Abstract
Eukaryotic cells utilize multiple mitogen-activated protein kinases (MAPKs) to transmit various extracellular stimuli to the nucleus. A subfamily of MAPKs that mediates environmental stress stimuli is also called stress-activated protein kinase (SAPK), which has crucial roles in cellular survival under stress conditions as well as inflammatory responses. Here we report that Cdc37, an evolutionarily conserved kinase-specific chaperone, is a positive regulator of Spc1 SAPK in the fission yeast Schizosaccharomyces pombe. Through a genetic screen, we have identified cdc37 as a mutation that compromises signaling through Spc1 SAPK. The Cdc37 protein physically interacts with Spc1, and the cdc37 mutation affects both the cellular level of the Spc1 protein and stress-induced Spc1 phosphorylation by Wis1 MAPK kinase (MAPKK). Consistently, expression of the stress response genes regulated by the Spc1 pathway is compromised in cdc37 mutant cells. On the other hand, a mutation in Hsp90, which often cooperates with Cdc37 in chaperoning protein kinases, does not affect Spc1 SAPK. These results suggest that Spc1 SAPK is a novel client protein for the Cdc37 chaperone, and the Cdc37 function is important to maintain the stability of the Spc1 protein and to facilitate stress signaling from Wis1 MAPKK to Spc1 SAPK.
A mitogen-activated protein kinase (MAPK) cascade is a signaling module ubiquitous among eukaryotes that transmits extracellular stimuli to the nucleus. A MAPK cascade is composed of three conserved kinases, MAPK, MAPK kinase (MAPKK), and MAPKK kinase (MAPKKK), and signals are transmitted through sequential activation of the three kinases by phosphorylation; stimulus-activated MAPKKK phosphorylates MAPKK, which in turn phosphorylates and activates MAPK. Activated MAPK phosphorylates downstream effector proteins, such as transcription factors, and modulates their function (6, 20, 27, 41). A subfamily of MAPKs dedicated for transmitting environmental stress stimuli, such as high-osmolarity stress, oxidative stress, heat shock, and genotoxic agents, are also known as stress-activated protein kinases (SAPKs). Among those are Hog1 in budding yeast Saccharomyces cerevisiae (4), Spc1 (also known as StyI) in fission yeast Schizosaccharomyces pombe (29, 43), and mammalian p38 (19, 24). Genetic studies of yeast SAPKs indicate that SAPKs play key roles in cellular stress resistance (18, 34), and mammalian SAPKs are also implicated in inflammation and the response of cancer cells to cytotoxic treatments (23, 53).
The structures and functions of SAPK cascades are highly conserved throughout evolution; like the mammalian p38 pathway, the fission yeast Spc1 cascade, which consists of Wis4 and Win1 MAPKKKs, Wis1 MAPKK, and Spc1 MAPK, is activated by a wide range of stress, including osmostress, oxidative stress, and heat shock (34). Interestingly, the highly homologous Hog1 SAPK cascade in budding yeast is responsive mostly to high-osmolarity stress (42), although a recent report also indicates heat shock-induced activation of Hog1 (55). Upon environmental stress, fission yeast Spc1 phosphorylates and activates the Atf1 transcription factor (44, 54), a fission yeast ortholog of mammalian ATF2, which is also a key substrate of the mammalian SAPKs (17, 39). Atf1 is responsible for expression of a number of stress resistance genes, including those in osmoregulation and oxidative stress responses (9, 38, 44, 54). Because of the high similarity between the fission yeast and mammalian SAPK cascades, the genetic study of the Spc1 pathway provides an excellent opportunity to identify and analyze novel elements in stress signaling by SAPK cascades.
CDC37 was first identified in budding yeast as a gene essential for cell cycle progression in G1 (13, 40), and structurally related proteins were subsequently identified in Drosophila melanogaster and mammalian cells (7, 8, 50). Although the function of Cdc37 was not apparent from its amino acid sequence, recent studies revealed that Cdc37 is a part of the chaperone complex required for the stability and/or activity of some protein kinases (reviewed in reference 21). Budding yeast Cdc28, a cyclin-dependent kinase (Cdk), shows significantly shorter half-lives in cdc37 mutants (12). Cdc37 is also required for maintaining the oncogenic tyrosine kinase v-Src in a soluble, biologically active form when expressed in budding yeast (11). Mammalian Cdc37 was found as a 50-kDa protein that forms a complex with diverse protein kinases, such as v-Src, Raf, and Cdk (16, 37, 50), while no MAPK has been identified as a target of Cdc37. Complexes of Cdc37 with different protein kinases often contain a molecular chaperone, Hsp90, and it has been proposed that Cdc37 is a kinase-targeting subunit of Hsp90 (50). On the other hand, in vitro experiments using budding yeast Cdc37 suggest that Cdc37 itself has a Hsp90-like chaperone activity (22) and, therefore, Cdc37 may also function independently of Hsp90.
Here we report a genetic screen in fission yeast to identify novel components of the Spc1 SAPK cascade. We have identified cdc37 as a mutation that inhibits the Spc1 function in response to the ectopic expression of activated Wis1 MAPKK. The Cdc37 protein physically interacts with Spc1 MAPK, and the cdc37 mutation brings about significant decreases in both the abundance and stress-induced phosphorylation of Spc1. Our study suggests that Cdc37 functions as a molecular chaperone to maintain the stability of Spc1 MAPK as well as to facilitate the phosphorylation of Spc1 by Wis1 MAPKK.
MATERIALS AND METHODS
Yeast strains and general techniques.
S. pombe strains used in this study are listed in Table 1. Growth media and basic techniques for S. pombe have been described previously (2, 30). S. pombe cells were grown in YES yeast extract medium and EMM2 synthetic medium.
TABLE 1.
S. pombe strains used in this study
| Strain | Genotypea | Source or reference |
|---|---|---|
| PR109 | h− | Laboratory stock |
| CA6 | h−his7-366 ade6-M216 | Laboratory stock |
| CA7 | h+his7-366 ade6-M216 | Laboratory stock |
| CA14 | h−spc1::ura4+ | Laboratory stock |
| CA39 | h−atf1::ura4+ | Laboratory stock |
| CA76 | h−spc1:HA6H(ura4+) | Laboratory stock |
| CA178 | h−wis1:myc(ura4+) | 14 |
| CA211 | h−his7-366 ade6-M216 atf1::ura4+ | Laboratory stock |
| CA212 | h+his7-366 ade6-M210 atf1::ura4+ | Laboratory stock |
| CA1150 | h+his7-366::his7+:spc1+ade6-M210 | This study |
| CA1388 | h−cdc37-681 | This study |
| CA1390 | h−spc1:HA6H(ura4+) cdc37-681 | This study |
| CA1412 | h+his7-366 ade6-M210 cdc37-681 | This study |
| CA1417 | h−cdc37-681 atf1::ura4+ | This study |
| CA1496 | h+swo1-26 | 3 |
| CA1450 | h+his7-366 wis1:myc(ura4+) cdc37-681 | This study |
| CA1621 | h−cdc37:HA6H(ura4+) | This study |
| CA1623 | h−cdc37:GFP(ura4+) | This study |
| CA1653 | h−cdc37:HA6H(ura4+) spc1:myc(ura4+) | This study |
| CA1725 | h−his7-366::his7+:spc1+ade6-M216 atf1::ura4+ | This study |
| CA1726 | h+his7-366::his7+:spc1+ade6-M210 atf1::ura4+ | This study |
| CA1727 | h+/−his7-366/his7 ade6-M210/ade6-M216 cdc37::ura4+ | This study |
All strains are leu1-32 ura4-D18.
