Abstract
Thrombospondin 2 (TSP2) is a matricellular protein controlling the apoptosis-proliferation balance in endothelial cells. Little is known about its transcriptional regulation compared with that of TSP1. We found that overexpression of a constitutively active mutant of Rac (RacV12) specifically increases TSP2 mRNA levels without affecting TSP1 in human aortic endothelial cells (HAEC). Moreover, TSP2 induction by RacV12 is dependent upon reactive oxygen species (ROS) production, as gp91ds-tat peptide, an inhibitor of NADPH oxidase, and the flavoprotein inhibitor diphenylene iodinium (DPI) block TSP2 synthesis. Furthermore, we found that increasing RacV12 expression results in a biphasic proliferative curve, with proliferation initially increasing as RacV12 expression increases and then returning to levels less than that of control cells at higher expression. This growth inhibition is mediated by TSP2, as either DPI treatment, which blocks TSP2 synthesis, or pan-TSP blocking antibodies restore the proliferative ability of HAEC with high expression. Mechanistically, we show that the effect of TSP2 on cell proliferation is independent of the antiangiogenic TSP2 Hep1 sequence, which is capable of altering actin cytoskeletal reorganization but not proliferation in our experimental conditions. Finally, we show in vivo that Rac-induced TSP2 expression is observed in the aorta of transgenic mice selectively expressing RacV12 in smooth muscle cells. These results identify Rac-induced ROS as a new pathway involved in the regulation of TSP2 expression.
Thrombospondins (TSPs) are a family of homotrimeric multidomain glycoproteins that on the cellular level modulate adhesion, proliferation, and migration (19) and have been implicated clinically in the development of atherosclerosis, angiogenesis, and oncogenesis. While initially identified in platelet alpha-granules, TSPs are also secreted by a variety of cells, including endothelial cells, smooth muscle cells, fibroblasts, astrocytes, keratinocytes, macrophages, and melanoma cells. The family of TSPs consists of five members (TSP1, -2, -3, -4, and -5) encoded by distinct genes that demonstrate a high degree of sequence homology with platelet TSP (TSP1) (35). TSP1 and -2 form one subgroup of trimeric proteins with a role that is likely distinct from that of the pentameric TSP3, -4, and -5 subgroup (21). Unlike other extracellular matrix molecules that form fibers or basement membrane, TSP1 and TSP2 act as modulators of cell-matrix interaction for a variety of cells. They appear to control the proliferation and migration of smooth muscle cells, as well as chemotaxis of melanoma cells, yet they inhibit endothelial cell proliferation and angiogenesis (27). The complex actions of TSPs are mediated by a number of putative receptors, including heparan sulfate proteoglycan, CD36, integrins, CD47/IAP, latent transforming growth factor β, and LRP (lipoprotein receptor-related protein) (5). Little, however, is known about the molecular pathways that regulate TSP2 production. Various reports have suggested that the synthesis of TSPs is increased by platelet-derived growth factor (PDGF) and interleukin-1β and during pathological conditions such as atherosclerosis and vascular injury (32, 36). Furthermore, TSP1 and -2 isoforms have been shown to induce focal adhesion labilization and to suppress stress fibers and focal contacts in endothelial cells (26). We hypothesized that the Rho family GTPase, Rac1, as a key mediator of similar cellular and physiologic processes (34) including cytoskeletal rearrangement, transduction of growth factors such as PDGF, atherosclerosis, and oncogenesis, might regulate TSP2 expression.
The Rho family of small GTPases are molecular switches controlling cytoskeletal actin reorganization and cellular proliferation. Rac activates many downstream effectors leading to protein synthesis and proliferation (15, 31). Specifically, activated Rac binds to p67phox and induces activation of the NADPH oxidase complex, which in turn produces superoxide ions and reactive oxygen species (ROS) in phagocytes (10). A NADPH oxidase-like activity has now been demonstrated in many nonphagocytic cells, including smooth muscle cells, endothelial cells, fibroblasts, thyrocytes, and normal or cancerous colon epithelial cells (1, 4, 18). Moreover, ROS have been implicated in Rac-induced proliferation (11, 16). Mechanistically, Rac-induced ROS production has been shown to activate NF-κB and also plays a role in the synthesis of collagenase (17). Since the recent identification of different isoforms of the catalytic subunit of the NADPH oxidase (Nox1, -2, -3, -4, and -5) in nonphagocytic cells (6, 20), the involvement of NADPH oxidase in cell signal transduction pathways has represented an intensive and attractive area of research. Recent microarray experiments have shown that overexpression of the Nox1 isoform controls around 200 different proteins related to the control of cytoskeletal structures, extracellular matrix, protein synthesis, transcription, and metabolism (3). To date, a direct regulation of NADPH oxidase activity by Rac has only been evaluated for Nox2 (formerly gp91-phox). This isoform, in addition to all the classical subunits of the NADPH oxidase, is expressed and functional in endothelial cells (22, 23).
In this report, we investigated the involvement of Rac1-induced superoxide production on TSP1 and TSP2 expression in human aortic endothelial cells (HAEC) and the consequent effect on cellular proliferation and actin reorganization. We found that overexpression of RacV12 in cultured endothelial cells results in specific up-regulation of TSP2 mRNA without affecting TSP1. Moreover, TSP2 production appears to result from Rac-regulated superoxide production, as the up-regulation may be blocked by the NADPH oxidase peptide inhibitor, gp91ds-tat, or the flavoprotein inhibitor diphenylene iodinium (DPI) but not by N-nitro-l-arginine methyl ester (l-NAME). Furthermore, Rac induction of TSP2 may be physiologically important, acting as an autocrine loop to stop Rac-induced proliferation. We found that increased Rac expression results in increased TSP2 production that in turn correlates with an inhibition of Rac-induced proliferation. Convincingly, this inhibition could be reversed by using anti-TSP blocking antibodies or DPI to restore the proproliferative capacity of cells expressing Rac at high levels. Finally, we confirmed in vivo, using a transgenic mouse model with smooth muscle selective expression of RacV12, that TSP2 expression is increased in the aorta. These results confirm the previously reported difference in the regulations of expression between TSP1 and TSP2 by identifying Rac-induced ROS as a pathway regulating TSP2 expression in endothelial cells.
MATERIALS AND METHODS
Reagents.
