Abstract
MC-1 is a marine, magnetotactic bacterium that is phylogenetically associated with the alpha subclass of the Proteobacteria and is the first and only magnetotactic coccus isolated in pure culture to date. By using a TBLASTN search, a lexA gene was identified in the published genome of MC-1; it was subsequently cloned, and the protein was purified to >90% purity. Results from reverse transcription-PCR analysis revealed that the MC-1 lexA gene comprises a single transcriptional unit with two open reading frames encoding proteins of unknown function and with a rumA-like gene, a homologue of the Escherichia coli umuD gene. Mobility shift assays revealed that this LexA protein specifically binds both to its own promoter and to that of the umuDC operon. However, MC-1 LexA does not bind to the promoter regions of other genes, such as recA and uvrA, that have been previously reported to be regulated by LexA in bacterial species belonging to the alpha subclass of the Proteobacteria. Site-directed mutagenesis of both the lexA and umuDC operator regions demonstrated that the sequence CCTN10AGG is the specific target motif for the MC-1 LexA protein.
The term “SOS response” was introduced in the early 1970s by Miroslav Radman, who described a network of genes induced as a consequence of DNA damage (29). In Escherichia coli, this regulon consists of more than 40 genes involved in DNA replication, DNA repair, mutagenesis, and control of the cell cycle (6, 11, 15, 17). Under normal conditions the SOS network remains repressed by the product of the lexA gene, its negative regulator (3, 19, 23, 36). LexA binds specifically to an operator region located at the promoters of the SOS genes, reducing their expression to basal level (15, 36). As a consequence of DNA damage or stalling of the replication fork, the positive regulator of the SOS regulon, RecA, achieves its active conformation (RecA∗) after binding to the generated single-stranded DNA, thereby creating a nucleofilament. RecA∗ mediates the autohydrolysis of the LexA repressor between residues Ala84 and Gly85, giving rise to two nonfunctional products, the N-terminal DNA binding domain and the C-terminal domain, the latter of which is involved in the dimerization of the repressor (21, 22). During this autocatalytic cleavage, residues Ser119 and Lys156 are responsible for the hydrolysis of the Ala-Gly bond through a biochemical reaction characteristic of serine proteases (24). As a consequence of the cleavage, LexA no longer represses the SOS genes, whose expression is enhanced, and the SOS proteins participate directly in repairing DNA lesions, thereby aiding cell survival. Recently, another regulator has been added to the SOS system. It has been demonstrated that the product of the dinI gene is involved in the arrest of RecA activation, preventing its binding to single-stranded DNA (35). Once DNA damage is repaired, RecA∗ loses its active conformation, LexA hydrolysis stops, and the repressor accumulates again, thus inhibiting the transcriptional expression of the SOS network.
The three-dimensional structure of the E. coli LexA protein has recently been determined (24). Two different E. coli LexA conformations exist: a cleavable form and a noncleavable conformation in which the catalytic center is distant from the cleavage site. In this model, RecA∗ is suggested to be involved in the stabilization of the cleavable conformation (24).
The presence of the lexA gene represents a distinct difference between species of the domains Archaea and Bacteria. The occurrence of the lexA gene is widespread among members of the domain Bacteria and is exclusive to it. However, no lexA homologues have been found by BLAST queries in the completely sequenced genomes of several bacterial species including Aquifex aerolicus, Borrelia burgdorferi, Chlamydia pneumoniae, Mycoplasma pneumoniae, Campylobacter jejuni, Helicobacter pylori, Porphyromonas gingivalis, and others. lexA-like genes appear to be completely absent in members of the domain Archaea: no lexA homologues have been identified in the 21 archaeal genomes currently available for examination (9).
