Abstract
A variety of environmental and metabolic cues trigger the transient activation of the alternative transcription factor SigB of Bacillus subtilis, which subsequently leads to the induction of more than 150 general stress genes. This general stress regulon provides nongrowing and nonsporulated cells with a multiple, nonspecific, and preemptive stress resistance. By a proteome approach we have detected the expression of the SigB regulon during continuous growth at low temperature (15°C). Using a combination of Western blot analysis and SigB-dependent reporter gene fusions, we provide evidence for high-level and persistent induction of the sigB operon and the SigB regulon, respectively, in cells continuously exposed to low temperatures. In contrast to all SigB-activating stimuli described thus far, induction by low temperatures does not depend on the positive regulatory protein RsbV or its regulatory phosphatases RsbU and RsbP, indicating the presence of an entirely new pathway for the activation of SigB by chill stress in B. subtilis. The physiological importance of the induction of the general stress response for the adaptation of B. subtilis to low temperatures is emphasized by the observation that growth of a sigB mutant is drastically impaired at 15°C. Inclusion of the compatible solute glycine betaine in the growth medium not only improved the growth of the wild-type strain but rescued the growth defect of the sigB mutant, indicating that the induction of the general stress regulon and the accumulation of glycine betaine are independent means by which B. subtilis cells cope with chill stress.
As a soil bacterium, Bacillus subtilis is frequently exposed to nutrient limitations and often experiences a variety of environmental insults in this habitat (69). The ability of the cell to thrive under such stressful circumstances depends on sensing of these environmental changes and response to them via highly integrated adaptational networks. The regulation of gene expression by alternative sigma factors is a particularly important facet of the adaptation of B. subtilis to its changing environment (30, 35). One of the alternative transcription factors is SigB (31), which is now known to control a large general stress regulon (34, 53). The induction of the SigB regulon provides nongrowing and nonsporulated cells with a multiple, nonspecific, and preemptive stress resistance (4, 25, 26, 63).
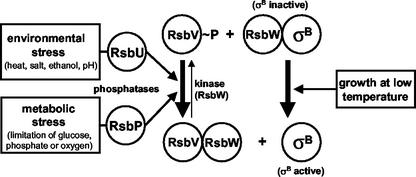
Proteome studies (3, 20) and transcriptional profiling (36, 52, 54) have demonstrated that SigB coordinates the expression of at least 150 genes in B. subtilis. Members of this regulon are transiently induced following heat shock; salt, ethanol, or acid stress; or limitation of glucose, phosphate, or oxygen (34, 53). SigB activity is subject to tight genetic and biochemical regulation to prevent the untimely expression of the SigB regulon in the absence of stress or starvation and to allow for the coordinated transcription of SigB-dependent genes in response to a wide spectrum of inducing stimuli. The biochemical activity of SigB as a transcription factor is controlled by a partner-switching mechanism that involves the anti-sigma factor RsbW and the antagonist protein RsbV (Fig. 1) (2, 10, 23, 53, 71). In exponentially growing cells, high-level expression of general stress genes is not required and SigB activity is prevented through the binding of RsbW to SigB (10). Release of SigB from this inhibitory SigB-RsbW complex requires RsbV, whose biochemical activity in turn critically depends on its phosphorylation status (2, 23, 71). During exponential growth, RsbV is rapidly phosphorylated by the kinase activity of RsbW and is thereby inactivated (23, 64, 71). Following the imposition of environmental or metabolic stress, RsbV is dephosphorylated by either of two phosphatases, RsbU or RsbP (61, 71), permitting RsbV to attack the SigB-RsbW complex and substitute for SigB in the complex (Fig. 1) (2, 22, 23). Free SigB can now bind to core RNA polymerase and promote the transcription of SigB-dependent genes. Basal cellular levels of SigB and most of its regulatory proteins are provided by transcription starting at a vegetative promoter located upstream of the sigB operon (rsbR-rsbS-rsbT-rsbU-rsbV-rsbW-sigB-rsbX) (40, 70).A SigB-dependent promoter positioned upstream of rsbV is activated by all SigB-inducing stimuli and accounts for an amplification of the SigB-mediated general stress response (13, 18).
FIG. 1.
Model for the regulation of SigB activity in B. subtilis. The environmental- and metabolic-stress-sensing branches of the signal transduction cascade conveying their output to RsbV are depicted (34, 53). Chill stress activates SigB via a new mechanism that operates independently of RsbV, RsbU, and RsbP.
Environmental and metabolic stresses activate SigB via two different signal transduction pathways (65), both of which converge on the control of the phosphorylation status of the antagonist protein RsbV (Fig. 1). The environmental stimuli heat, salt, and ethanol influence the activity of the environmental-stress-responsive phosphatase RsbU via additional regulatory proteins (41, 71). Perception of the various environmental stimuli seems to involve the ribosome (58, 72), but the actual details of this signal transduction process have not yet been elucidated. Limitation of glucose, phosphate, or oxygen, in turn, requires the activity of the metabolic-stress-responsive phosphatase RsbP (61). This branch of the signal transduction cascade likely constitutes a response to energy limitation, which possibly involves a reduction in the energy charge of the cell (2, 22, 49, 65).
The minimal growth temperature of B. subtilis is approximately 11°C (51), which is not unusual for a soil temperature in the Northern hemisphere. The stress response of B. subtilis to a sudden drop in temperature has been investigated, revealing the importance of cold shock proteins for cellular survival (28, 68) and the role of a fatty acid desaturase (1, 21, 67) and anteiso-branched fatty acid biosynthesis (45) in maintaining an appropriate degree of fluidity of the cytoplasmic membrane. In contrast, the long-term cellular adaptation of B. subtilis to continued growth at low temperatures has been less well explored. We have therefore carried out proteome analysis of B. subtilis cells that were continuously cultured under chill stress (15°C) conditions. Surprisingly, this study revealed high-level production of SigB-dependent general stress proteins during growth at a low temperature. Our data suggest that low-temperature induction of the SigB regulon involves a new signal transduction pathway that operates independently of RsbU, RsbP, and RsbV. The strongly impaired growth of a sigB mutant under chill stress conditions demonstrates the physiological relevance of general stress proteins for the effective adaptation of B. subtilis to low-temperature environments.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The experiments conducted in this study were performed with B. subtilis strain 168 (48). Mutations in sigB and the genes encoding SigB-regulatory proteins were transferred into the B. subtilis strain 168 background by transformation (33). The parent strains and the resulting mutants are listed in Table 1. Bacteria were routinely grown under vigorous agitation (220 rpm) in Spizizen's minimal medium (SMM) with 0.5% (wt/vol) glucose as the carbon source, l-tryptophan (20 mg/liter), and a solution of trace elements (32). When indicated the cells were grown in either Difco sporulation medium (DSM) or Luria Bertani (LB) medium. Precultures of B. subtilis strains were inoculated from exponentially growing overnight cultures propagated in SMM to a final optical density (OD) at 540 or 578 nm of 0.1. These precultures were allowed to grow to an OD at 540 or 578 nm of 0.5, diluted to an OD at 540 or 578 nm of 0.1, and subsequently transferred to the lower growth temperatures indicated below for the individual experiments. Ethanol stress was imposed on the cells by the addition of ethanol to a final concentration of 4% (vol/vol). Glycine betaine was purchased from Sigma Chemie (Steinheim, Germany) and used at a final concentration of 1 mM. For drug resistance selection in B. subtilis, antibiotics were used at the following final concentrations: chloramphenicol, 5 μg/ml; spectinomycin, 200 μg/ml; kanamycin, 20 μg/ml; erythromycin, 1 μg/ml.