The recombinant replication-incompetent adenovirus Ad.N17 Rac1 and Ad.V12 Rac1, containing the c-myc-tagged dominant-negative and c-myc-tagged constitutively activated forms of Rac1, respectively, were constructed by homologous recombination in 293 cells by using the adenovirus-based plasmid JM17 as previously described (31). The control adenovirus, dl312, which lacks the cDNA insert (Adnull), was used as a control for adenoviral infection. Hep1 peptide (ELTGAARKGSGRRLVKGPD), Hep1 scrambled peptide (RSKAGTLGERDLKPGARVG), gp91ds-tat peptide (RKKRRQRRRCSTRIRRQL), and scrambled-tat peptide (RKKRRQRRRCLRITRQSR) were obtained from AnaSpec, Inc. [3H]thymidine was obtained from Amersham Biosciences, Inc. Rabbit myc-tagged polyclonal antibody and mouse myc-tagged monoclonal antibody were obtained from Cell Signaling Technology, Inc., and Santa Cruz Biotechnology, respectively. Mouse monoclonal anti-TSP Ab-4 (clone A6.1), Ab-1 (clone A4.1), Ab-3 (clone C6.7), and Ab-9 (clone MBD 200.1) were obtained from Labvision Corp. Alexa Fluor 488 goat anti-rabbit immunoglobulin G (IgG)-conjugated antibody, Alexa Fluor 594 goat anti-mouse antibodies, Hoechst 33342, and dihydroethidium (DHE) were obtained from Molecular Probes. Horseradish peroxidase-conjugated goat anti-mouse antibody was obtained from HyClone Labs. Texas Red-conjugated IgG was obtained from Jackson ImmunoResearch Laboratories. IgG1 and IgM isotype controls were obtained from R&D.
Cell culture.
HAEC were cultured in EGM2 medium provided by the manufacturer (Clonetics). Experiments were performed between passage 4 and passage 10, and the serum-deprived conditions were obtained by overnight incubation in EBM2 medium containing 1% fetal bovine serum (FBS). Cells were seeded 24 h before infection. Unless otherwise specified, cells were plated at a density of 120 cells/mm2 in all experiments. Adenoviral infection was performed by diluting the viral vector in serum-deprived medium at the desired multiplicity of infection (MOI) overnight, after which the virus-containing medium was removed and replaced by fresh EGM2 medium for 32 h. The control adenovirus dl312 (Adnull), which lacks the cDNA insert, was used as a control for adenovirus infection. Before all experiments, cells were maintained in deprivation medium for at least 16 h.
Immunoblot analysis.
Cells were lysed in 500 μl of lysis buffer (15 mM HEPES [pH 7.0], 145 mM NaCl, 0.1 mM MgCl2, 10 mM EGTA, 1% Triton X-100) supplemented with a mixture of protease inhibitors [leupeptin, chymostatin, antipain, pepstatin, and 4-(2-aminoethyl)benzenesulfonyl fluoride]. The cells were harvested on ice and sonicated twice for 15 s, and the debris was pelleted in a microcentrifuge at maximum speed for 10 min at 4°C. Protein concentration was estimated by using the bicinchoninic acid assay (Sigma). A total of 10 μg of protein from the cell lysates was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4 to 20% acrylamide). Gels were transferred to a Hybond ECL nitrocellulose membrane (Amersham). Rac1 mutant expression was detected using a monoclonal mouse anti-c-myc epitope primary antibody, and TSP expression was detected using a mouse monoclonal anti-TSP known to recognize TSP1 and TSP2 (clone A6.1). A horseradish peroxidase-conjugated goat anti-mouse (HyClone Labs) was used as a secondary antibody. The chemiluminescent signal was detected on radiographic film and/or a phosphorimager using Super-signal reagent (Pierce Chemical Co.) as instructed by the manufacturer.
RNA isolation and real-time RT-PCR.
After 16 h of serum deprivation, total RNA was extracted using an RNeasy minikit (Qiagen). The amount of RNA isolated was estimated spectrophotometrically and adjusted for each sample to 2 μg/μl in diethyl pyrocarbonate water. Reverse transcription was performed on 2 μg of total RNA with the superscript preamplification system for first-strand cDNA synthesis (GIBCO BRL). Quantification of TSP1 and TSP2 mRNA was performed using real-time reverse transcriptase PCR (RT-PCR; TaqMan PCR, ABI Prism 7700 sequence detection system; Perkin-Elmer Applied Biosystems). We used 5′-GTGGAAGAGCATCACCCTGT-3′ and 5′-GGACGTCCAACTCAGCATTC-3′ as primers and 6FAM-GGACGTCCAACTCAGCATTC-TAMRA as the fluorescent probe to amplify a 100-bp fragment of TSP1, and we used 5′-AAGGATAACTGCCCCCATCT-3′ and 5′-CCGTCATTGTCATCGTCATC-3′ as primers and 6FAM-CCGTCATTGTCATCGTCATC-TAMRA as the fluorescent probe to amplify a 100-bp fragment of TSP2. The 18S mitochondrial mRNA was used with the primers 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′ and the fluorescent probe VIC-TGCTGGCACCAGACTTGCCCTC-TAMRA for internal calibration. The assay was optimized for efficiency relative to the target primers and the internal calibration primers by using 50 nM concentrations of TSP1 or TSP2 primers, 25 nM concentrations of 18S primers, and 100 nM concentrations of each fluorescent probe. The thermal conditions used were 15 s at 95°C and 1 min at 60°C, with a total of 40 cycles. Real-time RT-PCR was also performed on some total RNA extracts as the control for possible contamination by genomic or exogenous DNA. All results were normalized to the content of Rac cDNA in the control adenovirus.
Superoxide measurements. (i) Lucigenin assay.
Cells were harvested and resuspended in Krebs-HEPES buffer. NADPH (1 mM) was added at time zero, and the cells were incubated for 15 min at 37°C. A total of 105 cells was then added to 25 μM lucigenin in Krebs-HEPES buffer, and chemiluminescence was measured for 60 s using a Lumat Berthold LB 9507 luminometer. Measurements were done in the absence or presence of DPI (10 μmol/liter), and the results were expressed as DPI-inhibited relative light units per 105 cells.
(ii) DHE assay.
Superoxide generation in adherent cells was estimated by DHE staining as previously described (30). When oxidized to ethidium, upon reaction with superoxide (.O2−), ethidium binds to DNA, resulting in an increase in quantum yield. Briefly, infected serum-deprived cells were loaded with DHE at a concentration of 10 μmol/liter in HEPES buffer (2 mM HEPES-50 mM glucose in Hanks balanced salt solution) for 30 min at 37°C in 5% CO2. At the end of incubation, the cells were rinsed with Hanks balanced salt solution and examined alive with an excitation/emission wavelength of 560/660 nm. Digital images were recorded on a SenSys digital camera. The integrated fluorescence intensity (expressed in arbitrary units) was quantified on a gray scale of 0 to 255 and measured with the MetaMorph image analysis system. The relative fluorescence intensity was calculated by dividing the total integrated optical density by the total number of cells in each field. Measurements were done in the absence or presence of DPI (10 μmol/liter), and the results were expressed as DPI-inhibited integrated optical density. Mean fluorescence intensity measurements were obtained from three separate experiments in each group.