In E. coli, LexA binds specifically to a DNA motif known as the SOS box or LexA box (36). Both comparative analyses of the 31 E. coli LexA binding sites and site-directed mutagenesis experiments confirm that a 16-bp consensus sequence, CTGN10CAG, is the target for LexA in this organism (11, 37). This sequence also represents the LexA box in other species of the Enterobacteriaceae (16), as well as in other members of the gamma subclass of the Proteobacteria including Vibrionaceae, Pasteurellaceae, and Pseudomonaceae (16, 30). Nuclear magnetic resonance (NMR) studies of the N-terminal DNA binding domain of E. coli LexA suggest that the amino acids Asn41, Glu44, and Glu45 are directly involved in the recognition of the SOS box (13). In addition to the E. coli SOS box, three more LexA boxes have been identified in the Bacteria domain. The direct repeat GTTCN7GTTC is recognized by LexA proteins of members of the alpha subclass of the Proteobacteria including Rhodobacter sphaeroides, Paracoccus denitrificans, and Rhizobium etli (8, 12, 34). The consensus sequence CGAACRNRYGTTYG is the target for LexA in gram-positive bacteria such as Bacillus subtilis and Mycobacterium tuberculosis, among others (4, 38). Strikingly, the same motif has been described recently for Dehalococcoides ethenogenes, the first non-gram-positive bacterial species whose LexA repressor specifically recognizes the DinR box (10). The third LexA box was found in a member of the gamma subclass of the Proteobacteria; the sequence TTAGN6TACTA was identified as the target for the LexA repressor in Xylella fastidiosa (5).
Strain MC-1 is an incompletely characterized, unnamed, obligately microaerophilic gram-negative bacterium that represents the first and only magnetotactic coccus isolated in pure culture (26). Phylogenetically, this microorganism is affiliated with the alpha subclass of the Proteobacteria, in which it and the other (all uncultured) magnetotactic cocci appear to form a distinct group (7, 32). Although the genome of strain MC-1 is fully sequenced and available for examination (http://www.jgi.doe.gov/JGI_microbial/html/magnetococcus/magneto_homepage.html), it has not been completely annotated and mapped. Sequencing of the DNA repair genes under LexA regulation in the alpha subclass of the Proteobacteria (such as recA, uvrA, and ssb) (12, 34) did not reveal the presence either of the direct repeat GTTCN7GTTC (the LexA box of this phylogenetic group, as mentioned above) or of the other LexA box sequences described previously. In order to gain insight into the composition of the MC-1 LexA regulon and to characterize its LexA binding site, we identified, cloned, and purified the product of its lexA gene.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and general techniques.
The bacterial strains and plasmids used in this study are listed in Table 1 together with their relevant features. Strain MC-1 was grown autotrophically with 10 mM sodium thiosulfate as the electron donor as previously described (14). E. coli strains were grown in Luria-Bertani medium (Difco) at 37°C. Ampicillin at 100 μg/ml and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Roche, Mannheim, Germany) were added to growth media when required.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant features | Source or reference |
|---|---|---|
| Strains | ||
| MC-1 | Coccoid, marine, magnetotactic bacterium | 7 |
| Escherichia coli | ||
| DH5α | supE4 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Clontech |
| BL21(DE3) Codon plus | E. coli B F−ompT hsdSB(rB− mB−) dcm+ Tcrgalλ (DE3) endA Hte[argU ileY leuW Camr] | Stratagene |
| Plasmids | ||
| pGEM-T | PCR cloning vector; Apr | Promega |
| pET15b | His6 tag expression vector; Apr | Novagen |
| pUA1011 | pGEM-T derivative carrying the 678-bp PCR fragment containing the coding region of the MC-1 lexA gene; amplified with primers JC5 and JC6 | This work |
| pUA1013 | pET15b derivative carrying the 678-bp NdeI-BamHI fragment containing the coding region of MC-1 lexA | This work |
Cells of MC-1 used for genomic DNA extraction were harvested by centrifugation at 10,000 × g for 20 min at 4°C, resuspended in 20 mM Tris-HCl in a dilute artificial seawater (pH 7.0) (1), and recentrifuged. Cells were then suspended in TE buffer (10 mM EDTA-10 mM Tris-HCl [pH 8.0]) for DNA extraction. Genomic DNA from strain MC-1 was obtained by using a modification of the method of Marmur (25) as described elsewhere (18).
