Abstract
Aeromonas salmonicida subsp. salmonicida is a facultatively intracellular gram-negative bacterium that is the etiological agent of furunculosis, a bacterial septicemia of salmonids that causes significant economic loss to the salmon farming industry. The mechanisms by which A. salmonicida evades intracellular killing may be relevant in understanding virulence and the eventual design of appropriate treatment strategies for furunculosis. We have identified two open reading frames (ORFs) and related upstream sequences that code for two putative superoxide dismutases (SODs), sodA and sodB. The sodA gene encoded a protein of 204 amino acids with a molecular mass of approximately 23.0 kDa (SodA) that had high similarity to other prokaryotic Mn-SODs. The sodB gene encoded a protein of 194 amino acids with a molecular mass of approximately 22.3 kDa that had high similarity to other prokaryotic Fe-SODs. Two enzymes with activities consistent with both these ORFs were identified by inhibition of O2−-catalyzed tetrazolium salt reduction in both gels and microtiter plate assays. The two enzymes differed in their expression patterns in in vivo- and in vitro-cultured bacteria. The regulatory sequences upstream of putative sodA were consistent with these differences. We could not identify other SOD isozymes such as sodC either functionally or through data mining. Levels of SOD were significantly higher in virulent than in avirulent strains of A. salmonicida subsp. salmonicida strain A449 when cultured in vitro and in vivo.
Microorganisms have several highly specific and effective enzymatic pathways that confer protection from reactive oxygen species (ROS). Oxidant inactivation relies upon a variety of enzymes including glutathione peroxidase, glutathione reductase, catalase-peroxidase, and the metalloenzymes superoxide dismutases (SODs). The pathway involving SOD is one of the most extensively studied. SOD is responsible for the first step in the detoxification of the superoxide anion (O2−) to H2O and O2, via hydrogen peroxide (H2O2) (4). In addition to protecting cells against O2−-mediated toxicity the removal of O2− prevents the O2−-mediated reduction of Fe and the subsequent production of ·OH via the Haber-Weiss reaction (26).
SOD isozymes are classified into groups depending on their required prosthetic metal. These groups include manganese-cofactored SodA encoded by sodA, iron-cofactored SodB encoded by sodB, and copper-zinc-cofactored SodC encoded by sodC. In gram-negative bacteria both SodA and SodB are usually cytoplasmic (7). SOD functions to remove endogenously oxidants produced during normal oxidative metabolism. It has also been considered to protect cells from exogenously generated oxidants. The SodB of Mycobacterium tuberculosis is located in the glycocalyx or capsule secreted by this organism. M. tuberculosis also secretes SodB (20). Recently a variety of other forms of SOD have been identified in bacteria. These include a nickel-containing isozyme (24) and hybrid isoforms containing iron and zinc (23).
SOD expression is under the control of environmental stimuli. In all bacterial species, SodB, the iron-cofactored isozyme, is constitutively expressed under iron-replete conditions. The manganese-cofactored SodA is produced when bacteria are exposed to oxidative stress or under iron-limited conditions (2, 6, 26). Host phagocytic cells exploit the deleterious biological effects of ROS in their nonspecific host defense against pathogens (9, 26). It is unsurprising given the role of SOD that it has been implicated in intracellular pathogen survival and as a virulence factor in numerous species of bacteria including M. tuberculosis, (20, 30), Salmonella enterica serovar Typhimurium (14), Shigella flexneri (15), Streptococcus agalactiae (32), and Escherichia coli (3).
Aeromonas salmonicida is a gram-negative bacterium from the gamma class of the Proteobacteria phylum. There are four subspecies of A. salmonicida; the subspecies salmonicida is the etiological agent of a systemic infectious disease of fish called furunculosis. It is a facultative intracellular pathogen with the intracellular stage thought to allow this species to avoid temporarily the host immune system (18). Although the mechanisms by which this species survives within macrophages are not fully understood, the presence of the S-layer protein and SodA expression are thought to be important (2, 16). Both the role of ROS in the anti-Aeromonas immune response and the role of SOD in the response of A. salmonicida subsp. salmonicida to the host are unclear (33). The importance of these enzymes in the avoidance of host responses in this species has not been fully elucidated, although the suggestion has been made that it is not important, as both virulent and avirulent strains of A. salmonicida subsp. salmonicida possess the enzyme (2, 12, 16).
In this paper, we describe the genes encoding SodA and SodB in A. salmonicida subsp. salmonicida strain A449. We also quantitatively compared SOD activities in virulent and avirulent strains grown in vitro as well as in vivo to demonstrate that the role of SOD in this bacterium's virulence may depend upon the level of gene expression rather than a gene deficiency.
MATERIALS AND METHODS
Identification of the A. salmonicida subsp. salmonicida sodA and sodB genes.
The complete sequences of sodA and sodB were identified from a genomic DNA sequence database for A. salmonicida subsp. salmonicida strain A449 (R. K. Singh et al., unpublished data) by using BLAST searches (1).
Two-dimensional electrophoresis.
Outer membrane proteins were isolated by glycine extraction (29) from A. salmonicida subsp. salmonicida at stationary phase following growth in tryptic soy broth (Difco, Detroit, Mich.) at 17°C. The extracted outer membrane proteins were diluted with 7 M urea, 2 M thiourea, 4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 40 mM Tris, 2 mM tributylphosphine, and 0.05% carrier ampholytes. Isoelectric focusing was performed on an 18-cm Immobiline dry strip, pH 3 to 10, controlled with an IPGphor isoelectric focusing unit (AP-Biotech, Montreal, Quebec, Canada) with a voltage profile of 1 h at 500 V, 1 h at 2,000 V, and 8 h at 8,000 V. After electrophoresis the strip was equilibrated with 50 mM Tris-HCl (pH 8.8)-6 M urea-2% sodium dodecyl sulfate (SDS)-65 mM dithiothreitol-0.05% bromophenol blue-135 mM iodoacetamide for 30 min and then loaded onto an SDS-14% polyacrylamide gel and electrophoresed at 100 V until the dye front was within 5 mm of the bottom of the gel. Proteins were silver stained (34) and excised from the gel.