Quantification of DNA synthesis.
HAEC infected with the different adenoviruses were serum deprived for 16 h and then pulsed labeled with [3H]thymidine (2 μCi/ml) for 4 h, the DNA was precipitated, and the amount of [3H]thymidine incorporation was determined by liquid scintillation counter. In some experiments, TSP antibodies (150 μg/ml) Ab-1 (clone A4.1), Ab-3 (clone C6.7), and Ab-9 (clone MBD 200.1) or pharmacologic treatment was applied during the 16 h of serum deprivation, and then the [3H]thymidine incorporation assay was performed.
Cell proliferation assay.
HAEC infected with the different adenoviruses were plated in triplicate, and the total cell number was quantified after 16, 24, and 48 h of serum deprivation by using a hematocytometer and an Olympus CK2 inverted microscope. Cell viability was assessed by using trypan blue.
Cell death assay.
Programmed cell death was evaluated using a cell death detection ELISA-Plus kit (Roche Molecular Biochemicals). HAEC infected with different adenoviruses were plated in triplicate in a 6-well plate. Cells were serum deprived for 16 or 24 h and then lysed, and the cytoplasmic histone-associated DNA fragmentation (mono- and oligonucleosomes) was detected by spectrophotometry according to the manufacturer's instructions.
Cell cycle analysis.
To analyze cell cycle progression, HAEC (106 cells) infected with either RacV12 or Adnull were serum deprived for 24 h in EBM2. The cells were harvested by trypsinization, resuspended in ice-cold phosphate-buffered saline, and fixed in ethanol at −20°C overnight. The cells were spun down and resuspended in 1 ml of phosphate-buffered saline at room temperature and treated with 2 μl of RNase A (10 mg/ml) for 30 min at 37°C. The cells were then incubated with 10 μl of propidium iodine (10 mg/ml) and analyzed for DNA fragmentation by a flow cytometer with a FACScan (Becton-Dickinson). The percentage of cells in sub-G1, G1, S, or G2/M was determined by analyzing the data using ModFitLT software (Verity Software).
Immunostaining in RacV12 transgenic mice.
Transgenic mice overexpressing the constitutively active mutant form of the human Rac1 gene (RacV12, cDNA; gift from Alan Hall, London, England), with a glycine 12-to-valine substitution, were generated in FVB/N mice by using the smooth muscle α-actin promoter (gift from Arthur Strauch, Ohio State University, Columbus, Ohio). The genome of the mice incorporated the cDNA of RacV12, including its polyadenylation site. Founder mice were selected on the basis of Southern blot analysis, and the mice confirmed to have the highest number of human RacV12 gene copies were used to establish a stable transgenic line by breeding with nontransgenic FVB/N mates. To examine the distribution of Rac and TSP expression in transgenic aortas, we performed immunohistochemistry (IHC). IHC was performed on 4-μm-thick sections from formalin-fixed and paraffin-embedded aorta by using a mouse monoclonal anti-TSP (clone A6.1) or anti-c-myc (clone 9E10) and a standard streptavidin-biotin immunoperoxidase method (M.O.M kit; Vector) according to the manufacturer's instructions. The slides were developed by use of a Vectastain ABC kit and a DAB substrate kit (both from Vector).
Statistical analysis.
Data are presented as means ± standard deviation (SD). The results were compared by the Student t test wherever appropriate. Statistical analysis was performed using STAT-View software (SAS Institute, Inc.). Significance was defined as a P value of <0.05.
RESULTS
Increased TSP2 expression by Rac1-induced superoxide production.
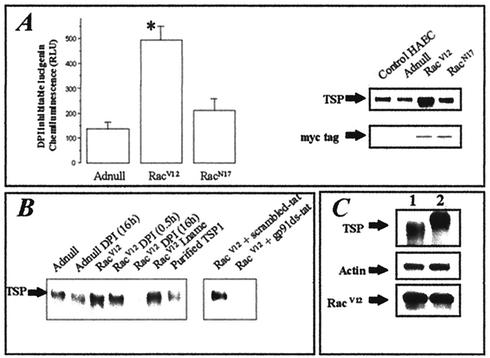
Rac1 may induce the production of superoxide through activation of the NADPH oxidase system involving the Nox2 isoform (1). We first analyzed whether the infection of HAEC with the constitutively active mutant of Rac1, RacV12, resulted in increased superoxide production through NADPH oxidase activity by using a lucigenin assay and DPI chloride (DPI is a known inhibitor of NADPH oxidase). Figure 1A shows that the DPI-inhibitable production of superoxide increased in HAEC infected with RacV12 (at a MOI of 300), while there was no significant increase in the amount of superoxide inhibited by DPI in cells expressing adenovirus control (Adnull) or dominant-negative Rac (RacN17). The production of ROS has been reported to mediate signaling pathways leading to the expression of numerous proteins (3). Thus, we assessed the effect of RacV12 overexpression on the level of expression of TSP by immunoblotting using an antibody recognizing TSP1 and -2 isoforms. RacV12 overexpression drastically increased TSP expression, whereas RacN17-expressing cells showed no change (Fig. 1A). Next, we tested whether the increase in TSP was dependent on Rac-dependent ROS production. We found that flavoprotein inhibition with DPI suppressed both Rac-induced superoxide production and the increase in TSP protein expression. In contrast, TSP production was not dependent on endothelial nitric oxidase synthase (eNOS), since short-term as well as long-term treatment with l-NAME, a specific inhibitor of eNOS, had no effect on TSP expression in RacV12-infected cells (Fig. 1B). In addition, TSP expression induced by Rac was inhibited by gp91ds-tat, a peptide reported to specifically inhibit the NADPH oxidase, compared to what was seen with the corresponding scrambled peptide (33). Comparison of TSP expressions between trypsinized cells and adherent cells showed that TSP expression is mainly extracellular. This localization was demonstrated by the appearance of a lower-molecular-weight form of TSP in trypsinized cells, consistent with the known ability of TSP to be partially cleaved by trypsin when located extracellularly (Fig. 1C).
FIG. 1.
