Abstract
Fabry disease is a compelling target for gene therapy as a treatment strategy. A deficiency in the lysosomal hydrolase α-galactosidase A (α-gal A; EC 3.2.1.22) leads to impaired catabolism of α-galactosyl-terminal lipids such as globotriaosylceramide (Gb3). Patients develop vascular occlusions that cause cardiovascular, cerebrovascular, and renal disease. Unlike for some lysosomal storage disorders, there is limited primary nervous system involvement in Fabry disease. The enzyme defect can be corrected by gene transfer. Overexpression of α-gal A by transduced cells results in secretion of this enzyme. Secreted enzyme is available for uptake by nontransduced cells presumably by receptor-mediated endocytosis. Correction of bystander cells may occur locally or systemically after circulation of the enzyme in the blood. In this paper we report studies on long-term genetic correction in an α-gal A-deficient mouse model of Fabry disease. α-gal A-deficient bone marrow mononuclear cells (BMMCs) were transduced with a retrovirus encoding α-gal A and transplanted into sublethally and lethally irradiated α-gal A-deficient mice. α-gal A activity and Gb3 levels were analyzed in plasma, peripheral blood mononuclear cells, BMMCs, liver, spleen, heart, lung, kidney, and brain. Primary recipient animals were followed for up to 26 weeks. BMMCs were then transplanted into secondary recipients. Increased α-gal A activity and decreased Gb3 storage were observed in all recipient groups in all organs and tissues except the brain. These effects occurred even with a low percentage of transduced cells. The findings indicate that genetic correction of bone marrow cells derived from patients with Fabry disease may have utility for phenotypic correction of patients with this disorder.
Lysosomal storage disorders (LSDs) constitute an important group of conditions in which the potential of gene manipulation as therapy should be assessed. They are monogenic defects, often with severe manifestations for which there are limited treatment options at this time. For some LSDs, target cells for therapy are accessible. Overexpression of the lysosomal hydrolase by gene-corrected cells results in secretion of some of the enzyme and its uptake by uncorrected bystander cells (metabolic cooperativity). This phenomenon may occur through receptor-mediated endocytosis and may play a role in gene transfer approaches by lessening the proportion of target cells that must be corrected to obtain clinical benefit. A number of reports have been recently published concerning the design and implementation of gene therapy strategies (1–6), as well as enzyme therapy (7), and bone marrow (BM) transplantation (8) for Fabry disease. A variety of gene delivery methods have been used to overexpress α-galactosidase A (α-gal A; EC 3.2.1.22), including recombinant retroviruses (1, 2, 4, 6), plasmids (3), and recombinant adenoviruses (5).
One of the sources of accumulating globotriaosylceramide (Gb3) in Fabry disease may be erythrocytorrhexis in macrophages (9, 10). Thus, gene modification of hematopoietic cells is perceived to be an appropriate objective for the amelioration of this disorder. Primitive hematopoietic cells and offspring also have the capability to become long-lived and to supply enzyme to other cell types through metabolic cooperativity. We have previously shown that the deficiency of α-gal A in enriched bone marrow mononuclear cells (BMMCs) from Fabry patients can be corrected by gene transfer (4). Sustained correction and metabolic cooperativity resulted in a significant reduction in accumulated Gb3. In a separate study, we demonstrated that transduced and transplanted BMMCs from normal mice could secrete an α-gal A/FLAG fusion protein for extended periods of time in vivo (6).
An animal model for Fabry disease has recently been developed by targeted gene disruption (11). These animals exhibit negligible α-gal A activity and demonstrate a progressive accumulation of Gb3 in various organs and tissues. Transplantation of normal murine BMMCs into these α-gal A-deficient mice leads to an increase in α-gal A activity and a concomitant reduction in Gb3 levels in some organs (8). However, because of high morbidity and mortality associated with BM transplantation, it has not been used for the treatment of patients with Fabry disease. The goal of the present study was to provide a rationale for autologous transplantation of gene-corrected cells for the therapy of Fabry disease. We demonstrate the effects of retrovirus-mediated transduction of BMMCs followed by transplantation of corrected cells into α-gal A-deficient mice. We transplanted both sublethally and lethally irradiated α-gal A-deficient animals with corrected cells. We analyzed enzyme activity and the quantity of Gb3 in the liver, spleen, heart, lung, kidney, and brain. We also performed secondary transplantations using cells from animals that had received transduced cells 26 weeks previously and evaluated correction in these recipients. We observed significant increases in α-gal A enzyme activity and reductions in Gb3 in all organs of recipient animals except for the brain. In some organs of transplant groups, enzyme activity levels and Gb3 storage approached normal levels. These in vivo results indicate that this approach may eventually lead to long-term correction for patients with Fabry disease.