TABLE 1.
B. subtilis strains and plasmids
| Strain or plasmid | Relevant genotype or features of plasmid | Construction or referenced |
|---|---|---|
| Strains | ||
| 168 | trpC2 | 48 |
| BSA46 | PY22 SPβ ctc::lacZ cat86 erma | 11 |
| BSA70 | PY22 rsbU::kan SPβ ctc::lacZa | 11 |
| BSA73 | PY22 rsbU::kan rsbV312 SPβ ctc::lacZ | 11 |
| BSA159 | PY22 rsbV312 rsbX::pWH25-spcb | 65 |
| BSM21 | trpC2 rsbU::kan | BSA70⇒168 |
| BSM22 | trpC2 rsbU::kan rsbV312 | BSA73⇒168 |
| BSM24 | trpC2 rsbV312 rsbX::pWH25-spc | BSA159⇒168 |
| BSM29 | trpC2 sigBΔ2::spc | FSB5⇒168 |
| BSM30 | trpC2 rsbP::spc | BSM201⇒168 |
| BSM149 | trpC2 rsbP::spc rsbU::kan | BSM30⇒BSM21 |
| BSM151 | trpC2 SPβ ctc::lacZ | BSA46⇒168 |
| BSM152 | trpC2 rsbP::spc SPβ ctc::lacZ | BSA46⇒BSM30 |
| BSM153 | trpC2 rsbP::spc rsbU::kan SPβ ctc::lacZ | BSA46⇒BSM149 |
| BSM154 | trpC2 rsbU::kan SPβ ctc::lacZ | BSA46⇒BSM21 |
| BSM155 | trpC2 rsbV312 rsbX::pWH25-spc SPβ ctc::lacZ | BSA46⇒BSM24 |
| BSM156 | trpC2 sigBΔ2::spc SPβ ctc::lacZ | BSA46⇒BSM29 |
| BSM201 | PY22 rsbP::spc | pAV01⇒PY22 |
| BSM202 | PY22 rsbP::spc rsbU::kan | BSA70⇒BSM201 |
| BSM227 | PY22 amyE::pGK30-gsiB::gfp cat86c | pGK30⇒PY22 |
| BSM267 | trpC2 rsbP::spc rsbU::kan rsbV312SPβ ctc::lacZ | BSA73⇒BSM152 |
| BSM269 | trpC2 amyE::pGK30-gsiB::gfp cat86c | BSM227⇒168 |
| BSM275 | trpC2 rsbV312 rsbX::pWH25-spc amyE::pGK30-gsiB::gfp | BSM227⇒BSM24 |
| cat86c | ||
| BSM276 | trpC2 sigBΔ2::spc amyE::pGK30-gsiB::gfp cat86c | BSM227⇒BSM29 |
| BSM277 | trpC2 rsbU::kan amyE::pGK30-gsiB::gfp cat86c | BSM227⇒BSM21 |
| BSM278 | trpC2 rsbP::spc amyE::pGK30-gsiB::gfp cat86c | BSM227⇒BSM30 |
| BSM279 | trpC2 rsbP::spc rsbU::kan rsbV312 | BSM202⇒BSM22 |
| BSM280 | trpC2 rsbP::spc rsbU::kan rsbV312 amyE::pGK30-gsiB::gfp cat86c | BSM227⇒BSM279 |
| FSB5 | trpC2 pheA1 sigBΔ2::spc | 59 |
| PY22 | trpC2 | P. Youngman, University of Georgia |
| Plasmids | ||
| pAV01 | AprrsbP::spc | This work |
| pDG1726 | Apr Spcr | 29 |
| pGK30 | AprgsiB::gfp cat86c | G. Kuhnke and U. Völker, unpublished data |
| pFSB79 | Low-copy-number gfp transcriptional fusion vector for integration into the amyE locus | F. Spiegelhalter and E. Bremer, unpublished data |
The ctc-lacZ fusion in the SPβ prophage is linked to the chloramphenicol (cat86) and erythromycin (erm) resistance genes.
The integrative plasmid pWH25 contains a 2-kb EcoRI-SphI fragment, including the 3′ end of rsbX and 1.9 kb downstream of rsbX.
The strain contains part of plasmid pGK30 inserted into the amyE locus of the chromosome via a double-crossover event, thus linking the transcriptional gsiB-gfp fusion to the chloramphenicol resistance gene (cat86).
The arrow indicates the construction of the strain by transformation.
Construction of plasmids.
To construct an rsbP disruption mutation, the rsbP structural gene was amplified by PCR using primers 5′-GAGAGAGCTCTTCAAACCATCCGAGT-3′ and 5′-GAGAGAATTCAAATATTTTGGTCGC-3′ and was subsequently cloned into the EcoRI- and SacI-digested multicopy vector pBlue2SKP, resulting in plasmid pAV02. For the disruption of the rsbP gene, a spectinomycin resistance cassette was amplified with primers 5′-GAGACAATTGGTAAAACGACGGCCAGT-3′ and 5′-GAGAGGATCCAACAGCTATGACCATGAT-3, with plasmid pDG1726 (29) as a template. After digestion with BamHI and MunI, this spectinomycin resistance cassette was inserted into plasmid pAV02 digested with the same enzymes, generating plasmid pAV01. Plasmid pAV01 linearized with ScaI was then transformed into B. subtilis strain PY22. Transformants were selected for their resistance to spectinomycin, and the integration of the rsbP::spc mutation into the chromosome via a double-crossover event was verified by PCR (Table 1, strain BSM201). Plasmid pFSB79 is a low-copy-number vector that can be used for constructing transcriptional fusions to a promoterless, fluorescence-optimized gfp gene (57) and subsequently transferring them into the amyE locus of the B. subtilis chromosome via a double-crossover event (F. Spiegelhalter and E. Bremer, unpublished data). For the construction of a gsiB-gfp fusion, a gsiB promoter fragment was amplified by PCR using primers 5′-GAGACCCGGGTGATGTTGTCGGCAAAAGAT-3′ and 5′-AATTGGTACCGTTGGTGGTTGTATTCCCG-3′ and was cloned into pFSB79cut with SmaI and KpnI, yielding plasmid pGK30 (Table 1). Linearized plasmid pGK30 was transformed into B. subtilis PY22. Transformants were selected with chloramphenicol and screened for an AmyE− phenotype. One of the resulting strains in which the correct recombination of the gsiB-gfp fusion into the amyE gene was verified by PCR was named BSM227 (Table 1).