Mass spectrometry (MS) analysis.
Protein spots were processed using the Investigator ProGest automated digestion unit (Genomic Solutions, Ann Arbor, Mich.). Briefly, the procedure involved spot destaining with potassium ferricyanide solution (15 mM potassium ferricyanide, 50 mM sodium thiosulfate). Gel pieces were then rinsed three times with Milli-Q water and were shrunk with high-pressure liquid chromatography-grade acetonitrile. They were reswelled with 20 μl of sequencing-grade modified trypsin solution (Promega, Madison, Wis.) prepared as 0.01 μg/μl in 50 mM ammonium bicarbonate. An additional 50 μl of 50 mM ammonium bicarbonate was added to cover the gel pieces prior to overnight incubation at 37°C. To recover the peptides, an organic solution (50 μl of 50% methanol-5% acetic acid) was added to the digestion solution and the mixture was separated from the gel pieces onto a clean 96-well plate. Peptide extracts were evaporated to dryness on a SpeedVac concentrator (Thermo Savant, Holbrook, N.Y.), and they were then reconstituted uniformly with 20 μl of 5% acetonitrile-1% acetic acid solution.
Partial peptide sequencing was achieved using liquid chromatography coupled to electrospray MS. The online liquid chromatography-electrospray MS experiments were carried out using a CapLC high-pressure liquid chromatography system coupled to a Q-ToF 2 hybrid quadrupole-time-of-flight instrument (Micromass, Manchester, United Kingdom). Peptide extract solution was loaded onto a 5-mm by 300-μm μ-Precolumn PepMap C18 cartridge (LC Packings, Amsterdam, The Netherlands) at 30 μl/min and eluted using a fast gradient of 5 to 70% acetonitrile-0.2% formic acid in 4 min at 1 μl/min. Mass spectral data were recorded using the automated data-dependent switching function. The MS survey scan was acquired over the mass range m/z 400 to 1,500 with a scan duration of 1 s. When the survey scan detected doubly or triply charged precursor ions with an intensity higher than 10 counts/s, the automated MS to MS-MS switching function was activated. A total of two channels for recording MS-MS spectra were selected, and the mass range was acquired from m/z 50 to 2,000 with a scan duration of 2 s. Data were acquired and processed in the MassLynx Windows NT-based data system version 3.4.
Bacterial strains and growth conditions.
Five strains of A. salmonicida subsp. salmonicida were used (Table 1): two virulent strains, A449 and 80204, and three avirulent strains, 80204-1, 84222-5, and SS70-1. Strain 80204-1 is an S-layer-negative mutant of 80204. Each strain was cultured to mid-exponential phase of growth from a glycerol stock in tryptic soy broth with agitation at 17°C. Strain A449 was also cultured in an iron-free minimal salts medium, a modification of simplified Griffin's medium (27) with iron sulfate added to give final iron concentrations between 0 and 40 mM. Bacterial number was estimated by A600 with a spectrophotometer (Ultrospec 2000; Pharmacia). A more accurate estimate of bacterial number was achieved by direct colony counts on tryptic soy agar (Difco) after incubation at room temperature for 24 h.
TABLE 1.
A. salmonicida subsp. salmonicida strains used in this studya
| Strain | S-layer | Virulence | Origin |
|---|---|---|---|
| A449 | + | + | Natural epizootic |
| 80204 | + | + | Natural epizootic |
| 80204-1 | − | − | Laboratory-derived mutant of 80204 |
| 84222-5 | − | − | Laboratory-derived mutant |
| SS70-1 | + | − | Laboratory-derived mutant |
A449 and 80204 were both derived from naturally occurring salmonid epizootics. 80204-1, 84222-5, and SS70-1 are all laboratory-derived avirulent mutants. The phenotype for A449 came from the late Julian Thornton (Microtek International, Saanichton, British Columbia, Canada, personal communication), and the phenotypes for 80204, 80204-1, 84222-5, and SS70-1 came from Gilles Olivier (Department of Fisheries and Oceans, Moncton, New Brunswick, Canada, personal communication).
In vivo culture of A. salmonicida.
The in vivo culture procedure was approved by the Dalhousie University Committee for Laboratory Animals and the National Research Council—Halifax Local Animal Care Committee. In vivo culture was performed as per the work of Garduño et al. (17) with some modifications. The in vivo growth chambers were lengths of autoclaved 12- to 14-kDa-molecular-mass-cutoff dialysis tubing (Spectrapor; Spectrum Laboratories, Rancho Dominguez, Calif.) ligated at both ends (10). Chambers were filled with a bacterial suspension at an A600 of 0.5 in 1× Hanks' balanced salt solution and implanted in the abdominal cavities of buffered tricaine methanosulfonate (Syndel Laboratories, Vancouver, British Columbia, Canada)-anesthetized juvenile Atlantic salmon (Salmo salar Linnaeus 1758; mean weight, 50.3 ± 12.0 g) via a ventral midline incision. The incision was closed with 4-0 polypropylene sutures on a three-eighths reverse cutting needle placed in a simple interrupted pattern. After recovery the fish were maintained in flowthrough, dechlorinated fresh water at 12°C. Fish were euthanized 24 h postsurgery with a lethal overdose of tricaine methylsulfonate. The implants were retrieved by dissection, the implant contents were removed, and their volume was noted. Bacterial numbers within the implants were determined by direct colony counts as described above. We have previously demonstrated that when cultured in this manner the bacterial cultures were in the exponential phase of growth at the time of harvest (A. Dacanay, unpublished observations).
