Abstract
Two distinct benzodiazepine binding sites have been identified, (i) a central site restricted to brain and (ii) a ubiquitously expressed mitochondrial binding site, the so-called peripheral-type benzodiazepine receptor (PBR). In this paper, we show that a benzazepine referred to as BBL22 (2-amino 9-chloro-7-(2-fluorophenyl)-5H-pyrimidol[5,4-d][2]benzazepine), which is classified as a PBR ligand based on structure, induces arrest in G2/M phase of the cell cycle in human tumor cell lines of both epithelial and hematopoietic cellular origin. After G2/M arrest, several tumor types, notably prostate and certain breast cancer lines exhibited significant apoptosis. Ideally, cancer therapies should selectively target tumor cells while sparing normal cell counterparts. BBL22 exhibited such selectivity, as it did not affect the growth and survival of nonmalignant breast and prostate epithelial lines. Moreover, BBL22 demonstrated structural requirements for this selective antitumor activity as 11 structurally related PBR ligands, including high-affinity ligands Ro5–4864 and PK11195, failed to induce tumor cell growth arrest or apoptosis. The in vivo antitumor activity of BBL22 was examined in a human xenograft model of androgen-independent prostate cancer where BBL22 significantly reduced the growth of PC3 prostate tumors without eliciting overt toxicity. Identification of BBL22 represents a tumor selective therapeutic strategy for a variety of human tumors.
Adenocarcinoma of the prostate and breast remain two of the most commonly diagnosed malignancies in North America and Europe. Whereas most prostate carcinomas are initially responsive to androgen ablation, with time they lose their hormone dependence and become refractory to hormonal manipulations as well as unresponsive to aggressive chemotherapeutic agents (1, 2). At that point, most men ultimately will die from progressive prostate cancer. Similarly, with the exception of erbB2-overexpressing breast cancers, where a humanized anti-erbB2 monoclonal antibody has impacted on survival (3), there have been relatively few therapeutic advances made over the past five decades that have prolonged overall survival in the setting of metastatic breast cancer. The chronic use of diazepam, a member of the benzodiazepine (BZD) family, has been associated with a more favorable outcome in breast cancer (4). Primary tumors among women who have used therapeutic doses of diazepam for more than 6 months tended to be smaller in size with a reduced incidence of lymph node involvement. These findings are intriguing in light of the growth regulatory effects of certain BZDs on murine tumor lines (5, 6).
BZDs interact with two distinct loci, a central and peripheral-type BZD binding site. The two sites differ not only in their tissue distribution and intracellular localization, but also in their ligand affinity (7). The central-type receptor is restricted to brain and mediates the therapeutic anxiolytic, anticonvulsant, and muscle-relaxant effects associated with commonly prescribed BZDs such as clonazepam, imparting allosteric effects on γ-aminobutyric acid-regulated chloride channels (8, 9). A second, more ubiquitously expressed BZD binding site is found on the outer mitochondrial membranes of peripheral tissues and cells (6, 10–12). This latter site, the so-called peripheral-type BZD receptor (PBR), is characterized by its affinity for another BZD ligand, Ro5–4864, and the isoquinoline derivative PK11195, rather than for clonazepam (6, 13, 14). A number of PBR ligands have been shown to influence a variety of cell processes, including adrenal steroidogenesis (15–22), mitochondrial respiration (23), and the growth and differentiation of murine cell lines (5, 6, 24, 25). However, the role of PBR in mediating these effects is unknown, as ligand binding affinity has not always correlated with biological activity (13, 25).
Although the mechanism(s) responsible for the growth and differentiative effects of various peripheral BZDs are unknown, one possibility is their effect on the expression of growth regulatory genes such as the c-fos protooncogene. c-fos is induced during nerve growth factor-stimulated neuronal differentiation of PC12 pheochromocytoma cells (26). Coculturing various peripheral-acting BZDs with nerve growth factor not only modified growth factor-mediated neuronal differentiation, but also resulted in the superinduction of c-fos (26). A second possibility is to modulate cell growth through their effects on the cytoskeleton, a key target of growth regulatory signals (27). And third, PBR and their high-affinity exogenous ligands PK11195 and Ro5–4864 enhance intracellular cholesterol transport (21, 22) and steroid biosynthesis (15–22), key processes in regulating cell growth. In addition, diazepam binding inhibitor, the putative endogenous PBR ligand (28), also has been shown to stimulate mitochondrial steroidogenesis (20).