Materials and Methods
α-gal A-Deficient Mice.
α-gal A-deficient male mice (11) were used in all of the present studies. Mice were maintained in a mouse facility accredited by the American Association for the Accreditation of Laboratory Animal Care and were fed autoclaved diet and water. All mice (both donors and recipients) were genotyped by PCR before the experiment (8).
Virus-Producing Cell Lines.
Two recombinant retroviral vectors with different backbones containing the full-length human α-gal A cDNA, pUMFG/α-gal A/FLAG (6) and pG1T/α-gal A (4), were used. For production of the pUMFG/α-gal A/FLAG retroviral vector, the E86/pUMFG/α-gal A/FLAG #13 recombinant ecotropic virus-producing cell line (6) was used. For production of pG1T/α-gal A retroviral vector, E86/pG1T/α-gal A ecotropic virus-producing cell clones were established as described previously (2) and assayed for intracellular α-gal A activities. A single-cell clone, E86/pG1T/α-gal A #16, that showed the highest intracellular α-gal A activity was selected for use in these studies. For these virus-producing cell lines, titer estimation along with marker rescue assays to ensure that replication-competent virus was not present were performed as described previously (2).
Isolation and Transduction of Murine BM Cells for in Vitro Experiments.
BM cells were obtained from the femurs and tibiae of 10-week-old α-gal A-deficient mice. BMMCs were isolated and transduced with E86/pUMFG/α-gal A/FLAG or E86/pG1T/α-gal A as described (6). Four once-a-day transductions using filtered supernatant in the presence of protamine sulfate (4 μg/ml; Elkins-Sin, Cherry Hill, NJ), recombinant mouse interleukin-6 (10 ng/ml; R & D Systems, Minneapolis, MN), and recombinant mouse stem cell factor (100 ng/ml; R & D Systems) were performed. Mock transductions were performed similarly by using supernatant from naive E86 packaging cells.
After transduction, aliquots of cells were taken and assayed for α-gal A activity and the remainder were plated in supplemented methylcellulose semisolid support medium (Methocult GF M3434; StemCell Technologies, Vancouver) to allow differentiation and colony formation. Two weeks later, hematopoietic progenitor colonies were collected as described (4) and α-gal A activity was measured.
Murine Long-Term Bone Marrow Cultures (LTBMCs).
LTBMCs were initiated by seeding isolated BMMCs into 25-cm2 flasks containing MyeloCult M5300 medium (StemCell Technologies) supplemented with 1 μM hydrocortisone (Sigma) and penicillin/streptomycin/glutamine (Life Technologies, Gaithersburg, MD) with 0.5 μg/ml Fungizone (Life Technologies). Approximately 108 cells were seeded into flasks and maintained in a total volume of 10 ml as described (4). The cultures were initiated at 37°C and then transferred after 3 days to 33°C.
For transductions, either the E86/pUMFG/α-gal A/FLAG or E86/pG1T/α-gal A retroviral vector was used. Starting on the first day of culture, transductions were repeated four times for each culture over a 4-day period. This was done by removal of one-half of the LTBMC medium, recovery of cells by centrifugation, resuspension of the collected cells in filtered overnight retroviral vector supernatant made with the above LTBMC medium, and reintroduction back into the original flasks. Mock-transduced cells were incubated with medium conditioned on wild-type E86 cells only. Each week thereafter, one-half of the LTBMC medium was removed and replaced with fresh LTBMC medium. Collected nonadherent cells and medium were then assayed for α-gal A activity. At the end of 15 weeks of LTBMCs, the adherent cells were collected by gentle trypsinization and the α-gal A activity in the various samples was measured.
Isolation, Transduction, and Transplantation of Murine BM Cells for in Vivo Experiments.
BM cells were obtained from the femurs and tibiae of nonconditioned 10-week-old donor mice, and BMMCs were isolated as described previously (6). The BMMCs were transduced with the E86/pUMFG/α-gal A/FLAG retroviral vector according to the method described previously (6) with some modifications. For this transduction, plates coated with RetroNectin (Takara, Otsu, Japan) at a concentration of 4 μg/cm2 were used according to the manufacturer's instructions. The BMMCs were resuspended at 2 × 106 cells per ml in pUMFG/α-gal A/FLAG vector-containing supernatant supplemented with 10 ng/ml recombinant mouse interleukin-6 (R & D Systems), 100 ng/ml recombinant mouse stem cell factor (R & D Systems), and cultivated in RetroNectin-coated plates. Four once-a-day transductions were performed. Mock transductions were performed similarly by using filtered supernatant from naive E86 packaging cells.