Bacterial preparation.
Suspensions of both broth- and in vivo-cultured bacteria were pelleted at 4°C for 10 min at 3,000 × g. The culture supernatant was carefully removed, and the pellet was stored at −20°C prior to extraction. Cell-free lysates of the bacteria were obtained by resuspending the bacterial pellets in a small volume of ice-cold double-distilled water and disrupting the cells by passage through a French press (American Instrument Company, Silver Spring, Md.) with an internal cell pressure of >18,000 lb/in2. The resulting lysate was centrifuged for 10 min at 10,000 × g at 4°C to pellet any cell debris, frozen at −20°C, and then freeze-dried to a powder that was stored at −20°C until use.
SOD: zymography.
Zymography was conducted using 100- by 105- by 1-mm 12% nondenaturing, nonreducing (native) polyacrylamide gels (polyacrylamide gel electrophoresis ). Loading volumes were adjusted to the equivalent of 108 CFU per well. Samples were mixed with nondenaturing, nonreducing loading buffer (125 mM Tris [pH 6.8], 20% glycerol, 0.4% bromophenol blue) and were not heated before loading. The running buffer was a 190 mM Tris-25 mM glycine solution that was unadjusted for pH. Electrophoresis was carried out at 200-V constant voltage in a gel apparatus maintained at 4°C. SOD activity was visualized as inhibition of the reduction of the tetrazolium salt 2,2′-bis(4-nitrophenyl)-5,5′-diphenyl-3,3′-(3,3′-dimethoxy-4,4′-diphenylene)ditetrazolium chloride(NBT) according to the method of Beauchamp and Fridovich (4). Commercially available E. coli SOD (Fe-SOD or Mn-SOD, EC 1.15.1.1; Sigma-Aldrich, Oakville, Ontario, Canada) was used as a positive control for activity only. Conventional molecular weight standards were not run. An assessment of migration was made by determining the migration of any zones of clearance observed relative to the migration of the dye front (Rf = distance migrated by band/distance migrated by dye front).
SOD: quantitative analysis.
SOD activity was quantitatively determined using a commercially available colorimetric microtiter plate method (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's protocol. SOD activity was assayed colorimetrically at 450 nm as the inhibition of the reduction of the tetrazolium salt 2-(4-iodophenyl) 3-4-(nitrophenyl)-5(2,4-disulfophenyl)-2H tetrazolium (WST-1) by O2−. Sample volumes were adjusted so that a protein amount equivalent to 107 CFU of A. salmonicida was loaded per well. Total SOD activities were determined kinetically by the initial rate of the reaction, vo. The reaction rate was determined using a Thermomax microplate reader and the Softmax Pro suite software (Molecular Devices Corp., Sunnyvale, Calif.). Commercially available E. coli Fe-SOD was used to construct standard curves. The identity of the SOD isozyme was determined by the ability of either 10 mM KCN or 10 mM H2O2 to inhibit the colorimetric change. Inhibitors were added to the reaction mixture and incubated for 20 min at room temperature before assay. Enzyme activity, defined as percent inhibition of WST-1 reduction, was determined as {[(reagent control vo − buffer blank vo) − (sample vo − sample blank vo)]/(reagent control vo − buffer blank vo)} × 100 for both samples and standards. SOD activity per well was determined from the standard curve, and the amount of SOD per cell was calculated. Data were transformed, and any difference in SOD activity was determined using one-way analyses of variance (ANOVAs) and Tukey's multiple comparisons with Prism 3.0 (Graphpad Software Inc., San Diego, Calif.).
Nucleotide sequence accession numbers
The sodA and sodB sequences have been deposited in GenBank under accession no. AY321354 and AY321353, respectively.
RESULTS
Sequence analysis of A. salmonicida subsp. salmonicida sodA gene.
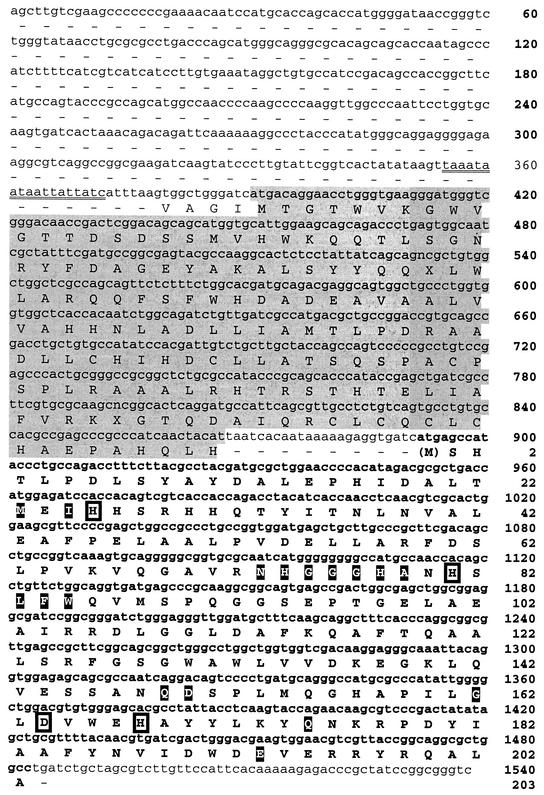
The deduced amino acid sequence of the putative manganese-cofactored SOD (SodA) is shown in Fig. 1. This protein consisted of 204 amino acids and had a predicted molecular mass of 22.9 kDa and a predicted pI of 5.47. The first methionine is removed in the active enzyme, and the amino acid numbering system used reflects this. The four conserved metal-binding residues His23, His81, Asp164, and His168 (A. salmonicida SodA numbering) are present along with residues that predominate in Mn-cofactored SODs: Met23, Ile25, Asn73, His74, Gly75, Gly76, Gly77, His78, Ala79, Gln149, Asp150, Gly158, Gln175, and Glu193 (22). Immediately upstream of sodA there was a 477-bp open reading frame (ORF) that encoded a protein of 159 amino acids with a predicted molecular mass of 17.8 kDa and a predicted pI of 6.6 (Fig. 2). Comparison of the deduced amino acid sequence with other sequences revealed a 33% identity (49% similarity) to a hypothetical 17-kDa protein (OrfX) from Pseudomonas syringae (accession number AF121078) and a slightly lower similarity to OrfX region deduced amino acid sequences from Pseudomonas putida (33% identity, 45% similarity) and Pseudomonas aeruginosa (28% identity, 40% similarity) (accession numbers AF1022780 and JC4983, respectively). A putative iron box region of 18 nucleotides (positions 358 to 375) upstream from OrfX was identified using the consensus GATATTGATAATCATTATC iron box sequence of E. coli given in the work of Calderwood and Mekalanos (8). This region has a 15-of-19-base match with the E. coli consensus sequence.