We now report on the identification of a benzazepine that selectively arrests a variety of both epithelial and hematological human tumors in G2/M phase of the cell cycle without affecting normal cell counterparts. After cell cycle arrest, several tumor types, notably prostate and breast, undergo apoptosis. Optimal cancer therapies should target tumor cells without adversely affecting normal cell counterparts, and BBL22 does not affect nonmalignant breast and prostate epithelial cell lines. To indicate the structural requirements of BBL22 antitumor activity, 11 additional PBR ligands, some with higher PBR affinity than is found in BBL22, had no effect on tumor cell lines. To demonstrate its in vivo antitumor activity, BBL22 was administered to mice bearing a highly aggressive, androgen-independent human prostate cancer xenograft. The growth of established tumors was significantly reduced in the PC3 xenograft after treatment with BBL22. Moreover, overt toxicity was not observed. The data and the clinical implications of these findings will be discussed.
Materials and Methods
Reagents.
Peripheral-acting BZDs (Ro5–4864, Ro5–5115, Ro22–3793, Ro11–6896, Ro7–5220, Ro7–3371, Ro5–6900, Ro23–4566, and Ro22–9370) were kindly provided by Hoffman–La Roche. Diazepam and PK11195 were purchased from Sigma. BBL22 (2-amino 9-chloro-7-(2-fluorophenyl)-5H-pyrimidol[5,4-d][2]benzazepine) was kindly provided by Bessor and Bessor Laboratories. For cell culture work, BBL22 was dissolved in PBS, pH 7.4, supplemented with 0.1% (vol/vol) DMSO.
Cell Culture.
BPH-1 cell line was kindly provided by Simon Hayward (Univ. of California, San Francisco). All additional cell lines were obtained from the American Type Culture Collection (Rockville, MD). MCF7 and MCF10A cells were grown in Eagle's minimum essential medium containing nonessential amino acids, 2 mM l-glutamine, 2 mg/ml bovine insulin, 10% heat-inactivated FCS, and 5 mg/ml penicillin/streptomycin. MDA-MB-468 cells were grown in l-15 medium containing 10% heat-inactivated FCS. HS478t cells were grown in DMEM supplemented with 10% heat-inactivated FCS. BT474, K562, Nalm6, and Nalm16 cells were grown in RPMI medium 1640 containing 10% heat-inactivated FCS and 5 mg/ml penicillin/streptomycin. HST578BST, LNCaP, DU145, PC3, BPH-1, and T98 cells were grown in DMEM supplemented with 10% heat-inactivated FCS. All cells were cultured in a humidified incubator at 37°C under a 5% CO2 atmosphere except MDA-MB-468, which was cultured under atmospheric air.
Protein Electrophoresis and Immunoblot Analysis.
Cells were harvested and lysed in buffer containing 20 mM Hepes (pH 8), 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, and 25% (vol/vol) glycerol. Protein concentrations were determined, and equal amounts of protein were run on a 10% SDS/PAGE gel under reducing conditions. After electrophoresis, proteins were transferred to an Immobilon P membrane where efficiency and equal loading of proteins were evaluated by Ponceau S staining. The membrane was blocked for 1 h with Tris-buffered saline (pH, 7.4) containing 150 mM NaCl and 5% (wt/vol) low-fat milk (TBS), and subsequently was incubated for 1 h at room temperature with an anti-human caspase-3 monoclonal antibody (PharMingen) at a 1:2,000 dilution in TBS containing 0.2% (vol/vol) Tween 20 (TBST). Blots then were washed three times in TBST, incubated with a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution in TBST), and then visualized by enhanced chemiluminescence (Amersham Pharmacia).
Cell Cycle Analysis.
Cells were harvested and fixed with 70% ethanol in PBS. Cell pellet was then resuspended in 0.5 ml PBS containing propidium iodide (50 μg/ml) and DNase-free RNase (100 μg/ml). Cell cycle analysis was performed by using a FACStar Plus (Becton Dickinson).
Tumor Xenografts.
PC3 cells (5 × 106) were injected s.c. into the flank of male nude athymic mice. BBL22 was dissolved in PBS (pH 7.4) supplemented with 0.1% (vol/vol) DMSO. At 17 days after inoculation with tumor cells, animals were divided into three groups, (i) vehicle (PBS/DMSO) alone; (ii) BBL22 50 mg/kg per day; and (iii) BBL22 250 mg/kg per day. Either vehicle or BBL22 was injected s.c. two times a day for 4 consecutive days (days 17 through 20 posttumor inoculation). Each group consisted of five animals. The mice were weighed every other day, and bidimensional tumor measurements were obtained twice a week with vernier calipers.
Results
BBL22 Arrests Human Tumor Cells in G2/M Phase of the Cell Cycle.