Effect of Rac1-induced NADPH oxidase activity on TSP expression. (A) HAEC were infected with different adenoviruses at a MOI of 300. After cells were detached, NADPH oxidase activity measurements were made using the lucigenin assay and are expressed as DPI-inhibitable lucigenin chemiluminescence, obtained by subtracting the value with DPI from that without DPI. Expression of both c-myc-tagged RacV12 and RacN17 overexpressed proteins was probed by immunoblotting using an anti-c-myc antibody. TSP expression was probed by immunoblotting using a pan-TSP antibody. (B) The effect of inhibition of NADPH oxidase activity on TSP expression was tested by immunoblotting as previously described. (C) Localization of TSP on RacV12-expressing cells was assessed by comparison of cell lysates with trypsinized cells (lane 1) or adherent cells (lane 2). RacV12 expression, actin content, and TSP were probed by immunoblotting.
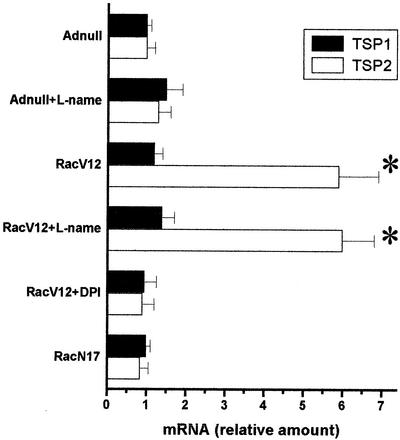
To assess the transcriptional up-regulation of TSP1 and -2 specifically, we quantified the mRNA levels of TSP1 and TSP2 isoforms. Real-time quantitative RT-PCR showed that RacV12 overexpression increased TSP2 mRNA levels by more than sixfold (P < 0.05). Moreover, this increase could be suppressed by DPI but not l-NAME (Fig. 2). TSP1 levels, in contrast, were not affected by RacV12 expression or redox manipulation.
FIG. 2.
Transcriptional up-regulation of TSP2 by RacV12. TSP1 and TSP2 mRNA quantification was performed by TaqMan RT-PCR on HAEC infected with different adenoviruses at a MOI of 300. RacV12-infected cells demonstrate a sixfold increase in TSP2 expression compared to Adnull- and RacN17-infected cells, whereas TSP1 expression was not affected. Results are expressed as relative values compared to control conditions for each specific DNA. Each point represents the mean of a triplicate set of experiments ± SD. ∗, P ≤ 0.05 versus control.
Effects of Rac-dependent TSP2 up-regulation on endothelial cell proliferation.
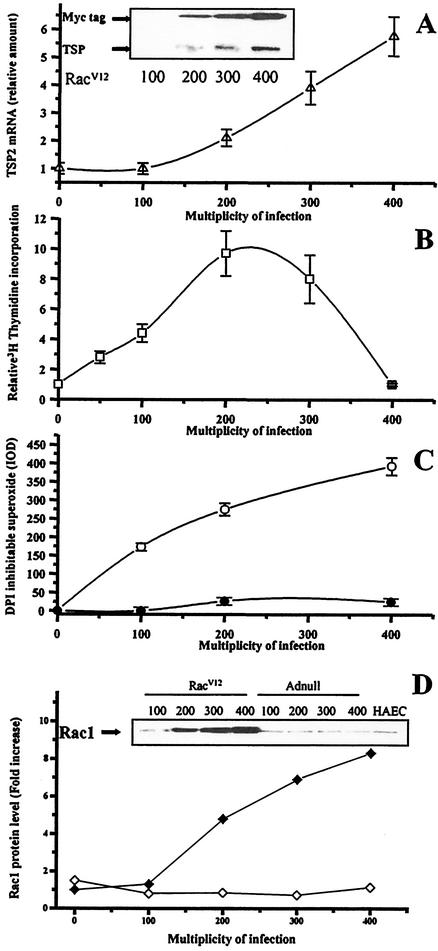
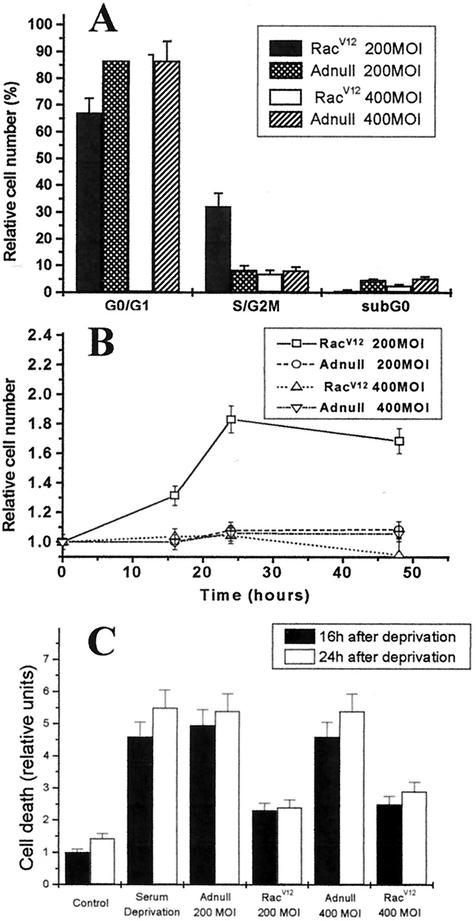
TSP1 and TSP2 are well known regulators of cell growth. TSPs are known to inhibit endothelial cell proliferation, yet they stimulate cell growth in aortic smooth muscle cells (24, 39). We evaluated the incorporation of [3H]thymidine in HAEC infected with Adnull or RacV12 adenovirus at different MOIs. [3H]thymidine incorporation shows a biphasic curve with increasing expression of RacV12 (Fig. 3B). Initially, proliferation increases with greater Rac expression, peaking with a ninefold increase in proliferation at a MOI of 200; however, at a MOI over 200, proliferation was less, returning to baseline by a MOI of 400. Interestingly, the decrease in DNA synthesis in RacV12-infected cells at a MOI of 400 correlated with an increase in TSP2 mRNA and superoxide levels assessed by the DHE assay (Fig. 3A and C). Indeed, the effect of RacV12 on TSP2 mRNA and TSP protein levels demonstrates a MOI-dependent increase up to a MOI of 400 (Fig. 3A) compared with what was seen with control cells. Moreover, by cell cycle analysis and direct cell counts, we confirmed that the increase in DNA synthesis at a MOI of 200 reflects a proliferative index. Cell cycle analysis showed an increased proportion of cells in the S/G2M phase at a RacV12 MOI of 200 compared with an Adnull MOI of 200 (32.1% ± 4.9% versus 8.4% ± 1.6%; P < 0.05). In contrast, cells infected with RacV12 at a MOI of 400 showed a reduced proportion of cells in the S/G2M phase compared to those infected with RacV12 at a MOI of 200 (7.1% ± 1.2% versus 32.1% ± 4.9%; P < 0.01 [Fig. 4A]). Direct cell counts of RacV12-infected cells also demonstrated an increase in cell number at a MOI of 200 compared to cells infected with Adnull at MOIs of 200 and 400 and with RacV12 at a MOI of 400 (Fig. 4B). This decrease in DNA synthesis and cell number observed with high RacV12 level could result from an arrest of proliferation or an increase in cell death index. We found, however, that RacV12 overexpression at MOIs of 200 and 400 protected against cell death to a similar degree (Fig. 4C).