FIG. 1.
Comparison of derived amino acid sequence of A. salmonicida subsp. salmonicida strain A449 SodA with other similar sequences of proteobacteria. Amino acids involved in metal binding are indicated by reversed type. Amino acids characteristic for Mn-containing enzymes are shaded (see the work of Jackson and Cooper [22]). Sequences were aligned using the Genomatix DiAlign progam (http://genomatix.gsf.de/). Only uppercase letters are considered to be aligned. The tryptic peptide is underlined.
FIG. 2.
Nucleotide sequence and deduced amino acid sequence of the manganese-cofactored SOD (sodA, SodA) region of A. salmonicida subsp. salmonicida strain A449 (nucleotides and amino acids in boldface) and its upstream flanking region. The shaded region upstream of sodA is an unidentified gene similar to orfX of Pseudomonas syringae (AF121078). Residues in filled boxes are conserved residues of sodA (see the work of Jackson and Cooper [22]). Residues in open boxes are conserved ligands for the metal cofactor. The putative iron box is double underlined.
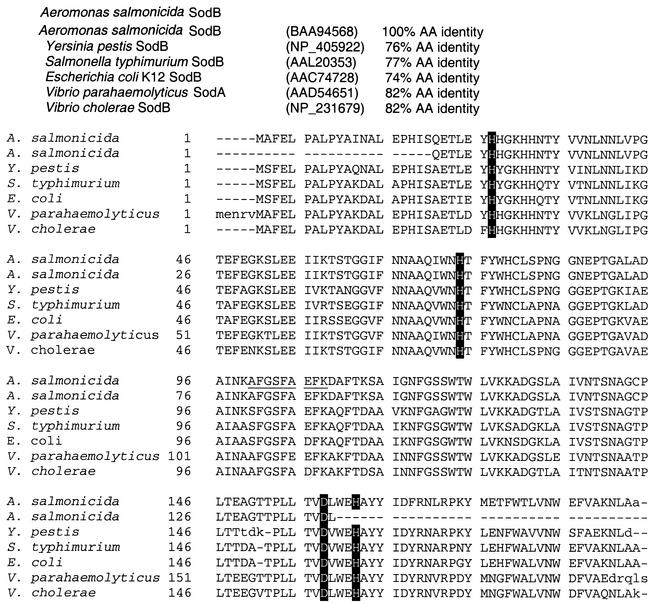
Sequence analysis of A. salmonicida subsp. salmonicida sodB gene.
The deduced amino acid sequence for a putative iron-cofactored SOD (SodB) is shown in Fig. 3. This protein consisted of 194 amino acids and had a predicted molecular mass of 21.6 kDa and a calculated pI of 5.55. The four conserved metal-binding residues His27, His74, Asp158, and His162 (A. salmonicida subsp. salmonicida sodB numbering) characteristic of SodB are present. This sequence was 100% similar to the partial sequence for A. salmonicida SodB (accession number BAA94568, subspecies not given). Comparison of the deduced amino acid sequence with those of other bacterial SodB proteins from different genera revealed the highest similarity (82% identity) to a manganese SOD gene product from Vibrio parahaemolyticus (accession number AAD54651). Comparisons to other SOD sequences revealed high similarities (>73% identity) only to SodB from a variety of bacterial species outside the genus Aeromonas.
FIG. 3.
Comparison of derived amino acid sequence of A. salmonicida subsp. salmonicida strain A449 iron-cofactored SOD (sodB, SodB) with other similar sequences of gamma-proteobacteria. Amino acids involved in metal binding are indicated by reversed type. Sequences were aligned using the Genomatix DiAlign program. Only uppercase letters are considered to be aligned. The tryptic peptide is underlined
Identification of SOD peptides.
Two tryptic peptides were identified during proteomic screening of A449 cultured under iron-replete and iron-limiting conditions.
A tryptic peptide with an amino acid sequence of RFGSGWAWLVVDK was obtained from a cytosolic protein with a molecular mass of 22.3 kDa and a pI of 5.74 from strain A449 cultured in low-iron medium. This partial sequence was 100% identical to the putative SodA amino acid sequence (positions 126 to 138, Fig. 1).