Table 1 shows the growth regulatory effects of BBL22 on a variety of human tumor lines of epithelial and hematopoietic cell origin. A dose–response curve was established by using BBL22 at final concentrations ranging from 10 pM to 1 mM. Exponentially growing, unsynchronized tumor cell lines were cocultured with BBL22. At various time points before and after treatment with BBL22, flow cytometric cell cycle analysis was performed with propidium iodide staining. BBL22 induced tumor-cell growth arrest in a dose- and time-dependent manner (data not shown). Relatively little growth inhibition was seen when tumor lines were cocultured with BBL22 at concentrations less than 1 μM for 24 h. When treated with 10 μM BBL22 for 24 h, there was approximately 15–20% inhibition of tumor cell growth as determined by absolute cell number (data not shown). Maximal growth inhibition of tumor lines was observed using 50 μM BBL22 for 24 h (Table 1). Growth inhibition was associated with significant cell cycle arrest in G2/M phase (Table 1). Most of the tumor lines examined exhibited doubling times of approximately 16 to 24 h, which corresponded to the optimal incubation period required for BBL22-induced cell cycle arrest. Arrest in G2/M was associated with a concomitant decrease in the percentage of cells in G1 phase, with a smaller reduction in the S phase population (Table 1). Induction of G2/M arrest by BBL22 consistently was observed regardless of tumor type, hormone receptor status of breast cancer cells, or androgen-dependent or -independent status of prostate cancer cells. Further examination of the phenotype of BBL22-arrested cells by using morphological and biochemical analyses (i.e., mitotic spindle formation, cyclin-dependent kinase 1 activity) revealed that cells were blocked in mitosis rather than in the G2 interphase (W.X., S.S., and N.L.S., unpublished work).
Table 1.
Cell cycle effects of BBL22
| Tissue type | % G1
|
% S phase
|
% G2/M
|
|||
|---|---|---|---|---|---|---|
| Control | BBL22 | Control | BBL22 | Control | BBL22 | |
| Breast cancer (estrogen receptor status) | ||||||
| MCF7 (+) | 68 | 46 | 12 | 10 | 10 | 40 |
| HS578t (−) | 73 | 26 | 10 | 17 | 16 | 54 |
| HBL100 (−) | 46 | 25 | 30 | 18 | 24 | 57 |
| MDA-MB-568 (−) | 47 | 28 | 17 | 20 | 29 | 50 |
| BT474 (+) | 67 | 25 | 20 | 15 | 12 | 60 |
| Prostate cancer (androgen status) | ||||||
| PC3 (independent) | 64 | 33 | 16 | 8 | 20 | 54 |
| DU145 (independent) | 71 | 34 | 15 | 20 | 9 | 32 |
| LNCaP* (dependent) | 51 | 17* | 22 | 22* | 26 | 11* |
| Other epithelial carcinomas | ||||||
| HeLa, cervical cancer | 71 | 19 | 15 | 14 | 12 | 56 |
| T98, glioblastoma | 70 | 22 | 15 | 14 | 14 | 64 |
| Hematological malignancies | ||||||
| K562, M6 (AML) | 37 | 9 | 32 | 15 | 24 | 71 |
| Nalm16, B cell (ALL) | 38 | 15 | 38 | 17 | 22 | 62 |
| Nalm6, B cell (ALL) | 53 | 31 | 31 | 17 | 15 | 43 |
| Nonmalignant breast epithelium | ||||||
| MCF10A | 37 | 33 | 25 | 25 | 36 | 38 |
| HS578BST | 39 | 40 | 21 | 18 | 38 | 41 |
| Nonmalignant prostate epithelium | ||||||
| BPH1 | 70 | 66 | 17 | 15 | 12 | 15 |
Cells in exponential log growth phase were treated with 50 μM BBL22, and cell cycle kinetics were determined by propidium iodide staining and fluorescence-activated cell sorter analysis 24 h later. These results are representative of four independent experiments. AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia.
The sub-G1 apoptotic population comprised 49% of BBL22-treated LNCaP cells.
BBL22 Cell Cycle Arrest Is Sustained and Not Abrogated by Saturation of PBR Binding Sites.
To determine whether BBL22-induced G2/M arrest was transient, MCF7 breast cancer cells were treated for 24 h with 50 μM BBL22, after which the compound was washed out and cells were recultured in drug-free medium for an additional 96 h before cell cycle analysis. As shown, the percentage of cells blocked in G2/M phase increased to 70%, consistent with BBL22 activity being long-lived even in the absence of the compound (Table 2). Similar results were obtained with other tumor lines (e.g., HS578t, BT474; data not shown).
Table 2.
BBL22 cell cycle arrest is cumulative and not abrogated by saturation of PBR binding sites
| Culture conditions in MCF7 cells | % G1 | % S phase | % G2/M |
|---|---|---|---|
| Control | 68 | 12 | 10 |
| BBL22, 24 h | 46 | 10 | 40 |
| BBL22, 24 h followed by 72-h washout period* | 20 | 10 | 70 |
| Saturation of PBR (preincubation with 50 μM Ro5-4864) before BBL22, 24 h | 43 | 16 | 38 |
These results are representative of four independent experiments.