Recipient mice were divided into five groups and irradiated as follows: group 1, untouched; groups 2, 4, and 5, 1,100 rads; group 3, 550 rads. Two hours after irradiation, 2 × 106 mock-transduced BMMCs were injected intravenously into group 2 recipient mice and 2 × 106 pUMFG/α-gal A/FLAG-transduced BMMCs were injected into each recipient mouse from groups 3, 4, and 5. Untouched wild-type mice (age and strain matched) were used as a control.
At either 12 or 26 weeks after the transplantation, recipient mice were killed and analyzed. For sample analysis, BMMCs, peripheral blood mononuclear cells (PBMCs), and plasma were collected and pooled for each group and for controls as previously described (6), and the α-gal A activity in each group was measured. The liver, spleen, heart, lung, kidney, and brain were harvested from each transduced and transplanted mouse along with those from control mice. The organs were thoroughly rinsed in PBS and homogenized, and the α-gal A activity and the amount of Gb3 were measured.
Secondary Transplantation.
Secondary transplantations were performed to confirm transduction of more primitive hematopoietic cells and to look for corrective effects. BMMCs from the 26-week group transplanted with transduced cells were isolated and transplanted into lethally irradiated (1,100 rads) recipient α-gal A-deficient mice as described above. Analyses of the BMMCs, PBMCs, plasma, and organs of these animals were performed 6 weeks after the secondary transplantation.
Progenitor Colony PCR.
To provide an estimation of gene transfer efficiency in these in vivo experiments, a PCR-based assay was used to test for the presence of the provirus. Well-isolated progenitor colonies derived from transduced and control BMMCs were collected into colony PCR buffer and assayed by PCR as described (6). Primers specific to a region in the promoter of the murine P1–450 gene were included in separate analyses as a control for the “fitness” of the genomic DNA preparations.
α-gal A Assay and HPLC Quantitation of Gb3 Lipid Levels.
α-gal A activity was measured in cell extracts, culture medium, and isolated plasma with 5 mM 4-methylumbelliferyl α-d-galactopyranoside (Research Products International) with 0.1 M N-acetyl-d-galactosamine (Sigma) used as an inhibitor of α-N-acetylgalactosaminidase as described (2). HPLC analyses of Gb3 levels in organs of the mice were performed as described previously (7). The α-gal A activity and Gb3 level from each sample were measured at least three times, and the values are expressed as the mean ± standard deviation. The protein concentration of assay samples was determined by the method of Lowry et al. (12).
Results
α-gal A Activities in Transduced BMMCs, Progenitor Colonies, and LTBMCs.
Before the in vivo testing of the effectiveness of therapeutic gene transduction and transplantation of BMMCs in the α-gal A-deficient mouse, we sought to compare the effectiveness of two different recombinant vectors in an in vitro system. Vectors were replication-incompetent and were of similar titer (data not shown). One vector, pG1T/α-gal A (4), was derived from the LN series of retroviral vectors (13). The other vector, pUMFG/α-gal A/FLAG (6), was derived from the MFG series (from R. Mulligan, Harvard Medical School, Boston) and contains internal splice donor and acceptor sites that lead to the generation of both a full-length and a truncated mRNA that may increase the efficiency of translation. Other studies have been reported in which a different therapeutic reporter has shown greater expression with MFG-based constructs in comparison to the N2 series of vectors (13, 14).
For the suspension culture transduction using the supplemented BM medium, we analyzed α-gal A activity 24 hr after the last transduction. We also plated hematopoietic progenitor colonies into semisolid support medium and collected colony cells after 14 days. Table 1 shows the α-gal A activities in transduced BMMCs and hematopoietic progenitor colony cells in comparison with values obtained from mock-transduced α-gal A-deficient mouse cells and mock-transduced wild-type mouse control cells. Significant increases in intracellular α-gal A activity were seen both in transduced primary BMMCs and in collected transduced progenitor colony cells. When the MFG-based recombinant vector was used to transduce the α-gal A-deficient mice, these increases surpassed the values obtained from the mock-transduced normal controls.
Table 1.
α-gal A enzyme activities in transduced murine BM-derived cells
| Murine phenotype | Treatment | α-gal A activity, nmol/h per mg protein
|
||
|---|---|---|---|---|
| BM cells (24 hr) | Progenitor colony cells (14 d) | Adherent LTBMCs (15 wk) | ||
| Fabry | None (mock) | 7 ± 0 | 6 ± 0 | 19 ± 0 |
| Fabry | pG1T/α-gal A | 1,630 ± 30* | 42 ± 2* | 290 ± 7* |
| Fabry | pUMFG/α-gal A/FLAG | 2,310 ± 70*† | 720 ± 16*† | 790 ± 18*† |
| Wild type | None (mock) | 2,170 ± 50 | 3,600 ± 140 | NE |
Activities were measured 24 hr, 14 days, or 15 weeks after the last transduction, as shown. NE, not examined. *, P < 0.0001 vs. Fabry (mock) group; †, P < 0.0001 vs. pG1T/α-gal A group.