A second tryptic nonapeptide with an amino acid sequence of AFGSFAEFK was obtained from an outer membrane protein with a molecular mass of 22.3 kDa and a pI of 5.0 from strain A449 cultured under iron-replete conditions. This partial sequence was 100% identical to the putative SodB amino acid sequence (positions 100 to 108, Fig. 3) and also to the existing database sequence.
Bacterial strain and growth condition expression of SOD. (i) Zymography (NBT reduction).
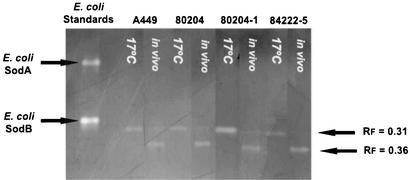
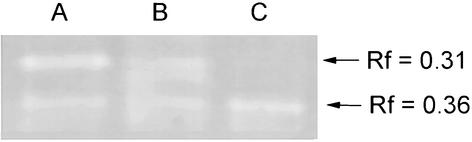
Cell-free lysates produced from the virulent strain (80204) and two avirulent strains (80204-1 and 84222-5) grown in vitro under low aeration to log phase at 17°C produced a single zone of clearance on native PAGE zymography with an Rf value of 0.31 (Fig. 4). This band was sensitive to 10 mM H2O2 (Fig. 5). Cell-free lysates from the same strains cultured in vivo produced a zone of clearance on native PAGE zymography with an Rf value of 0.36 (Fig. 4). This band was insensitive to both 10 mM H2O2 and 10 mM KCN (Fig. 5). Lysates from strain A449 cultured in modified Griffin's medium with differing amounts of iron sulfate were also analyzed by zymography. The Rf 0.31 zone of clearance was seen at all concentrations of iron sulfate whereas the Rf 0.36 zone of clearance was seen only at a medium concentration of 0 mM iron sulfate (lane A, Fig. 5).
FIG. 4.
Zymograms of cell-free lysates of a virulent strain (80204) and two avirulent strains (80204-1 and 84222-5) of A. salmonicida subsp. salmonicida cultured in vitro at 17°C in vitro and in vivo. Note the presence of SOD activity with an Rf value of 0.31 in all strains cultured in vitro. Strains cultured in vivo show SOD activities with Rf values of 0.36.
FIG. 5.
Zymograms of cell-free lysates of A. salmonicida subsp. salmonicida strain A449, a virulent strain, cultured in vitro in iron-free simplified Griffin's medium. Lysates were incubated for 30 min prior to zymography with either buffer (A), 10 mM KCN (B), or 10 mM H2O2 (C).
(ii) Quantitative analysis (WST-1 reduction).
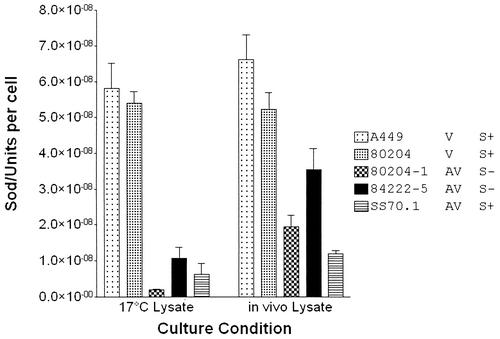
SOD activity was quantified in two virulent (A449 and 80204) and three avirulent (80204-1, 84222-5, and SS70-1) strains cultured to mid-exponential phase in vitro in tryptic soy broth or cultured for 24 h in vivo. Under both growth conditions SOD activity was present in the cell-free lysates of all strains (Fig. 6). Following in vitro culture there were significant differences in SOD activity in the lysates of the different strains (one-way ANOVA; P < 0.001): SOD activity was significantly higher in the virulent strains than in the avirulent strains (Tukey's test; P < 0.01). There was no significant difference between the levels of SOD activity of the virulent strains in vitro. With in vivo-cultured bacteria there were significant differences in lysate SOD activity between the different strains (one-way ANOVA; P < 0.001) (Fig. 6). There was no significant difference in the levels of SOD activity between the two virulent strains. However, there was significantly higher SOD activity in the virulent strains than in avirulent strains 80204-1 and SS70-1 (Tukey's test; P < 0.01) The level of SOD activity in 84222-5 was not significantly different from that of 80204, but it was significantly lower than the levels of SOD activity in A449 (Tukey's test; P < 0.01). Inhibitor analyses were performed on A449 lysates from in vitro- and in vivo-cultured cells. In the in vitro lysates, 10 mM hydrogen peroxide significantly reduced vo (Tukey's test; P = 0.01) but 10 mM KCN did not. In the in vivo lysates there were no significant differences in vo with the addition of either 10 mM H2O2 or 10 mM KCN (data not shown).
FIG. 6.
Quantitative analysis of SOD in cell-free lysates for two virulent strains (A449 and 80204) and three avirulent strains (80204-1, 84222-5, and SS70-1) of A. salmonicida subsp. salmonicida cultured in vitro and in vivo. V, virulent strain; AV, avirulent strain; S+, S-layer-positive strain; S−, S-layer-deficient strain. Values are the mean (± standard deviation) SOD units per cell (n = 3). One unit of SOD (EC 1.15.1.1) is defined as that required to inhibit reduction of cytochrome c by 50% in a coupled system with xanthine oxidase at pH 7.8 at 25°C in a 3.0-ml reaction volume.
DISCUSSION
Sequence analysis.
As the result of genomic sequencing of A. salmonicida subsp. salmonicida strain A449 we have identified two ORFs with high identities to prokaryotic SodA and SodB. The putative SodB sequence was identical to the partial sodB product sequence for A. salmonicida (accession number BAA94568). Our sequence also has high levels of amino acid identity to SodB from other Aeromonas species. We report a high similarity of the putative SodB sequence to the SodA gene sequence of V. parahaemolyticus (accession number AAD54651); however, this similarity appears to be due to a misannotation of that gene as sodA. We feel that this is the case as the reported sequence for V. parahaemolyticus sodA shares high identities with numerous SodB sequences but not with other SodA sequences. No other SOD or SOD-like genes were identified.
