from the wild type (P < 0.092) (Fig. 5A). Both wild-type and mutant cells were sensitive to the addition of 100 pM exogenous recombinant TGF-β, showing severely reduced levels of proliferation under these conditions (Fig. 5A). The addition of TGF-β reduced cell proliferation of Sno mutant cells to the level of unstimulated control cultures, whereas wild-type cells were not as completely inhibited from proliferating (Fig. 5A), consistent with increased TGF-β sensitivity in mutant cells. High doses of exogenous IL-2 could not completely reverse the suppressive effect of added TGF-β in either wild-type or mutant cells (Fig. 5A), suggesting that inhibition of IL-2 production alone does not completely account for the inhibitory capacity of TGF-β at this concentration.
IL-2 and IL-4 mRNAs are reduced in Sno-null splenocytes and are induced to lower levels after T-cell receptor stimulation.
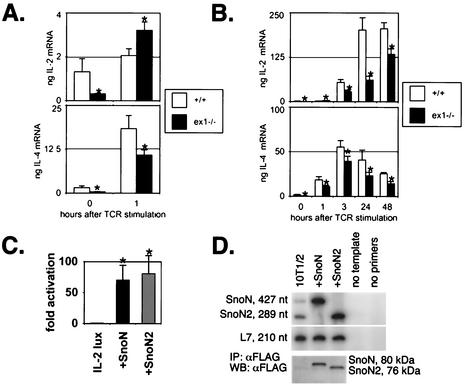
IL-2 and IL-4 mRNA levels were found to be lower in unstimulated Sno-null than in wild type splenic T-cell populations (Fig. 6A), suggesting that steady-state levels of T-cell activation in vivo are decreased in mutant mice. Although IL-2 mRNA levels were higher 1 h after αCD3 stimulation in mutant cells than in wild-type controls, by 3 h (and at all subsequent time points) mutant cells expressed significantly less IL-2 mRNA than control cells (Fig. 6B). Upregulation of a second cytokine, IL-4, after CD3 ligation was less in Snoex1−/− splenic T cells than in the wild type at all time points tested (Fig. 6A and B).
FIG. 6.
(A and B) Sno mutant T cells express reduced IL-2 and IL-4 mRNAs prior to and after T-cell receptor (TCR) stimulation. Spleen cells from littermates of each genotype were seeded in αCD3-coated 10-cm-diameter plates or harvested without plating (zero time point). The cells were incubated for the indicated times at 37°C and harvested, and RNA was purified, reverse transcribed, and subjected to real-time TaqMan PCR in quadruplicate, using commercially available primer sets (Applied Biosystems, Inc.) and making a template dilution curve for quantification of the results. The asterisks indicate results that were statistically significantly (P < 0.05) different from the wild type. Amplifications of the 18S rRNA internal control were similar in all samples (data not shown). Rescaled data from 0 and 1 h in panel B are presented in panel A. (C) Sno induces IL-2 promoter activity. 10T1/2 fibroblasts were transiently transfected with 1 μg of IL-2-luciferase reporter, plus or minus 2 μg of expression construct driving either SnoN or SnoN2 expression. An amount of each cell lysate corresponding to 50 μg of protein was used for each luciferase assay. The results are expressed as n-fold activation above the level of the IL-2 luciferase reporter alone, which was set to onefold. The error bars correspond to the calculated standard deviations for the triplicate dishes and duplicate measurements. The asterisks indicate results that were statistically significantly (P ≤ 0.02) different from the IL-2 luciferase reporter transfected alone. (D) 32P RT-PCR shows that SnoN and SnoN2 are present at endogenous levels in untransfected 10T1/2 cells and that the corresponding Sno isoform is expressed at high levels in transfected cells. Immunoprecipitation (IP) followed by Western blotting (WB) using the FLAG epitope tag on the Sno expression constructs confirmed that Sno proteins were overexpressed in transfected cell lysates.
Sno trans-activates the IL-2 promoter in non-T cells.
Since Sno deletion reduces the amount of IL-2 mRNA produced in response to αCD3 stimulation, we tested whether Sno could regulate IL-2 gene expression. 10T1/2 fibroblasts were transiently cotransfected with an IL-2 promoter element (632 bp [28, 54]) driving a luciferase reporter (IL-2-lux) and expression constructs driving FLAG-tagged SnoN or SnoN2. Control transfections were performed with IL-2-lux alone. IL-2-lux activity, in the absence of Sno constructs, was very low, as expected. Cotransfection with either SnoN or SnoN2 increased luciferase activity >60-fold (Fig. 6C). Anti-FLAG immunoprecipitates were used to confirm expression of the transfected Sno constructs (Fig. 6D). 32P RT-PCR demonstrated low-level expression of endogenous SnoN and SnoN2 in untransfected 10T1/2 cells, while the levels were >50-fold higher in transfected cells (Fig. 6D). No additional T-cell-specific factors were cotransfected, suggesting that Sno expression is sufficient to induce activation of IL-2 expression even in a non-T cell.
Sno-deficient embryo-derived fibroblasts also exhibit reduced antagonism of TGF-β signaling.