Cells were cocultured with 50 μM BBL22 for 24 h, then replaced in drug-free medium, and cell cycle analysis was performed 72 h later.
To determine whether BBL22 cell cycle arrest was mediated by PBR, tumor cells were pretreated with either Ro5–4864 or PK11195 at 50 μM, both high-affinity PBR ligands, at concentrations known to saturate PBR binding sites (5, 6, 13). Blocking PBR sites did not abrogate BBL22-induced cell cycle arrest (Table 2).
BBL22 Induces Tumor Cell Apoptosis After G2/M Arrest.
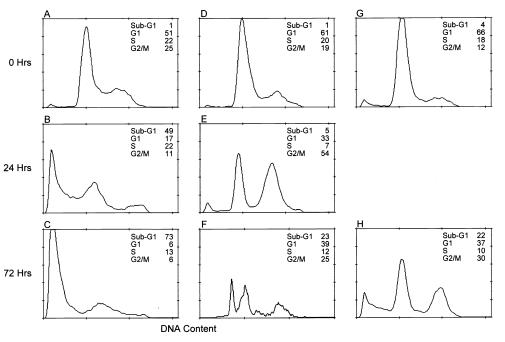
After G2/M arrest, many of the tumor lines, notably prostate and certain breast cancer lines, exhibited morphologic changes consistent with apoptosis, i.e., membrane blebbing, the appearance of a rounded morphology, and eventually detachment from the surface of the tissue culture dish where these epithelial tumor lines normally grow as an adherent monolayer. The percentage of apoptotic cells was quantitated with propidium iodide staining and flow cytometric analysis where the sub-G1 peak represents apoptotic cells. The proapoptotic effects of BBL22 on tumor cells were best illustrated in prostate (PC3 and LNCaP) and breast cancer (HBL100) lines. LNCaP is an androgen-dependent human prostate cancer line. As shown in Fig. 1, there was a marked increase in the sub-G1 peak from 1% in untreated cells (Fig. 1A) to 73% in 72-h BBL22-treated cells (Fig. 1C). In addition to LNCaP, the androgen-independent prostate cancer line PC3 also was sensitive to BBL22-induced apoptosis. The spontaneous rate of apoptosis in untreated PC3 cells was 1% (Fig. 1D). By 24 h after treatment with BBL22, the G2/M population had increased 3-fold as compared with controls, with a corresponding reduction in both G1 and S phase populations (Fig. 1E). On prolonged exposure to BBL22 for 72 h, the sub-G1 apoptotic peak increased to 23%, with a concomitant decrease in the G2/M fraction (Fig. 1F). Similar to prostate cancer cells, the estrogen-receptor-negative breast cancer line HBL100 also underwent apoptosis in response to BBL22. After exposure to BBL22 for 72 h, 22% of HBL100 cells underwent apoptosis as compared with 4% in untreated controls (Fig. 1 G and H). Leukemic cells also were sensitive to BBL22 apoptosis. K562, a Philadelphia-chromosome-positive erythroblastic leukemia cell line underwent significant apoptosis after G2/M arrest (data not shown).
Figure 1.
BBL22 induces human tumor cell apoptosis. DNA histograms indicate cell cycle kinetics of androgen-dependent LNCaP prostate cancer cells (A–C), androgen-independent PC3 prostate cancer cells (D–F), and the estrogen-receptor-negative HBL100 breast carcinoma line (G and H) before (0 h) and at various time points (24 h and 72 h) after treatment with 50 μM BBL22. The percentage of cells in G1, S phase, and G2/M are indicated. The sub-G1 peak represents the apoptotic population. Data shown is representative of five independent experiments.
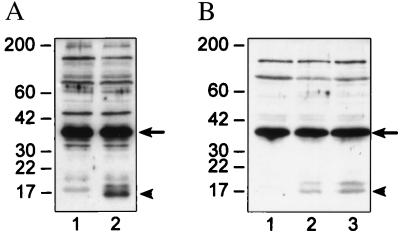
In the case of both LNCaP and HBL100, we were able to demonstrate caspase activation concomitant with the onset of apoptosis. In untreated controls, caspase-3 was expressed predominantly in its inactive 32-kDa procaspase state (p32) (Fig. 2, arrow). Conversion of p32 to the active 17-kDa form of caspase-3 (p17) was observed after treatment with BBL22 for 72 h (Fig. 2, arrowhead). In HBL100 cells, we showed a dose–response effect with regard to caspase-3 activation (Fig. 2B, compare lanes 2 and 3). PC3 cells had constitutively low levels of caspase-3, making it difficult to demonstrate caspase activation in response to BBL22 (data not shown).