Iron metabolism genes in bacteria such as E. coli are under the control of the ferric uptake regulation protein (Fur), which functions as a transcriptional regulator. Fur both represses and activates the expression of certain target genes (11, 13). Its regulatory role in the control of E. coli SodA has been well studied (21). In P. aeruginosa, iron-limiting conditions appear to increase SodA activity (6). An 891-bp sequence upstream from sodA was examined to identify putative regulatory elements. Immediately upstream of sodA there was a 477-bp ORF that encoded a protein similar to the hypothetical 17-kDa protein (orfX) of Pseudomonas spp. that is also upstream of the P. aeruginosa sodA gene (31). Using the consensus iron box sequence for E. coli, we identified a putative iron box region of 18 nucleotides upstream from orfX that has a 15-of-19-base match with the E. coli consensus sequence (8).
Expression studies.
Zymography demonstrated a SOD activity with an Rf of 0.36 in cell-free lysates of A449 cultured in vitro in iron-deficient medium and in all five strains cultured in vivo (Fig. 4). Both zymographic (Fig. 5) and colorimetric (data not shown) inhibitor studies demonstrated that this activity was H2O2 and KCN insensitive, which suggested that this activity was due to SodA. A protein identified as SodA by MS was identified in the A449 proteome when the strain was cultured in iron-deficient medium. SodA levels were significantly higher in virulent than in avirulent strains in in vivo-cultured bacteria with the exception of 84222-5 (Fig. 6). The molecular mass as determined by reducing-denaturing SDS-PAGE for this isozyme was estimated to be 22.3 kDa, which is close to the predicted molecular mass of 22.9 kDa based on the sequence data. Barnes et al. (2) have previously reported a SodA produced under iron-limited conditions with a molecular mass of 45.6 kDa in another virulent strain of A. salmonicida subsp. salmonicida. SodA, therefore, likely exists as a homodimer.
Zymography identified a SOD activity with an Rf of 0.31 in virulent and avirulent strains of A. salmonicida subsp. salmonicida cultured in vitro in iron-replete medium (Fig. 4). The same activity was obtained when the virulent strain A449 was grown in modified Griffin's medium with high or low iron levels. Both zymographic (Fig. 5) and colorimetric (data not shown) inhibitor studies demonstrated that this activity was H2O2 sensitive and KCN insensitive, which suggested that this activity was due to SodB. A protein identified as SodB by MS was identified in the A449 proteome when the strain was cultured in iron-replete medium. SodB levels were significantly higher in virulent than in avirulent strains in broth-cultured bacteria (Fig. 6). The estimated size of SodB under native conditions is 50.9 kDa (2). The molecular mass of A. salmonicida subsp. salmonicida SodB was estimated to be 22.3 kDa under reducing and denaturing conditions. This figure is close to the deduced molecular mass for the sodB ORF product of 21.6 kDa. As with SodA, SodB would appear to exist as a homodimer.
These data suggested that the virulent strains had an enhanced antioxidative capacity over that of the avirulent strains with respect to both SodA and SodB. There are two possible interpretations as to the biological relevance of this finding. SOD has been implicated in the detoxification of O2− produced by phagocytic cells as an antimicrobial, the so-called respiratory burst (3, 14, 15, 20, 30, 32). In contrast to these data a recent study showed no significant difference in SOD levels between virulent and avirulent P. aeruginosa strains (6). This was linked to the unimportance of ROS-mediated killing in neutrophils, the primary leukocyte subpopulation involved in the immune response against P. aeruginosa. It is also clear that salmonid mononuclear phagocytic cells capable of a robust respiratory burst play an important role in the immune response to A. salmonicida subsp. salmonicida (33); therefore, up-regulation of SOD might be more relevant in A. salmonicida subsp. salmonicida pathogenesis. However, the antimicrobial action of exogenous O2− is likely through reactions with nitric oxide to produce peroxynitrous acid, which unlike O2− can penetrate cell membranes. It has been suggested that the cytosolic subcellular localization of both SodA and SodB in many species of bacteria (7) posits a role in detoxifying endogenous, rather than exogenous, O2− (5, 19). This latter interpretation offers a new view of the A. salmonicida-S. salar relationship. The literature (reviewed in reference 33) has assumed that SOD acts on exogenous O2−. Certainly the periplasmic localization of SodB (2) supports this. However, under in vivo conditions, when it might be more likely to encounter O2−, especially if it is facultatively intracellular (18), the predominant form of SOD is cytoplasmically located SodA (2). Given the inability of O2− to cross lipid membranes, the significant increase in SodA in vivo reported here might be seen as irrelevant. Indeed this may be the case if one considers only oxidative stress as O2− applied as an antimicrobial. However, we argue that the avirulent strains may have been attenuated in their ability to cope with endogenously generated aerobic stress.