Synthetic oligonucleotides (Tib Molbiol or Genosys) used in this study for PCR amplification of different DNA fragments are listed in Table 2. When required, PCR fragments were cloned into the pGEM-T vector (Promega) and both strands of the insert were sequenced by the dideoxynucleotide method, by labeling DNA samples with the fmol DNA Cycle Sequencing system (Promega) and using an ALF Sequencer (Amersham Pharmacia Biotech).
TABLE 2.
Oligonucleotide primers used in this work
| Primer | Sequencea | Positionb | Application |
|---|---|---|---|
| JC5 | 5′-CATATGAAACCAGGACGGAGACCCACAGAAGGG-3′ | +1 | Upper primer for cloning coding region of Magnetococcus lexA gene and amplifying lexA fragment in RT-PCR experiments |
| JC6 | 5′-GGATCCGTCGACTTATTCATTATTGTTGGCGGCCCCGTGG-3′ | +672 | Lower primer for cloning coding region of Magnetococcus lexA gene and amplifying lexA fragment in RT-PCR experiments |
| JC7 | 5′-GAGGTGGAAGGCCATTCCTGTTGCC-3′ | −261 | Upper primer to amplify LexA1 probe |
| JC8DIG | 5′-DIG-CCAGTTTCTGCACCTGTTCATGGGC-3′ | +468 | Lower primer to amplify DIG-labeled probe fragments of lexA promoter |
| JC8 | 5′-CCAGTTTCTGCACCTGTTCATGGGC-3′ | +468 | Lower primer to amplify unlabeled probe fragments of lexA promoter |
| JC13 | 5′-CAGATCCATATTGGTTACTTACCG-3′ | −178 | Upper primer to amplify LexA2 probe |
| JC14 | 5′-AACTTACACCTTATTAAAATAAGG-3′ | −100 | Upper primer to amplify LexA3 probe |
| JC15 | 5′-TAAAATAAGGTCACAAGAGAGC-3′ | −86 | Upper primer to amplify LexA4 probe |
| JC16 | 5′-AACTTACGGGGTATTAAAATAAGG-3′ | −100 | JC14 with ACCT-to-GGGG change in LexA3 probe |
| JC22 | 5′-AACTTACACCTTATTAAAAATAAGGTCAC-3′ | −100 | JC14 with addition of an A at position −84 in LexA3 probe |
| JC23 | 5′-AACTTACACCTTATTAAAAAATAAGGTCAC-3′ | −100 | JC14 with addition of two A's at position −84 in LexA3 probe |
| JC24 | 5′-AACTTACACCTTATTAAAAAAATAAGGTCAC-3′ | −100 | JC14 with addition of three A's at position −84 in LexA3 probe |
| JC26 | 5′-AACTTACGCCTTATTAAAATAAGG-3′ | −100 | JC14 with A-to-G change at position −93 in LexA3 probe |
| JC27 | 5′-AACTTACAGCTTATTAAAATAAGG-3′ | −100 | JC14 with C-to-G change at position −92 in LexA3 probe |
| JC28 | 5′-AACTTACACGTTATTAAAATAAGG-3′ | −100 | JC14 with C-to-A change at position −91 in LexA3 probe |
| JC29 | 5′-AACTTACACCGTATTAAAATAAGG-3′ | −100 | JC14 with T-to-A change at position −90 in LexA3 probe |
| JC30 | 5′-AACTTACACCTGATTAAAATAAGG-3′ | −100 | JC14 with T-to-G change at position −89 in LexA3 probe |
| JC31 | 5′-AACTTACACCTTGTTAAAATAAGG-3′ | −100 | JC14 with A-to-G change at position −88 in LexA3 probe |
| JC32 | 5′-AACTTACACCTTAGTAAAATAAGG-3′ | −100 | JC14 with T-to-G change at position −87 in LexA3 probe |
| JC33 | 5′-AACTTACACCTTATGAAAATAAGG-3′ | −100 | JC14 with T-to-G change at position −86 in LexA3 probe |
| JC34 | 5′-AACTTACACCTTATTGAAATAAGGTCAC-3′ | −100 | JC14 with A-to-G change at position −85 in LexA3 probe |