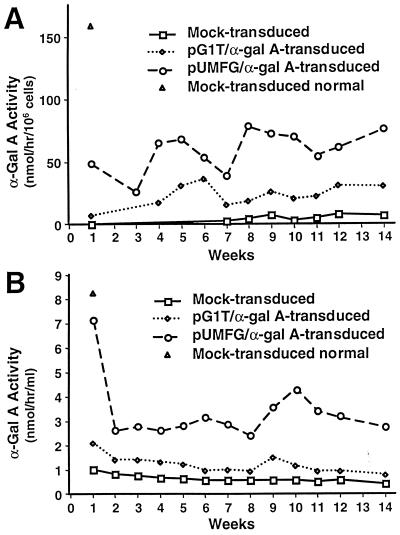
LTBMCs demonstrated sustained increases in both intracellular and secreted α-gal A activity throughout the period of observation (Fig. 1). Both intracellular and secreted α-gal A activities were significantly higher in the E86/pUMFG/α-gal A/FLAG-transduced cells than in the E86/pG1T/α-gal A-transduced cells even though the viral titers of the vectors were similar. In nonadherent cells (Fig. 1A), the level of intracellular α-gal A activity from the E86/pUMFG/α-gal A/FLAG-transduced cells was increased on average 2-fold over the E86/pG1T/α-gal A-transduced cells. A similar increase was observed in secreted enzyme. The mean enzyme activity for E86/pUMFG/α-gal A/FLAG-transduced cells was approximately 40% of that obtained for mock-transduced, strain-matched wild-type murine control cells (Fig. 1A). The level of secreted α-gal A activity from the E86/pUMFG/α-gal A/FLAG-transduced cells was consistently 3- to 4-fold higher than that from the E86/pG1T/α-gal A-transduced cells over the culture period (Fig. 1B). In adherent cells isolated from the LTBMCs at the end of 15 weeks, the level of intracellular α-gal A activity in the E86/pUMFG/α-gal A/FLAG-transduced cells also showed a 3-fold increase in activity over the E86/pG1T/α-gal A-transduced cells (Table 1). We therefore selected the more efficient E86/pUMFG/α-gal A/FLAG vector (6) for in vivo experiments.
Figure 1.
Intracellular α-gal A activity of transduced and control nonadherent LTBMC cells derived from α-gal A-deficient mice (A) and secreted α-gal A activity in the culture medium (B).
α-gal A Activities in BMMCs, PBMCs, and Plasma of Transplanted Mice.
Because in vitro responses may not accurately predict a positive therapeutic outcome, we examined the effects of primary and secondary transplantations in the α-gal A-deficient mouse. Transplanted animals received 2 × 106 cells that were estimated to be ≈35% positive for the provirus on the basis of our previous transductions with this vector (6). No untoward effects from either the irradiation procedure or the transplantation of transduced cells were observed.
Groups of recipient animals and controls were euthanized and tissues were collected for enzyme and lipid analyses 12 and 26 weeks after transplantation. Table 2 shows the α-gal A enzyme activities in pooled BMMCs, PBMCs, and plasma from each group of experimental animals. Enzyme activities increased significantly (P < 0.0001, unpaired t test) in all treated groups compared with that of the mock-transduced group. Both the lethally irradiated and sublethally irradiated recipient animals showed increases in α-gal A activity in collected BMMCs. In PBMCs and in plasma for both treated groups, α-gal A activity was greater than that observed from age and strain-matched normal animals. Sustained α-gal A activity in the plasma may make the corrective enzyme available for distribution and uptake into nontransduced cells. Most importantly, all of these enzyme increases in tissue persisted from 12 to 26 weeks (Table 2), indicating that this correction is not a short-term, transient effect. Furthermore, these findings demonstrate that the selected retroviral vector backbone can generate long-term therapeutic expression in vivo.
Table 2.