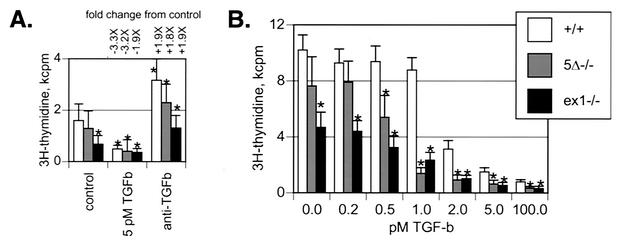
MEFs derived from embryonic skin express Sno at high levels. Snoex1−/− MEFs grow more slowly than wild-type MEFs in the absence of added TGF-β (P < 0.002) (Fig. 7). Furthermore, Sno5Δ−/− and Snoex1−/− cells show reduced proliferation rates at lower TGF-β concentrations than wild-type cells (0.5 and 1.0 pM TGF-β) (Fig. 7B). Both results indicate increased sensitivity to TGF-β. Sno5Δ−/− proliferation rates were reproducibly intermediate between the wild type and Snoex1−/− at 0.5 pM TGF-β and below, possibly indicating a protein dosage effect (Fig. 7). Culture in the presence of anti-TGF-β boosted the levels of proliferation of both mutant and wild-type MEFs almost twofold over untreated cells (Fig. 7A). The observation that neither Sno5Δ−/− nor Snoex1−/− proliferation was equal to that of the wild type in the presence of anti-TGF-β suggests that additional factors may be suppressing growth in Sno mutant MEFs independently of TGF-β signaling pathways.
FIG. 7.
(A and B) Wild-type and mutant MEFs show different DNA synthetic rates (A) and Sno mutant cells are more sensitive to TGF-β (B). MEFs were isolated from litters of embryos derived from intercrossed mice that were either both wild type or both homozygous mutant. The genotype of each MEF preparation was verified by PCR; multiple preparations gave the same results in these experiments. Equal numbers of cells were plated in quadruplicate sets of microwells and untreated or treated with increasing concentrations of TGF-β or panspecific anti-TGF-β antibody at 2 μg/ml for 24 h. The cells were metabolically labeled for the final 3 h with 1 μCi of [3H]thymidine per well and harvested onto glass fiber filters to determine the [3H]thymidine incorporation. The asterisks above the control bars (panel A, control; panel B, 0.0 pM) indicate results that were statistically significantly (P < 0.05) different from the wild-type control. In panel A, the asterisks above the other bars indicate results that were statistically significantly (P < 0.05) different from the corresponding untreated control cells. In panel B, the asterisks at 100 pM TGF-β indicate significant (P < 0.02) difference from the wild type; the other asterisks indicate significant difference (P < 0.009) from the wild type. Incorporation into mutant cells was statistically significantly different from the wild type in the presence of anti-TGF-β antibody (Sno5Δ−/−, P < 0.013; Snoex1−/−, P < 0.001), whereas incorporation in mutants and the wild type was not significantly different in the presence of 5 pM TGF-β. The genotypes were wild type (Sno+/+), Sno5Δ−/−, and Snoex1−/−. Two independent experiments are shown with different absolute [3H]thymidine incorporation levels in the controls.
Both Sno5Δ and Snoex1 mutations modulate the response of the TGF-β-responsive reporters 3TP-lux and A3-lux.
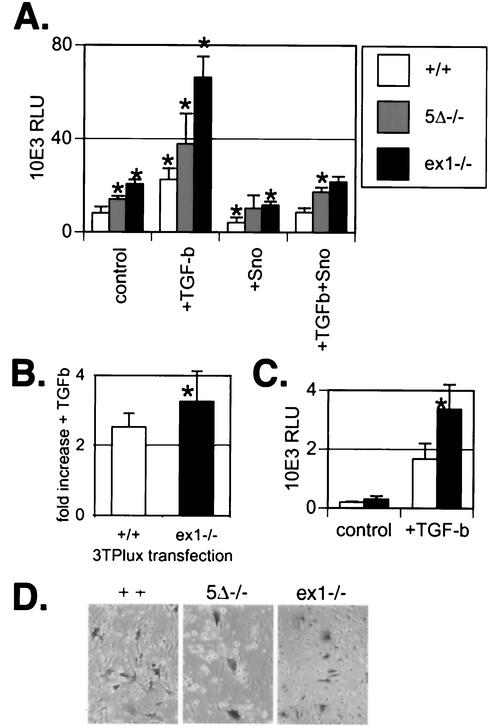
To confirm that Sno levels modulate TGF-β sensitivity, we tested the activity of a TGF-β-responsive promoter in MEFs with the three different genotypes. 3TP-lux is a composite reporter used by other investigators to measure cellular TGF-β responses (70); it contains an element from the human PAI-1 promoter (30) together with a 3× multimerized tetradecanoyl phorbol acetate response element from the human collagenase gene (15) coupled with a luciferase coding sequence. 3TP-lux expression is increased relative to the wild type in Sno5Δ−/− and Snoex1−/− cells in the absence of added TGF-β (P < 0.001) (Fig. 8A, control). Exogenous TGF-β increases 3TP-lux expression in cells of all three genotypes, demonstrating that TGF-β signaling is intact in each case (Fig. 8A). Compilation of data from five separate experiments showed a small but statistically significantly higher activation of 3TP-lux by TGF-β in Snoex1−/− cells than in wild-type cells (Fig. 8B). Luciferase activity in Sno5Δ−/− cells was indistinguishable from that in the wild type in this compilation and is not shown. Cotransfection of the reporter construct with SnoN reduced the level of 3TP-lux expression to below pretransfection levels (Fig. 8A), although the luciferase activity in Snoex1−/− cells remained higher than in wild-type cells. Transfection of FLAG-tagged SnoN reversed the TGF-β-induced increase in activity of the reporter construct (Fig. 8A). In order to confirm that the transfection efficiencies were comparable for all genotypes and preparations of MEFs, we transfected a pSVβgal construct in parallel and stained for beta-galactosidase activity (Fig. 8C). Snoex1−/− cells express LacZ in their nuclei because of the knock-in design. However, cytoplasmic staining is seen only in cells exposed to the pSVβgal construct during the transfection procedure and appears with comparable frequencies for all three cell types (Fig. 8C). Similar results were obtained with the A3-lux reporter construct, in which the activin response elements are activated by Smad2/Smad4/FAST-1 or FAST-2 (for forkhead activin signal transducer) (73) (Fig. 8D). Each of these transfections included a FAST-2 expression plasmid. Snoex1−/− cells once again exhibited significantly increased luciferase activity in the presence of TGF-β. In this instance, neither the control levels without added TGF-β nor the 8- to 10-fold increase after addition of TGF-β was significantly different from the wild type, suggesting that although Sno can repress TGF-β signaling through Smad2/Smad4, it also works through Smad3.