2-DE.
Crude protein extracts for separation by two-dimensional protein gel electrophoresis (2-DE) were prepared from 150-ml cultures grown in 1-liter flasks to an OD at 578 nm of 1.0. High-resolution 2-DE with immobilized pH gradients (pH 4 to 7) in the first dimension was performed as previously described (37). Analytical gels were stained with silver nitrate according to the work of Bloom et al. (16). After scanning, 2-DE gel images were analyzed with the Melanie 3.0 software package (Bio-Rad Laboratories GmbH, Munich, Germany). Three separate gels of each condition were analyzed, and only changes in the protein pattern appearing on all three parallel gels were considered significant.
Western-blot analysis.
Western blot analysis was carried out as described previously (12, 65). The monoclonal antibodies raised against RsbS, RsbU, RsbV, RsbW, SigB, and RsbX have been described previously (12, 23, 24).
Determination of β-galactosidase activity.
For determination of the β-galactosidase activities of ctc-lacZ fusion strains, cultures were propagated as described above. At appropriate time points, 1-ml aliquots were harvested by centrifugation in an Heraeus tabletop centrifuge at 4°C. β-Galactosidase enzyme assays were conducted as described previously (50, 65).
Visualization of the fluorescence of gsiB-gfp fusion strains.
For the determination of gsiB-gfp fluorescence, 1-ml aliquots of the cultures were removed at an OD at 540 nm of 1.0, mixed with 10 μl of erythromycin (200 mg/ml), harvested by centrifugation in an Heraeus tabletop centrifuge at room temperature, and subsequently resuspended in 200 μl of SMM. After immobilization of the cells with 1% SeaPlaque GTG agarose (Biozym, Hessisch Oldendorf, Germany) on microscope slides, the samples were observed with a Zeiss fluorescence microscope using a 450 to 490/FT510/LP520 filter set. Images were recorded and processed for publication by using Adobe Photoshop.
RESULTS
Chill stress-induced changes in the protein profile of B. subtilis.
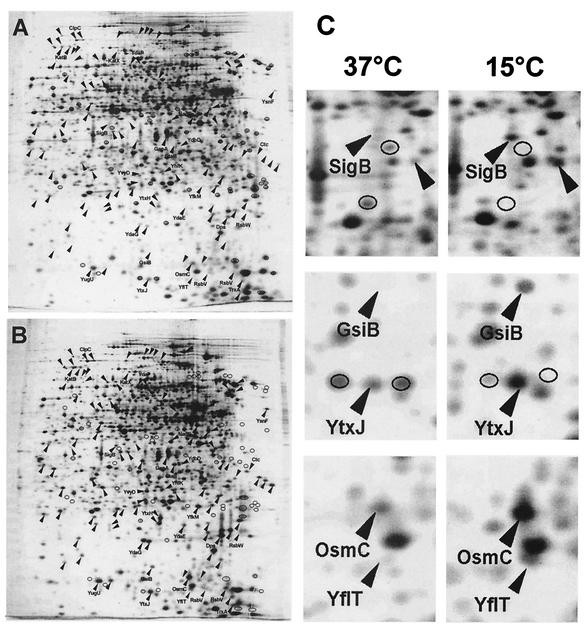
We prepared crude protein extracts of soluble proteins from exponentially growing cultures of B. subtilis strain 168 that were propagated in SMM at 37 or 15°C and separated them by 2-DE. We chose B. subtilis strain 168 for our study because the DNA sequence of this isolate was determined in the B. subtilis genome project (48) and it also was used to establish the Sub2D proteome database of B. subtilis (http://microbio2.biologie.uni-greifswald.de:8880/sub2d.htm) (20). A comparison of the protein profiles of silver-stained gels revealed extensive differences between the two cultivation conditions. A representative set of two-dimensional (2D) gels prepared from cells grown at 37 or 15°C is shown in Fig. 2A and B. At least 171 proteins displayed significantly different expression levels at 15 versus 37°C, indicating that adaptation to continuous growth at low temperatures requires massive changes in cellular protein composition. Through a software-aided comparison of representative sets of gels from the two growth conditions, we noted 61 protein spots displaying higher intensities on gels prepared from cultures grown at 37°C and 110 protein spots that were present at higher levels in gels derived from cultures propagated at 15°C. A comparison of both protein patterns with the Sub2D database (3, 20) revealed the strong expression of the entire SigB-dependent general stress regulon under chill stress conditions. Sections of 2D gels displaying the expression levels of selected members of this regulon are depicted in Fig. 2C. We emphasize that the differences between the protein profiles of cultures grown at high and low temperatures are not restricted to the expression of the SigB regulon (Fig. 2A and B). However, high-level expression of the SigB-controlled general stress regulon in cells that were continuously growing in batch cultures at the low temperature was a highly unexpected finding, since it is well established that most SigB-activating conditions (e.g., heat, salt, ethanol, acid) lead only to a transient induction of the general stress genes (13, 18, 62, 65). We therefore focused our further analysis on the chill stress induction of the SigB regulon.
FIG. 2.
Influence of growth at a low temperature on the protein profile of B. subtilis 168. (A and B) Cells were grown in SMM at either 37°C (A) or 15°C (B) to an OD at 578 nm of 1.0. Crude protein extracts were prepared and separated by 2-DE. After being stained with silver nitrate, the gels were scanned with an imaging system and analyzed with the Melanie 3.0 software package. Proteins induced or repressed by growth at the low temperature are marked with arrowheads or circles, respectively. (C) Enlarged sections of gels under both conditions. Representative members of the SigB-dependent general stress regulon are labeled.
Induction of the sigB operon during growth at a low temperature.
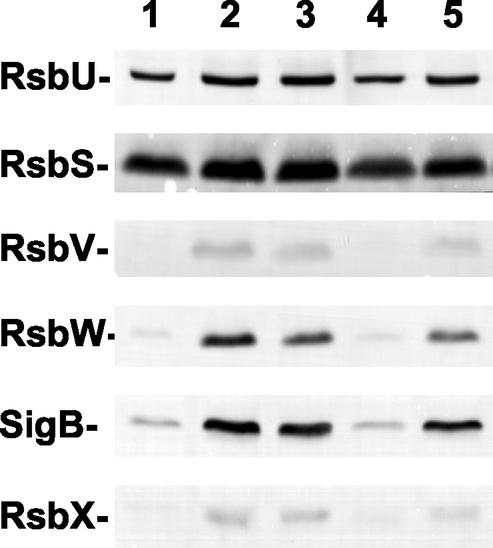
The downstream part (rsbV-rsbW-sigB-rsbX) of the sigB operon is subjected to positive autoregulation by SigB due to the presence of a SigB-dependent promoter positioned in front of rsbV (13, 18), whereas the upstream part (rsbR-rsbS-rsbT-rsbU) of this operon is expressed from another, presumably SigA-dependent promoter (24, 70). If chill stress indeed leads to high-level expression of the SigB regulon, as suggested by the proteome experiments described above (Fig. 2), one would expect increased cellular levels of the RsbV, RsbW, SigB, and RsbX proteins under this growth condition. To test this prediction experimentally, we used a set of monoclonal antibodies directed against these proteins in Western blot analysis of crude protein extracts prepared from B. subtilis 168 cultures propagated at either 37 or 15°C. As controls, we used monoclonal antibodies directed against RsbU and RsbS, whose cellular levels should not increase during chill stress. The data documented in Fig. 3 (lanes 4 and 5) demonstrate increased production of the RsbV, RsbW, SigB, and RsbX proteins at the low temperature, whereas no such increase was observed for the RsbU and RsbS proteins. The accumulation of RsbV, RsbW, SigB, and RsbX observed under chill stress reached a cellular level similar to that observed after treatment of the cells with ethanol (Fig. 3, lanes 1 to 3), a well-known and strong inducer of the SigB regulon (18, 65). Consequently, these data provide solid evidence for a chill-induced increase in the synthesis of SigB, the master regulator of the general stress response, and its primary regulatory proteins.