α-gal A activity in BMMCs, PBMCs, and plasma in mice after ex vivo transduction and transplantation of syngeneic BMMCs
| Group (no. of mice) | α-gal A activity
|
||
|---|---|---|---|
| BMMCs, nmol/hr/mg protein | PBMCs, nmol/hr/mg protein | Plasma, nmol/ hr/ml | |
| Wild type: 12 weeks | |||
| Untouched (5) | 420 ± 18 | 17 ± 1 | 2.4 ± 0.1 |
| Knockout: 12 weeks | |||
| Untouched (4) | 2 ± 1 | 1 ± 0 | 0.5 ± 0.1 |
| 1,100 rads–mock (5) | 4 ± 1 | 1 ± 1 | 0.5 ± 0.1 |
| 550 rads–transduced (5) | 360 ± 15* | 34 ± 2* | 9.4 ± 0.5* |
| 1,100 rads–transduced (6) | 75 ± 6* | 370 ± 22* | 3.2 ± 0.2* |
| Knockout: 26 weeks | |||
| 1,100 rads–transduced (6) | 190 ± 10* | 250 ± 19* | 3.7 ± 0.2* |
*, P < 0.0001 vs. 1,100 rads–mock group (unpaired t test).
α-gal A Activity and Gb3 Levels in Organs of Transplanted α-gal A-Deficient Mice.
We determined α-gal A activity in various organs from each of the recipient and control mice and analyzed the Gb3 levels in these organs. In all vector-treated groups, α-gal A activity in the liver increased significantly, and Gb3 levels decreased to levels seen in wild-type age- and strain-matched mice (Table 3). α-gal A activity reached approximately 30% of normal. The enzymatic correction and Gb3 reduction were maintained over time. Comparison of Gb3 levels between untouched animals and mock-transduced animals suggest that lethal irradiation itself may have increased the overall lipid load. This lipid increase might be predicted as a result of the large number of hematopoietic cells that would be expected to die as a result of exposure to this dose of radiation.
Table 3.
α-gal A activity and Gb3 levels in organs derived from mice sacrificed 12 or 26 weeks after transplantation
| Organ | Group (no. of mice) | α-gal A activity, nmol/hr/ mg protein | Gb3, nmol/mg protein |
|---|---|---|---|
| Liver | Wild type: 12 weeks | ||
| Untouched (5) | 69 ± 4 | 0.1 ± 0.1 | |
| Knockout: 12 weeks | |||
| Untouched (4) | 0.7 ± 0.1 | 3.1 ± 1.1 | |
| 1,100 rads–mock (5) | 1.2 ± 0.3 | 7.5 ± 1.2 | |
| 550 rads–transduced (5) | 20 ± 12* | 0.1 ± 0.2† | |
| 1,100 rads–transduced (6) | 19 ± 6 | 0.1 ± 0.0† | |
| Knockout: 26 weeks | |||
| 1,100 rads–transduced (6) | 27 ± 20* | 0.1 ± 0.0† | |
| Spleen | Wild type: 12 weeks | ||
| Untouched (5) | 453 ± 50 | 0.1 ± 0.1 | |
| Knockout: 12 weeks | |||
| Untouched (4) | 9 ± 1 | 15 ± 4 | |
| 1,100 rads–mock (5) | 18 ± 2 | 15 ± 1.2 | |
| 550 rads–transduced (5) | 390 ± 190* | 1.1 ± 0.9† | |
| 1,100 rads–transduced (6) | 210 ± 96† | 1.4 ± 0.3† | |
| Knockout: 26 weeks | |||
| 1,100 rads–transduced (6) | 300 ± 220* | 3.7 ± 1.4* | |
| Heart | Wild type: 12 weeks | ||
| Untouched (5) | 10 ± 1.3 | 0.2 ± 0.1 | |
| Knockout: 12 weeks | |||
| Untouched (4) | 0.2 ± 0.1 | 1.1 ± 0.2 | |
| 1,100 rads–mock (5) | 0.3 ± 0.1 | 2.1 ± 0.5 | |
| 550 rads–transduced (5) | 2.0 ± 1.1* | 0.4 ± 0.2† | |
| 1,100 rads–transduced (6) | 3.3 ± 1.2† | 0.3 ± 0.1† | |
| Knockout: 26 weeks | |||
| 1,100 rads–transduced (6) | 4.2 ± 3.3* | 0.9 ± 0.3* | |
| Lung | Wild type: 12 weeks | ||
| Untouched (5) | 81 ± 5 | 0.5 ± 0.1 | |
| Knockout: 12 weeks | |||
| Untouched (4) | 1.5 ± 0.5 | 5.3 ± 0.3 | |
| 1,100 rads–mock (5) | 1.8 ± 0.2 | 4.2 ± 0.2 | |
| 550 rads–transduced (5) | 14 ± 8* | 2.7 ± 0.9* | |
| 1,100 rads–transduced (6) | 9.2 ± 2.7† | 1.9 ± 0.5† | |
| Knockout: 26 weeks | |||
| 1,100 rads–transduced (6) | 15 ± 15 | 1.7 ± 1.0† | |
| Kidney | Wild type: 12 weeks | ||
| Untouched (5) | 48 ± 6 | 1.4 ± 7.4 | |
| Knockout: 12 weeks | |||
| Untouched (4) | 1.0 ± 0.1 | 8.7 ± 2.3 | |
| 1,100 rads–mock (5) | 1.1 ± 0.1 | 10.7 ± 1.4 | |
| 550 rads–transduced (5) | 2.2 ± 0.6* | 7.3 ± 0.7† | |
| 1,100 rads–transduced (6) | 3.0 ± 1.5* | 8.0 ± 1.9* | |
| Knockout: 26 weeks | |||