| JC35 | 5′-AACTTACACCTTATTAGAATAAGGTCAC-3′ | −100 | JC14 with A-to-G change at position −84 in LexA3 probe |
| JC36 | 5′-AACTTACACCTTATTAAGATAAGGTCAC-3′ | −100 | JC14 with A-to-G change at position −83 in LexA3 probe |
| JC37 | 5′-AACTTACACCTTATTAAAGTAAGGTCAC-3′ | −100 | JC14 with A-to-G change at position −82 in LexA3 probe |
| JC38 | 5′-AACTTACACCTTATTAAAAGAAGGTCAC-3′ | −100 | JC14 with T-to-G change at position −81 in LexA3 probe |
| JC39 | 5′-AACTTACACCTTATTAAAATGAGGTCACAAGAGAG-3′ | −100 | JC14 with A-to-G change at position −80 in LexA3 probe |
| JC40 | 5′-AACTTACACCTTATTAAAATAGGGTCACAAGAGAG-3′ | −100 | JC14 with A-to-G change at position −79 in LexA3 probe |
| JC41 | 5′-AACTTACACCTTATTAAAATAACGTCACAAGAGAG-3′ | −100 | JC14 with G-to-C change at position −78 in LexA3 probe |
| JC42 | 5′-AACTTACACCTTATTAAAATAAGCTCACAAGAGAG-3′ | −100 | JC14 with G-to-C change at position −77 in LexA3 probe |
| JC43 | 5′-AACTTACACCTTATTAAAATAAGGGCACAAGAGAG-3′ | −100 | JC14 with T-to-G change at position −76 in LexA3 probe |
| JC11 | 5′-GGTTTTCCGTGAAAAAGTCTCATAACCGCG-3′ | −308 | Upper primer to amplify umuDC promoter region |
| JC12 | 5′-AGCCGGTACCGCCGGATGCACCTGC-3′ | +228 | Lower primer to amplify umuDC promoter region |
| JC46 | 5′-ACATCCCCATCAATCCGCACCATGG-3′ | −342 | Upper primer to amplify recA promoter region |
| JC47 | 5′-CAGTGCTTTGCTCTTATCTTTATCC-3′ | +32 | Lower primer to amplify recA promoter region |
| JC48 | 5′-CCAATGGGGCTCTAACTGCGTGGGG-3′ | −271 | Upper primer to amplify uvrA promoter region |
| JC49 | 5′-CAGGTTGTGTTCCCGGGCACCACGG-3′ | +48 | Lower primer to amplify uvrA promoter region |
| JC50 | 5′-AACCGCCCCATTGCGGTGCCCGGCG-3′ | −270 | Upper primer to amplify recN promoter region |
| JC51 | 5′-CGACATGCTTCAATGGTGAGTTGACG-3′ | +30 | Lower primer to amplify recN promoter region |
| JC19 | 5′-ACCGCGATACCTAATATTTATTAGG-3′ | −55 | Upper primer to amplify umuD probe |
| JC12DIG | 5′-AGCCGGTACCGCCGGATGCACCTGC-3′ | +228 | Lower primer to amplify DIG-labeled umuD probe |
| JC57 | 5′-ACCGCGATGCCTAATATTTATTAGG-3′ | −55 | JC19 with A-to-G change at position −48 in umuD probe |
| JC58 | 5′-ACCGCGATAGCTAATATTTATTAGG-3′ | −55 | JC19 with C-to-G change at position −47 in umuD probe |
| JC59 | 5′-ACCGCGATACGTAATATTTATTAGG-3′ | −55 | JC19 with C-to-G change at position −46 in umuD probe |
| JC60 | 5′-ACCGCGATACCGAATATTTATTAGG-3′ | −55 | JC19 with T-to-G change at position −45 in umuD probe |
| JC61 | 5′-ACCGCGATACCTGATATTTATTAGG-3′ | −55 | JC19 with A-to-G change at position −44 in umuD probe |
| JC62 | 5′-ACCGCGATACCTAGTATTTATTAGG-3′ | −55 | JC19 with A-to-G change at position −43 in umuD probe |
| JC63 | 5′-ACCGCGATACCTAAGATTTATTAGG-3′ | −55 | JC19 with T-to-G change at position −42 in umuD probe |
| JC64 | 5′-ACCGCGATACCTAATGTTTATTAGGTATCGCTGC-3′ | −55 | JC19 with A-to-G change at position −41 in umuD probe |