Figure 2.
Activation of caspase-3 by BBL22. (A) Immunoblot analysis of caspase-3 in LNCaP cells before (lane 1) and 72 h after (lane 2) treatment with 50 μM BBL22. The 32-kDa procaspase-3 form (arrow) is the predominant species in untreated cells. After treatment with BBL22, procaspase-3 is cleaved to the active 17-kDa product (arrowhead). (B) Proteolytic cleavage of procaspase-3 (arrow) to the active caspase-3 form (arrowhead) after treatment of HBL100 cells with BBL22 (as described previously). Untreated controls (lane 1), 25 μM BBL22 for 72 h (lane 2), and 50 μM BBL22 for 72 h (lane 3). Equal amounts of total cell lysate were loaded to each lane in both A and B.
It must also be pointed out that many breast cancer cells did not undergo apoptosis although they remained blocked in the G2/M phase.
BBL22 Does Not Affect Normal Breast and Prostate Tissue.
Incubation with 50 μM BBL22 for 24 h did not significantly affect the growth of nonmalignant prostate (BPH-1) and breast epithelial cell lines (MCF10A and HS578BST). The apparent tumor-selective nature of BBL22-induced growth arrest was best illustrated in two cell lines established from the same individual. HS578t and HS578BST are neoplastic and nonmalignant breast epithelial lines, respectively. Under identical treatment conditions, only neoplastic HS578t cells arrested in G2/M phase in response to BBL22 (Table 1).
Antitumor Effects of BBL22 Are Not Conserved Among Structurally Related PBR Ligands.
It has been shown previously that there are distinct structure–function relationships associated with various growth and differentiative effects exerted by BZDs on murine cell lines (6, 25). To determine whether the antitumor effects observed with BBL22 were ubiquitous for PBR ligands, we examined the effects of 11 structurally related PBR ligands on the same tumor lines listed in Table 1. Among the compounds examined were the high-affinity PBR ligands Ro5–4864 and PK11195. None of the 11 compounds had any significant effects on tumor cell cycle kinetics or apoptosis. As an example, coculturing PC3 cells with either Ro5–4864 or PK11195 under identical conditions to those described for BBL22 had no effect on tumor cell growth or viability (Table 3).
Table 3.
Structural requirements for antitumor effects of peripheral-acting BZD
| Culture condition | % Sub-G1 | % G1 | % S phase | % G2/M |
|---|---|---|---|---|
| Control | <1 | 70 | 14 | 16 |
| Ro5-4864 | <1 | 68 | 17 | 13 |
| PK11195 | <1 | 69 | 13 | 19 |
PC3 cells in exponential log growth phase were cocultured with vehicle alone (control), Ro5-4864 (50 μM), or PK11195 (50 μM) for 24 h. Subsequent cell cycle analysis at 72 h revealed no further effect of either compound on cell cycle kinetics. These results are representative of five independent experiments. No effect on cell cycle was observed when tumor cells were cocultured with the following peripheral-acting BZD compounds (50 μM for 72 h): Ro22-3793, Ro5-6900, Ro5-5120, Ro11-6896, Ro5-5115, Ro7-3371, Ro5-3463, Ro7-5220, and diazepam.
BBL22 Elicits in Vivo Antitumor Activity.
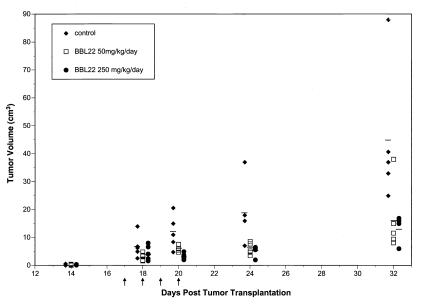
In light of the in vitro antitumor effects of BBL22, we proceeded to test its efficacy in the PC3 xenograft model of human androgen-independent prostate cancer. Tumors were established by inoculating nude athymic mice s.c. with 5 × 106 PC3 cells. At 17 days after tumor inoculation, at which time tumors were palpable and well established, mice were injected s.c. with (i) vehicle alone (n = 5), (ii) BBL22 50 mg/kg per day for 4 consecutive days (n = 5), or (iii) BBL22 250 mg/kg per day for 4 consecutive days (n = 5). Administration of BBL22 at both 50 and 250 mg/kg per day significantly inhibited the growth of tumors as compared with controls treated with vehicle alone (Fig. 3). At day 32 after injection of PC3 cells, the mean tumor volume in animals treated with 50 and 250 mg/kg per day BBL22 was approximately 150 and 100 mm3, respectively, as compared with 450 mm3 for controls. Although histological analysis of normal tissue was not performed, BBL22 at both doses appeared to be well tolerated, as there was no evidence of overt gross toxicity (i.e., weight loss, neurological deficits).