Integration of the spc1+ gene at the his7 locus.
The 2.9-kb EcoRI-SpeI genomic DNA fragment containing the spc1+ gene was cloned into the NotI site of pBluescript II (Stratagene), to which the his7+ marker gene was inserted at the SmaI site. The resultant plasmid was used to transform a wild-type (CA7) S. pombe strain. Stable His+ transformants were selected, and the integration of the plasmid construct at the his7 locus was verified by Southern hybridization analysis. The integrated spc1+ gene was able to suppress the osmosensitivity and the temperature sensitivity of the Δspc1 strain (data not shown).
Isolation of mutants resistant to Wis1 overexpression.
Strains CA1725 and CA1726 were transformed with the pREP1-WIS1DD-HA6H plasmid, which expresses Wis1DD (46), a constitutive active mutant of Wis1 MAPKK under the control of the thiamine-repressible nmt1 promoter (28). The transformants were grown in liquid EMM2 lacking thiamine (EMM2−T) at 30°C for ∼16 h and plated onto EMM2−T at the concentration of ∼2 × 107 cells per plate. After incubation at 30°C for 7 days, growing colonies were picked up and streaked to confirm their growth on EMM2−T. Resultant colonies were subjected to anti-hemagglutinin (anti-HA) immunoblotting to select mutants that had not lost the ability of Wis1 overexpression.
Cloning of the cdc37+ gene.
A temperature-sensitive strain, CA1388, isolated from the above screen was transformed with an S. pombe genomic library and plated onto EMM2 medium. After incubation at 25°C for 7 days, transformants were replica plated onto YES medium and incubated at 37°C for 1 day. The transformants were further replica plated onto fresh YES medium, which was followed by incubation at 37°C for 2 days. Forty-one out of ∼80,000 transformants showed colony formation after the second replica plating at 37°C. DNA sequencing and PCR analyses of the recovered plasmids from all of the ts+ colonies showed that they all contained the cdc37+ gene.
A mutation in the cdc37 gene in the isolated mutants was further confirmed by determining the mutation site. Wild-type and mutant cdc37 gene sequences were PCR amplified using genomic DNA from wild-type and the ts mutant strains as templates. In all the mutant sequences, the 1,285th T in the cdc37+ open reading frame was mutated to C, resulting in the replacement of Leu-285 in the Cdc37 protein with proline.
Construction of cdc37 null mutant, cdc37:HA6H, and cdc37:GFP strains.
For cdc37 gene disruption, 0.5-kb sequences immediately upstream and downstream of the cdc37+ open reading frame were amplified by PCR with pairs of primers (SpCdC37disrpN5, GGCGTGGGAAGTAATAGAGT, and SpCdC37disrpN3,TCGCCCTATAGTGAGTCGTACTCGAAGCAAATTATAATTTCAA, forthe upstream sequence; SpCdC37disrpC5, CCAGCTTTTGTTCCCTTTAGTATTTTTCTGCTTACGGGTGTG, and SpCdC37disrpC3, AATGACTATGTACCTCACTAC, for the downstream sequence). The 1.8-kb HindIII fragment of the ura4+ marker gene was amplified by PCR using the aforementioned 0.5-kb fragments upstream and downstream of cdc37+ as primers. The resultant PCR product, a ura4+ fragment flanked with the genomic sequences adjacent to cdc37+, was used to transform a diploid strain constructed by mating strains CA6 and CA7. Stable Ura+ transformants were isolated, and the disruption of one of the cdc37 loci was confirmed by Southern hybridization experiments, which were followed by tetrad analysis.
For constructing strains in which the chromosomal cdc37+ gene is tagged with the sequences encoding two copies of the HA epitope followed by six consecutive histidines (HA6H) (45) or the green fluorescent protein (GFP), a NotI site was introduced by PCR at the 3′ end of the cdc37+ sequence. The resultant PCR fragment was digested at the HpaI site within the cdc37+ sequence and the 3′-end NotI site, and the resultant 1.8-kb HpaI-NotI fragment was cloned to construct pBluescript-ΔNCdc37-HA2His6 and pBluescript-ΔNCdc37-GFP with the ura4+ marker gene. These plasmids were used to transform a wild-type (PR109) strain, and the integration of the plasmids at the cdc37+ locus was confirmed by Southern blotting analysis.
Immunoblotting of Spc1 and Wis1 in crude lysate.
For Western blotting analyses, S. pombe cell lysate was prepared as described previously (52). Cells grown to the mid-log phase were harvested by filtration in the presence of 10% trichloroacetic acid (TCA). Harvested cells were then suspended in 10% TCA and vortexed vigorously with 0.5-mm-diameter glass beads for 5 min at 4°C. After breakage, cell suspensions were centrifuged at 800 × g for 10 min, and the supernatant was discarded. The remaining pellets were suspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer containing 0.5 M Tris-HCl (pH 8.0) and boiled for 5 min, followed by centrifugation at 16,100 × g for 15 min. The solubilized proteins in the supernatant were subjected to immunoblotting with rabbit polyclonal anti-Spc1 antibodies and mouse monoclonal anti-myc antibodies (9E10; BAbCO).
Stress treatments of S. pombe cells.
Treatments of S. pombe cultures with high-osmolarity stress and oxidative stress have been described previously (47). The phosphorylation state of Spc1 in stressed cells was monitored by immunoblotting using anti-phospho-p38 antibodies (Cell Signaling Technology Inc.) that recognize active Spc1 phosphorylated on both Thr-171 and Tyr-173 (45). Northern hybridization analyses of gpd1+, pyp2+, ctt1+, and leu1 have been described previously (32, 44).
Immunoprecipitation.
Immunoprecipitation was performed following the procedure described previously (33). Anti-myc rabbit polyclonal antibodies (A14; Santa Cruz Biotech.) conjugated to protein A-Sepharose (Pharmacia Biotech) were used to precipitate the Spc1-myc or Wis1-myc proteins from the lysate, which was followed by immunoblotting with mouse monoclonal anti-myc antibodies (9E10; BAbCO), mouse monoclonal anti-HA antibodies (12CA5; Boehringer Mannheim), or rabbit polyclonal anti-Spc1 antibodies.
Fluorescence microscopy.
To examine the cellular localization of the Cdc37 protein, the cdc37:GFP strain (CA1623) grown to the early mid-log phase in YES medium was observed with an Eclipse E600 microscope (Nikon) equipped with a 100× objective lens and a digital charge-coupled device camera (Hamamatsu). Chromosomal DNA was stained with Hoechst 33342 (Sigma) as described previously (5). Images were captured by using the Openlab software (Improvision) and transferred to Adobe Photoshop (Adobe Systems) for figure preparation.
RESULTS
Isolation of mutants resistant to the overexpression of Wis1 MAPKK.