FIG. 3.
Effect of RacV12-dependent TSP2 induction on HAEC proliferation. (A) TSP2 mRNA quantification was performed by use of TaqMan RT-PCR on cells infected with RacV12 adenovirus at different MOIs. TSP2 mRNA levels demonstrate a MOI-dependent increase through a MOI of 400. The inset shows an immunoblot for TSP and RacV12 (myc-tagged) expression with different MOIs. (B) [3H]thymidine incorporation was measured in cells infected with RacV12 and Adnull adenoviruses at different MOIs. Results are expressed as the ratio of thymidine incorporation (RacV12 divided by Adnull) for each MOI considered. RacV12-infected cells showed a ninefold increase in [3H]thymidine incorporation at a MOI of 200, and values returned to less than those of noninfected cells at a MOI of 400. Each point represents the mean of at least three experiments ± SD. (C) DPI-inhibited superoxide production assessed by DHE staining in HAEC infected with increasing concentrations of RacV12 (open circles) or Adnull (filled circles) adenovirus. (D) Quantification of Rac1 protein level by immunoblotting with RacV12 (open diamonds) or Adnull (filled diamonds) adenovirus at different MOIs. Inset shows the corresponding immunoblot that is representative of three independent experiments.
FIG. 4.
Effect of RacV12 on cell cycle and cell growth in HAEC. (A) HAEC were infected with RacV12 or Adnull at MOIs of 200 and 400 and deprived of serum for 24 h. DNA content was measured with propidium iodide staining of the nuclei by using flow cytometry. HAEC infected with RacV12 at a MOI of 200 show an increase in cells at S/G2M phase in contrast to those infected with Adnull at a MOI of 200 or RacV12 at a MOI of 400. Cells infected with RacV12 at a MOI of 400 have a similar ability to arrest HAEC in the G1/G0 phase of the cell cycle compared with those infected with Adnull at a MOI of 400. (B) HAEC infected with RacV12 or Adnull at MOIs of 200 and 400 were plated at a density of 120 cells/mm2 in triplicate. Cell were trypsinized and counted after 16, 24, and 48 h of serum deprivation. Data are expressed as relative cell numbers obtained by dividing the cells counted at each time point by the initial cell numbers at 0 h. Cells infected with RacV12 at a MOI of 200 showed a significant increase in relative cell numbers at all time points analyzed compared with those infected with Adnull at MOIs of 200 and 400 and RacV12 at a MOI of 400. (C) Histone-associated DNA fragmentation was assessed by cell death enzyme-linked immunosorbent assay in control cells and adenovirus-infected HAEC for 16 and 24 h. Increased apoptosis was noted in serum-deprived and Adnull-infected cells compared to control cells, whereas RacV12 at MOIs of 200 and 400 significantly reduced apoptosis compared to serum-deprived and Adnull-infected cells. Data are expressed as means of arbitrary units ± SD from at least one experiment performed in triplicate.
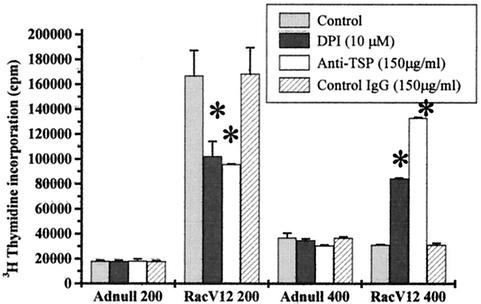
Biphasic control of proliferation by ROS and particularly exogenous H2O2 has been reported previously (7, 13). Under the conditions in our study, endogenous ROS production was increased by RacV12 overexpression. We similarly found that [3H]thymidine incorporation was inhibited by DPI in HAEC expressing RacV12 at a MOI of 200, yet DPI increased [3H]thymidine incorporation in HAEC expressing RacV12 at a MOI of 400 (Fig. 5). This suggests that the biphasic proliferation pattern seen with RacV12 overexpression is dependent on ROS production. This restoration of DNA synthesis might be explained by the suppression of the RacV12-induced TSP2 overexpression by DPI. Indeed, TSP2 has been reported to inhibit endothelial cell proliferation, and the inhibition of RacV12-induced DNA synthesis correlates well with TSP2 expression. To further confirm the involvement of TSP2, we used TSP blocking antibodies in RacV12- and Adnull-infected cells. Interestingly, these antibodies, like ROS inhibition, increased DNA synthesis at high levels of RacV12 (133,141 ± 620 cpm versus 31,264 ± 278 cpm; P < 0.05) yet inhibited proliferation in cells with lower levels of Rac expression (95,820 ± 436 cpm versus 167,000 ± 20,223 cpm; P < 0.05) (Fig. 5). In these experimental conditions, the blocking TSP antibodies did not affect the level of Rac expression (data not shown).
FIG. 5.
Rac1-induced proliferation modulation by redox status and TSP in HAEC. [3H]thymidine incorporation was evaluated in cells infected with low (MOI of 200) or high (MOI of 400) adenoviral levels in the presence or absence of the NADPH oxidase inhibitor DPI or blocking anti-TSP antibodies. [3H]thymidine incorporation was inhibited by DPI and anti-TSP antibodies in RacV12-infected cells at a MOI of 200, whereas both treatments increased the [3H]thymidine incorporation in RacV12-infected cells at a MOI of 400. Each point represents the mean of at least one experiment performed in triplicate ± SD. ∗, P ≤ 0.05 versus control.
