Abstract
St. John's wort (Hypericum perforatum) is an herbal remedy used widely for the treatment of depression. Recent clinical studies demonstrate that hypericum extracts increase the metabolism of various drugs, including combined oral contraceptives, cyclosporin, and indinavir. In this report, we show that hyperforin, a constituent of St. John's wort with antidepressant activity, is a potent ligand (Ki = 27 nM) for the pregnane X receptor, an orphan nuclear receptor that regulates expression of the cytochrome P450 (CYP) 3A4 monooxygenase. Treatment of primary human hepatocytes with hypericum extracts or hyperforin results in a marked induction of CYP3A4 expression. Because CYP3A4 is involved in the oxidative metabolism of >50% of all drugs, our findings provide a molecular mechanism for the interaction of St. John's wort with drugs and suggest that hypericum extracts are likely to interact with many more drugs than previously had been realized.
St. John's wort is an ancient herbal remedy that has gained widespread popularity as “nature's Prozac.” Several clinical studies provide evidence that St. John's wort is as effective as conventional, synthetic antidepressants (1–3). Hypericum extracts are active in several behavioral models of depression and inhibit the uptake of serotonin, dopamine, norepinephrine, and γ-aminobutyric acid in rodent synapses (4–8). St. John's wort is a complex mixture of over two dozen constituents. Recent evidence indicates that the acylphloroglucinol derivative hyperforin is responsible for much of the antidepressant activity of the herb. Hyperforin inhibits the reuptake of neurotransmitters in synapses (6–8). Moreover, the clinical effects of St. John's wort on depression correlate with its hyperforin content (9).
As an herbal remedy, St. John's wort has not been subjected to the rigorous clinical testing of modern drug candidates. Several recent reports provide evidence that St. John's wort promotes the metabolism of coadministered drugs, including the HIV protease inhibitor indinavir, the immunosuppressant cyclosporin, and oral contraceptives (10–14). Because each of these drugs is metabolized by cytochrome P450 (CYP) 3A4, a monooxygenase involved in the metabolism of many xenobiotics, these findings suggested that St. John's wort might induce CYP3A4 expression. Transcription of CYP3A4 is known to be induced by a range of xenobiotics, including drugs such as the antibiotic rifampicin, the antimycotic clotrimazole, the insulin-sensitizer troglitazone, and the barbiturate phenobarbital (15, 16). Increases in CYP3A4 expression represent the basis for a number of established drug–drug interactions (15).
We and others recently described a new member of the steroid/thyroid hormone receptor family, termed the pregnane X receptor (PXR), that serves as a key regulator of CYP3A4 transcription (17–21). PXR binds to the CYP3A4 promoter and is activated by the range of xenobiotics known to induce CYP3A4 expression. In this report, we show that St. John's wort activates PXR and induces CYP3A4 expression in human hepatocytes. Moreover, we have identified hyperforin as the component of St. John's wort responsible for PXR activation. Our data demonstrate that PXR can be activated by plant products and suggest that St. John's wort should be used with caution by patients taking drugs metabolized by CYP3A4.
Methods
Reagents and St. John's Wort Extract Preparation.
Commercial capsule preparations of St. John's wort were obtained from Nature's Way Products (Springville, UT; 300 mg); Nature's Plus (Melville, NY; 300 mg); and Nutraceutical (for Solaray, Park City, UT; 900 mg). Capsule contents were transferred to amber glass vials and extracted with 1 ml of absolute ethyl alcohol (Aaper Alcohol and Chemical, Shelbyville, KY) by gentle agitation for 30 min. Extracts were centrifuged at 300 × g for 5 min, and the alcohol layer was transferred to a clean centrifuge tube and recentrifuged at 425 × g for 30 min. Ethanol extracts were decanted and stored in amber glass vials at −20°C. Chemicals and their sources are as follows: hyperforin (Apin Chemicals Limited, Abingdon, Oxon, U.K.); amentoflavone and isoquercitrin (Indofine Chemical Company, Belle Mead, NJ); hyperoside (Research Plus, Bayonne, NJ); kaempferol, luteolin, myricetin, quercetin, quercitrin, rifampicin, β-sitosterol (from soybeans), scopoletin, and umbelliferone (Sigma); rutin (Acros Organics, subsidiary of Fisher Scientific); hypericin and pseudohypericin (Calbiochem); and SR12813 was synthesized in house. Because of the sensitivity of many of the components of Saint John's wort to light, ethanol extractions and the preparation and dilution of compounds were done in darkness or in reduced light.
Transfection Assays.
Transient transfection experiments and chloramphenicol acetyltransferase (CAT) assays were performed in a 96-well format with the pSG5-hPXR expression plasmid and (CYP3A1)2-tk-CAT reporter as previously described (21).