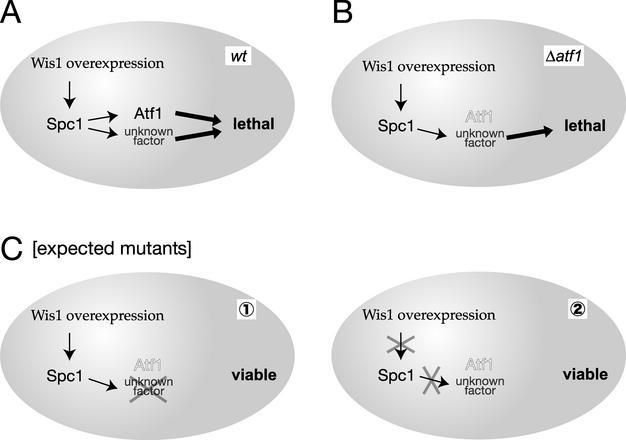
Aiming to identify new components of the SAPK cascade, we performed a genetic screen in S. pombe, the rationale of which is illustrated in Fig. 1. Overexpression of Wis1 MAPKK results in a lethal phenotype accompanied by cell lysis, partly due to a defect in cellular osmoregulation (43, 44). This lethal phenotype is caused by deregulated hyperactivation of Spc1 MAPK and is suppressed by the spc1 null (Δspc1) mutation. However, Wis1 overexpression brings about a severe growth defect even in the absence of Atf1, the only known target transcription factor for Spc1, although the cell lysis phenotype is suppressed by the Δatf1 mutation (Fig. 1B) (44). This observation implies that hyperactivated Spc1 MAPK phosphorylates an unknown factor in addition to Atf1, leading to a growth defect. Therefore, we screened for mutations that rescue the lethality of Wis1 overexpression in Δatf1 strains (Fig. 1C), because they would include mutations in (i) the unknown factor downstream of Spc1, or (ii) novel factors required for activation or function of Spc1 MAPK.
FIG. 1.
Strategy of the genetic screen. (A) In wild-type cells, Wis1 overexpression leads to hyperactivation of Spc1, which causes cellular lethality through Atf1 and an unknown factor. (B) In Δatf1 mutant cells, Spc1 activated by Wis1 overexpression brings about cellular lethality through an unknown factor. (C) The toxicity of Wis1 overexpression is expected to be suppressed in mutants defective in (i) the unknown target of Spc1 MAPK or (ii) factors required for activity and/or function of Spc1 MAPK.
Three classes of unwanted mutants could be isolated in the suppressor mutation screen described above. First, mutants defective in transcription from the Wis1 overexpression plasmid would be isolated. We used HA epitope-tagged Wis1 for overexpression and eliminated this class of mutants by measuring the Wis1-HA protein level by anti-HA immunoblotting. Second, mutations in upstream components that positively regulate Wis1, such as Wis4 and Win1 MAPKKKs, would weaken the toxicity of Wis1 overexpression. To eliminate this possibility, we overexpressed the constitutively active form of Wis1, Wis1DD, in which the MAPKKK phosphorylation sites in Wis1 are substituted with aspartic acid residues that mimic phosphorylation (46). Third, as mentioned above, spc1 mutations completely repress the lethality by Wis1 overexpression (43). To avoid isolating spc1 mutations, we used strains in which an additional copy of the spc1+ gene was integrated in the genome, as it is unlikely that two copies of spc1+ are mutated at the same time when we screen for spontaneous mutations.
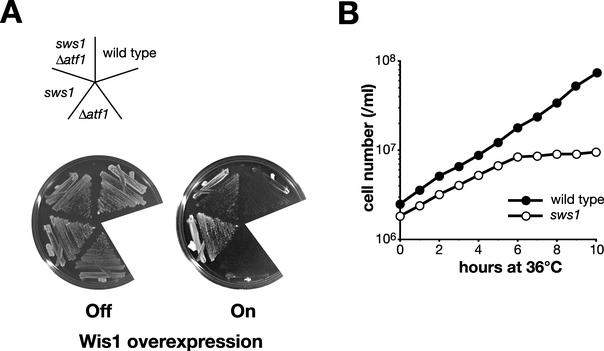
Out of ∼109 cells plated, about 1,000 viable colonies appeared spontaneously under the Wis1 overexpression condition. When 184 isolates were examined by anti-HA immunoblotting, 61 of them were confirmed for the overexpression of Wis1DD-HA. Subsequent genetic analyses showed that a mutant, named sws1-681 (suppressor of Wis1 overexpression), exhibited a temperature-sensitive (ts) growth phenotype (see Fig. 3B) in addition to the resistance to Wis1 overexpression. This report focuses on the detailed study of the sws1 mutation.
FIG. 3.
(A) The sws1 mutation suppresses the lethality of Wis1 overexpression even in the presence of Atf1. Wild-type (PR109), Δatf1 (CA211), sws1-681 (CA1388), and sws1-681 Δatf1 (CA1417) strains were transformed with the pREP1-WIS1DD plasmid, which expresses the constitutively active mutant form of Wis1 under the regulation of the thiamine-repressible nmt1 promoter. Transformants were streaked on EMM2 with (Off) or without (On) thiamine and grown for 3 days at 30°C. The sws1-681 mutant grew as well as the sws1-681 Δatf1 double mutant even when Wis1 was overexpressed. (B) Temperature-sensitive growth of the sws1-681 mutant. Wild-type (PR109) and sws1-681 (CA1388) strains were grown to the early log phase at 30°C in YES medium. At time zero, the cultures were shifted to 36°C and the cell number was monitored along the time course using a Coulter counter (Beckman Coulter).
Atf1-dependent gene expression is defective in the sws1 mutant.
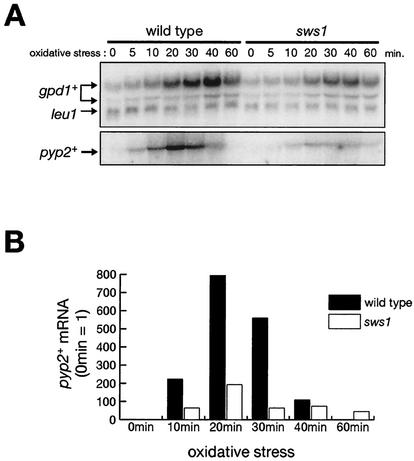
As described above, the sws1 mutation may represent an unknown, Atf1-independent branch downstream of Spc1 MAPK or a novel factor required for activation and/or function of Spc1 (Fig. 1C). In order to distinguish these two possibilities, we examined whether the sws1-681 mutation affects the activity of Atf1 in stress-induced gene expression. Three genes of which transcription is induced by the Spc1-Atf1 pathway upon stress were studied by Northern blotting analyses: gpd1+(encoding glycerol-3-phosphate dehydrogenase), pyp2+(encoding tyrosine-specific phosphatase, which dephosphorylates and inactivates Spc1 MAPK), and ctt1+ (encoding catalase, an enzyme which decomposes hydrogen peroxide) (10, 32, 44, 54). In wild-type cells, gpd1+ mRNA was induced within 10 min of oxidative stress by 0.3 mM H2O2 and reached the maximum level at 40 min (Fig. 2A). On the other hand, in the sws1-681 mutant, the level of gpd1+ mRNA was reduced to approximately 50% of wild type, while the kinetics of induction was similar to that in wild-type cells. The effect of the sws1 mutation was more obvious in the pyp2+ expression. In the wild-type strain, pyp2+ expression was detected at 5 min after exposure to the stress, with maximum induction at 20 min. In the sws1 mutant, pyp2+ expression upon stress was significantly compromised, and the maximum level of pyp2+ mRNA was approximately 25% of that in wild-type cells (Fig. 2). We observed that the stress-induced expression of ctt1+ was also defective in sws1-681 cells (data not shown). These results indicate that expression of the Atf1-dependent genes is compromised in the sws1-681 mutant.
FIG. 2.
The sws1 mutant is defective in the expression of the stress-response genes regulated by Atf1. (A) Wild-type (PR109) and sws1-681 (CA1388) strains were grown to the mid-log phase at 30°C in YES medium and treated with oxidative stress induced by 0.3 mM H2O2. Aliquots of cells were taken at the indicated times, and total RNA was extracted for the Northern blot analysis of gpd1+ and pyp2+ mRNA. The leu1 probe served as a loading control. (B) The levels of pyp2+ mRNA in the experiment shown in panel A were quantified and normalized by the leu1 mRNA levels using the Storm system (Molecular Dynamics). Numbers are in arbitrary units.