Figure 3.
Antitumor activity of BBL22 in PC3 xenografts. Palpable tumors were established by inoculating nude athymic mice s.c. with 5 × 106 PC3 cells. At day 17 after tumor cell inoculation, animals were injected s.c. with vehicle alone (filled diamonds), BBL22 50 mg/kg per day (open squares), or BBL22 250 mg/kg per day (filled circles) for 4 consecutive days (days 17–20, indicated by arrows). Tumor volumes were determined on days 14, 18, 20, 24, and 32. Horizontal lines indicate mean values for each treatment group.
Discussion
We have identified an agent with tumor-selective cell cycle and proapoptotic effects on both epithelial and hematological human tumor lines without affecting normal cell counterparts. The in vitro antitumor effects of BBL22 were confirmed in vivo, by using a human xenograft model of androgen-independent prostate cancer where BBL22 significantly inhibited tumor growth without eliciting overt toxicity.
Although BBL22 is classified as a PBR ligand based on structure, its antitumor effects seem to be mediated independently of mitochondrial PBR. First, mitochondrial PBR are ubiquitously expressed in both neoplastic and normal tissue, a distribution inconsistent with the tumor-selective effects of BBL22. Second, high-affinity PBR ligands Ro5–4864 and PK11195 did not demonstrate antiproliferative or proapoptotic effects on tumor lines. Third, blocking PBR binding sites did not abrogate subsequent BBL22-induced G2/M arrest of tumor cells (Table 2). And finally, in breast cancer cells, BBL22 localizes to the nucleus and not to mitochondria, whereas other PBR ligands are present in cytoplasm (W.X., S.S., and N.L.S., unpublished work).
The mechanism by which BBL22 exerts its selective effects on neoplastic cells is unknown. Mitochondrial PBRs are widely distributed in a variety of normal and neoplastic tissues (7, 10–12, 29). Although we did not perform PBR binding assays on tumor cell lines, other groups have shown the presence of these binding sites in MCF7, HS578t, and MDA-MB-468 cell lines (21, 30). To date, there have been conflicting reports on the effects of high-affinity PBR ligands PK11195 and Ro5-4864 on the growth of tumor cell lines. Papadopoulos et al. (21) recently showed that PK11195 stimulated the growth of breast cancer lines concomitant with increased nuclear transport of cholesterol. In contrast, Gavish et al. (30) showed that PK11195 and Ro5-4864 exerted antiproliferative effects on MCF7 cells by inducing them to undergo G0/G1 cell cycle arrest. These apparent discrepancies may be concentration dependent. In contrast, our data consistently show BBL22 inducing G2/M cell cycle arrest in a variety of tumor types of both epithelial and hematologic cell origin, thereby minimizing bias toward a particular cell line or tumor type. Camins et al. (31) showed that PK11195, Ro5-4864, and diazepam induced mitotic arrest in Chinese hamster lung cells, although we did not see an effect of these compounds on human tumor lines.
BBL22 appears to exert its antitumor effects through a mechanism independent of the mitochondrial PBR. In this context, Papadopoulos et al. recently identified a form of PBR localized to nuclei of breast cancer cells but not normal breast tissue (21). In their report, they showed that PK11195 acted as an agonist at the nuclear PBR site by stimulating nuclear transport of cholesterol. Therefore, if BBL22 binds the nuclear PBR described by Papadopoulos and colleagues (21), then it represents the first reported antagonist of that binding site. Examples of other nuclear receptors regulating tumor cell growth have been reported. For example, peroxisome proliferator-activated receptor-γ (PPARγ) nuclear receptors are expressed in a number of tumor types (32, 33). Treating PPARγ-expressing tumor cells with PPARγ agonists has been shown to inhibit cell growth, although in some cases inducing subsequent terminal cell differentiation (33). Because PPARγ receptors also are expressed on normal tissue, the clinical use of PPARγ agonists likely will be associated with toxicity. Restriction of the BBL22 binding site to tumors provides an explanation for its specificity of action.
BBL22 also exerts proapoptotic effects on tumor cells. There have been conflicting reports on the effects of PBR ligands on apoptosis. Bono et al. (34) reported that Ro5-4864 protected U937 lymphoblastoid cells against tumor necrosis factor-α (TNF-α)-induced apoptosis, whereas PK11195 sensitized U937 cells to TNF-α-induced killing. Similarly, PK11195 was shown to sensitize lymphocytic cell lines to the proapoptotic effects of chemotherapeutics, γ irradiation, and ceramide (35). These differences may be related to the particular cell type and the apoptotic stimulus. However, each of these studies failed to show an effect of either PK11195 or Ro5-4864 by itself. In contrast, our data indicate that BBL22 exerts proapoptotic effects on a variety of tumor lines when used alone, while sparing normal cell counterparts.