FIG. 8.
MEFs from Snoex1−/− embryos show increased activity of the 3TP-lux and A3-lux TGF-β-responsive promoters, either with or without TGF-β supplementation of the cultures. (A) The activity of a TGF-β-responsive promoter element, 3TP-lux, was tested in transfected MEFs. The genotypes were wild type (Sno+/+), Sno5Δ−/−, and Snoex1−/−. “Control” indicates the level of luciferase activity of transfected 3TP-lux reporter alone. +TGF-b, TGF-β (100 pM) was added; +Sno, pCMV-SnoN expression construct was cotransfected. Sixty-millimeter-diameter dishes were transfected in triplicate, and the relative light units (RLU) emitted by the luciferase reporter were measured in duplicate in a luminometer. The error bars indicate the calculated standard deviations from each group of six values measured. The asterisks above the control bars indicate results that were statistically significantly (P < 0.05) different from the wild type. The asterisks above the other bars indicate results that were statistically significantly (P < 0.05) different from the corresponding untreated control cells. The results for the mutants were significantly different from the corresponding wild-type results under each condition (Snoex1−/−, P < 0.0007; Sno5Δ−/−, P < 0.034). (B) The increase with added TGF-β was plotted for Sno+/+ and Snoex1−/− cells from combined data from five experiments. Sno5Δ−/− cells had the same 2.7-fold increase as Sno+/+ cells and were not plotted. The asterisk indicates that the 3.2-fold increase in luciferase in the presence of TGF-β was statistically significantly (P ≤ 0.016) higher in Snoex1−/− cells than the 2.7-fold increase in the wild type. (C) The activity of a different TGF-β-responsive promoter element, A3-lux, was tested in wild-type and Snoex1−/− MEFs cotransfected with a FAST-2 expression vector. The asterisk indicates that the activity in the presence of TGF-β was statistically significantly higher in Snoex1−/− than in Sno+/+ MEFs (P < 0.005). (D) To confirm that MEFs of the three genotypes were transfected with similar efficiencies, a pSVβgal construct was transfected in parallel in the same experiment, and the dishes were stained and photographed. The transfection efficiencies were similar among the threegenotypes. The Snoex1−/− cells expressed lacZ from the knock-in construct, seen in the nuclear staining in the figure. The transfected pSVβgal gave cytoplasmic staining and was thus distinguishable from the nuclear-staining Snoex1−/− background.
The TGF-β response of an endogenous gene, JunB, confirms differential effects of the Sno5Δ and Snoex1 mutations on TGF-β signaling.
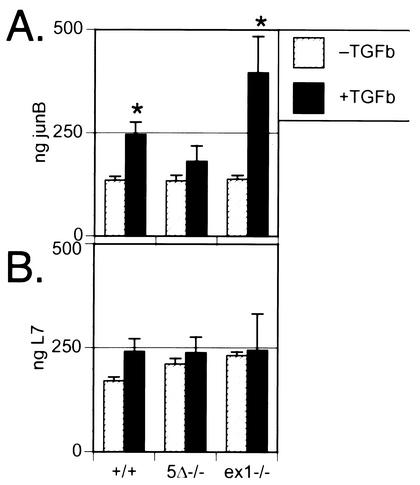
We used quantitative real-time RT-PCR to examine expression of JunB, a major target for induction by TGF-β (35, 38), in MEFs. The elevation of JunB levels in Snoex1−/− MEFs was significantly (P < 0.019) above that seen in wild-type MEFs 2 h after TGF-β administration (Fig. 9A). L7 ribosomal protein mRNA levels were similar in all samples, showing that equivalent amounts of template were used in all examples (Fig. 9B). In some repetitions of this experiment, JunB was elevated in Snoex1−/− cells in the absence of added TGF-β, with an additional increase as shown in the presence of TGF-β, but we consistently observed no effect in Sno5Δ−/− mutant cells. This suggests that JunB expression in MEFs is relatively insensitive to TGF-β signaling, since it appears that the low residual Sno protein levels in Sno5Δ−/− mutant cells may provide enough TGF-β opposition to keep JunB at “wild-type” levels. The observation that a TGF-β-responsive endogenous gene is expressed at elevated levels in response to TGF-β in Snoex1−/− cells provides additional evidence that Sno is a significant modulator of TGF-β signals.
FIG. 9.