| JC65 | 5′-ACCGCGATACCTAATAGTTATTAGGTATCGCTGC-3′ | −55 | JC19 with T-to-G change at position −40 in umuD probe |
| JC66 | 5′-ACCGCGATACCTAATATGTATTAGGTATCGCTGC-3′ | −55 | JC19 with T-to-G change at position −39 in umuD probe |
| JC67 | 5′-ACCGCGATACCTAATATTGATTAGGTATCGCTGC-3′ | −55 | JC19 with T-to-G change at position −38 in umuD probe |
| JC68 | 5′-ACCGCGATACCTAATATTTGTTAGGTATCGCTGC-3′ | −55 | JC19 with A-to-G change at position −37 in umuD probe |
| JC69 | 5′-ACCGCGATACCTAATATTTAGTAGGTATCGCTGC-3′ | −55 | JC19 with T-to-G change at position −36 in umuD probe |
| JC70 | 5′-ACCGCGATACCTAATATTTATGAGGTATCGCTGC-3′ | −55 | JC19 with T-to-G change at position −35 in umuD probe |
| JC71 | 5′-ACCGCGATACCTAATATTTATTGGGTATCGCTGC-3′ | −55 | JC19 with A-to-G change at position −34 in umuD probe |
| JC72 | 5′-ACCGCGATACCTAATATTTATTACGTATCGCTGC-3′ | −55 | JC19 with G-to-C change at position −33 in umuD probe |
| JC73 | 5′-ACCGCGATACCTAATATTTATTAGCTATCGCTGCTC-3′ | −55 | JC19 with G-to-C change at position −32 in umuD probe |
| JC74 | 5′-ACCGCGATACCTAATATTTATTAGGGATCGCTGCTC-3′ | −55 | JC19 with T-to-G change at position −31 in umuD probe |
| 637rv | 5′-CCATCCATGTCACTACAGGTC-3′ | +1542 | Lower primer to amplify lexA-ORF637 fragment in RT-PCR experiments |
| 637fwd | 5′-CTTGGATTGGGTGAAGCTTTGCC-3′ | +1542 | Upper primer to amplify ORF637-rumA and ORF637-ORF640 fragments in RT-PCR experiments |
| 639rv | 5′-ATCGGCTCCAATGATCGATCC-3′ | +3120 | Lower primer to amplify ORF637-rumA fragment in RT-PCR experiments |
| 640rv | 5′-ATGAAGGTATTCAACCCGGCCC-3′ | +3787 | Lower primer to amplify ORF637-ORF640 fragment in RT-PCR experiments |
Added restriction sites are italicized; nucleotide changes are underlined.
Position of the 5′ end of the oligonucleotide with respect to the proposed translational starting point of each Magnetococcus MC-1 gene.
Cloning of the MC-1 lexA gene.
The MC-1 lexA gene sequence was identified by performing a TBLASTN search of its unfinished genome at the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/Microb_blast/unfinishedgenome.html) with E. coli LexA protein as the query. The comparison yielded a region containing significant homology in part of contig 371, suggesting the presence of a 672-bp lexA gene in strain MC-1 (223 amino acid residues with an estimated molecular size of 24,453 kDa). Using BLAST analyses we also obtained the sequence of about 1 kb of DNA flanking each side of the lexA coding sequence. With this information, primers JC5 and JC6 (Table 2) were designed and used to PCR amplify the entire MC-1 lexA gene. The temperature profile employed was 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The resulting DNA fragment was then cloned into pGEM-T (Promega). This plasmid, pUA1011, was used as a template for further studies.