Competition Ligand Binding Assays.
Scintillation proximity competition binding assays were performed with purified human PXR ligand-binding domain and [3H]SR12813 as previously described (21).
Primary Culture of Human Hepatocytes and Northern Blot Analysis.
Primary human hepatocytes were obtained from Steve Strom (University of Pittsburgh). Cells were cultured in Matrigel-coated six-well plates in serum-free Williams' E medium (Life Technologies, Rockville, MD) supplemented with 100 nM dexamethasone and insulin-transferrin-selenium (ITS-G; Life Technologies). At 48 h after isolation, hepatocytes were treated with crude ethanol extracts of St. John's wort, rifampicin (10 μM), or hyperforin (1 μM), which were added to the culture medium as 1,000× stocks in either ethanol or dimethyl sulfoxide. Control cultures received vehicle alone. Cells were cultured for a further 30 h before harvest, and total RNA was isolated by using a commercially available reagent (Trizol; Life Technologies) according to the manufacturer's instructions. Total RNA (10 μg) was resolved on a 1% agarose/2.2 M formaldehyde denaturing gel and transferred to a nylon membrane (Hybond N+; Amersham Pharmacia). Blots were hybridized sequentially with 32P-labeled CYP3A4 [bases 790 to 1,322 of the published cDNA sequence (GenBank accession no. M18907)] and β-actin (CLONTECH) cDNA probes.
Results
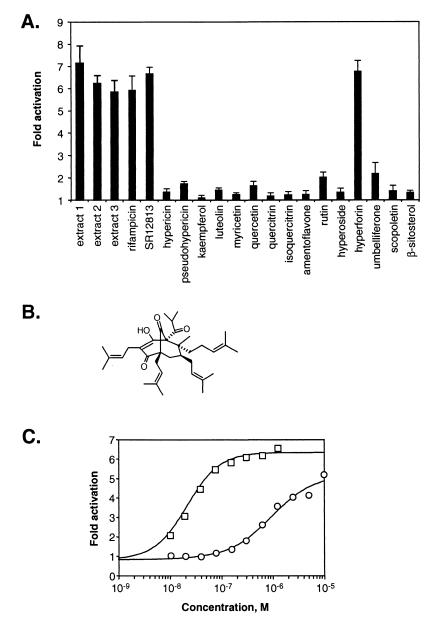
Because there is accumulating evidence that St. John's wort interacts with a variety of drugs, we used a standard cell-based reporter assay to investigate whether hypericum extracts would activate the orphan nuclear receptor PXR, a key transcriptional regulator of CYP3A4. CV-1 cells were cotransfected with an expression vector for human PXR and a reporter plasmid containing two copies of a CYP3A PXR binding site upstream of the thymidine kinase promoter and the CAT gene. Three different commercial preparations of St. John's wort were extracted with ethanol, and the extracts were used to treat transfected cells. Serial dilutions first were tested, because each extract was toxic to the cells at higher concentrations (data not shown). All three extracts at optimal dilution resulted in PXR activation comparable to that achieved with rifampicin, an established activator of PXR and CYP3A4 expression (Fig. 1A).
Figure 1.
Hyperforin is a potent activator of PXR. (A) CV-1 cells were transfected with expression plasmids for human PXR and the (CYP3A1)2-tk-CAT reporter. Cells were treated with extracts prepared from three different commercial preparations of St. John's wort [extract 1, Nature's Way (9 μg/ml); extract 2, Nature's Plus (75 μg/ml); extract 3, Solaray (7 μg/ml)] or with 10 μM of the indicated pure compounds, except for hyperforin, which was tested at 1 μM. Hyperforin was toxic to cells at concentrations >1 μM. Cell extracts subsequently were assayed for CAT activity. Data represent the mean of assays performed in quadruplicate ± SE and are plotted as -fold activation relative to transfected cells treated with vehicle alone. (B) Chemical structure of hyperforin. (C) CV-1 cells were transfected as in A and treated with increasing concentrations of hyperforin (squares) or rifampicin (circles). Data points represent the mean of assays performed in triplicate.
Hypericum extracts contain over two dozen bioactive constituents, including naphtodiantrons, flavonoids, phloroglucinols, proanthocyanidins, and tannins. We tested a number of the isolated components of hypericum extracts for activity in the PXR transfection assay. Most of these compounds had little or no PXR activity (Fig. 1A). Notably, hyperforin (Fig. 1B), which has antidepressant activity, was an efficacious activator of PXR (Fig. 1A). Full dose–response analysis showed that hyperforin activated PXR with a half-maximal effective concentration (EC50) of 23 nM (Fig. 1C). Thus, hyperforin is the most potent PXR activator to be reported to date. The concentrations of hyperforin required to activate PXR are well below those that are achieved in humans: Plasma levels of hyperforin peak at ≈380 nM in individuals taking the standard regimen of St. John's wort (3 × 300 mg daily), with steady-state plasma levels estimated to be ≈200 nM (22). Thus, it is likely that St. John's wort activates PXR in humans.