Snoex1−/− MEFs show enhanced activation of endogenous JunB in response to TGF-β. (A) Real-time PCR measured levels of JunB endogenous mRNAs in wild-type and Sno mutant MEFs with (+TGFb) or without (−TGFb) incubation with 100 pM TGF-β for 2 h. The quantified levels calculated for each sample are presented in the histograms. Each sample was measured in quadruplicate and standardized against a dilution curve generated in the same experiment, using the same JunB primers and twofold serial dilutions of template (not shown). The genotypes are indicated below panel B. The asterisks indicate results that were statistically significantly (P < 0.015) different from the corresponding untreated cells. The difference in the presence of added TGF-β between Snoex1−/− and the wild type was significant (P < 0.018). (B) L7 ribosomal protein loading control real-time PCR results are presented as a histogram, showing that the samples contained comparable levels of RT-RNA; the profiles were not normalized. The error bars indicate the calculated standard deviations.
DISCUSSION
In this report, we show that Sno plays an important role in opposing TGF-β signaling in both lymphocytes and fibroblasts. Sno mRNA is expressed in the thymus as early as embryonic day 13.5 postcoitum, and Sno expression continues through adulthood. Sno mRNA is also expressed in the spleen at all developmental stages from fetal to adult, with upregulation in primary mouse splenocytes within 1 h of T-cell receptor stimulation in vitro. We examined the role of Sno in T-lymphocyte function using two targeted deletions in mice: a hypomorphic mutation with a 1.7-kb deletion upstream of the ATG codon (Sno5Δ) and a null mutation with deletion of exon 1 (Snoex1) that removes the coding sequence for the entire domain responsible for most known biological activities of Sno. Both Sno5Δ−/− and Snoex1−/− mice have a defect in T-cell activation as measured by splenocyte proliferation in response to T-cell receptor stimulation. The defect is partially reversed by supplementation with exogenous IL-2, supporting the idea that Sno functions early in the pathway that activates IL-2 expression. This idea is further supported by the observation of reduced IL-2 and IL-4 levels in unstimulated and αCD3-stimulated T cells and the demonstration that Sno trans-activates the IL-2 promoter in transient-transfection assays. SnoN and SnoN2 isoforms, which differ in the presence of a 46-amino-acid internal sequence, can both stimulate IL-2 upregulation. The T-cell proliferative defect is also reversed by incubation with panspecific anti-TGF-β antibody during stimulation. Along with evidence for enhanced TGF-β signaling in MEFs from Sno mutants using both luciferase reporter constructs and an endogenous TGF-β target gene, JunB, our results confirm that Sno is an important negative regulator of the known effects of TGF-β signaling during T-cell activation, as suggested by previous investigators (reviewed in reference 22). In addition to this “reactive” role for Sno, our data further suggest that Sno functions constitutively to prevent tonic TGF-β-mediated suppression of T-cell activation during the early steps in an antigen-specific immune response. We saw no evidence that Sno activity plays a role in opposing the postulated role for TGF-β in maintaining homeostatic control of the T-cell population size in vivo (22), since splenic-T-cell numbers from mutant mice were not different from those of wild-type controls on an inbred (C57BL/6) genetic background.
Analysis of any mutant allele must exclude the possibility that the mutant phenotype is due to the influence of the deletion on a gene closely linked to the primary target, as was noted for Arf/Ink4a (51), or to an unintended second mutation site. The similarity of the phenotypes in T cells for our two independent mutations is good evidence not only that it is a target-specific effect but also that it does not result from a second mutation at an unknown site.
Mice with another null mutation in the Sno gene were reported to die in early embryogenesis (52), whereas our null mice are viable. It is puzzling that our two mutations differ so dramatically from a third mutation at the same locus (52). The results with our mutants are strongly substantiated; two out of two ES clone-derived lines of Snoex1−/− mice and the single line of Sno5Δ−/− mice have similar phenotypes. We have also excluded possible second-site mutations, unless they are tightly linked to Sno, through our backcrossing regimen. The report of an earlier attempt to create Sno-null mice described intercrossing two different ES clone-derived germ line Sno lines to exclude the possibility of second-site mutations (52). However, this does not exclude the possibility that a lethal second-site mutation had arisen in their ES cell population prior to or at the time of targeting. Other potential sources for changes in the outcome of Sno mutation can be postulated, including differences in the deletion boundaries, the genetic backgrounds used, the location and direction of neomycin resistance cassettes, or effects on nearby gene expression. However, such unintended secondary consequences of homologous recombination, including deletion or deregulation of nearby genes, are more likely to play a role in the more dramatic phenotype reported earlier. Alternatively, we must consider the possibility that our Snoex1 mutation allows expression of a partial gene product with partial function.