FIG. 3.
Effect of low temperature and ethanol stress on the levels of products of the sigB operon. Crude protein extracts were prepared from cultures of B. subtilis strain 168 grown at either 37°C (lanes 1 to 4) or 15°C (lane 5). To activate SigB, cells were treated for either 30 min (lane 2) or 60 min (lane 3) with 4% ethanol during exponential growth (OD at 540 nm, 0.5). Cultures were propagated either in LB medium (lanes 1 to 3) or in SMM (lanes 4 and 5). Samples collected for lanes 1, 4, and 5 were harvested during exponential growth (OD at 540 nm, 0.5). After separation by sodium dodecyl sulfate-polyacrylamide protein gel electrophoresis and transfer of the proteins to a nitrocellulose membrane, the proteins were reacted with a set of monoclonal antibodies, and specific antibody binding was detected by an alkaline phosphatase-conjugated goat anti-mouse secondary antibody.
Chill induction of the SigB-dependent ctc and gsiB genes.
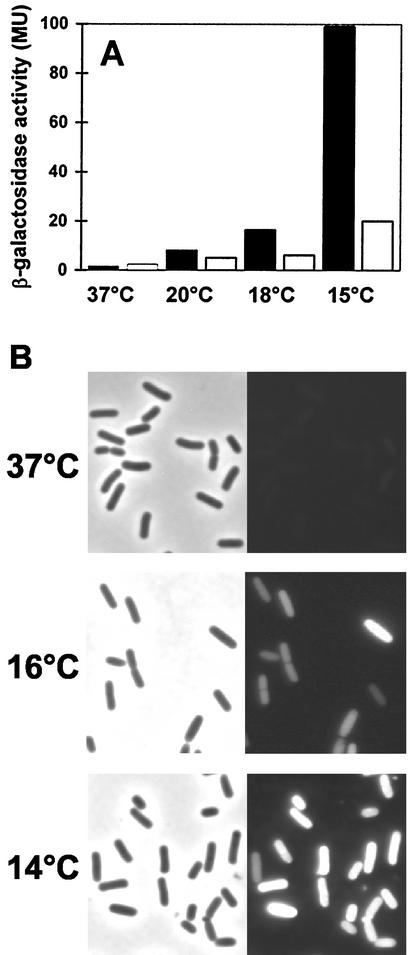
Increased production of SigB in cells grown at a low temperature suggests that the transcription of the entire SigB regulon is induced by chill stress. We tested this hypothesis by growing cells in SMM and monitoring the expression of two members of the SigB regulon: ctc, a traditional reporter for SigB activity (38), and gsiB, a gene that is transcribed exclusively from a SigB-dependent promoter (49). To monitor ctc expression, we used strain BSA46 (Table 1), which carries a ctc-lacZ promoter fusion integrated as a single chromosomal copy into the SPβ prophage. The activity of the gsiB promoter was monitored by means of a gsiB-gfp transcriptional fusion construct integrated as a single copy into the amyE locus in strain BSM269 (Table 1). There was only a low basal level of ctc-lacZ expression in cells grown at 37°C, but β-galactosidase activity was strongly increased (approximately 50-fold) when this fusion strain was propagated at 15°C (Fig. 4A). Intermediate induction levels were observed when the cells of the BSA46 reporter strain were grown at 20 or 18°C (Fig. 4A). Likewise, there was no detectable expression of the gsiB-gfp reporter fusion when strain BSM269 was grown at 37°C, but green fluorescent protein activity was readily detected in cells grown at 16°C and increased further when the growth temperature was dropped to 14°C (Fig. 4B). In agreement with the proteome analysis (Fig. 2), the reporter gene fusion experiments with ctc and gsiB strongly indicate the induction of the entire SigB regulon under chill stress conditions.
FIG. 4.
Temperature-dependent expression of SigB-controlled ctc-lacZ (A) and gsiB-gfp (B) fusions. (A) The B. subtilis ctc-lacZ fusion strain BSA46 was grown in SMM at the indicated temperatures either in the absence (filled bars) or in the presence (open bars) of 1 mM glycine betaine. Samples were removed for β-galactosidase assays during exponential growth (OD at 578 nm, 0.5). β-Galactosidase activities are expressed in Miller units (MU). (B) The B. subtilis gsiB-gfp fusion strain BSM269 was grown in SMM at the indicated temperatures, and samples were removed for phase-contrast and fluorescence microscopy during exponential growth (OD at 540 nm, 1.0). Left and right panels display phase-contrast and fluorescence images, respectively, of the same cells.
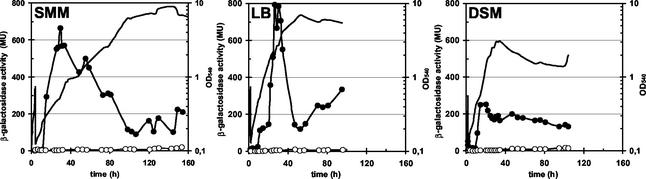
To test whether the chill-stress-mediated induction of the ctc gene was medium dependent, we monitored the transcription of the ctc-lacZ fusion in cells that were propagated at 16°C in LB medium or DSM. Induction of the ctc-lacZ fusion was observed in both of these complex media and was strictly dependent on an intact sigB gene (Fig. 5). In all three media tested (SMM, LB, DSM), ctc-lacZ expression peaked during mid-exponential growth and then declined again over a long period to a level that was higher than that detected prior to the shift of the cells to 16°C (Fig. 5). Induction of the gene fusion did not occur until the cells had completed two cell doublings subsequent to the temperature downshift (Fig. 5). This delayed induction of ctc-lacZ expression upon the shift to 16°C contrasts with the induction of SigB-dependent genes, which generally occurs within a few minutes after the imposition of salt, heat, or ethanol stress (18, 65).
FIG. 5.