| 1,100 rads–transduced (6) | 3.1 ± 1.3* | 2.9 ± 1.7† | |
| Brain | Wild type: 12 weeks | ||
| Untouched (5) | 130 ± 3 | 0.2 ± 0.1 | |
| Knockout: 12 weeks | |||
| Untouched (4) | 1.2 ± 0.4 | 0.3 ± 0.1 | |
| 1,100 rads–mock (5) | 1.4 ± 0.2 | 0.4 ± 0.0 | |
| 550 rads–transduced (5) | 2 ± 0 | 0.3 ± 0.1 | |
| 1,100 rads–transduced (6) | 2 ± 0 | 0.2 ± 0.1 | |
| Knockout: 26 weeks | |||
| 1,100 rads–transduced (6) | 0.8 ± 0.2 | 0.2 ± 0.1 |
*, P < 0.05; †, P < 0.01 vs. 1,100 rads–mock group.
In the spleen, α-gal A activities increased and Gb3 levels decreased significantly in vector-treated groups. Enzyme activities increased to more than 80% of normal in the sublethally irradiated recipient group at 12 weeks. Gb3 levels were significantly reduced but never reached the levels observed in normal animals. α-gal A activity in the spleen was sustained in the 12- to 26-week groups of animals, although a slight increase in the Gb3 level occurred over the additional 3 months in the spleens of the 26-week recipient group.
α-gal A activities increased and Gb3 levels decreased significantly in the heart in all vector-treated groups. This finding is in contrast to earlier observations, where wild-type, non-genetically modified cells were transplanted into α-gal A-deficient animals. In that study, no enzymatic differences were seen in the heart in comparison with nontransplanted animals (8). In our present study, the α-gal A activity at 12 weeks was 30% of normal and remained elevated in the long-term 26-week group. Significant increases in α-gal A activity in the lung were found in both 12-week recipient groups, and Gb3 levels decreased significantly in all vector-treated groups. In this organ, however, the enzyme activity increases never reached 20% of normal. α-gal A activity in the kidney increased and Gb3 levels decreased significantly in all vector-treated groups. Here the overall increase in the measured α-gal A activity was the lowest of all organs, as no treatment group reached 10% of normal levels. The slight, but significant, increase was maintained from 12 to 26 weeks. Moreover, the 26-week recipient group had the lowest amount of Gb3, indicating that a sustained effect of the production of α-gal A was to reduce the overall lipid load in this organ. Finally, we could not detect any effect of treatment in the brain in any of the recipient or control groups. Taken together, these results demonstrate that transduction of BM cells followed by transplantation leads to sustained increases in enzyme activity and a reduction of Gb3 levels over time in numerous organs. No untoward effects were observed in any recipient animals, either from the transduction and transplantation protocol or from the overexpression of human α-gal A.
Secondary Transplantation.
To determine whether our transduction protocol leads to a correction of very primitive murine hematopoietic cells and to further prove that the corrected BM cells could provide for systemic metabolic cooperativity for the therapy of Fabry disease, we performed secondary BM transplantation of BMMCs collected from the 26-week recipient group into naïve, lethally irradiated α-gal A-deficient animals. To gauge the percentage of marked BM hematopoietic cells contributing to the above corrective effects (knockout 26-week recipient group; Tables 2 and 3) and to estimate the percentage of vector-positive cells undergoing secondary transplantation, we assayed progenitor colonies plated from the collected BMMCs for the presence of the human α-gal A-containing provirus by PCR (6). Sixty colonies were assayed and all were positive for the control murine P1–450 genomic sequence. However, only two colonies were found to be positive for the α-gal A proviral sequence (data not shown).