The design of our null mutation, in which we excised exon 1 but not exons 2 to 6, raises the possibility that a truncated carboxyl-terminal protein species could be expressed. We detected transcripts including these downstream exons using Northern blots (Fig. 4A) and RT-PCR (not shown). We carefully examined protein products of the predicted size and found such a truncated protein (SnoN2 isoform but not SnoN), but it is expressed at low levels in wild-type as well as mutant cells (Fig. 4B). This carboxyl-terminal fragment of Sno carries the lysine residues that are responsible for ubiquitination and degradation of Sno (55) and thus might turn over rapidly. This domain, including only the carboxyl-terminal half of Sno, would lack SKIP binding (13); N-CoR binding (44); TAFII110 binding (11); Smad 2, 3, or 4 binding (56, 58); transcriptional activation or repression (11); oncogenic transformation; and muscle differentiation-promoting activities (12). However, such a 34-kDa carboxyl-terminal Sno species would be predicted to retain the ability to heterodimerize with Ski (12, 25, 41) and thus could have dominant-negative activity. If truncated Sno protein in these cells has dominant-negative activity, we would expect even heterozygous cells to have phenotypes approaching that of the homozygous mutant. However, we have not observed heterozygote phenotypes that are comparable to homozygous mutant phenotypes, strongly suggesting the absence of such dominant-negative activity. Also, when the Sno5Δ mutation is combined with the Ski-null mutation, double-homozygous mutant mice die early in embryogenesis, a full 12 days earlier than Ski−/− mice in the absence of the Sno5Δ mutation (3; S. Pearson-White and C. Colmenares, unpublished data). This shows that Sno and Ski compensate for some of each other's functions. The Sno5Δ mutation alone confers a mild phenotype, but this is altered dramatically when Ski is deleted, showing that there is no dominant-negative effect of the Sno5Δ mutation or, by analogy, of the Snoex1 mutation with a similar mild phenotype. The Sno-null mouse described elsewhere also had only exon 1 deleted, and experiments that would have detected a downstream mRNA species or truncated protein were not reported (52). Because of its lethality in the homozygous state, all of the experiments with this mutation were obligatorily performed in heterozygous mice (52). One possible explanation for the effects reported with this mutation might be a dominant-negative effect on Ski function.
Sno is one of many proteins with a negative regulatory influence on TGF-β signaling and is likely, therefore, to have a modulatory rather than a binary controlling influence. Evi-1 (34), oncogenic Ras (33), Ski (1, 36, 57, 71), c-Jun (16), lefty (64), TGIF1 (68, 69), and TGIF2 (39) also participate in the inhibition of TGF-β signaling. In the face of so many alternative modulators of TGF-β, it is significant that phenotypic changes in TGF-β effects in lymphocytes are measurable in the absence of Sno. This suggests that Sno is a very important modulator of TGF-β signaling in certain cell types and that Sno may be the critical factor regulating TGF-β activity in T cells.
In primary T cells, TGF-β does more than just inhibit IL-2 production to suppress T-cell activation, since the addition of IL-2 does not rescue T-cell proliferation in the presence of high doses of TGF-β (Fig. 5A). TGF-β also enhances IL-2R expression by IL-2 (19) and plays a significant role in T-cell development and terminal differentiation (22, 31, 66). Sno deletion appears not to affect T-cell-developmental aspects of TGF-β signaling, since immunophenotyping revealed no differences among T-cell subpopulations in Sno mutant mice (data not shown). Furthermore, it appears to play no role in homeostatic regulation of the peripheral T-cell pool.
Sno5Δ−/− and Snoex1−/− mice show some differences in phenotype. It is only in MEFs, in the regulation of the JunB response to TGF-β, that the two mutants show completely distinct responses: Snoex1−/− cells hyperactivate JunB in response to TGF-β, whereas Sno5Δ−/− cells do not (Fig. 9). Subtle differences were also seen in other assay systems. Both the activation of the TGF-β-responsive promoter 3TP-lux and the [3H]thymidine incorporation rate are intermediate in Sno5Δ−/− cells between wild-type and Snoex1−/− cells (Fig. 7 and 8), and reversal of proliferative defects in splenocytes by both IL-2 and anti-TGF-β antibodies was less complete in Sno5Δ−/− than in Snoex1−/− cells. In these cases, the Sno5Δ mutation appears to be more resistant to complete reversal than Snoex1. While these differences may result from a subtle dose effect for Sno, it is also possible that compensatory developmental changes in interacting pathways not seen in the Sno5Δ−/− hypomorph alter expression in the Snoex1−/− null mutant.
There is also evidence in data from both mutants that Sno regulates functions other than those induced by TGF-β. Anti-TGF-β antibody was not able to completely rescue proliferation of Sno5Δ−/− or Snoex1−/− fibroblasts to the level attained by the wild type with added anti-TGF-β antibody (Fig. 7A). It is not known which additional pathway requires Sno function. Sno and Ski are linked to several other signaling pathways through the Smad proteins, which are also targets of the Ras/MAPK, Wnt/β-catenin, and nuclear hormone signaling pathways (27, 37). Ski and Sno can also interact with Gli3, which responds to Sonic hedgehog signaling (14). Our results show that Sno is important in the negative regulation of TGF-β signals in vivo in T-cell activation but also suggest that Sno may modulate signaling through another pathway still to be identified.
Acknowledgments
We thank Rowena Crittenden for outstanding technical support and Margaret Ober of the University of Virginia Transgenic Mouse Core Facility for construction of the 5Δ targeting construct and all blastocyst injections to make germ line-transmitting male chimeras. We thank Kerrington Ramsey for the IL-2 transfection experiments when she rotated in the laboratory. We thank Tim Bender, Bill Pearson, and David Wotton for comments on the manuscript. We thank the University of Virginia Child Health Research Center Molecular Genetics Core for plasmid purifications, the Biomolecular Core Facility for DNA sequencing, and the FACS core facility for flow cytometry. We thank Shunsuke Ishii for the anti-Sno monoclonal antibody mixture. We thank Mike Rudnicki, Shahragim Tajbakhsh, Margaret Buckingham, Tim Bender, and Klaus Rajewsky for plasmids.
This work was supported by the Muscular Dystrophy Association and Public Health Service grant HD-35130 from the National Institute of Child Health and Human Development to S.P.W.