Time-resolved low-temperature induction of ctc-lacZ in different growth media. The B. subtilis ctc-lacZ fusion strains BSM151 (SigB+) (filled circles) and BSM156 (SigB−) (open circles) were precultured in SMM at 37°C and used to inoculate cultures in either SMM, LB medium, or DSM. The cultures were then propagated at 16°C. Samples were removed for β-galactosidase assays at the indicated time points. β-Galactosidase activities are expressed in Miller units (MU). Solid lines indicate the growth of the wild-type strain (BSM151); growth of the sigB mutant strain (BSM156) did not differ from that of the wild-type strain under these growth conditions.
Growth of a sigB mutant is strongly impaired at a low temperature and is rescued by the compatible solute glycine betaine.
To test the physiological role of SigB under chill stress conditions, we propagated the wild-type B. subtilis strain 168 and its isogenic sigB mutant BSM29 (sigB::spc) at 15°C in SMM. We found that the growth of the sigB mutant was strongly impaired in comparison to that of its sigB+ parent at this temperature (Fig. 6). We noted that the induction of the general stress regulon is particularly important in a rather narrow temperature range close to 15°C, since there was no substantial growth difference between a SigB+ and a SigB− strain when the cells were cultivated at or above 16°C (data not shown).
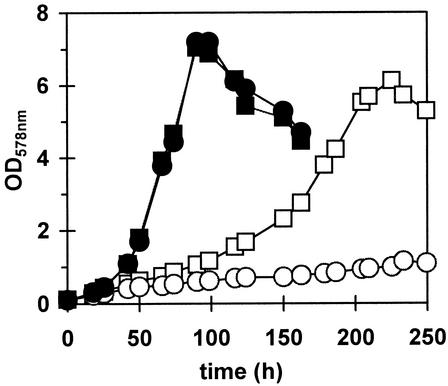
FIG. 6.
Influence of sigB and glycine betaine on growth at a low temperature. The B. subtilis wild-type strain 168 (squares) and its isogenic sigB mutant BSM29 (circles) were precultured in SMM at 37°C and used to inoculate 25-ml cultures in 100-ml Erlenmeyer flasks with (filled symbols) or without (open symbols) 1 mM glycine betaine. The cultures were then propagated at 15°C.
Glycine betaine has a well-established role in the osmoprotection of microbial cells (19), but additional experiments suggest a function for this compatible solute in chill stress protection as well (6, 15, 46). We therefore tested the influence of glycine betaine on the growth of the wild-type B. subtilis strain 168 and its isogenic sigB mutant at a low temperature. Addition of 1 mM glycine betaine to the culture of the wild-type strain greatly increased its growth rate at 15°C and was able to fully rescue the sigB mutant strain BSM29 from growth inhibition at this low temperature (Fig. 6).
Compatible solutes such as glycine betaine not only have a protective function for B. subtilis at high osmolality (17) but are also known to have a modulating influence on the expression of osmotically responsive genes such as opuE, encoding a high-affinity uptake system for the osmoprotectant proline (59). Similarly, glycine betaine not only increased the growth of B. subtilis at a low temperature (Fig. 6) but also strongly reduced the chill induction of the SigB-dependent ctc-lacZ fusion (Fig. 4A).
RsbV-independent chill induction of a gsiB-gfp fusion.
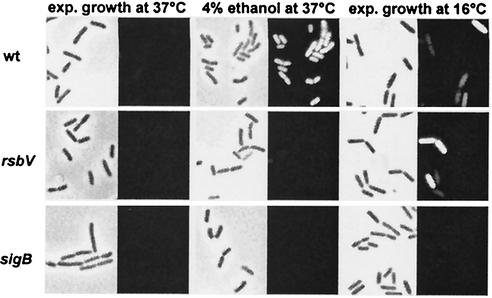
The environmental and metabolic branches of the SigB-activating signal transduction cascade share the main regulatory protein RsbV. Its phosphorylation status is critical for SigB activation by mediating the release of SigB from the RsbW-SigB inhibitory complex. Consequently, the SigB regulon is not inducible by any environmental or metabolic cues described thus far in an rsbV mutant (34, 53). We tested whether this was also true for the chill induction of the SigB regulon by using a gsiB-gfp fusion construct and comparing its induction by ethanol treatment versus chill stress. Induction of the fusion by ethanol treatment occurred in a wild-type strain, and as expected, it was no longer detectable in either an rsbV or a sigB mutant background (Fig. 7). However, to our great surprise, chill stress triggered gsiB-gfp expression not only in a wild-type strain but also in an rsbV mutant (Fig. 7). This chill stress-mediated induction of the reporter gene fusion was completely abolished in a sigB mutant (Fig. 7), demonstrating that the chill induction of gsiB is critically dependent on SigB activity.
FIG. 7.
An RsbV-independent signal transduction cascade controls chill induction of gsiB-gfp. The isogenic set of B. subtilis strains BSM269 (RsbV+ SigB+), BSM275 (RsbV− SigB+), and BSM276 (RsbV+ SigB−) was precultured in SMM at 37°C and used to inoculate cultures in SMM that were subsequently either exposed to ethanol stress or grown at 16°C. Samples were removed for phase-contrast and fluorescence microscopy from cultures growing exponentially (exp.) at 37 or 16°C (OD at 540 nm, 1.0) or 60 min after cells grown at 37°C had been exposed to 4% ethanol.
Chill induction of the SigB regulon in mutants lacking regulators of SigB activity.
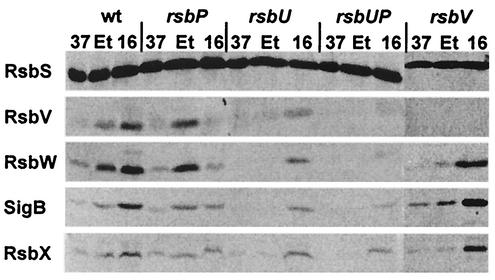
The low-temperature induction of the gsiB-gfp fusion in the rsbV mutant suggests the existence of a new facet in the control of SigB activity during chill stress. To assess the contribution of the environmental and metabolic signal transduction pathways to chill activation of SigB, we performed Western blot analysis using monoclonal antibodies directed against SigB and its regulatory proteins (see Fig. 3). As a result of the positive autoregulatory loop controlling the expression of the downstream part (rsbV-rsbW-sigB-rsbX) of the sigB operon, we observed strong increases in the levels of the RsbV, RsbW, SigB, and RsbX proteins in the wild-type strain following ethanol treatment or exposure to a low temperature (Fig. 8). Inactivation of the environmental branch of the signal transduction cascade through the introduction of an rsbU mutation abolished ethanol induction but not induction by chill stress (16°C). Likewise, inactivation of RsbP, a phosphatase that is critical for the sensing of metabolic stress, did not effect ethanol induction but reduced induction by chill stress. Even in the absence of both stress-responsive phosphatases (RsbU and RsbP), induction by a low temperature was not abolished while induction mediated by ethanol treatment was completely prevented (Fig. 8). In agreement with the data reported above for the expression of the gsiB-gfp fusion in an rsbV mutant background (Fig. 7), we also observed a very strong increase in the levels of these proteins during growth at 16°C but found no induction of RsbW, SigB, or RsbX by ethanol treatment (Fig. 8). As a control we monitored the RsbS protein, whose structural gene is not under the control of SigB. As expected, we found no significant variations in the cellular level of RsbS after treatment of the various strains with either ethanol or a low temperature (Fig. 8).