Lysates from A. salmonicida subsp. salmonicida cultured in iron-limited medium or in vivo had SodA activity, but its expression was abolished by the addition of 5 mM iron sulfate. Upstream of sodA we identified a putative iron box consistent with this and also an ORF of unknown function that is similarly located with respect to sodA of P. aeruginosa. Barnes et al. (2) reported the expression of only SodA when A. salmonicida was cultured in iron-deficient medium. The presence of SodA activity in cells cultured in vivo suggested either that the in vivo culture condition is limited in the amount of available iron or that other mechanisms play a role in stimulating SodA activity, as SodA is also known to be produced under conditions of oxidative stress (26). The presence of SodA due to the bacteria being in stationary phase growth as in Aeromonas hydrophila (25) is unlikely as in vivo-cultured bacteria at 24 h in the system used in this study are in exponential growth (data not shown). The molecular mass cutoff of the in vivo culture chamber was chosen to both retain bacteria within the chamber and exclude host cells from it. It is likely, therefore, that SodA expression was a response to low-iron conditions in the peritoneal cavity. Garduño et al. (17) demonstrated that virulent A. salmonicida subsp. salmonicida strain A450 when grown in vivo with a similar system acquired resistance to bacteriolysis, phagocytosis, and oxidative killing. The differential expression of SodA in vivo, in an arguably more authentic culture condition than in vitro broth culture, suggests that further studies of A. salmonicida subsp. salmonicida, especially knockout mutants of SodA, would be instructive in the study of the A. salmonicida-S. salar host-pathogen relationship. When cultured in vivo, SOD activity was partially restored to the three avirulent strains. This was especially noticeable in the S-layer-deficient strains 80204-1 and 84222-5 (Fig. 5). This suggests that there may be some regulatory hurdle to SOD expression that is overcome in vivo in these strains. It also suggests that, in 80204-1 and 84222-5, S-layer and SOD expression may be linked. This is noteworthy, as the S-layer has often been implicated as a virulence factor in A. salmonicida subsp. salmonicida (28). The nature of this link may seem intuitive given that Barnes et al. (2) have clearly demonstrated that SodB, the enzyme predominantly expressed in vitro, has a periplasmic localization. However, as it was SodA activity, with a demonstrated cytosolic localization (2), that was restored in vivo, the nature of any possible link between SOD and the S-layer is obscure. Given that 80204-1 and 84222-5 are considered avirulent, the partial restoration of SOD activity in vivo would not seem to be concomitant with a restoration of virulence.
In summary we have demonstrated quantitative differences in SOD levels between virulent and avirulent strains of A. salmonicida subsp. salmonicida by the inhibition of O2−-catalyzed tetrazolium salt reduction. We have identified two ORFs that code for two SOD isozymes, SodA and SodB, that are consistent with the observed SOD activities. The sodA of A. salmonicida subsp. salmonicida was a cytoplasmic dimer of a predicted 22.9-kDa protein, with an Rf of 0.36 by zymography, and was insensitive to inhibition by 10 mM H2O2 or KCN. Its activity appeared to be at least partly restored in vivo in two S-layer-deficient strains. The sodB of A. salmonicida subsp. salmonicida was a periplasmic dimer of a predicted 21.6-kDa protein, with an Rf of 0.31 by zymography, and was insensitive to inhibition by 10 mM H2O2 but not KCN. These two isozymes differ in their expression patterns under in vivo and in vitro culture conditions. No other SOD or SOD-like molecules were identified by data mining or functionally. This does not, however, preclude their existence. The role of SOD in the pathogenesis of A. salmonicida subsp. salmonicida is unclear. These data do suggest a role in virulence for SOD, although the role has not been fully elucidated. These data together with the subcellular localization of SOD by Barnes et al. (2) suggest that avirulence might be homeostasis rather than defense related. A more rigorous assessment of the role of SOD in virulence would require both sodA- and sodB-knockout strains. Much has been made of the inability to correlate virulence in A. salmonicida subsp. salmonicida with putative virulence factors such as the S-layer or a number of secreted toxins (12, 28, 35). However, the view that virulence will be linked to a single gene is overly simplistic and the data presented here suggest that other putative virulence factors should be reassessed quantitatively.
Acknowledgments
This work was supported by the Genomics and Health Initiative from the National Research Council of Canada (NRC).
The A. salmonicida strains used in this study were the kind gifts of the late Julian Thornton (Microtek International Ltd., Saanichton, British Columbia) and Gilles Olivier (Department of Fisheries and Oceans, Moncton, New Brunswick). We acknowledge the contributions of Sandra Sperker, Krista Melville, Marshall Greenwell, and Kelly Boutilier from NRC-IMB. The DNA sequences were provided by the DNA sequencing team at NRC-IMB. The protein sequences were from Pierre Thibault and the protein sequencing team at NRC-IBS and the proteomics unit at NRC-IMB. We also acknowledge John Batt from the Dalhousie University Aquatron and Rafael Garduño and Chris Harvey-Clarke from Dalhousie University, Halifax, Nova Scotia.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, A. C., M. T. Horne, and A. E. Ellis. 1996. Effect of iron on expression of superoxide dismutase by Aeromonas salmonicida and associated resistance to superoxide anion. FEMS Microbiol. Lett. 142:19-26. [Google Scholar]
- 3.Battistoni, A., F. Pacello, S. Folcarelli, M. Ajello, G. Donnarumma, R. Greco, M. G. Ammendolia, D. Touati, G. Rotilio, and P. Valenti. 2000. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect. Immun. 68:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. [DOI] [PubMed] [Google Scholar]
- 5.Benov, L., and I. Fridovich. 1995. Superoxide dismutase protects against aerobic heat shock in Escherichia coli. J. Bacteriol. 177:3344-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britigan, B. E., R. A. Miller, D. J. Hasset, M. A. Pfaller, M. L. McCormick, and G. T. Rasmussen. 2001. Antioxidant enzyme expression in clinical strains of Pseudomonas aeruginosa: identification of an atypical form of manganese superoxide dismutase. Infect. Immun. 69:7396-7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton, L., and I. Fridovich. 1977. Intracellular localization of the superoxide dismutases of Escherichia coli: a re-evaluation. J. Bacteriol. 131:815-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderwood, S. B., and J. J. Mekalanos. 1987. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J. Bacteriol. 169:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, S., and C. J. Secombes. 1988. Analysis of events occurring within teleost macrophages during the respiratory burst. Comp. Biochem. Physiol. B 89:39-54.