The conclusion above implies that the sws1 mutation represents a factor required for activation and/or function of Spc1 MAPK, rather than an Atf1-independent pathway downstream of Spc1. Although the sws1-681 mutation was originally isolated in the Δatf1 background, this model predicts that, like spc1 mutations, the sws1 mutation suppresses the phenotypes of Wis1 MAPKK overexpression even in the presence of atf1+. As expected, the sws1-681 atf1+ strain formed viable colonies even when Wis1 was overexpressed (Fig. 3A). Taken altogether, these results imply that the sws1 mutant is defective in the activation and/or function of Spc1 MAPK.
sws1 is allelic to cdc37+.
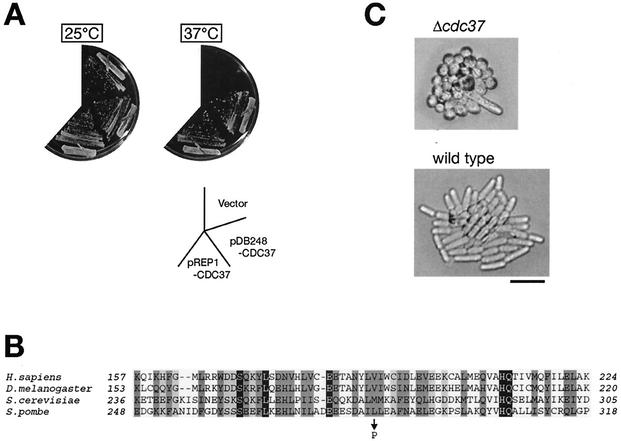
In addition to the resistance to Wis1 overexpression, sws1-681 cells exhibited a ts growth phenotype, and they stopped dividing at temperatures above 36°C (Fig. 3B). Heterozygous diploids constructed by mating wild-type and sws1-681 strains showed neither of the phenotypes, indicating that sws1-681 is a recessive mutation (data not shown). In order to identify the sws1+ gene, haploid sws1-681 cells were transformed with an S. pombe genomic library, and the plasmid clones that complement the sws1 ts phenotype were isolated. Among ∼80,000 transformants screened, 41 of them showed growth at 37°C, and all the plasmids recovered from the ts+ colonies were found to contain the cdc37+ gene (GenBank accession number AJ132376). As shown in Fig. 4A, one of the plasmids isolated from the library, pDB248-CDC37, which contains cdc37+ and an adjacent open reading frame, SPBC9B6.09c, suppressed the sws1 ts phenotype. We subcloned the open reading frame of cdc37+ into the S. pombe expression vector pREP1 (28), and the resultant plasmid, pREP1-CDC37, was also capable of suppressing the ts phenotype of sws1 cells (Fig. 4A), indicating the complementation of sws1-681 by the cdc37+ gene. In addition, a 1-bp substitution that changes Leu-285 of the Cdc37 protein to proline was found in the cdc37 gene cloned from the sws1-681 mutant (Fig. 4B). Replacement of this mutated sequence with the wild-type cdc37+ sequence by homologous recombination rescued the sws1 ts phenotype (data not shown). Taken together, we concluded that sws1+ is identical to cdc37+ and, hereafter, we refer to sws1-681 as cdc37-681.
FIG. 4.
sws1 is allelic to fission yeast cdc37+. (A) The temperature-sensitive (ts) growth phenotype of the sws1-681 mutant is suppressed by the cdc37+ gene. A sws1-681 strain (CA1388) was transformed with the empty vector, pDB248-CDC37, or pREP1-CDC37 plasmids, and the transformants were incubated at the permissive (25°C) or restrictive (37°C) temperature for the sws1-681 mutant. The pDB248-CDC37 plasmid was obtained from the S. pombe genomic library for its ability to complement the sws1-681 ts phenotype, and it contains the cdc37+ gene and an adjacent gene, SPBC9B6.09c. The pREP1-CDC37 expression plasmid contains only the open reading frame of cdc37+ under the regulation of the nmt1 promoter. (B) Leu-285 of Cdc37 is changed to proline in the sws1-681 mutant. The mutation site and the surrounding sequences of S. pombe Cdc37 are aligned with the corresponding regions of Cdc37 orthologs in the human (Homo sapiens), fruit fly (D. melanogaster), and budding yeast (S. cerevisiae) sequences by a multiple alignment program, ClustalW (http://clustalw.genome.ad.jp/). (C) Heterozygous diploid cells of cdc37+/Δcdc37 were sporulated, and the spores dissected on a YES plate were incubated at 25°C for 3 days. Δcdc37 mutant cells show a growth arrest phenotype with abnormally short cell lengths. Bar, 10 μm.
Fission yeast cdc37+ encodes a 466-amino-acid, 53-kDa protein with a significant sequence similarity to orthologs in humans, mice, chickens, flies, worms, and budding yeasts. To investigate the cellular function of fission yeast Cdc37, we performed a gene disruption experiment. The entire open reading frame of one of the cdc37+ genes in wild-type diploid cells was replaced with the ura4+ marker gene by homologous recombination (see Materials and Methods). Sporulation of the resultant heterozygous diploid followed by tetrad analysis revealed that each tetrad produced two viable segregants of the wis4 mutant and two inviable segregants, indicating that the cdc37+ gene is essential for cellular viability. Most of the mutant cdc37 haploid segregants divided several times after germination to form microcolonies of very short cells with one or two elongated cells (Fig. 4C). Thus, Cdc37 has functions essential for vegetative cell growth of S. pombe, which contrasts with the fact that the Spc1 MAPK cascade is dispensable for cell viability in the absence of environmental stress (29, 43).
Both the amount of and the stress-induced phosphorylation of Spc1 are reduced in the cdc37 mutant.