FIG. 8.
Effects of a low growth temperature and ethanol stress on the levels of products of the sigB operon in a wild-type strain and in SigB regulatory mutants. Crude protein extracts were prepared from cultures of an isogenic set of B. subtilis strains grown in SMM at either 37 or 16°C or treated with 4% ethanol (Et) at 37°C for 60 min. After separation by sodium dodecyl sulfate-polyacrylamide protein gel electrophoresis and transfer of the proteins to a nitrocellulose membrane, the proteins were reacted with a set of monoclonal antibodies, and specific antibody binding was detected by an alkaline phosphatase-conjugated goat anti-mouse secondary antibody. The following strains were used: BSM151 (wild type), BSM152 (rsbP), BSM154 (rsbU), BSM153 (rsbU rsbP), and BSM155 (rsbV).
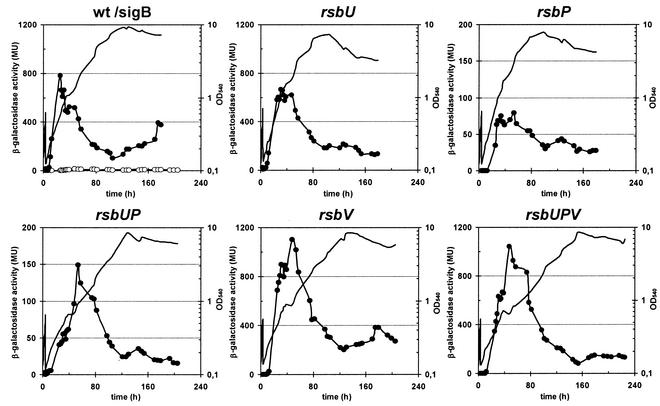
To test whether the expression profile just described for the rsbV-rsbW-sigB-rsbX operon also extends to the SigB regulon, we monitored the transcription of a SigB-dependent ctc-lacZ fusion in genetic backgrounds with defects in the major SigB-regulatory proteins (Fig. 9). We made the following observations. (i) In all mutants tested except the SigB− strain, there was an increase in ctc-lacZ activity that peaked during mid-exponential growth after the cells were shifted to a low temperature. (ii) Loss of the environmental-stress-responsive phosphatase RsbU did not significantly influence the expression of the fusion. (iii) Inactivation of the metabolic-stress-responsive phosphatase RsbP alone or in combination with an rsbU mutation reduced ctc-lacZ expression but did not prevent its chill induction. (iv) Disruption of the rsbV gene did not reduce chill stress-mediated ctc transcription but resulted in an induction of the fusion even stronger than that found in a wild-type background. (v) In contrast to the rsbU rsbP double mutant, a strain lacking all three regulatory proteins (RsbU, RsbP, and RsbV) displayed ctc-lacZ expression levels that exceeded those found in a wild-type strain (Fig. 9).
FIG. 9.
Time-resolved chill-stress-induced expression of ctc-lacZ in a wild-type strain and a set of isogenic SigB regulatory mutants. A set of B. subtilis ctc-lacZ fusion strains was precultured in SMM at 37°C and used to inoculate cultures that were then propagated at 16°C. Samples were removed for β-galactosidase assays at the indicated time points. β-Galactosidase activities are expressed in Miller units (MU). Solid lines indicate the growth of the various strains. Filled circles display β-galactosidase activity. In the panel showing the wild-type strain we additionally included the β-galactosidase activity of the sigB mutant, which is represented by open circles. The following strains were used: BSM151 (wild type), BSM156 (sigB), BSM154 (rsbU), BSM152 (rsbP), BSM153 (rsbU rsbP), BSM155 (rsbV), and BSM267 (rsbV rsbP rsbU).
DISCUSSION
When B. subtilis cells are exposed to severe stress or starvation, up to one-third of their residual protein-synthesizing capability is engaged in producing SigB-controlled general stress proteins (14). Disruption of the gene encoding the master regulator SigB abolishes induction of the entire regulon and results in sensitivity of the cells to a variety of stress factors such as heat, high salt, ethanol, low or high pH, and free radicals (4, 25, 26, 63), suggesting an important physiological function for general stress proteins in natural settings (34, 53). Our data now add a new facet to the physiological function of the SigB regulon by demonstrating through a proteome approach the high-level production of general stress proteins in cells that continuously grow at a low temperature (Fig. 2). Additionally, we show that the growth of a sigB mutant is strongly impaired under these cultivation conditions (15°C) (Fig. 6).
Our chill stress experiments were performed at a temperature that occurs frequently in the upper layers of soil. Thus, one can readily envision that the continued high-level synthesis of general stress proteins in cold-adapted and actively growing cells can yield cellular levels sufficient for protection in the soil. Hence, the chill-triggered induction of the SigB-dependent general stress regulon is likely to be of ecophysiological relevance for the growth of B. subtilis. This notion is supported by the observation that B. subtilis cells do not sporulate effectively at low temperatures (A. Bashir and U. Völker, unpublished data). While the structure of the general stress regulon has been rather well described (3, 20, 34, 36, 53), the contribution of its individual members to cellular protection against specific stresses has been defined for only a few regulon members (5, 47, 56, 60, 66). A comparison of the members of the B. subtilis SigB regulon to databases did not reveal any particular proteins that might serve a specific function during continuous growth at low temperatures.
The compatible solute glycine betaine is present at substantial concentrations (40 to 400 μmol g−1 [dry weight]) in plants (55), and some of it will eventually reach the habitat of B. subtilis through root exudates and decomposing plant material. The function of glycine betaine as an osmoprotectant for microbial cells has been well documented (19); in addition, recent experiments with the food-borne pathogen Listeria monocytogenes demonstrate that it also confers chill protection (6, 15, 46). The growth experiments that we conducted (Fig. 6) show that this compatible solute serves a strong chill-protective function for B. subtilis as well. This soil bacterium possesses three high-affinity uptake systems (OpuA, OpuC, and OpuD) for glycine betaine (42-44), and mutant studies have shown that each of these transporters is involved in glycine betaine acquisition under chill stress conditions (T. Hoffmann and E. Bremer, unpublished data). Inclusion of 1 mM glycine betaine in the culture medium not only improved the growth of the wild-type strain but also completely rescued the growth defect of a sigB mutant observed at a low temperature (15°C) (Fig. 6). This observation indicates that the induction of the general stress regulon and the accumulation of a compatible solute are independent means for B. subtilis cells to cope with chill stress. Similarly, alternative protective functions for the accumulation of glycine betaine and the general stress response have already been described for the cellular adaptation to growth-preventing salt concentrations (63). A connection has also been proposed between the accumulation of compatible solutes and the SigB-controlled general stress response in L. monocytogenes, where SigB contributes to cold shock adaptation in a growth-phase-dependent manner and to the efficient accumulation of glycine betaine and carnitine as cryoprotectants (7, 8).