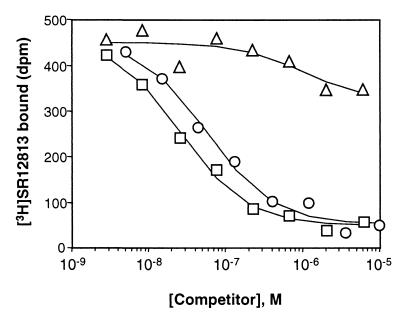
We next tested whether hyperforin mediates its effects through direct binding to PXR. An established scintillation proximity competition binding assay was used with the purified ligand binding domain of PXR and the high-affinity PXR radioligand [3H]SR12813 (21). Hyperforin competed efficiently with [3H]SR12813 for binding to PXR with a half-maximal inhibitory concentration (IC50) of 27 nM (Fig. 2). These data are in good agreement with the results of the transient transfection assay (Fig. 1C). Umbelliferone, a component of St. John's wort that has only weak activity in the PXR activation assay (Fig. 1A), competed only weakly for radioligand binding to PXR (Fig. 2). These data establish hyperforin as a high-affinity PXR ligand.
Figure 2.
Hyperforin binds to PXR with high affinity. Competition binding assays were performed with purified human PXR LBD and 10 nM of the high-affinity PXR radioligand [3H]SR12813 in the presence of the indicated concentrations of hyperforin (squares), SR12813 (circles), or umbelliferone (triangles). Each data point represents the mean of assays performed in triplicate. The Ki value of hyperforin was calculated to be 27 nM by nonlinear regression analysis.
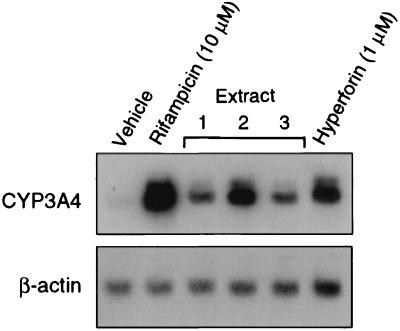
Because PXR regulates CYP3A4 expression, we examined whether hypericum extracts and hyperforin would induce CYP3A4 expression in liver cells. Primary human hepatocytes were treated with each of the three St. John's wort extracts or with hyperforin, and total RNA was prepared from the cells and analyzed by Northern blot analysis. As predicted by the PXR activation assay, each of the treatments resulted in a marked increase in CYP3A4 mRNA levels (Fig. 3). Hyperforin is a labile compound, which may account for its reduced efficacy relative to rifampicin. These data demonstrate that St. John's wort induces CYP3A4 expression in hepatocytes and that hyperforin is responsible for much, if not all, of this activity.
Figure 3.
St. John's wort extracts and hyperforin induce CYP3A4 expression in human hepatocytes. Northern blot analysis was performed with total RNA (10 μg) prepared from primary cultures of human hepatocytes treated for 30 h with extracts prepared from three different commercial preparations of St. John's wort [extract 1, Nature's Way (9 μg/ml); extract 2, Nature's Plus (75 μg/ml); extract 3, Solaray (7 μg/ml)], 1 μM hyperforin, or vehicle alone (0.1% ethanol). The blot was probed sequentially with 32P-labeled fragments of CYP3A4 and β-actin.
Conclusions
In this report, we have demonstrated that St. John's wort activates the orphan nuclear receptor PXR and consequently induces the expression of CYP3A4, a monooxygenase that plays a central role in the metabolism of most drugs. Our finding that hyperforin, an abundant, lipophilic component of St. John's wort, is a high-affinity PXR ligand provides evidence that a single constituent of hypericum extracts contributes to both the therapeutic effects and the side effects of this herb. PXR previously was shown to be activated by a structurally diverse collection of xenobiotics and natural steroids, including various pregnanes (17–21). Our findings extend the range of PXR ligands to include the herbal constituent hyperforin and raise the intriguing possibility that this orphan receptor evolved as part of the body's defense mechanism against potentially toxic ingested plant products. From a medical perspective, our results indicate that St. John's wort is likely to interact with the many drugs that are metabolized by CYP3A4. Indeed, there are already reports in the literature that hypericum extracts enhance the metabolism of combined oral contraceptives, cyclosporin, and indinavir (10–14). In some instances, these herb–drug interactions can be life-threatening (13, 14). Because it is an herb, St. John's wort is not subject to the rigorous testing and regulation of modern prescription drugs. Our findings indicate that St. John's wort should be used with caution by people taking medications, especially those metabolized by CYP3A4. Moreover, our data illustrate the value of testing herbal remedies as well as drug candidates in PXR binding and activation assays as a means for assessing their potential to induce hepatic monooxygenase activity and interact with other drugs. Finally, our results suggest that it may be possible to identify and develop safer hyperforin analogs that retain antidepressant activity but that do not activate PXR and promote drug metabolism.