REFERENCES
- 1.Akiyoshi, S., H. Inoue, J. Hanai, K. Kusanagi, N. Nemoto, K. Miyazono, and M. Kawabata. 1999. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J. Biol. Chem. 274:35269-35277. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Berk, M., S. Y. Desai, H. C. Heyman, and C. Colmenares. 1997. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial patterning, and skeletal muscle development. Genes Dev. 11:2029-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonni, S., H. R. Wang, C. G. Causing, P. Kavsak, S. L. Stroschein, K. Luo, and J. L. Wrana. 2001. TGF-beta induces assembly of a Smad2-Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat. Cell Biol. 3:587-595. [DOI] [PubMed] [Google Scholar]
- 5.Brabletz, T., I. Pfeuffer, E. Schorr, F. Siebelt, T. Wirth, and E. Serfling. 1993. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol. Cell. Biol. 13:1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Bradley, A. 1987. Production and analysis of chimaeric mice, p. 113-151. In E. J. Robertson (ed.), Teratocarcinomas and embryonic stem cells, a practical approach. IRL Press, Oxford, United Kingdom.
- 8.Buskin, J. N., D. L. Gregory, W. A. La-Framboise, and S. D. Hauschka. 1996. Harvest protocol to reduce variability of soluble enzyme yield from cultured cells. BioTechniques 20:92-94. [DOI] [PubMed] [Google Scholar]
- 9.Caubit, X., R. Thangarajah, T. Theil, J. Wirth, H. G. Nothwang, U. Ruther, and S. Krauss. 1999. Mouse Dac, a novel nuclear factor with homology to Drosophila dachshund shows a dynamic expression in the neural crest, the eye, the neocortex, and the limb bud. Dev. Dyn. 214:66-80. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, S. B., R. Nicol, and E. Stavnezer. 1998. A domain necessary for the transforming activity of SnoN is required for specific DNA binding, transcriptional repression and interaction with TAF(II)110. Oncogene 17:2505-2513. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, S. B., G. Zheng, H. C. Heyman, and E. Stavnezer. 1999. Heterodimers of the SnoN and Ski oncoproteins form preferentially over homodimers and are more potent transforming agents. Nucleic Acids Res. 27:1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahl, R., B. Wani, and M. J. Hayman. 1998. The Ski oncoprotein interacts with Skip, the human homolog of Drosophila Bx42. Oncogene 16:1579-1586. [DOI] [PubMed] [Google Scholar]
- 14.Dai, P., T. Shinagawa, T. Nomura, J. Harada, S. C. Kaul, R. Wadhwa, M. M. Khan, H. Akimaru, H. Sasaki, C. Colmenares, and S. Ishii. 2002. Ski is involved in transcriptional regulation by the repressor and full-length forms of Gli3. Genes Dev. 16:2843-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Groot, R. P., and W. Kruijer. 1990. Transcriptional activation by TGF beta 1 mediated by the dyad symmetry element (DSE) and the TPA responsive element (TRE). Biochem. Biophys. Res. Commun. 168:1074-1081. [DOI] [PubMed] [Google Scholar]
- 16.Dennler, S., C. Prunier, N. Ferrand, J. M. Gauthier, and A. Atfi. 2000. c-Jun inhibits transforming growth factor beta-mediated transcription by repressing smad3 transcriptional activity. J. Biol. Chem. 275:28858-28865. [DOI] [PubMed] [Google Scholar]
- 17.Diaco, R. 1995. Practical considerations for the design of quantitative PCR assays, p. 84-108. In M. A. Innis, D. H. Gelfand, and J. J. Sninsky (ed.), PCR strategies. Academic Press, Inc., San Diego, Calif.
- 18.Engert, J. C., S. Servaes, P. Sutrave, S. H. Hughes, and N. Rosenthal. 1995. Activation of a muscle-specific enhancer by the Ski proto-oncogene. Nucleic Acids Res. 23:2988-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espevik, T., A. Waage, A. Faxvaag, and M. R. Shalaby. 1990. Regulation of interleukin-2 and interleukin-6 production from T-cells: involvement of interleukin-1 beta and transforming growth factor-beta. Cell. Immunol. 126:47-56. [DOI] [PubMed] [Google Scholar]
- 20.Field, S. J., F. Y. Tsai, F. Kuo, A. M. Zubiaga, W. G. Kaelin, Jr., D. M. Livingston, S. H. Orkin, and M. E. Greenberg. 1996. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell 85:549-561. [DOI] [PubMed] [Google Scholar]
- 21.Givol, I., P. L. Boyer, and S. H. Hughes. 1995. Isolation and characterization of the chicken c-sno gene. Gene 1565:271-276. [DOI] [PubMed] [Google Scholar]
- 22.Gorelik, L., and R. A. Flavell. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2:46-53. [DOI] [PubMed] [Google Scholar]
- 23.Gu, H., Y.-R. Zou, and K. Rajewsky. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 73:1155-1164. [DOI] [PubMed] [Google Scholar]
- 24.Heanue, T. A., R. Reshef, R. J. Davis, G. Mardon, G. Oliver, S. Tomarev, A. B. Lassar, and C. J. Tabin. 1999. Synergistic regulation of vertebrate muscle development by dach2, eya2, and six1, homologs of genes required for Drosophila eye formation. Genes Dev. 13:3231-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyman, H. C., and E. Stavnezer. 1994. A carboxyl-terminal region of the ski oncoprotein mediates homodimerization as well as heterodimerization with the related protein SnoN. J. Biol. Chem. 269:26996-27003. [PubMed] [Google Scholar]