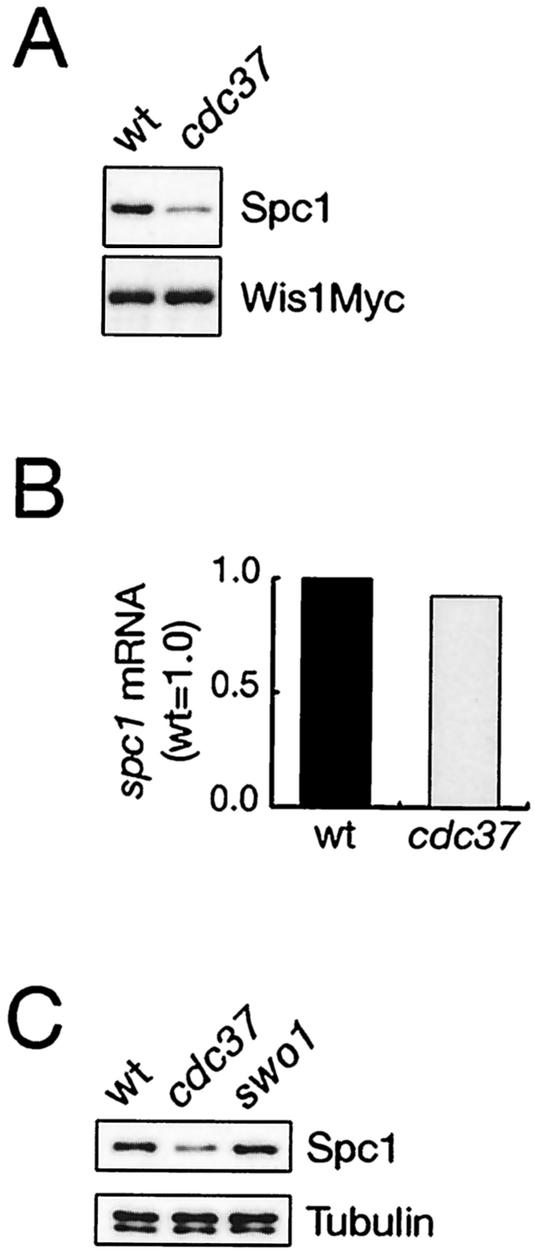
Cdc37 is important for the stability and/or activity of several kinases, such as v-Src, Raf, and Cdk (see introduction). Therefore, it is possible that Cdc37 may also regulate Spc1 MAPK or Wis1 MAPKK in fission yeast, although no MAPK or MAPKK has been reported to require Cdc37 for the kinase function. To examine this possibility, we compared wild-type and cdc37 mutant cells for the amounts of Spc1 and Wis1 as well as Spc1 activation in response to stress. The cell lysate was prepared from wis1:myc and cdc37-681 wis1:myc strains, in which chromosomal wis1+ is tagged with the sequence encoding the myc epitope (14), and the protein levels of Spc1 and Wis1 were evaluated by anti-Spc1 and anti-myc immunoblotting, respectively (Fig. 5A). In the cdc37 mutant, the amount of Spc1 protein was reduced to 30 to 50% of that in wild-type cells, whereas the level of Wis1 MAPKK showed little difference between the two strains. Northern blotting experiments showed that the spc1+ mRNA was not affected by the cdc37-681 mutation (Fig. 5B), and Cdc37 may affect the stability of the Spc1 protein, as previously reported for other kinases (12, 50). On the other hand, while Cdc37 is known to function together with Hsp90 in the chaperoning of Raf1, Cdk, and other kinases, we found that Spc1 MAPK was not affected by a defect in Hsp90. S. pombe has only one Hsp90 gene, of which mutation, swo1-26, brings about the destabilization of the protein kinase Wee1 in cell cycle regulation (3); Swo1 Hsp90 binds to the Wee1 kinase and probably functions as a molecular chaperone. As shown in Fig. 5C, the protein level of Spc1 was affected by the cdc37 mutation but not by swo1-26, implying that Cdc37 regulates Spc1 MAPK independently of Hsp90.
FIG. 5.
The cellular level of Spc1 MAPK, but not Wis1 MAPKK, is reduced in the cdc37 mutant. (A) Wild-type (CA178) and cdc37-681 (CA1450) strains, in which chromosomal wis1+ is tagged with the sequence encoding the myc epitope, were grown to the mid-log phase at 30°C in YES medium, and their cell lysate was subjected to immunoblotting with anti-Spc1 and anti-myc antibodies to examine the protein levels of Spc1 and Wis1myc, respectively. (B) Wild-type (PR109) and cdc37-681 (CA1388) cells exponentially growing in YES medium at 30°C were harvested and subjected to a Northern hybridization analysis to quantify the spc1+ mRNA levels. The results were quantified and normalized by the leu1 mRNA levels using the Storm system (Molecular Dynamics). (C) The cellular levels of Spc1 MAPK in wild-type (PR109), cdc37-681 (CA1388) and swo1-26 (CA1496) strains were examined by anti-Spc1 immunoblotting as described for panel A. The protein level of Spc1 is not affected by the Hsp90 mutation, swo1-26. Immunoblotting with anti-tubulin antibodies (lower panel) showed that the amounts of the cell lysate loaded to each lane were comparable.
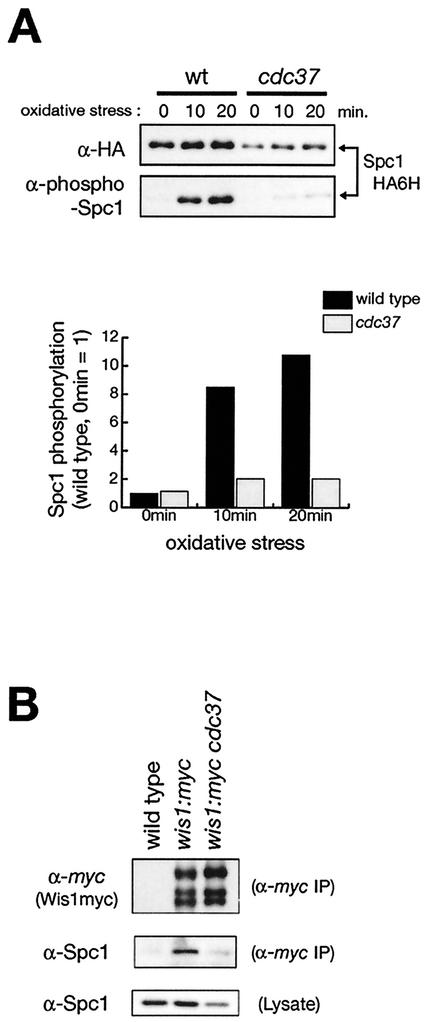
In order to examine whether cdc37 affects the stress-induced activation of Spc1 MAPK, phosphorylation of Spc1 was monitored in wild-type and cdc37-681 mutant strains exposed to high osmolarity and oxidative stress. In these strains, the chromosomal spc1+ gene was tagged with the sequence encoding the HA epitope followed by six consecutive histidine residues (HA6H), so that Spc1 was easily purified by Ni-nitrilotriacetic acid beads and analyzed by immunoblotting with anti-HA antibodies as well as antibodies that cross-react with the phosphorylated, active form of Spc1 (45). As shown in Fig. 6A, stress-induced phosphorylation of Spc1 dramatically decreased in the cdc37 mutant. Quantification of Spc1 phosphorylation followed by normalization with the amount of Spc1 protein indicated that the level of Spc1 phosphorylation upon oxidative stress in cdc37-681 was only 20% of that in wild-type cells (Fig. 6A, lower panel). A significant reduction in Spc1 phosphorylation was also detected in cdc37-681 cells exposed to high-osmolarity stress (data not shown). Consistently, immunofluorescence microscopy with anti-Spc1 antibodies showed that osmostress-induced nuclear accumulation of Spc1, which is dependent on Spc1 phosphorylation (14), was also significantly compromised in cdc37-681 cells (data not shown).
FIG. 6.
Stress-induced phosphorylation of Spc1 MAPK is impaired in the cdc37 mutant. (A) Wild-type (CA76) and cdc37-681 (CA1390) strains carrying the spc1:HA6H allele were grown to the mid-log phase at 30°C in YES medium and treated with oxidative stress induced by 0.3 mM H2O2. Aliquots of cells were harvested at the indicated times, and the Spc1HA6H protein was purified by Ni-nitrilotriacetic acid chromatography followed by immunoblotting with anti-phospho-p38 MAPK to detect phosphorylated Spc1 as well as with anti-HA antibodies. Signals of anti-phospho-p38 antibodies were quantified by the Storm system (Molecular Dynamics) and plotted after normalization to signals of anti-HA antibodies. (B) The physical interaction between Spc1 MAPK and Wis1 MAPKK is compromised in the cdc37 mutant. Anti-myc immunoprecipitation was performed with the cell lysate from wild-type (PR109), wis1:myc (CA178), and wis1:myc cdc37-681 (CA1450) strains, and the precipitates were analyzed by immunoblotting with anti-myc antibodies (top panel) to detect Wis1myc and anti-Spc1 antibodies (middle panel). The protein levels of Spc1 in the cell lysate used in this experiment were also measured by anti-Spc1 immunoblotting (bottom panel).