BBL22 demonstrated structural requirements for cell cycle arrest and apoptosis as 11 structurally related molecules, including the high-affinity PBR ligands Ro5–4864 and PK11195, failed to modulate tumor cell growth or apoptosis (Table 3). Other examples of distinct structural requirements for the biological activities of PBR ligands have been reported. For example, the structural requirements for BZDs to induce hemoglobin synthesis in Friend erythroleukemia cells are similar to those associated with their ability to inhibit nerve growth factor-induced neurite outgrowth in PC12 cells (25). These structural requirements, however, differ from those associated with BZD induction of ornathine decarboxylase activity in PC12 cells (25). Identification of the structural requirements for BBL22 tumor-selective G2/M cell cycle arrest and apoptosis will lead to a new generation of tumor-selective agents for treating a variety of epithelial and hematological malignancies.
BBL22 demonstrated in vivo antitumor activity against the PC3 human xenograft, a widely accepted model of highly aggressive, androgen-independent prostate cancer. Clinically, androgen-independent prostate tumors generally are refractory to standard cytotoxic chemotherapeutic agents (1, 2). Administration of BBL22 for only 4 consecutive days inhibited the growth of established PC3 tumors (Fig. 3). The degree of tumor inhibition was similar in the 50 and 250 mg/kg per day treatment groups, suggesting that lower doses should elicit antitumor activity. No overt toxicity was demonstrated in either BBL22 treatment group. These results indicate that BBL22 treatment is an effective inhibitor of the growth of established tumors without eliciting overt toxicity. Further research will need to be done to optimize the dose and schedule of BBL22 administration.
Therapeutic doses of diazepam result in plasma concentrations of between 1 μM and 50 μM (36). Therefore, the plasma concentrations associated with antitumor effects in vitro should be safely achievable in vivo, enabling BBL22 to be used (i) in combination with conventional cytotoxic chemotherapeutic agents, (ii) in a minimal disease state as a long-term adjuvant maintenance therapy, or (iii) as a chemopreventative agent in high-risk groups. Importantly, similar to other small-molecule PBR ligands (37), BBL22 (molecular weight, 338.7) should cross the blood–brain barrier, making it suitable for treating both primary and metastatic central nervous system tumors (see Table 1 for activity against glioblastoma cells). Because BBL22 does not bind central-type BZD receptors, it should not cause central nervous system depression, the dose-limiting side effect associated with centrally acting BZDs. In conclusion, BBL22 offers a strategy for developing a tumor-specific therapy, with minimal toxicities, that will be amenable to both epithelial and hematological malignancies.
Abbreviations
- BZD
benzodiazepine
- PBR
peripheral benzodiazepine receptor
- PPARγ
peroxisome proliferator-activated receptor-γ
- BBL22
(2-amino 9-chloro-7-(2-fluorophenyl)-5H-pyrimidol[5,4-d][2]benzazepine)
References
- 1.Nasu Y, Timme T L, Yang G, Bangma C H, Li L, Ren C, Park S H, DeLeon M, Wang J, Thompson T C. Nat Med. 1998;4:1062–1064. doi: 10.1038/2048. [DOI] [PubMed] [Google Scholar]
- 2.Oesterling J, Fuks Z, Lee C T, Scher H I. In: Cancer Principles and Practice of Oncology. 5th Ed. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott-Raven; 1997. pp. 1322–1386. [Google Scholar]
- 3.Cobleigh M A, Vogel C L, Tripathy D, Robert N J, Scholl S, Fehrenbacher L, Wolter J M, Paton V, Shak S, Lieberman G, Slamon D J. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 4.Kleinerman R A, Brinton L A, Hoover R, Fraumeni J F., Jr Cancer Res. 1984;44:1223–1225. [PubMed] [Google Scholar]
- 5.Wang J K T, Morgan J I, Spector S. Proc Natl Acad Sci USA. 1984;81:753–756. doi: 10.1073/pnas.81.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J K T, Morgan J I, Spector S. Proc Natl Acad Sci USA. 1984;81:3770–3772. doi: 10.1073/pnas.81.12.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braestrip C, Squires R F. Proc Natl Acad Sci USA. 1977;74:3805–3805. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tallman J F, Paul S M, Skolnick P, Gallager D W. Science. 1980;207:274–281. doi: 10.1126/science.6101294. [DOI] [PubMed] [Google Scholar]