In contrast to the SigB-activating stimuli studied thus far (e.g., a sudden rise in temperature from 37 to 48°C, the sudden addition of 0.7 M NaCl, or treatment of the cells with 4% ethanol), low temperature triggers not a short and transient (34, 53) but a slow and long-lasting induction of the general stress regulon in actively growing cells (Fig. 2 and 5). Induction following transfer of the cells to a low temperature is also delayed for several hours (Fig. 5 and 9), which explains why increased transcription of SigB-dependent genes was not observed in proteome and transcriptome studies analyzing the initial response of B. subtilis to cold shock (9, 27, 39).
Two complex SigB-activating signal transduction pathways responding to either environmental or metabolic stress, both of which converge on the antagonist protein RsbV, have been discovered in B. subtilis (Fig. 1) (34, 53). The regulatory output of both pathways is the dephosphorylation of RsbV (2, 61, 64, 71), which, in turn, in its nonphosphorylated form can free the transcription factor SigB from the inhibitory SigB-RsbW complex (22, 23). Consequently, both the metabolic- and environmental-stress pathways require an intact RsbV protein in order to function (Fig. 1), and loss of the antagonist in an rsbV mutant completely prevents induction by all of the SigB-activating stimuli described thus far (65).
The data presented in this communication reveal important differences from the traditional pattern of SigB activation (Fig. 1), because chill activation of the SigB response does not depend on either RsbV or the two phosphatases RsbU and RsbP (Fig. 8 and 9). Accordingly, we observed high-level expression of the SigB-dependent general stress genes ctc and gsiB (Fig. 7 and 9) and high-level accumulation of the autoregulated RsbW, SigB, and RsbX proteins (Fig. 8) in an rsbV mutant strain. Even in an rsbU rsbP rsbV triple mutant we found high-level expression of a ctc-lacZ fusion, clearly demonstrating that neither the antagonist protein RsbV nor the environmental (RsbU) or the metabolic (RsbP) phosphatase is required for ctc expression under chill stress conditions (Fig. 9). These data strongly argue for the presence of an additional branch of signal transduction leading to SigB activation in cells continuously growing at a low temperature (Fig. 1). Although the mechanism of this SigB activation has not been explored yet, two alternative possibilities for such a new signal transduction come immediately to mind. New signaling proteins that talk independently from RsbV to RsbW could disrupt the inhibitory RsbW-SigB complex and allow chill activation of SigB. Alternatively, key physical interactions between RsbW and SigB or between SigB and core RNA polymerase might change at low temperatures. The latter chill-induced changes in the protein properties might be intrinsic to the proteins or might be modulated by temperature-responsive accessory proteins such as the chaperones. It will be a challenge for the future to distinguish between these different molecular mechanisms, to unravel their components, and to integrate them into the already complex picture of SigB activation by metabolic and environmental stress (34, 53).
Acknowledgments
We appreciate the help of J. Gade and S. Hövel in cell cultivation and 2-DE. We are grateful to W. G. Haldenwang for providing the monoclonal antibodies directed against SigB and its regulatory proteins, to F. Spiegelhalter and G. Kuhnke for the construction of plasmids, and to M. Niederweis for providing an optimized GFP variant. We thank V. Koogle for editing the manuscript.
Financial support for this study was provided by the Deutsche Forschungsgemeinschaft through the Graduiertenkolleg “Proteinfunktion auf atomarer Ebene” and the SFB-395, the Max-Planck-Society, and the Fonds der Chemischen Industrie (to E.B. and U.V.).
REFERENCES
- 1.Aguilar, P. S., A. M. Hernandez-Arriaga, L. E. Cybulski, A. C. Erazo, and D. de Mendoza. 2001. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper, S., A. Dufour, D. A. Garsin, L. Duncan, and R. Losick. 1996. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J. Mol. Biol. 260:165-177. [DOI] [PubMed] [Google Scholar]
- 3.Antelmann, H., J. Bernhardt, R. Schmid, H. Mach, U. Völker, and M. Hecker. 1997. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis 18:1451-1463. [DOI] [PubMed] [Google Scholar]
- 4.Antelmann, H., S. Engelmann, R. Schmid, and M. Hecker. 1996. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 178:6571-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antelmann, H., S. Engelmann, R. Schmid, A. Sorokin, A. Lapidus, and M. Hecker. 1997. Expression of a stress- and starvation-induced dps/pexB homologous gene is controlled by the alternative sigma factor σB in Bacillus subtilis. J. Bacteriol. 179:7251-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 7.Becker, L. A., M. S. Cetin, R. W. Hutkins, and A. K. Benson. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker, L. A., S. N. Evans, R. W. Hutkins, and A. K. Benson. 2000. Role of σB in adaptation of Listeria monocytogenes to growth at low temperature. J. Bacteriol. 182:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckering, C. L., L. Steil, M. H. Weber, U. Völker, and M. A. Marahiel. 2002. Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis. J. Bacteriol. 184:6395-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson, A. K., and W. G. Haldenwang. 1993. Bacillus subtilis σB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc. Natl. Acad. Sci. USA 90:2330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benson, A. K., and W. G. Haldenwang. 1992. Characterization of a regulatory network that controls σB expression in Bacillus subtilis. J. Bacteriol. 174:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson, A. K., and W. G. Haldenwang. 1993. Regulation of σB levels and activity in Bacillus subtilis. J. Bacteriol. 175:2347-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson, A. K., and W. G. Haldenwang. 1993. The σB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhardt, J., U. Völker, A. Völker, H. Antelmann, R. Schmid, H. Mach, and M. Hecker. 1997. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology 143:999-1017. [DOI] [PubMed] [Google Scholar]
- 15.Beumer, R. R., M. C. Te Giffel, L. J. Cox, F. M. Rombouts, and T. Abee. 1994. Effect of exogenous proline, betaine, and carnitine on growth of Listeria monocytogenes in a minimal medium. Appl. Environ. Microbiol. 60:1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloom, H., H. Beier, and H. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 17.Boch, J., B. Kempf, and E. Bremer. 1994. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176:5364-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bremer, E., and R. Krämer. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79-97. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 20.Büttner, K., J. Bernhardt, C. Scharf, R. Schmid, U. Mader, C. Eymann, H. Antelmann, A. Völker, U. Völker, and M. Hecker. 2001. A comprehensive two-dimensional map of cytosolic proteins of Bacillus subtilis. Electrophoresis 22:2908-2935. [DOI] [PubMed] [Google Scholar]
- 21.Cybulski, L. E., D. Albanesi, M. C. Mansilla, S. Altabe, P. S. Aguilar, and D. de Mendoza. 2002. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol. Microbiol. 45:1379-1388. [DOI] [PubMed] [Google Scholar]
- 22.Delumeau, O., R. J. Lewis, and M. D. Yudkin. 2002. Protein-protein interactions that regulate the energy stress activation of σB in Bacillus subtilis. J. Bacteriol. 184:5583-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dufour, A., and W. G. Haldenwang. 1994. Interactions between a Bacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV). J. Bacteriol. 176:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dufour, A., U. Völker, A. Völker, and W. G. Haldenwang. 1996. Relative levels and fractionation properties of Bacillus subtilis σB and its regulators during balanced growth and stress. J. Bacteriol. 178:3701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelmann, S., and M. Hecker. 1996. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol. Lett. 145:63-69. [DOI] [PubMed] [Google Scholar]