Wis1 has a MAPK-docking sequence, and the interaction between Wis1 and Spc1 contributes to the efficient phosphorylation of Spc1 by Wis1 (31). Since the cdc37 mutant showed reduced phosphorylation of Spc1 MAPK by Wis1 MAPKK, we examined the physical interaction between Wis1 and Spc1 in the cdc37 mutant by coprecipitation experiments. When Wis1 was immunoprecipitated with anti-myc antibodies from the cell lysate of a wis1:myc strain, copurified Spc1 was detected by anti-Spc1 immunoblotting (Fig. 6B). On the other hand, very little Spc1 was coprecipitated with Wis1 from a wis1:myc cdc37-681 strain, while a detectable level of Spc1 was present in the cell lysate (Fig. 6B, bottom panel). Thus, the physical interaction between Wis1 MAPKK and Spc1 MAPK seems to be reduced in cdc37-681, and this defect is likely to contribute to the decrease in Spc1 phosphorylation in this mutant.
These results suggest that Cdc37 plays a significant role in the stable expression of the Spc1 protein and its interaction with Wis1, which are important for stress signaling from Wis1 MAPKK to Spc1 MAPK.
Cdc37 physically interacts with Spc1 MAPK in vivo.
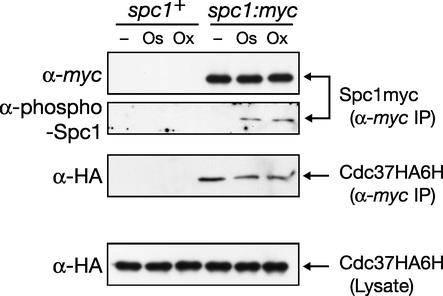
The Cdc37 protein binds to protein kinases, such as v-Src and Cdk, as a molecular chaperone important for the stability and/or activity of those kinases. Results described above suggest that Cdc37 is also important for the stable expression and function of Spc1 MAPK in S. pombe, implying a role for Cdc37 as a molecular chaperone for this SAPK. In order to examine whether Cdc37 interacts physically with Spc1, we tested the copurification of Cdc37 with Spc1 from the cell lysate. Spc1 was isolated by anti-myc immunoprecipitation from a spc1:myc cdc37:HA6H strain, in which chromosomal spc1+ and cdc37+ are tagged with the sequences encoding the myc epitope and HA6H, respectively. As shown in Fig. 7, anti-HA immunoblotting detected Cdc37HA6H coprecipitating with Spc1myc, while Cdc37HA6H was not detectable in the immunoprecipitates from a control strain expressing untagged Spc1 (Fig. 7, spc1+). Similar experiments using the lysate prepared from cells exposed to high osmolarity and oxidative stress were also performed; anti-myc antibodies precipitated phosphorylated Spc1myc and a slightly reduced amount of Cdc37HA6H, while the protein level of Cdc37 in the cell lysate showed little change before and after stresses (Fig. 7, bottom panel). Thus, Cdc37 physically interacts with Spc1 in vivo, a result consistent with the notion that Cdc37 functions as a molecular chaperone for Spc1 MAPK.
FIG. 7.
Cdc37 physically interacts with Spc1 MAPK. spc1+ (CA1621) and spc1:myc (CA1653) strains, in which chromosomal cdc37+ is tagged with the sequence encoding the HA6H tag, were grown to the mid-log phase at 30°C in YES medium. Aliquots of cells were harvested before (−) or after treatments with either high-osmolarity stress induced by 0.6 M KCl (Os) or oxidative stress induced by 0.3 mM H2O2 (Ox). Crude lysate was prepared, and the Spc1myc protein was precipitated with protein A-Sepharose beads conjugated with anti-myc antibodies, followed by immunoblotting with anti-myc, anti-phospho-p38, and anti-HA antibodies. The protein levels of Cdc37HA6H in the crude lysate used in this experiment were also examined by anti-HA immunoblotting (bottom panel). Cdc37HA6H was coprecipitated with Spc1myc but not from the lysate of spc1+ cells.
Cellular localization of the Cdc37 protein.
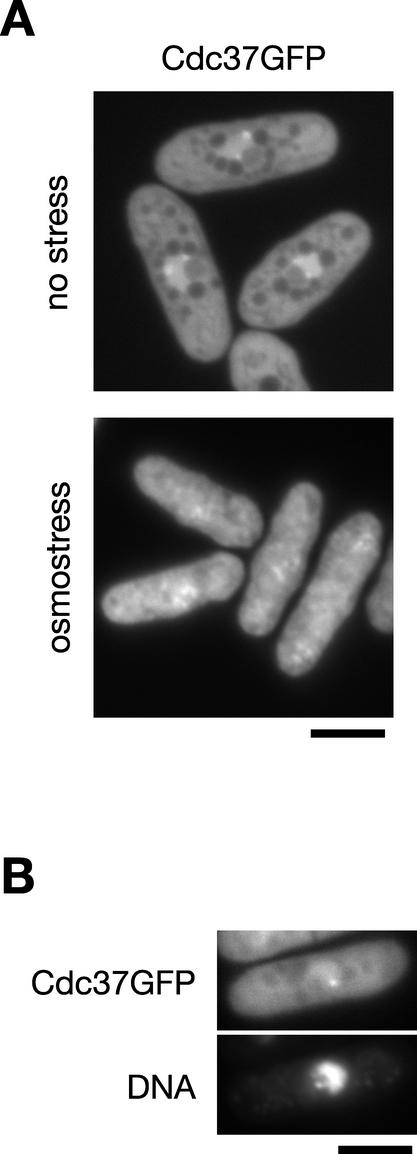
In response to stress, both Spc1 MAPK and Wis1 MAPKK show dynamic changes in their cellular localization (14, 15, 31). Under normal growth conditions, Spc1 is found throughout the cell, while Wis1 is found exclusively in the cytoplasm due to its nuclear export signal sequence. Once cells are exposed to osmostress, both proteins are translocated into the nucleus within a few minutes. Because of the detected interaction between Cdc37 and Spc1, the cellular localization of Cdc37 was studied both in the presence and absence of stress. We constructed an S. pombe strain in which the chromosomal cdc37+ gene was tagged with the sequence encoding green fluorescent protein (GFP). The resultant cdc37:GFP strain showed no apparent growth defect at different temperatures tested (data not shown), indicating that the Cdc37GFP fusion protein is functional. Fluorescence microscopy of Cdc37GFP in living cells showed that the Cdc37 protein was located throughout the cell, with prominent localization in the chromatin region of the nucleus (Fig. 8A, upper panel, and B); within the chromatin region, one or two bright dots of Cdc37GFP signal were consistently observed (Fig. 8A, upper panel, and B). The cytoplasmic staining of Cdc37GFP was somewhat uneven, probably due to subcellular compartments in the cytoplasm. In contrast to Spc1 and Wis1, we did not observe a dramatic change in the localization of Cdc37 when cells were treated by high-osmolarity stress (Fig. 8A, lower panel), although the Cdc37GFP signal in the chromatin region became less marked.
FIG. 8.
Cellular localization of Cdc37 in S. pombe. (A) Strain CA1623, of which the chromosomal cdc37+ gene was tagged with the sequence encoding GFP, was grown to the early log phase and observed by fluorescence microscopy in the presence (lower panel) or absence (upper panel) of high-osmolarity stress induced by 0.6 M KCl. (B) Chromosomal DNA in strain CA1623 was stained with Hoechst 33342, and cells were incubated in YES liquid medium. After 2 h of incubation, cells were observed with fluorescence microscopy for Cdc37GFP and DNA. Bar, 5 μm.