RT-PCR analysis of the lexA gene region.
To determine the transcriptional organization of the MC-1 lexA region, reverse transcriptase (Roche) was used to generate cDNA by reverse transcription-PCR (RT-PCR) using total RNA from MC-1 strain as a template and the pairs of primers indicated in Table 2. These oligonucleotides were designed to amplify PCR products of 1,542 and 1.578 bp if lexA and the three open reading frames found immediately downstream constituted a single transcription unit.
Total RNA from strain MC-1 was obtained as reported elsewhere (31). The RNA extracted was treated with RNase-free DNase I (Roche) to ensure the absence of contaminating DNA. The concentration and integrity of the RNA were determined by A260 measurements and 1% formaldehyde-agarose gel electrophoresis, respectively. In all RT-PCR experiments, the absence of contaminating DNA in RNA samples after treatment with RNase-free DNase I was confirmed by carrying out PCR amplification without reverse transcriptase.
Mobility shift assays.
Electrophoretic mobility shift assays (EMSA) were performed as previously described (12). Basically, probes were prepared by PCR amplification from MC-1 genomic DNA with one of the primers labeled at its 5′ end with digoxigenin (DIG) (Table 2), and the products were purified in a 2 to 3% low-melting-point agarose gel depending on DNA size. The thermal cycler profile was 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 30 s. Reaction mixtures (20 μl) containing 10 ng of a DIG-labeled DNA probe and 15 ng (final concentration, approximately 25 to 30 nM) of pure MC-1 LexA were incubated in a binding buffer containing 10 mM HEPES NaOH (pH 8), 10 mM Tris-HCl (pH 8), 5% glycerol, 50 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 μg of bulk carrier DNA, and 50 μg of bovine serum albumin/ml. After 30 min of incubation at 30°C, the mixture was loaded onto a 5 to 6% nondenaturing Tris-glycine polyacrylamide gel (prerun for 30 min at 10 V/cm in 25 mM Tris-HCl [pH 8]-250 mM glycine-1 mM EDTA). DNA-protein complexes were separated at 150 V for 2.5 h in a 20-cm-long gel, followed by transfer to a Biodine B nylon membrane (Pall Gelman Laboratory). DIG-labeled DNA-protein complexes were detected by following the manufacturer's protocol (Roche). Experiments were repeated a minimum of three times to ensure the reproducibility of the results.
Purification of MC-1 LexA protein.
MC-1 LexA protein was purified with the TALON purification kit (Stratagene), by Co2+ affinity chromatography taking advantage of the histidine tag placed in its N terminus. By use of primers JC5 and JC6, the coding sequence of the MC-1 lexA gene was amplified and cloned into the pGEM-T vector, generating plasmid pUA1011. To facilitate the cloning of lexA in the expression vector, primers JC5 and JC6 contain incorporated NdeI and BamHI restriction sites, respectively (Table 2). Subsequently, pUA1011 was digested with NdeI-BamHI, and the 0.7-kb DNA fragment was cloned into pET15b (Novagene) to express LexA with a hexahistidine tag at the N-terminal end (pUA1013). The latter plasmid was transformed into BL21 Codon plus RIL cells (Stratagene) to overproduce the protein. An overnight culture of BL21/pUA1013 was diluted 1/100 in 1 liter of Luria-Bertani medium and incubated at 37°C until an optical density at 600 nm of 0.5 was reached. At that time, IPTG was added to the culture to a final concentration of 1 mM, and the culture was incubated for three additional hours. Cells were recovered by centrifugation at 8,000 × g for 15 min and resuspended in 20 ml of extraction-wash buffer (pH 7.0) according to the recommendations of the manufacturer (Stratagene). The cell suspension was then sonicated for 7 min at 40 W by using a Braun LabsonicU (Braun Biotech International) and centrifuged at 18,000 × g for 30 min. The supernatant containing the soluble His-LexA protein was incubated for 2 h at 4°C in TALON metal affinity resin previously equilibrated in extraction-wash buffer containing 0.1% Triton X-100. The resin was washed three times with extraction-wash buffer-0.1% Triton X-100 and twice more with extraction-wash buffer without Triton X-100. Next, resin containing attached His-LexA protein was placed in a 2-ml column, and after a wash with 2 bed volumes of extraction-wash buffer containing 10 mM imidazole, the protein was eluted with 2 ml of elution buffer containing 150 mM imidazole (fraction A) and then with 2 more ml of elution buffer containing 200 mM imidazole (fraction B) (Fig. 1). MC-1 LexA was purified to approximately 80% in fraction A, whereas LexA in fraction B was judged to be more than 90% pure based on a sodium dodecyl sulfate-13% polyacrylamide gel (Fig. 1).