Six weeks after transplantation, we analyzed α-gal A activity and Gb3 levels in organs and tissues of the recipient animals. α-gal A activity in the plasma, BMMCs, and PBMCs of nontransplanted α-gal A-deficient mice was virtually undetectable. Secondarily transplanted animals had pooled enzyme activities that increased to 5.1 ± 0.4 nmol/hr per mg protein, 13.8 ± 0.9 nmol/hr per mg, and 4.8 ± 0.2 nmol/hr per ml, in BMMCs, PBMCs, and plasma, respectively. α-gal A activity increased in all organs of recipient animals except for the brain (Table 4). Gb3 levels decreased in all organs except for the kidney and brain. Enzyme activity in the hearts of recipient animals showed the highest increase, up to 31% of wild-type levels. Gb3 levels in the liver of these secondary recipients showed the greatest decrease, to levels seen in wild-type mice.
Table 4.
α-gal A activity and Gb3 levels in mice that received secondary transplantation
| Organ | α-gal A activity, nmol/hr/mg protein
|
Gb3 level, nmol/mg protein
|
||
|---|---|---|---|---|
| Mock (n = 5) | 2nd transplant (n = 3) | Mock (n = 5) | 2nd transplant (n = 3) | |
| Liver | 1.2 ± 0.3 | 15 ± 4.8* | 7.5 ± 1.2 | 0.1 ± 0.0† |
| Spleen | 18 ± 2 | 130 ± 37* | 15 ± 1.2 | 1.3 ± 0.2† |
| Heart | 0.3 ± 0.1 | 3.1 ± 0.9† | 2.1 ± 0.5 | 0.7 ± 0.0* |
| Lung | 1.8 ± 0.2 | 5.8 ± 0.6† | 4.2 ± 0.2 | 2.4 ± 0.2† |
| Kidney | 1.1 ± 0.1 | 2.0 ± 0.4* | 11 ± 1.4 | 8.0 ± 1.3 |
| Brain | 1.4 ± 0.2 | 0.8 ± 0.2 | 0.4 ± 0.0 | 0.2 ± 0.1 |
*, P < 0.01; †, P < 0.001 vs. 1,100 rads–mock group.
Discussion
We have demonstrated that cells derived from the BM of α-gal A-deficient mice can be effectively transduced ex vivo and that intracellular enzymatic correction and secretion occurs. The E86/pUMFG/α-gal A/FLAG vector was more effective in transduction than was the E86/pG1T/α-gal A vector. Transplantation of genetically modified hematopoietic cells in the murine model for Fabry disease leads to long-term enzymatic correction and reduction of Gb3 in a number of clinically relevant organs. The observation that correction of the metabolic defect in nontransduced cells (metabolic cooperativity) is effective and sustained in vivo is an indication of the potential of gene therapy for this disease. Especially encouraging is the fact that corrective effects are observed in vivo when these transduced BM cells are used as enzyme delivery vehicles even though a relatively low percentage of marked cells were detected. We were also encouraged by α-gal A increases and Gb3 reductions in the sublethally irradiated recipient animal group, as full myelo-ablation of patients is unlikely to be used in clinical gene therapy protocols for Fabry disease. The data comparing the enzyme activity increases in the BMMCs and the PBMCs in the sublethally and lethally irradiated groups (Table 2) are important, although we cannot explain the differences and the increased plasma activity in the 550-rads recipient group. Interestingly, these increases were also observed in some solid organs (Table 3). These effects warrant further study. The overall levels of increased enzyme activity that we saw in organs such as the liver, spleen, and heart are also important, as there are phenotypically normal individuals who are heterozygous for the genetic deficiency that have as little as 20–60% of normal α-gal A enzyme activity (15). In addition, as little as 5% of exogenous enzyme has been shown to be capable of normalizing Gb3 catabolism in cultured hemizygous fibroblasts (16). The results in heart are particularly encouraging. Involvement of this organ, particularly in “atypical or cardiac variants” of Fabry disease (16, 17), make this an important target for any potential therapeutic approach.
Studies employing this murine model for Fabry disease also provide a platform for future work toward optimization of gene transfer approaches for Fabry disease. Other vectors have been generated in our laboratory (G.Q. and J.A.M., unpublished work) that allow enrichment of transduced cells before transplantation (18). Not only will this increase the relative number of vector-positive cells that a recipient receives, it will also allow a definitive quantification of the numbers of transduced and persisting cells that contribute to corrective systemic effects over time. Further, by selecting an enrichment tag that does not cross-react with endogenous murine signals, we can also track and localize engrafted cells. For our ultimate goal of optimizing correction of the defect in patients, it will be important to determine the best cell source to generate metabolic cooperativity. It will also be important to determine which BM-derived cells are contributing to the long-term increases in α-gal A activity that we observed in the plasma of recipient animals. In addition to hematopoietic stem and progenitor cells, BM contains other populations of cells such as stromal cells (19) that can be readily transduced and could contribute to secretion of enzyme. The optimal producer cells may then become the prime targets for gene therapy for this disorder. Using other marking vectors (20), we can also determine whether therapeutically transduced cells have any growth advantage in vivo which would further increase the likelihood of a positive outcome in the clinic. It has recently been demonstrated that an infusion of recombinant α-gal A into Fabry patients leads to a reduction of Gb3 in plasma, urine sediment, and liver (7). Thus the present study in which a long-term continuous source of enzyme is provided is indicative of the potential of gene therapy for Fabry disease.