DISCUSSION
Previous studies strongly suggest that Cdc37 is an evolutionarily conserved molecular chaperone specific for protein kinases (21), and its functions are essential for cell growth in both budding yeast (40) and fission yeast (this study). Cdc37 is expressed at high levels in some cancer cells (51), and the ectopic overexpression of Cdc37 in mice promotes cellular transformation (49). However, only a limited number of protein kinases have been demonstrated as clients for the Cdc37 chaperone. In this study, we identified Cdc37 as a positive regulator of the fission yeast Spc1, a member of the evolutionarily conserved stress-activated MAPK subfamily.
In the present study, we have obtained genetic and biochemical data suggesting that Cdc37 plays an important role in the SAPK pathway in S. pombe. First, cdc37 was identified as a mutation that suppresses aberrant Spc1 signaling induced by overexpression of Wis1 MAPKK. Second, Cdc37 forms a complex with Spc1 in vivo, and in the cdc37 mutant the protein level of Spc1, but not the spc1+ mRNA, is reduced. Third, the interaction of Spc1 with Wis1 MAPKK is compromised in the cdc37 mutant, and stress-induced phosphorylation of Spc1 by Wis1 is significantly reduced. Consistently, the expression of gpd1+, ctt1+, and pyp2+ genes, which is induced upon stress by activated Spc1 through the Atf1 transcription factor, is also compromised in the cdc37 mutant. These results support the notion that Spc1 MAPK is a novel target for the Cdc37 chaperone. Interaction with Cdc37 may stabilize the Spc1 protein and maintain Spc1 in a properly folded state competent for the interaction with Wis1 MAPKK.
Some studies strongly suggest that the function of Cdc37 is to target the Hsp90 chaperone machinery to protein kinases by interacting with both Hsp90 and kinases (16, 50). In addition, mutational inactivation of Cdc37 and Hsp90 similarly affect the stability and/or function of v-Src and Ste11 MAPKKK in budding yeast (1, 11, 26, 56) and the sevenless receptor tyrosine kinase pathway in Drosophila (7), indicating the cooperative action of Cdc37 and Hsp90. The genome sequence of S. pombe contains only one Hsp90 gene, swo1+ (3); Swo1 binds to a protein kinase, Wee1, and the Wee1 protein is destabilized in the swo1-26 mutant, suggesting the chaperone function of the Swo1 Hsp90 for the Wee1 kinase. In contrast, no apparent defect in the stability and activation of Spc1 MAPK was observed in the swo1-26 mutant and, therefore, the Hsp90 function does not appear to be important for Spc1. Interestingly, an Hsp90-like chaperone activity of the Cdc37 protein has been detected in vitro, and Cdc37 is able to perform the chaperone function independently of Hsp90 at least when overexpressed in budding yeast (22, 25). It is possible that Cdc37 and Hsp90 have some distinct functions in vivo through the regulation of different protein kinases.
Although the amount of active Spc1 MAPK is dramatically reduced in the cdc37-681 mutant, the mutant cells do not show apparent growth defects under environmental stresses; cdc37-681 cells are not sensitive to the high osmolarity of 1 M KCl, and the viability of the mutant cells exposed to oxidative stress by H2O2 is comparable to that of wild-type cells (data not shown). Consistently, in the cdc37-681 mutant, Spc1-dependent phosphorylation of Atf1 is detectable (data not shown), and the stress response genes regulated by the Spc1-Atf1 pathway are induced upon stress, although the induction levels of those genes are lower than those in wild-type cells (Fig. 7). Thus, the remaining activity of Spc1 in the cdc37-681 mutant may be sufficient for the cellular survival of stress at least under the conditions tested. The stress sensitivity is also not obvious in the Wis4 MAPKKK null mutant, which is significantly compromised for Spc1 activation (45), and the full activation of the Spc1 pathway does not seem to be necessary for survival under the experimental stress conditions.
Whereas the Spc1 MAPK pathway is not essential unless cells are exposed to environmental stress, cdc37+ is absolutely required for cell viability and the Δcdc37 mutant is lethal. Therefore, Cdc37 must have functions other than regulating the Spc1 pathway. In budding yeast (12), flies (7), and mammals (36, 50), Cdc37 is important for the activity and/or stability of Cdk, and it is possible that the lethal phenotype of Δcdc37 in S. pombe is caused by the loss of functional Cdc2, an essential Cdk in the fission yeast cell cycle. However, in contrast to cdc2 mutants that show a highly elongated cell morphology caused by cell cycle arrest (35), most Δcdc37 cells stop dividing with short cell length, and cdc37-681 cells also do not show a cdc phenotype at the restrictive temperature (data not shown). Thus, the lethal phenotype of the cdc37 mutants cannot be explained solely by inactivation of Cdc2, and the Cdc37 targets essential for cell growth in fission yeast remain to be identified.
In summary, we have identified Cdc37 as a novel regulator of Spc1 MAPK. Although the chaperone function of Cdc37 has been described for MAPKKKs, Raf in higher eukaryotes (16, 48), and Ste11 in budding yeast (1), this is the first report that a MAPK requires Cdc37. Because of the high conservation of SAPKs between fission yeast and mammalian cells, it will be of interest to examine whether human p38 MAPKs are also clients of Cdc37. In addition to cdc37+, we have isolated another locus, named sws2 in the genetic screen described in this report, and the characterization of sws2 may also identify a novel, evolutionarily conserved regulator of SAPKs.
Acknowledgments
We are grateful to Mitsue Shiozaki, Doris Lui, Nhan Vo, and Akiko Seki for technical assistance. We also thank Aminah Ikner for her critical reading of the manuscript and Paul Russell for strains.
H.T. was a recipient of a JSPS (Japan Society for the Promotion of Science) Postdoctoral Fellowship for Research Abroad 2002. This research was supported by grants awarded to K.S. from NIH (GM59788) and from the University of California Cancer Research Coordinating Committee.