FIG. 1.
Purification of the MC-1 LexA protein. MC-1 His-LexA was purified >90% by Co2+ affinity chromatography using a TALON purification kit (see Materials and Methods for the detailed protocol). Each lane shown in the sodium dodecyl sulfate-13% polyacrylamide gel represents one of the different protein purification steps employed: MW, molecular weight marker; −, crude extract of BL21 Codon plus/pUA1013; +, crude extract of BL21 Codon plus/pUA1013 induced with 1 mM IPTG; Fraction A, MC-1 His-LexA eluted with 150 mM imidazole; Fraction B, purified MC-1 His-LexA protein after elution with 200 mM imidazole.
RESULTS AND DISCUSSION
Cloning of the MC-1 lexA gene.
Although the genome sequence of strain MC-1 is not completely annotated, BLAST analyses can be performed with contigs of the genome available at the U.S. Department of Energy (DOE) Joint Genome Institute (JGI) website (http://www.jgi.doe.gov/JGI_microbial/html/magnetococcus/magneto_homepage.html) and at NCBI (http://www.ncbi.nlm.nih.gov/Microb_blast/unfinishedgenome.html). The E. coli LexA 202-amino-acid sequence was used to query the MC-1 genome with the TBLASTN program, revealing the presence of a 223-amino-acid protein showing significant homology. The 223-residue protein conserves the two main domains of LexA repressors: an N-terminal DNA binding domain harboring a helix-turn-helix (HTH) motif and a C-terminal domain containing serine protease activity. When compared to current databases, MC-1 LexA shows high homology to the LexA proteins of Streptomyces clavuligerus, Clostridium perfringens, and Bacillus halodurans. MC-1 LexA protein shares the highest identity with Streptomyces clavuligerus LexA (44%), while the repressor is only 37 and 34% identical to the Rhodobacter sphaeroides and E. coli LexA proteins, respectively (Fig. 2). As deduced from CLUSTAL W alignment of different LexA proteins (Mac Vector, version 6.5; Oxford Molecular), MC-1 LexA contains the four conserved key residues involved in repressor autocleavage (Ala104, Gly105, Ser138, and Lys175) (Fig. 2). However, dramatic structural differences can be observed when the MC-1 LexA three-dimensional structure is modeled and compared to that of E. coli (data not shown). For example, the second helix of the HTH motif is shorter than that of E. coli, and in the hinge region between the DNA binding and C-terminal domains of MC-1 LexA, there are 14 additional residues forming an extra double beta-sheet structure not present in E. coli (24).
FIG. 2.
CLUSTAL W alignment performed with Mac Vector (version 6.5; Oxford Molecular) comparing LexA proteins from strain MC-1 (Mag), S. clavuligerus (Scl) (residues 49 to 264), C. perfringens (Cpe), B. halodurans (Bha), R. sphaeroides (Rsp), and E. coli (Eco). Dark shading, identical conserved amino acids; light shading, similar conserved residues. I and S, percentages of identity and similarity, respectively, that each LexA sequence shares with the MC-1 LexA repressor. For better visualization of the figure, the first 49 residues of the S. clavuligerus LexA protein are not included in the alignment, because none of them matched in the comparison. Stars indicate the Ala, Gly, Ser, and Lys residues involved in the autocatalytic cleavage of the LexA protein. Accession numbers in the Entrez protein database at NCBI are as follows: S. clavuligerus, CAA12169; C. perfringens, BAB80867; B. halodurans, Q9KAD3; R. sphaeroides, Q9ZFA4; E. coli, P03033.