Abbreviations
- α-gal A
α-galactosidase A
- BM
bone marrow
- BMMCs
bone marrow mononuclear cells
- Gb3
globotriaosylceramide
- PBMCs
peripheral blood mononuclear cells
- LTBMCs
long-term bone marrow cultures
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120177997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120177997
References
- 1.Sugimoto Y, Aksentijevich I, Murray G J, Brady R O, Pastan I, Gottesman M M. Hum Gene Ther. 1995;6:905–915. doi: 10.1089/hum.1995.6.7-905. [DOI] [PubMed] [Google Scholar]
- 2.Medin J A, Tudor M, Simovitch R, Quirk J M, Jacobson S, Murray G J, Brady R O. Proc Natl Acad Sci USA. 1996;93:7917–7922. doi: 10.1073/pnas.93.15.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novo F J, Gorecki D C, Goldspink G, MacDermot K D. Gene Ther. 1997;4:488–492. doi: 10.1038/sj.gt.3300410. [DOI] [PubMed] [Google Scholar]
- 4.Takenaka T, Hendrickson C S, Tworek D M, Tudor M, Schiffmann R, Brady R O, Medin J A. Exp Hematol. 1999;27:1149–1159. doi: 10.1016/s0301-472x(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler R J, Yew N S, Li C, Cherry M, Berthelette P, Romanczuk H, Ioannou Y A, Zeidner K M, Desnick R J, Cheng S H. Hum Gene Ther. 1999;10:1667–1682. doi: 10.1089/10430349950017671. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka T, Qin G, Brady R O, Medin J A. Hum Gene Ther. 1999;10:1931–1939. doi: 10.1089/10430349950017293. [DOI] [PubMed] [Google Scholar]
- 7.Schiffmann R, Murray G J, Treco D, Daniel P, Sellos-Moura M, Myers M, Quirk J M, Zirzow G C, Borowski M, Loveday K, et al. Proc Natl Acad Sci USA. 2000;97:365–370. doi: 10.1073/pnas.97.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohshima T, Schiffmann R, Murray G J, Kopp J, Quirk J M, Stahl S, Chan C C, Zerfas P, Tao-Cheng J H, Ward J M, et al. Proc Natl Acad Sci USA. 1999;96:6423–6427. doi: 10.1073/pnas.96.11.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady R O, Gal A E, Bradley R M, Martensson E, Warshaw A L, Laster L. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 10.Dawson G, Sweeley C C. J Biol Chem. 1970;245:410–416. [PubMed] [Google Scholar]
- 11.Ohshima T, Murray G J, Swaim W D, Longenecker G, Quirk J M, Cardarelli C O, Sugimoto Y, Pastan I, Gottesman M M, Brady R O, et al. Proc Natl Acad Sci USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Miller A D, Rosman G J. BioTechniques. 1989;7:980–987. [PMC free article] [PubMed] [Google Scholar]
- 14.Krall W J, Skelton D C, Yu X J, Riviere I, Lehn P, Mulligan R C, Kohn D B. Gene Ther. 1996;3:37–48. [PubMed] [Google Scholar]
- 15.Desnick R J, Allen K Y, Desnick S J, Raman M K, Bernlohr R W, Krivit W. J Lab Clin Med. 1973;81:157–171. [PubMed] [Google Scholar]
- 16.Desnick R J, Ioannou Y A, Eng C M. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 2741–2784. [Google Scholar]
- 17.Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, et al. N Engl J Med. 1995;333:288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- 18.Medin J A, Karlsson S. Proc Assoc Am Physicians. 1997;109:111–119. [PubMed] [Google Scholar]
- 19.Prockop D J. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 20.Medin J A, Brandt J E, Rozler E, Nelson M, Bartholomew A, Li C, Turian J, Chute J, Chung T, Hoffman R. Ann NY Acad Sci. 1999;872:233–240. doi: 10.1111/j.1749-6632.1999.tb08468.x. [DOI] [PubMed] [Google Scholar]