- 26.Gaidenko, T. A., and C. W. Price. 1998. General stress transcription factor σB and sporulation transcription factor σH each contribute to survival of Bacillus subtilis under extreme growth conditions. J. Bacteriol. 180:3730-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graumann, P., K. Schröder, R. Schmid, and M. A. Marahiel. 1996. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178:4611-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graumann, P., T. M. Wendrich, M. H. W. Weber, K. Schröder, and A. Marahiel. 1997. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol. Microbiol. 25:741-756. [DOI] [PubMed] [Google Scholar]
- 29.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 30.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haldenwang, W. G., and R. Losick. 1980. Novel RNA polymerase sigma factor from Bacillus subtilis. Proc. Natl. Acad. Sci. USA 77:7000-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harwood, C. R., and A. R. Archibald. 1990. Growth, maintenance and general techniques, p. 1-26. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 33.Harwood, C. R., and S. M. Cutting. 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 34.Hecker, M., and U. Völker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35-91. [DOI] [PubMed] [Google Scholar]
- 35.Helmann, J. D., and C. P. Moran. 2002. RNA polymerase and sigma factors, p. 289-312. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 36.Helmann, J. D., M. F. Wu, P. A. Kobel, F. J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann, T., A. Schütz, M. Brosius, A. Völker, U. Völker, and E. Bremer. 2002. High-salinity-induced iron limitation in Bacillus subtilis. J. Bacteriol. 184:718-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igo, M., and R. Losick. 1986. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J. Mol. Biol. 191:615-624. [DOI] [PubMed] [Google Scholar]
- 39.Kaan, T., G. Homuth, U. Mäder, J. Bandow, and T. Schweder. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response. Microbiology 148:3441-3455. [DOI] [PubMed] [Google Scholar]
- 40.Kalman, S., M. L. Duncan, S. M. Thomas, and C. W. Price. 1990. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J. Bacteriol. 172:5575-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang, C. M., M. S. Brody, S. Akbar, X. F. Yang, and C. W. Price. 1996. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor σB in response to environmental stress. J. Bacteriol. 178:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappes, R. M., B. Kempf, S. Kneip, J. Boch, J. Gade, J. Meier-Wagner, and E. Bremer. 1999. Two evolutionarily closely related ABC transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 32:203-216. [DOI] [PubMed] [Google Scholar]
- 44.Kempf, B., and E. Bremer. 1995. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J. Biol. Chem. 270:16701-16713. [DOI] [PubMed] [Google Scholar]
- 45.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krüger, E., U. Völker, and M. Hecker. 1994. Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance. J. Bacteriol. 176:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, and A. Danchin. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 49.Maul, B., U. Völker, S. Riethdorf, S. Engelmann, and M. Hecker. 1995. σB-dependent regulation of gsiB in response to multiple stimuli in Bacillus subtilis. Mol. Gen. Genet. 248:114-120. [DOI] [PubMed] [Google Scholar]
- 50.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 51.Nichols, D. S., P. D. Nichols, and T. A. McMeekin. 1995. Ecology and physiology of psychrophilic bacteria from Antarctic saline lakes and ice-sea. Sci. Prog. 78:311-348. [Google Scholar]
- 52.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price, C. W. 2000. Protective function and regulation of the general stress response in Bacillus subtilis and related gram-positive bacteria, p. 179-197. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 54.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 55.Rhodes, D., and A. D. Hanson. 1993. Quarternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44:357-384. [Google Scholar]
- 56.Scharf, C., R. Riethdorf, H. Ernst, S. Engelmann, U. Völker, and M. Hecker. 1998. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J. Bacteriol. 180:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholz, Q., A. Thiel, W. Hillen, and M. Niederweis. 2000. Quantitative analysis of gene expression with an improved green fluorescent protein. Eur. J. Biochem. 267:1565-1570. [DOI] [PubMed] [Google Scholar]
- 58.Scott, J. M., J. L. Ju, T. Mitchell, and W. G. Haldenwang. 2000. The Bacillus subtilis GTP binding protein Obg and regulators of the σB stress response transcription factor cofractionate with ribosomes. J. Bacteriol. 182:2771-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiegelhalter, F., and E. Bremer. 1998. Osmoregulation of the opuE proline transport gene from Bacillus subtilis—contributions of the σA- and σB-dependent stress-responsive promoters. Mol. Microbiol. 29:285-296. [DOI] [PubMed] [Google Scholar]
- 60.Varon, D., S. A. Boylan, K. Okamoto, and C. W. Price. 1993. Bacillus subtilis gtaB encodes UDP-glucose pyrophosphorylase and is controlled by stationary-phase transcription factor σB. J. Bacteriol. 175:3964-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vijay, K., M. S. Brody, E. Fredlund, and C. W. Price. 2000. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the σB transcription factor of Bacillus subtilis. Mol. Microbiol. 35:180-188. [DOI] [PubMed] [Google Scholar]
- 62.Völker, U., T. Q. Luo, N. Smirnova, and W. Haldenwang. 1997. Stress activation of Bacillus subtilis σB can occur in the absence of the σB negative regulator RsbX. J. Bacteriol. 179:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Völker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Völker, U., A. Völker, and W. G. Haldenwang. 1996. Reactivation of the Bacillus subtilis anti-σB antagonist, RsbV, by stress- or starvation-induced phosphatase activities. J. Bacteriol. 178:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Völker, U., A. Völker, B. Maul, M. Hecker, A. Dufour, and W. G. Haldenwang. 1995. Separate mechanisms activate σB of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 177:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Blohn, C., B. Kempf, R. M. Kappes, and E. Bremer. 1997. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol. Microbiol. 25:175-187. [DOI] [PubMed] [Google Scholar]
- 67.Weber, M. H., W. Klein, L. Müller, U. M. Niess, and M. A. Marahiel. 2001. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 39:1321-1329. [DOI] [PubMed] [Google Scholar]
- 68.Weber, M. H., and M. A. Marahiel. 2002. Coping with the cold: the cold shock response in the Gram-positive soil bacterium Bacillus subtilis. Philos. Trans. R. Soc. Lond. Biol. Sci. 357:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wipat, A., and C. R. Harwood. 1999. The Bacillus subtilis genome sequence: the molecular blueprint of a soil bacterium. FEMS Microbiol. Ecol. 28:1-9. [Google Scholar]
- 70.Wise, A. A., and C. W. Price. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor σB in response to environmental signals. J. Bacteriol. 177:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang, X. F., C. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265-2275. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, S., J. M. Scott, and W. G. Haldenwang. 2001. Loss of ribosomal protein L11 blocks stress activation of the Bacillus subtilis transcription factor σB. J. Bacteriol. 183:2316-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]