REFERENCES
- 1.Abbas-Terki, T., O. Donze, and D. Picard. 2000. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 467:111-116. [DOI] [PubMed] [Google Scholar]
- 2.Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast: a laboratory course manual. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 3.Aligue, R., H. Akhavan-Niak, and P. Russell. 1994. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 13:6099-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewster, J. L., T. de Valoir, N. D. Dwyer, E. Winter, and M. C. Gustin. 1993. An osmosensing signal transduction pathway in yeast. Science 259:1760-1763. [DOI] [PubMed] [Google Scholar]
- 5.Chikashige, Y., D. Q. Ding, H. Funabiki, T. Haraguchi, S. Mashiko, M. Yanagida, and Y. Hiraoka. 1994. Telomere-led premeiotic chromosome movement in fission yeast. Science 264:270-273. [DOI] [PubMed] [Google Scholar]
- 6.Cobb, M. H., and E. J. Goldsmith. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843-14846. [DOI] [PubMed] [Google Scholar]
- 7.Cutforth, T., and G. M. Rubin. 1994. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 77:1027-1036. [DOI] [PubMed] [Google Scholar]
- 8.Dai, K., R. Kobayashi, and D. Beach. 1996. Physical interaction of mammalian CDC37 with CDK4. J. Biol. Chem. 271:22030-22032. [DOI] [PubMed] [Google Scholar]
- 9.Degols, G., and P. Russell. 1997. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 17:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degols, G., K. Shiozaki, and P. Russell. 1996. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 16:2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey, B., J. J. Lightbody, and F. Boschelli. 1996. CDC37 is required for p60v-src activity in yeast. Mol. Biol. Cell 7:1405-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell, A., and D. O. Morgan. 2000. Cdc37 promotes the stability of protein kinases Cdc28 and Cak1. Mol. Cell. Biol. 20:749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson, J., J. Y. Ho, T. A. Peterson, and S. I. Reed. 1986. Nucleotide sequence of the yeast cell division cycle start genes CDC28, CDC36, CDC37, and CDC39, and a structural analysis of the predicted products. Nucleic Acids Res. 14:6681-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaits, F., G. Degols, K. Shiozaki, and P. Russell. 1998. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 12:1464-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaits, F., and P. Russell. 1999. Active nucleocytoplasmic shuttling required for function and regulation of stress-activated kinase Spc1/StyI in fission yeast. Mol. Biol. Cell 10:1395-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grammatikakis, N., J. H. Lin, A. Grammatikakis, P. N. Tsichlis, and B. H. Cochran. 1999. p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell. Biol. 19:1661-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, S., D. Campbell, B. Dérijard, and R. J. Davis. 1995. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267:389-393. [DOI] [PubMed] [Google Scholar]
- 18.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, J., J.-D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 20.Herskowitz, I. 1995. MAP kinase pathways in yeast: for mating and more. Cell 80:187-197. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, T., and R. Y. C. Poon. 1997. Cdc37: a protein kinase chaperone? Trends Cell Biol. 7:157-161. [DOI] [PubMed] [Google Scholar]
- 22.Kimura, Y., S. L. Rutherford, Y. Miyata, I. Yahara, B. C. Freeman, L. Yue, R. I. Morimoto, and S. Lindquist. 1997. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 11:1775-1785. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, J. E. Stickler, M. M. McLaughlin, I. R. Siemens, S. M. Fisher, G. P. Livi, J. R. White, J. L. Adams, and P. R. Young. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 25.Lee, P., J. Rao, A. Fliss, E. Yang, S. Garrett, and A. J. Caplan. 2002. The Cdc37 protein kinase-binding domain is sufficient for protein kinase activity and cell viability. J. Cell Biol. 159:1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louvion, J.-F., T. Abbas-Terki, and D. Picard. 1998. Hsp90 is required for pheromone signaling in yeast. Mol. Biol. Cell 9:3071-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 28.Maundrell, K. 1990. nmt1 of fission yeast. J. Biol. Chem. 265:10857-10864. [PubMed] [Google Scholar]
- 29.Millar, J. B. A., V. Buck, and M. G. Wilkinson. 1995. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 9:2117-2130. [DOI] [PubMed] [Google Scholar]
- 30.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, A. N., A. D. Ikner, M. Shiozaki, S. M. Warren, and K. Shiozaki. 2002. Cytoplasmic localization of Wis1 MAPKK by nuclear export signal is important for nuclear targeting of Spc1/Sty1 MAPK in fission yeast. Mol. Biol. Cell 13:2651-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen, A. N., A. Lee, W. Place, and K. Shiozaki. 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11:1169-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen, A. N., and K. Shiozaki. 1999. Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 13:1653-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen, A. N., and K. Shiozaki. 2002. MAPping stress survival in yeasts: from the cell surface to the nucleus, p. 75-90. In K. B. Storey and J. M. Storey (ed.), Sensing, signaling and cell adaptation, vol. 3. Elsevier Science, Amsterdam, The Netherlands.
- 35.Nurse, P., P. Thuriaux, and K. Nasmyth. 1976. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 146:167-178. [DOI] [PubMed] [Google Scholar]
- 36.O'Keeffe, B., Y. Fong, D. Chen, S. Zhou, and Q. Zhou. 2000. Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated tat stimulation of HIV-1 transcription. J. Biol. Chem. 275:279-287. [DOI] [PubMed] [Google Scholar]
- 37.Perdew, G. H., H. Wiegand, J. P. Vanden Heuvel, C. Mitchell, and S. S. Singh. 1997. A 50 kilodalton protein associated with raf and pp60v-src protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry 36:3600-3607. [DOI] [PubMed] [Google Scholar]
- 38.Quinn, J., V. J. Findlay, K. Dawson, J. B. Millar, N. Jones, B. A. Morgan, and W. M. Toone. 2002. Distinct regulatory proteins control the graded transcriptional response to increasing H2O2 levels in fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 13:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Dérijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed, S. I. 1980. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics 95:561-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaeffer, H. J., and M. J. Weber. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuller, C., J. L. Brewster, M. R. Alexander, M. C. Gustin, and H. Ruis. 1994. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 13:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiozaki, K., and P. Russell. 1995. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378:739-743. [DOI] [PubMed] [Google Scholar]
- 44.Shiozaki, K., and P. Russell. 1996. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 10:2276-2288. [DOI] [PubMed] [Google Scholar]
- 45.Shiozaki, K., and P. Russell. 1997. Stress-activated protein kinase pathway in cell cycle control of fission yeast. Methods Enzymol. 283:506-520. [DOI] [PubMed] [Google Scholar]
- 46.Shiozaki, K., M. Shiozaki, and P. Russell. 1998. Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol. Biol. Cell 9:1339-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiozaki, K., M. Shiozaki, and P. Russell. 1997. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol. Biol. Cell 8:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverstein, A. M., N. Grammatikakis, B. H. Cochran, M. Chinkers, and W. B. Pratt. 1998. p50cdc37 binds directly to the catalytic domain of Raf as well as to a site on hsp90 that is topologically adjacent to the tetratricopeptide repeat binding site. J. Biol. Chem. 273:20090-20095. [DOI] [PubMed] [Google Scholar]
- 49.Stepanova, L., M. Finegold, F. DeMayo, E. V. Schmidt, and J. W. Harper. 2000. The oncoprotein kinase chaperone CDC37 functions as an oncogene in mice and collaborates with both c-myc and cyclin D1 in transformation of multiple tissues. Mol. Cell. Biol. 20:4462-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stepanova, L., X. Leng, S. B. Parker, and J. W. Harper. 1996. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 10:1491-1502. [DOI] [PubMed] [Google Scholar]
- 51.Stepanova, L., G. Yang, F. DeMayo, T. M. Wheeler, M. Finegold, T. C. Thompson, and J. W. Harper. 2000. Induction of human Cdc37 in prostate cancer correlates with the ability of targeted Cdc37 expression to promote prostatic hyperplasia. Oncogene 19:2186-2193. [DOI] [PubMed] [Google Scholar]
- 52.Tomonaga, T., K. Nagao, Y. Kawasaki, K. Furuya, A. Murakami, J. Morishita, T. Yuasa, T. Sutani, S. E. Kearsey, F. Uhlmann, K. Nasmyth, and M. Yanagida. 2000. Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 14:2757-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Widmann, C., S. Gibson, M. B. Jarpe, and G. L. Johnson. 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79:143-180. [DOI] [PubMed] [Google Scholar]
- 54.Wilkinson, M. G., M. Samuels, T. Takeda, W. M. Toone, J.-C. Shieh, T. Toda, J. B. A. Millar, and N. Jones. 1996. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10:2289-2301. [DOI] [PubMed] [Google Scholar]
- 55.Winkler, A., C. Arkind, C. P. Mattison, A. Burkholder, K. Knoche, and I. Ota. 2002. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. Cell 1:163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, Y., and S. Lindquist. 1993. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc. Natl. Acad. Sci. USA 90:7074-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]