TSPs control cell proliferation and migration by modulating the adhesive status of endothelial cells. Since TSP1 and -2 are deadhesive, cell proliferation might be controlled through modification of the adhesiveness or cytoskeletal organization. Indeed, TSP1 and -2 have been shown to labilize focal contact and actin stress fibers through a 19-amino-acid sequence (amino acids 17 to 35), referred to as the Hep1 peptide (9, 26). To evaluate whether the effect of TSP2 on the regulation of cell proliferation is dependent on its induction of cytoskeletal reorganization, we studied the effect of the Hep1 peptide on HAEC proliferation and cellular organization. Thymidine incorporation was not affected by Hep1 peptide treatment in either Adnull or RacV12 at a MOI of 200 compared to what was seen with the scrambled peptide (17,400 ± 1,200 cpm versus 17,500 ± 900 cpm for Adnull at a MOI of 200; 151,900 ± 4,200 cpm versus 149,500 ± 4,600 cpm for RacV12 at a MOI of 200; nonsignificant). In contrast, Hep1 peptide treatment in Adnull- and RacN17-infected cells mimicked RacV12, producing a slight rounding, a disappearance of stress fibers on the cell basement, and a suppression of paxillin localization along actin fibers (data not shown). These results demonstrate that while induction of full-length TSP2 by Rac activation controls cell proliferation, the Hep1 sequence of TSP2 controls only cytoskeletal reorganization and not cell proliferation.
TSP2 upregulation in RacV12 transgenic mice.
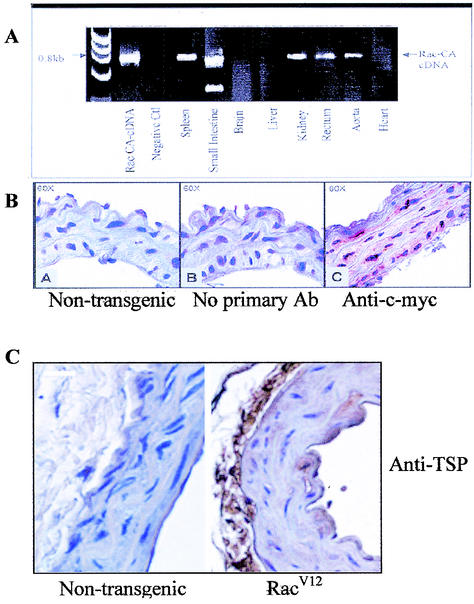
While the expressions of TSP1 and -2 are usually observed in cell culture, the appearance of these proteins in animals is seen only during development or in pathological conditions. For RacV12 transgenic mice, RT-PCR analysis confirmed expression of RacV12 in smooth muscle cells, including blood vessels, spleen, uterus, and intestine, whereas the transcript was not detected in control mice (Fig. 6A). Similarly, IHC demonstrated RacV12 expression in the aorta (Fig. 6B). To evaluate the physiological relevance of the Rac-induced TSP2 increase, we performed immunostaining for pan-TSP and TSP2 on RacV12 and nontransgenic aortas. The IHC studies demonstrated a marked increase in TSP protein expression in the aorta of RacV12 mice compared to what was seen with nontransgenic controls (Fig. 6C). In particular, the adventitia and intimal layer (endothelial cells) displayed strong staining for TSP. Similar results were found with fluorescence staining, which showed markedly increased TSP-2 protein expression in elastic laminae of RacV12 aortas in contrast to what was seen with nontransgenic mice (Fig. 6D). These findings confirm that, in vivo, overexpression of RacV12 results in an up-regulation of TSP2 protein in the aorta.
FIG. 6.
TSP expression in RacV12 transgenic mice. (A) RT-PCR analysis of mRNA from various tissues of RacV12 transgenic mice shows that spleen, intestine, kidney, rectum, and aorta overexpressed Rac V12 cDNA. (B) Immunohistochemistry for RacV12 using a monoclonal antibody against c-myc shows expression in the aorta of RacV12 transgenic mice. (C) Immunohistochemistry for TSP using a mouse monoclonal anti-TSP antibody shows positive TSP staining on the aorta (endothelium and adventitia) of RacV12 transgenic mice (right panel), whereas in nontransgenic mice, no staining for TSP was observed (left panel). Bar, 10 μm.
DISCUSSION
There is an extensive literature on the function of TSP1; however, very little is known about how the regulation and function of TSP2 differ. The major in vitro data often do not take into account the different developmental and spatial patterns of expression of these two proteins. Indeed, the two isoforms are encoded by different genes and recent work on knockout mice for TSP1 and TSP2 has shown distinct phenotypes with no detectable compensatory increase of the paralogous genes for either isoform (5). Although TSP1 and -2 have a high degree of homology, the regulations of expression for TSP1 and -2 are likely to be different. Both isoforms are reported to inhibit endothelial cell proliferation and to induce apoptosis (14, 28). However, while TSP1 induces cell growth arrest through caspase activation, TSP2 proliferation inhibition does not involve induction of apoptosis (2).
In this paper, we confirm that each isoform can be uniquely regulated by identifying the Rac pathway as a major regulator of TSP2 expression in HAEC. We show that Rac-regulated superoxide production specifically increases TSP2 mRNA levels, while TSP1 mRNA levels are not affected. Dose-escalating expression of RacV12 leads to increased ROS production and subsequent TSP2 expression in HAEC. TSP2 expression is also induced in vascular smooth muscle cells (VSMC) in the same manner (data not shown).
Both Rac and TSP2 have been reported to regulate cell proliferation and apoptosis in different cell types. We sought to determine the involvement of RacV12-induced TSP2 expression on the regulation of endothelial cell proliferation. To specifically study the Rac-dependent pathways, we expressed a recombinant adenovirus for RacV12 in endothelial cells without any growth factors and with small amounts of FBS (1%). While increasing expression of RacV12 leads to increased expression of TSP2, the thymidine incorporation first increases and then decreases at higher doses. We further confirmed that the increased thymidine incorporation reflects an increase in the proliferative index by using cell cycle analysis, which showed an increased number of cells in S/G2M phase among cells with low levels of RacV12 expression compared with control cells or cells with high levels of RacV12. In addition, direct cell counts showed an increase in the number of cells with low levels of RacV12 expression compared with control cells or cells with a higher level of RacV12 expression. The decreased proliferation at high levels of Rac expression does not, however, reflect a toxic effect with increased cell death. In contrast, RacV12 expression at both low and high levels similarly protected HAEC from apoptosis compared to control cells, consistent with the previous finding that TSP2 regulates proliferation independent of apoptosis (2).