- 26.Ikeda, K., Y. Watanabe, H. Ohto, and K. Kawakami. 2002. Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol. Cell. Biol. 22:6759-6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh, S., F. Itoh, M. J. Goumans, and P. Ten Dijke. 2000. Signaling of transforming growth factor-beta family members through Smad proteins. Eur. J. Biochem. 267:6954-6967. [DOI] [PubMed] [Google Scholar]
- 28.Jain, J., C. Loh, and A. Rao. 1995. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7:333-342. [DOI] [PubMed] [Google Scholar]
- 29.Jonk, L. J., S. Itoh, C. H. Heldin, P. ten Dijke, and W. Kruijer. 1998. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-beta, activin, and bone morphogenetic protein-inducible enhancer. J. Biol. Chem. 273:21145-21152. [DOI] [PubMed] [Google Scholar]
- 30.Keeton, M. R., S. A. Curriden, A. J. van Zonneveld, and D. J. Loskutoff. 1991. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J. Biol. Chem. 266:23048-23052. [PubMed] [Google Scholar]
- 31.Kehrl, J. H., L. M. Wakefield, A. B. Roberts, S. Jakowlew, M. Alvarez-Mon, R. Derynck, M. B. Sporn, and A. S. Fauci. 1986. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163:1037-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelder, B., C. Richmond, E. Stavnezer, E. O. List, and J. J. Kopchick. 1997. Production, characterization and functional activities of v-Ski in cultured cells. Gene 202:15-21. [DOI] [PubMed] [Google Scholar]
- 33.Kretzschmar, M., J. Doody, I. Timokhina, and J. Massague. 1999. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 13:804-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurokawa, M., K. Mitani, K. Irie, T. Matsuyama, T. Takahashi, S. Chiba, Y. Yazaki, K. Matsumoto, and H. Hirai. 1998. The oncoprotein Evi-1 represses TGF-β signalling by inhibiting Smad3. Nature 394:92-96. [DOI] [PubMed] [Google Scholar]
- 35.Li, L., J. S. Hu, and E. N. Olson. 1990. Different members of the jun proto-oncogene family exhibit distinct patterns of expression in response to type beta transforming growth factor. J. Biol. Chem. 265:1556-1562. [PubMed] [Google Scholar]
- 36.Luo, K., S. L. Stroschein, W. Wang, D. Chen, E. Martens, S. Zhou, and Q. Zhou. 1999. The Ski oncoprotein interacts with the Smad proteins to repress TGFβ signaling. Genes Dev. 13:2196-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massague, J. 2000. How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 1:169-178. [DOI] [PubMed] [Google Scholar]
- 38.Mauviel, A., K. Y. Chung, A. Agarwal, K. Tamai, and J. Uitto. 1996. Cell-specific induction of distinct oncogenes of the Jun family is responsible for differential regulation of collagenase gene expression by transforming growth factor-beta in fibroblasts and keratinocytes. J. Biol. Chem. 271:10917-10923. [DOI] [PubMed] [Google Scholar]
- 39.Melhuish, T. A., C. M. Gallo, and D. Wotton. 2001. TGIF2 interacts with histone deacetylase 1 and represses transcription. J. Biol. Chem. 276:32109-32114. [DOI] [PubMed] [Google Scholar]
- 40.Mimura, N., K. Ichikawa, A. Asano, T. Nagase, and S. Ishii. 1996. A transient increase of snoN transcript by growth arrest upon serum deprivation and cell-to-cell contact. FEBS Lett. 397:253-259. [DOI] [PubMed] [Google Scholar]
- 41.Nagase, T., N. Nomura, and S. Ishii. 1993. Complex formation between proteins encoded by the ski gene family. J. Biol. Chem. 268:13710-13716. [PubMed] [Google Scholar]
- 42.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicol, R., and E. Stavnezer. 1998. Transcriptional repression by v-Ski and c-Ski mediated by a specific DNA binding site. J. Biol. Chem. 273:3588-3597. [DOI] [PubMed] [Google Scholar]
- 44.Nomura, T., M. M. Khan, S. C. Kaul, H. D. Dong, R. Wadhwa, C. Colmenares, I. Kohno, and S. Ishii. 1999. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 13:412-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson-White, S., D. Deacon, R. Crittenden, G. Brady, N. Iscove, and P. J. Quesenberry. 1995. The ski/sno protooncogene family in hematopoietic development. Blood 86:2146-2155. [PubMed] [Google Scholar]
- 46.Pearson-White, S. H., and R. Crittenden. 1997. Proto-oncogene Sno expression, alternative isoforms, and immediate early serum response. Nucleic Acids Res. 25:2930-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelzer, T., G. E. Lyons, S. Kim, and R. W. Moreadith. 1996. Cloning and characterization of the murine homolog of the sno proto-oncogene reveals a novel splice variant. Dev. Dyn. 205:114-125. [DOI] [PubMed] [Google Scholar]
- 48.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 49.Rawls, A., J. H. Morris, M. Rudnicki, T. Braun, H. H. Arnold, W. H. Klein, and E. N. Olson. 1995. Myogenin's functions do not overlap with those of MyoD or Myf-5 during mouse embryogenesis. Dev. Biol. 172:37-50. [DOI] [PubMed] [Google Scholar]
- 50.Rudnicki, M. A., T. Braun, S. Hinuma, and R. Jaenisch. 1992. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene myf-5 and results in apparently normal muscle development. Cell 71:383-390. [DOI] [PubMed] [Google Scholar]