- 9.Mohler H, Okada T. Science. 1977;198:849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- 10.Marangos P J, Patel J, Boulenger J P, Clark-Rosenberg E. Mol Pharmacol. 1982;22:26–32. [PubMed] [Google Scholar]
- 11.Anholt R R H, Pedersen P L, DeSouza E B, Snyder S H. J Biol Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- 12.Schoenmaker H, Bliss M, Yamamura H I. Eur J Pharmacol. 1981;71:173–175. doi: 10.1016/0014-2999(81)90405-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang J K T, Taniguichi T, Spector S. Mol Pharmacol. 1984;25:349–351. [PubMed] [Google Scholar]
- 14.LeFur G, Perrier M L, Vancher N, Imbault F, Flamier A, Uzan A, Renault C, Dubroeucq M C, Gueremy C. Life Sci. 1983;32:1839–1847. doi: 10.1016/0024-3205(83)90062-0. [DOI] [PubMed] [Google Scholar]
- 15.Anholt R R H, DeSouza E B, Kuhar M J, Snyder S H. Eur J Pharmacol. 1985;110:41–46. doi: 10.1016/0014-2999(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 16.Ritta M N, Campos M D, Calandra R S. Life Sci. 1987;40:791–798. doi: 10.1016/0024-3205(87)90307-9. [DOI] [PubMed] [Google Scholar]
- 17.Mukhin A G, Papadopoulos V, Costa E, Krueger K E. Proc Natl Acad Sci USA. 1989;86:9813–9817. doi: 10.1073/pnas.86.24.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. J Biol Chem. 1997;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- 19.Costa E, Guidotti A. Life Sci. 1991;49:325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- 20.Papadopoulos V, Berkovich A, Krueger K E, Costa E, Guidotti A. Endocrinology. 1991;129:1481–1488. doi: 10.1210/endo-129-3-1481. [DOI] [PubMed] [Google Scholar]
- 21.Hardwick M, Fertikh D, Culty M, Li J, Vidic B, Papadopoulos V. Cancer Res. 1999;59:831–842. [PubMed] [Google Scholar]
- 22.Papadopoulos V, Mukhin A G, Costa E, Krueger K E. J Biol Chem. 1990;265:3772–3779. [PubMed] [Google Scholar]
- 23.Hirsch J D, Beyer C F, Mulkowitz L, Beer B, Blume A J. Mol Pharmacol. 1988;34:157–160. [PubMed] [Google Scholar]
- 24.Clarke G D, Ryan P J. Nature (London) 1980;287:160–161. doi: 10.1038/287160a0. [DOI] [PubMed] [Google Scholar]
- 25.Morgan J I, Johnson M D, Wang J K T, Sonnenfeld K H, Spector S. Proc Natl Acad Sci USA. 1985;82:5223–5226. doi: 10.1073/pnas.82.15.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran T, Morgan J I. Science. 1985;229:1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- 27.Bandman E, Walker C R, Strohman R C. Science. 1978;200:559–561. doi: 10.1126/science.565534. [DOI] [PubMed] [Google Scholar]
- 28.Guidotti A, Forchetti C M, Corda M G, Konkel D, Bennet C D, Costa E. Proc Natl Acad Sci USA. 1983;80:3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies L P, Huston V. J Pharmacol. 1981;73:209–211. doi: 10.1016/0014-2999(81)90092-3. [DOI] [PubMed] [Google Scholar]
- 30.Carmel I, Fares F A, Leschiner S, Scherubl H, Weisinger G, Gavish M. Biochem Pharmacol. 1999;15:273–278. doi: 10.1016/s0006-2952(99)00093-3. [DOI] [PubMed] [Google Scholar]
- 31.Camins A, Diez-Fernandez C, Pujadas E, Camarasa J, Escubedo E. Eur J Pharmacol. 1995;272:289–292. doi: 10.1016/0014-2999(94)00652-n. [DOI] [PubMed] [Google Scholar]
- 32.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Chambon P. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarraf P, Mueller E, Jones D, King F J, DeAngelo D J, Partridge J B, Holden S A, Chen L B, Singer S, Fletcher C, Spiegelman B M. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 34.Bono F, Lamarche I, Prabonnaud V, Le Fur G, Herbert J M. Biochem Biophys Res Commun. 1999;265:457–461. doi: 10.1006/bbrc.1999.1683. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch T, Decauden D, Susin S A, Marchetti P, Larochette N, Resche-Rigon M, Kroemer G. Exp Cell Res. 1998;241:426–434. doi: 10.1006/excr.1998.4084. [DOI] [PubMed] [Google Scholar]
- 36.Bowling A C, DeLorenzo R J. Science. 1982;216:1247–1250. doi: 10.1126/science.6281893. [DOI] [PubMed] [Google Scholar]
- 37.Starosta-Rubinstein S, Ciliax B J, Penney J B, McKeever P, Young A B. Proc Natl Acad Sci USA. 1987;84:891–895. doi: 10.1073/pnas.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]