Rac-dependent NADPH oxidase activation is known to regulate cell proliferation (16). We showed that DPI, an inhibitor of flavoprotein, inhibits the increased DNA synthesis observed at low RacV12 levels, while it both restores DNA synthesis and blocks TSP2 synthesis for higher RacV12 expression levels. Similarly, TSP blocking antibodies show the same effect as DPI, inhibiting proliferation at low levels of RacV12 expression while restoring the proliferative capacity of cells with high RacV12 expression. These data demonstrate that Rac-mediated ROS induce TSP2 expression, which in turn inhibits Rac-stimulated proliferation. Similar dose-dependent biphasic behavior has been reported on cell migration for TSP1 (39). Exogenous administration of ROS has also been reported to induce a biphasic control of cell proliferation, as well as to control the synthesis of many cell cycle and matrix-related proteins (3). Rac's proproliferative effect is known to be ROS dependent; however, the cellular targets of Rac-mediated superoxide generation involved in the regulation of growth are not well established (12). The antiproliferative effect of high ROS concentration is classically attributed to ROS-induced apoptosis (8). Based on the finding that Rac-regulated ROS induce TSP2, which in turn regulates proliferation in the absence of increased apoptosis, we propose that the Rac-mediated increase in TSP2 may represent a pathway by which ROS may inhibit proliferation in endothelial cells independently of apoptosis.
The effect of TSPs on cell proliferation is mediated by different domains of the protein that interact with different receptors (5). The overall effect of TSPs on endothelial cells is deadhesive, with the inhibition of proliferation and induction of apoptosis. TSPs have, additionally, been implicated in the regulation of cytoskeletal reorganization (29). In HAEC, the Hep1 peptide sequence present in TSP1 and TSP2 has been shown to induce focal contact labilization (26, 29). We sought to determine whether TSP2 might regulate proliferation by altering cytoskeletal organization through the HEP1 sequence. While we found that Hep1 was able to induce stress fiber disruption and relocalization of paxillin in HAEC expressing Adnull or RacN17 (data not shown), we did not see any effect of the Hep1 peptide on HAEC proliferation in Adnull or RacV12 at a MOI of 200. This result suggests that TSP2's control of cell proliferation is independent of the HEP1 sequence. The increase in TSP2 expression induced by Rac activation, however, might participate indirectly in the cytoskeletal reorganization characteristic of the Rac phenotype. Indeed, treatment of HAEC without increased Rac activation (i.e., Adnull or RacN17 without FBS and growth factors) with the HEP1 peptide led to the labilization of stress fibers and reorganization of focal contacts in our experimental conditions. Rac is well known to induce actin reorganization by remodeling focal contact and stress fibers to form lamellipodia and focal adhesion structures smaller than focal contact (38). These results further support the concept that besides the classical Rac pathway involving p21-activated kinase, LIM kinase, and cofilin for the control of actin organization, increased TSP2 induced by Rac via ROS production could be involved, in some specific conditions, in the control of cytoskeletal organization as has been previously suggested (25).
To confirm Rac-dependent TSP expression in vivo, we used a transgenic mouse model expressing RacV12 under the dependence of the smooth muscle α-actin. We found increased expression of TSP2, probably synthesized and secreted by VSMC expressing RacV12, in the elastic lamina of aortas from transgenic mice. Additionally, in our RacV12 transgenic model, where the total Rac protein expression was increased by 3.5-fold, we observed a trend toward hypertrophy of the medial layer (H. Hassanain and P. J. Goldschmidt-Clermont, unpublished data). In contrast to what occurs with HAEC, TSP1 is known to stimulate the proliferation of VSMC. There is, however, no clear information concerning the role of TSP2 in VSMC proliferation. Transgenic mice expressing RacV12 in cardiomyocytes have been shown to induce focal contact labilization and hypertrophy of the mouse heart (37), and such an effect might be linked to the Rac-dependent TSP2 expression. Further studies will be required to characterize the involvement of TSP2 overexpression in the hypertrophy of the aorta in the RacV12 background.
In conclusion, we demonstrate that production of ROS by Rac in HAEC leads to a specific increase in TSP2 mRNA expression levels without affecting TSP1 mRNA expression levels. Increased TSP2, in turn, inhibits cellular proliferation. In vivo, we show increased TSP2 in the aortas of transgenic mice expressing RacV12. The induction of TSP2 expression by Rac-dependent ROS production represents a controlled regulation of cell proliferation with low levels of ROS supporting cell proliferation, yet high levels of ROS inhibit proliferation through increased TSP2 expression. An increase in the levels of the antiangiogenic protein TSP2 by Rac1 might thus correspond to a counterbalance operating during sustained Rac1 activation and consequently high superoxide production, limiting the ROS-dependent proliferative effect on cells. The Rac activation levels used in our study correspond to a strong activation compared to those obtained with growth factors like PDGF. In addition, human adult endothelial cells express very low levels of TSP2 in regular conditions, while TSP2 is expressed during embryonic development and in some physiopathological conditions. Thus, Rac-induced expression of TSP2 might be particularly important in pathological conditions involving angiogenesis, like ischemia-reperfusion and cancer. These results confirm the idea evoked by Armstrong et al. (2) that TSP1 and TSP2, although closely homologous, are differentially regulated.
Acknowledgments
N. Lopes and D. Gregg contributed equally to this work.
This study was supported by NIH grant ROI HL-71536 to P. Goldschmidt-Clermont.
We thank Christopher D. Kontos for his helpful comments and suggestions.