- 51.Sherr, C. J. 2001. Parsing Ink4a/Arf: “pure” p16-null mice. Cell 106:531-534. [DOI] [PubMed] [Google Scholar]
- 52.Shinagawa, T., H. D. Dong, M. Xu, T. Maekawa, and S. Ishii. 2000. The sno gene, which encodes a component of the histone deacetylase complex, acts as a tumor suppressor in mice. EMBO J. 19:2280-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinagawa, T., T. Nomura, C. Colmenares, M. Ohira, A. Nakagawara, and S. Ishii. 2001. Increased susceptibility to tumorigenesis of ski-deficient heterozygous mice. Oncogene 20:8100-8108. [DOI] [PubMed] [Google Scholar]
- 54.Siebenlist, U., D. B. Durand, P. Bressler, N. J. Holbrook, C. A. Norris, M. Kamoun, J. A. Kant, and G. R. Crabtree. 1986. Promoter region of interleukin-2 gene undergoes chromatin structure changes and confers inducibility on chloramphenicol acetyltransferase gene during activation of T cells. Mol. Cell. Biol. 6:3042-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stroschein, S. L., S. Bonni, J. L. Wrana, and K. Luo. 2001. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 15:2822-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stroschein, S. L., W. Wang, S. Zhou, Q. Zhou, and K. Luo. 1999. Negative feedback regulation of TGF-β signaling by the SnoN oncoprotein. Science 286:771-774. [DOI] [PubMed] [Google Scholar]
- 57.Sun, Y., X. Liu, E. N. Eaton, W. S. Lane, H. F. Lodish, and R. A. Weinberg. 1999. Interaction of the Ski oncoprotein with Smad3 regulates TGF-β signaling. Mol. Cell 4:1-20. [DOI] [PubMed] [Google Scholar]
- 58.Sun, Y., X. Liu, E. Ng-Eaton, H. F. Lodish, and R. A. Weinberg. 1999. SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor beta signaling. Proc. Natl. Acad. Sci. USA 96:12442-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutrave, P., T. D. Copeland, S. D. Showalter, and S. H. Hughes. 1990. Characterization of chicken c-ski oncogene products expressed by retrovirus vectors. Mol. Cell. Biol. 10:3137-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tajbakhsh, S., E. Bober, C. Babinet, S. Pournin, H. Arnold, and M. Buckingham. 1996. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibres as well as early embryonic muscle. Dev. Dyn. 206:291-300. [DOI] [PubMed] [Google Scholar]
- 61.Tajbakhsh, S., and M. E. Buckingham. 1994. Mouse limb muscle is determined in the absence of the earliest myogenic factor myf-5. Proc. Natl. Acad. Sci. USA 91:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarapore, P., C. Richmond, G. Zheng, S. B. Cohen, B. Kelder, J. Kopchick, J. Kruse, A. E. Sippel, C. Colmenares, and E. Stavnezer. 1997. DNA binding and transcriptional activation by the Ski oncoprotein mediated by interaction with NFI. Nucleic Acids Res. 25:3895-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokitou, F., T. Nomura, M. M. Khan, S. C. Kaul, R. Wadhwa, T. Yasukawa, I. Kohno, and S. Ishii. 1999. Viral ski inhibits retinoblastoma protein (Rb)-mediated transcriptional repression in a dominant negative fashion. J. Biol. Chem. 274:4485-4488. [DOI] [PubMed] [Google Scholar]
- 64.Ulloa, L., and S. Tabibzadeh. 2001. Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-beta receptor. J. Biol. Chem. 276:21397-21404. [DOI] [PubMed] [Google Scholar]
- 65.Verrecchia, F., C. Tacheau, M. Schorpp-Kistner, P. Angel, and A. Mauviel. 2001. Induction of the AP-1 members c-Jun and JunB by TGF-beta/Smad suppresses early Smad-driven gene activation. Oncogene 20:2205-2211. [DOI] [PubMed] [Google Scholar]
- 66.Wahl, S. M., J. M. Orenstein, and W. Chen. 2000. TGF-beta influences the life and death decisions of T lymphocytes. Cytokine Growth Factor Rev. 11:71-79. [DOI] [PubMed] [Google Scholar]
- 67.Wakefield, L. M., and A. B. Roberts. 2002. TGF-β signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 12:22-29. [DOI] [PubMed] [Google Scholar]
- 68.Wotton, D., R. S. Lo, S. Lee, and J. Massague. 1999. A Smad transcriptional corepressor. Cell 97:29-39. [DOI] [PubMed] [Google Scholar]
- 69.Wotton, D., R. S. Lo, L. A. Swaby, and J. Massague. 1999. Multiple modes of repression by the smad transcriptional corepressor TGIF. J. Biol. Chem. 274:37105-37110. [DOI] [PubMed] [Google Scholar]
- 70.Wrana, J. L., L. Attisano, J. Carcamo, A. Zentella, J. Doody, M. Laiho, X. F. Wang, and J. Massague. 1992. TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71:1003-1014. [DOI] [PubMed] [Google Scholar]
- 71.Xu, W., K. Angelis, D. Danielpour, M. M. Haddad, O. Bischof, J. Campisi, E. Stavnezer, and E. E. Medrano. 2000. Ski acts as a co-repressor with Smad2 and Smad3 to regulate the response to type beta transforming growth factor. Proc. Natl. Acad. Sci. USA 97:5924-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamasaki, L., T. Jacks, R. Bronson, E. Goillot, E. Harlow, and N. J. Dyson. 1996. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85:537-548. [DOI] [PubMed] [Google Scholar]
- 73.Zhou, S., L. Zawel, C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1998. Characterization of human FAST-1, a TGF beta and activin signal transducer. Mol. Cell 2:121-127. [DOI] [PubMed] [Google Scholar]