Acknowledgments
We thank Dr. Steve Strom and Kelli Plunket for assistance with the primary human hepatocytes and Jennifer Lorenz for assistance with transfection assays.
Abbreviations
- PXR
pregnane X receptor
- CYP
cytochrome P450
- CAT
chloramphenicol acetyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.130155097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.130155097
References
- 1.Linde K, Ramirez G, Mulrow C D, Pauls A, Weidenhammer W, Melchart D. Br Med J. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volz H P. Pharmacopsychiatry. 1997;2:72–76. doi: 10.1055/s-2007-979522. [DOI] [PubMed] [Google Scholar]
- 3.Wheatley D. Pharmacopsychiatry. 1997;2:77–80. doi: 10.1055/s-2007-979523. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya S K, Chakrabarti A, Chatterjee S S. Pharmacopsychiatry. 1998;1:22–29. doi: 10.1055/s-2007-979342. [DOI] [PubMed] [Google Scholar]
- 5.Butterweck V, Wall A, Lieflander-Wulf U, Winterhoff H, Nahrstedt A. Pharmacopsychiatry. 1997;2:117–124. doi: 10.1055/s-2007-979531. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S S, Bhattacharya S K, Wonnemann M, Singer A, Muller W E. Life Sci. 1998;63:499–510. doi: 10.1016/s0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 7.Muller W E, Singer A, Wonnemann M, Hafner U, Rolli M, Schafer C. Pharmacopsychiatry. 1998;1:16–21. doi: 10.1055/s-2007-979341. [DOI] [PubMed] [Google Scholar]
- 8.Kaehler S T, Sinner C, Chatterjee S S, Philippu A. Neurosci Lett. 1999;262:199–202. doi: 10.1016/s0304-3940(99)00087-7. [DOI] [PubMed] [Google Scholar]
- 9.Laakmann G, Schule C, Baghai T, Kieser M. Pharmacopsychiatry. 1998;1:54–59. doi: 10.1055/s-2007-979346. [DOI] [PubMed] [Google Scholar]
- 10.Ernst E, Rand J I, Barnes J, Stevinson C. Eur J Clin Pharmacol. 1998;54:589–594. doi: 10.1007/s002280050519. [DOI] [PubMed] [Google Scholar]
- 11.Ernst E. Lancet. 1999;354:2014–2016. doi: 10.1016/S0140-6736(99)00418-3. [DOI] [PubMed] [Google Scholar]
- 12.Fugh-Berman A. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 13.Piscitelli S C, Burstein A H, Chaitt D, Alfaro R M, Falloon J. Lancet. 2000;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 14.Ruschitzka F, Meier P J, Turina M, Luscher T F, Noll G. Lancet. 2000;355:548–549. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 15.Maurel P. In: Cytochromes P450: Metabolic and Toxicological Aspects. Ioannides C, editor. Boca Raton, FL: CRC; 1996. pp. 241–270. [Google Scholar]
- 16.Ramachandran V, Kostrubsky V E, Komoroski B J, Zhang S, Dorko K, Esplen J E, Strom S C, Venkataramanan R. Drug Metab Dispos. 1999;27:1194–1199. [PubMed] [Google Scholar]
- 17.Kliewer S A, Moore J T, Wade L, Staudinger J L, Watson M A, Jones S A, McKee D D, Oliver B B, Willson T M, Zetterstrom R H, et al. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann J M, McKee D D, Watson M A, Willson T M, Moore J T, Kliewer S A. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Proc Natl Acad USA. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter C M, Ong E S, Evans R M. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S A, Moore L B, Shenk J L, Wisely G B, Hamilton G A, McKee D D, Tomkinson N C O, LeCluyse E L, Lambert M H, Willson T M, et al. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 22.Biber A, Fischer H, Romer A, Chatterjee S S. Pharmacopsychiatry. 1998;1:36–43. doi: 10.1055/s-2007-979344. [DOI] [PubMed] [Google Scholar]