- 10.Colquhoun, D., and H. Sørum. 1998. Outer membrane protein expression during in vivo cultivation of Vibrio salmonicida. Fish Shellfish Immunol. 8:367-377. [Google Scholar]
- 11.Dubrac, S., and D. Touati. 2000. Fur positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J. Bacteriol. 182:3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis, A. E., A. S. Burrows, and K. J. Stapleton. 1988. Lack of relationship between virulence of Aeromonas salmonicida and the putative virulence factors: A-layer, extracellular proteases and extracellular haemolysins. J. Fish Dis. 11:309-323. [Google Scholar]
- 13.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang, F. C., M. A. DeGroote, J. W. Foster, A. J. Baumler, U. Ochsner, T. Testerman, S. Bearson, J. C. Giard, Y. Xu, G. Campbell, and T. Laessig. 1999. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. USA 96:7502-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franzon, V. L., J. Arondel, and P. J. Sansonetti. 1990. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect. Immun. 58:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garduño, R. A., M. A. Kuzyk, and W. W. Kay. 1997. Structural and physiological determinants of resistance of Aeromonas salmonicida to reactive radicals. Can. J. Microbiol. 43:1044-1053. [Google Scholar]
- 17.Garduño, R. A., J. C. Thornton, and W. W. Kay. 1993. Aeromonas salmonicida grown in vivo. Infect. Immun. 61:3854-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garduno, R. A., A. R. Moore, G. Olivier, A. L. Lizama, E. Garduno, and W. W. Kay. 2000. Host cell invasion and intracellular residence by Aeromonas salmonicida: role of the S-layer. Can. J. Microbiol. 46:660-668. [DOI] [PubMed] [Google Scholar]
- 19.Gort, A. S., and J. A. Imlay. 1998. Balance between endogenous superoxide stress and antioxidant defenses. J. Bacteriol. 180:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harth, G., and M. A. Horwitz. 1999. Export of recombinant Mycobacterium tuberculosis superoxide dismutase is dependant upon both information in the protein and mycobacterial export machinery. J. Biol. Chem. 274:4281-4292. [DOI] [PubMed] [Google Scholar]
- 21.Hassan, H. M., and L. W. Schrum. 1994. Roles of manganese and iron in the regulation of the biosynthesis of manganese-superoxide dismutase in Escherichia coli. FEMS Microbiol. Rev. 14:315-324. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, S. M. J., and J. B. Cooper. 1998. An analysis of structural similarity in the iron and manganese superoxide dismutases based on known structure and sequences. Biometals 11:159-173. [DOI] [PubMed] [Google Scholar]
- 23.Kim, E.-J., H.-J. Chung, B. Suh, Y. C. Hah, and J.-H. Roe. 1998. Expression and regulation of the sodF gene encoding iron and zinc-containing superoxide dismutase in Streptomyces coelicolor Müller. J. Bacteriol. 180:2014-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, E.-J., H.-J. Chung, B. Suh, Y. C. Hah, and J.-H. Roe. 1998. Transcriptional and post-transcriptional regulation by nickel of sodN gene encoding nickel-containing superoxide dismutase from Streptomyces coelicolor Müller. Mol. Microbiol. 27:187-195. [DOI] [PubMed] [Google Scholar]
- 25.Leclère, V., A. Chotteau-Lelièvre, F. Gancel, M. Imbert, and R. Blondeau. 2001. Occurrence of two superoxide dismutases in Aeromonas hydrophila: molecular cloning and differential expression of the sodA and sodB genes. Microbiology 147:3105-3111. [DOI] [PubMed] [Google Scholar]
- 26.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Leary, M. O., C. Panos, and G. E. Helz. 1956. Studies on the nutrition of Bacterium salmonicida. J. Bacteriol. 72:673-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivier, G. 1990. Virulence of Aeromonas salmonicida: lack of correlation with phenotypic characteristics. J. Aquat. Anim. Health 2:119-127. [Google Scholar]
- 29.Phipps, B. M., T. J. Trust, E. E. Ishiguro, and W. W. Kay. 1983. Purification and characterization of the cell surface virulent A protein from Aeromonas salmonicida. Biochemistry 22:2934-2939. [DOI] [PubMed] [Google Scholar]
- 30.Piddlington, D. D., F. C. Fang, T. Laessig, A. M. Cooper, I. M. Orme, and N. A. Buchmeier. 2001. Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 69:4980-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polack, B., D. Dacheux, I. Delic-Attree, B. Toussaint, and P. M. Vignais. 1996. The Pseudomonas aeruginosa fumc and soda genes belong to an iron-responsive operon. Biochem. Biophys. Res. Commun. 226:555-560. [DOI] [PubMed] [Google Scholar]
- 32.Poyart, C., E. Pellegrini, O. Gaillot, C. Boumaila, M. Baptista, and P. Trieu-Cuot. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect. Immun. 69:5098-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secombes, C. J., and G. Olivier. 1997. Host-pathogen interactions in salmonids, p. 269-296. In E.-M. Bernoth, A. E. Ellis, P. J. Midtlyng, G. Olivier, and P. Smith (ed.), Furunculosis: multidisciplinary fish disease research. Academic Press, London, United Kingdom.
- 34.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 35.Vipond, R., I. R. Bricknell, E. Durant, T. J. Bowden, A. E. Ellis, M. Smith, and S. McIntyre. 1998. Defined deletion mutants demonstrate that the major secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect. Immun. 66:1990-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]