REFERENCES
- 1.Abo, A., E. Pick, A. Hall, N. Totty, C. G. Teahan, and A. W. Segal. 1991. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353:668-670. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, L. C., B. Bjorkblom, K. D. Hankenson, A. W. Siadak, C. E. Stiles, and P. Bornstein. 2002. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol. Biol. Cell 13:1893-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, R. S., J. Shi, E. Murad, A. M. Whalen, C. Q. Sun, R. Polavarapu, S. Parthasarathy, J. A. Petros, and J. D. Lambeth. 2001. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA 98:5550-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1374-1376. [PubMed] [Google Scholar]
- 5.Bornstein, P. 2001. Thrombospondins as matricellular modulators of cell function. J. Clin. Investig. 107:929-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, G., Z. Cao, X. Xu, E. G. van Meir, and J. D. Lambeth. 2001. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269:131-140. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande, S. S., P. Angkeow, J. Huang, M. Ozaki, and K. Irani. 2000. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 14:1705-1714. [DOI] [PubMed] [Google Scholar]
- 8.Droge, W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47-95. [DOI] [PubMed] [Google Scholar]
- 9.Goicoechea, S., A. W. Orr, M. A. Pallero, P. Eggleton, and J. E. Murphy-Ulrich. 2000. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J. Biol. Chem. 275:36358-36368. [DOI] [PubMed] [Google Scholar]
- 10.Gorzalczany, Y., N. Sigal, M. Itan, O. Lotan, and E. Pick. 2000. Targeting of Rac1 to the phagocyte membrane is sufficient for the induction of NADPH oxidase assembly. J. Biol. Chem. 51:40073-40081. [DOI] [PubMed] [Google Scholar]
- 11.Irani, K., Y. Xia, J. L. Zweier, S. J. Sollott, C. J. Der, E. R. Fearon, M. Sundaresan, T. Finkel, and P. J. Goldschmidt-Clermont. 1997. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275:1649-1652. [DOI] [PubMed] [Google Scholar]
- 12.Irani, K., and P. J. Goldschmidt-Clermont. 1998. Ras, superoxide and signal transduction. Biochem. Pharmacol. 55:1339-1346. [DOI] [PubMed] [Google Scholar]
- 13.Irani, K. 2000. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 87:179-183. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez, B., O. V. Volpert, S. E. Crawford, M. Febbraio, R. L. Silverstein, and N. Bouck. 2000. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 6:41-48. [DOI] [PubMed] [Google Scholar]
- 15.Joneson, T., M. McDonough, D. Bar-Sagi, and L. Van Aelst. 1996. Rac regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science 274:1374-1376. [DOI] [PubMed] [Google Scholar]
- 16.Joneson, T., and D. Bar-Sagi. 1998. A Rac1 effector site controlling mitogenesis through superoxide production. J. Biol. Chem. 273:17991-17994. [DOI] [PubMed] [Google Scholar]
- 17.Kheradmand, F., E. Werner, P. Tremble, M. Symons, and Z. Werb. 1998. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science 280:898-902. [DOI] [PubMed] [Google Scholar]
- 18.Kim, K. S., K. Takeda, R. Sethi, J. B. Pracyk, K. Tanaka, Y. F. Zhou, Z. X. Yu, V. J. Ferrans, J. T. Bruder, I. Kovesdi, K. Irani, P. Goldschmidt-Clermont, and T. Finkel. 1998. Protection from reoxygenation injury by inhibition of rac1. J. Clin. Investig. 101:1821-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahav, J. 1993. The functions of thrombospondin and its involvement in physiology and pathophysiology. Biochim. Biophys. Acta 1182:1-14. [DOI] [PubMed] [Google Scholar]
- 20.Lambeth, J. D., G. Cheng, R. S. Arnold, and W. A. Edens. 2000. Novel homologs of gp91phox. Trends Biochem. Sci. 25:459-461. [DOI] [PubMed] [Google Scholar]
- 21.Lawler, J. 2002. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell. Mol. Med. 6:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J. M., and A. M. Shah. 2002. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J. Biol. Chem. 277:19952-19960. [DOI] [PubMed] [Google Scholar]
- 23.Li, J. M., and A. M. Shah. 2001. Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc. Res. 52:477-486. [DOI] [PubMed] [Google Scholar]
- 24.Majack, R. A., S. C. Cook, and P. Bornstein. 1986. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc. Natl. Acad. Sci. USA 83:9050-9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldovan, L., K. Irani, N. I. Moldovan, T. Finkel, and P. J. Goldschmidt-Clermont. 1999. The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxid. Redox Signal. 1:29-43. [DOI] [PubMed] [Google Scholar]
- 26.Murphy-Ullrich, J. E., S. Gurusiddappa, W. A. Frazier, and M. Hook. 1993. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J. Biol. Chem. 268:26784-26789. [PubMed] [Google Scholar]
- 27.Murphy-Ullrich, J. E. 2001. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J. Clin. Investig. 107:785-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nor, J. E., R. S. Mitra, M. M. Sutorik, D. J. Mooney, V. P. Castle, and P. J. Polverini. 2000. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J. Vasc. Res. 37:209-218. [DOI] [PubMed] [Google Scholar]
- 29.Orr, A. W., M. A. Pallero, and J. E. Murphy-Ullrich. 2002. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J. Biol. Chem. 277:20453-20460. [DOI] [PubMed] [Google Scholar]
- 30.Pearlstein, D. P., M. H. Ali, P. T. Mungai, K. L. Hynes, B. L. Gewertz, and P. T. Schumacker. 2002. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler. Thromb. Vasc. Biol. 22:566-573. [DOI] [PubMed] [Google Scholar]
- 31.Pracyk, J. B., K. Tanaka, D. D. Hegland, K. S. Kim, R. Sethi, I. I. Rovira, D. R. Blazina, L. Lee, J. T. Bruder, I. Kovesdi, P. J. Goldschmidt-Clermont, K. Irani, and T. Finkel. 1998. A requirement for the rac1 GTPase in the signal transduction pathway leading to cardiac myocyte hypertrophy. J. Clin. Investig. 102:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed, M. J., L. Iruela-Arispe, E. R. O'Brien, T. Truong, T. LaBell, P. Bornstein, and E. H. Sage. 1995. Expression of thrombospondins by endothelial cells. Injury is correlated with TSP-1. Am. J. Pathol. 147:1068-1080. [PMC free article] [PubMed] [Google Scholar]
- 33.Rey, F. E., M. E. Cifuentes, A. Kiarash, M. T. Quinn, and P. J. Pagano. 2001. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ. Res. 89:408-414. [DOI] [PubMed] [Google Scholar]
- 34.Ridley, A. J. 2001. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11:471-477. [DOI] [PubMed] [Google Scholar]
- 35.Sheibani, N., and W. A. Frazier. 1999. Thrombospondin-1, PECAM-1, and regulation of angiogenesis. Histol. Histopathol. 14:285-294. [DOI] [PubMed] [Google Scholar]
- 36.Sorescu, D., D. Weiss, B. Lassegue, R. E. Clempus, K. Szocs, G. P. Sorescu, L. Valppu, M. T. Quinn, J. D. Lambeth, J. D. Vega, W. R. Taylor, and K. K. Griendling. 2002. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105:1429-1435. [DOI] [PubMed] [Google Scholar]
- 37.Sussman, M. A., S. Welch, A. Walker, R. Klevitsky, T. E. Hewett, R. L. Price, E. Shaefer, and K. Yager. 2000. Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J. Clin. Investig. 105:875-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tapon, N., and A. Hall. 1997. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol. 9:86-92. [DOI] [PubMed] [Google Scholar]
- 39.Taraboletti, G., D. Roberts, L. A. Liotta, and R. Giavazzi. 1990. Platelet thrombospondin modulates endothelial cell adhesion, motility and growth: a potential angiogenesis regulatory factor. J. Cell Biol. 111:765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]