An open reading frame in the opposite transcriptional direction, encoding a putative protein of unknown function consisting of 406 amino acids, was found 198 bp upstream of the lexA gene (Fig. 3A). Downstream of lexA, and in the same transcriptional direction, there are three open reading frames (Fig. 3A). The first two encode putative proteins of unknown function, whereas the third shows significant identity (58%) with the product of the rumA gene of the E. coli plasmid R391, which is a homologue of the umuD gene (20), encoding a subunit of the DNA damage-inducible polymerase V (33). The fact that the distance between lexA and these three open reading frames is very short (Fig. 3A) suggested that all of these genes are cotranscribed. To evaluate this hypothesis, RT-PCR analysis of total MC-1 RNA was carried out with primers designed to amplify a fragment of 1,542 bp or of 1,578 bp if a polycistronic mRNA was produced. Recovered PCR products demonstrated that transcription of these four genes is linked (Fig. 3B). It is worth noting that in the R391 plasmid a second gene called rumB, downstream of rumA, is cotranscribed with rumA (20), whereas in strain MC-1, neither gene downstream of the rumA-like gene is cotranscribed with it.
FIG. 3.
(A) Genetic organization of the MC-1 lexA region. The proposed translational starting point and stop codon of each of these genes are boldfaced and underlined. Arrows indicate positions of primers used to identify the transcripts. Numerical positions refer to the putative lexA translational start codon. (B) RT-PCR transcriptional analysis of the region surrounding the lexA gene using total RNA from MC-1 cells (RNA-RT-PCR). As a control, PCR experiments were carried out with the same primers but without reverse transcriptase and with either RNA (RNA-PCR) or DNA (DNA-PCR) as a template. The band sizes of the molecular mass marker used (HindIII-digested λ DNA) are shown at the left of the gel.
Identification of the MC-1 LexA-binding site.
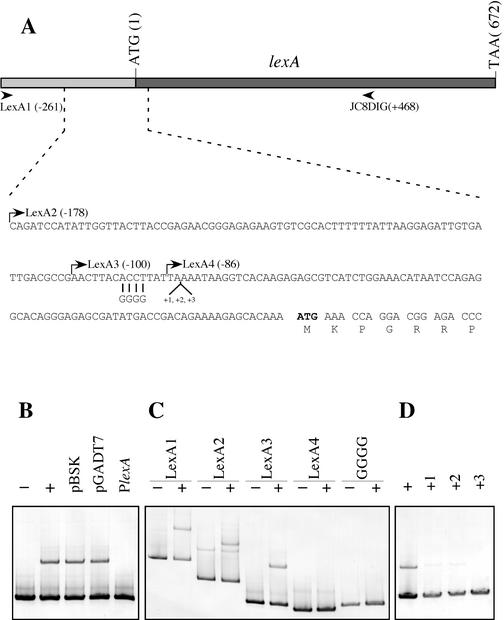
All of the LexA proteins characterized to date share a common feature: they negatively control their own transcriptional expression after binding specifically to a DNA motif located at their promoter regions (3, 5, 10, 12, 38). To identify this sequence in the MC-1 strain, a 261-bp region upstream of the putative translational start codon of the lexA gene (LexA1) was PCR amplified using oligonucleotide primers JC7 and JC8DIG (Fig. 4A). This LexA1 fragment was then utilized as a probe in EMSA experiments with purified LexA protein of this microorganism. MC-1 LexA shifted the mobility of the LexA1 probe (Fig. 4B). Furthermore, the retarded band did not disappear when 3 μg of nonspecific DNA was included in the same reactions (Fig. 4B). However, the shifted band was eliminated by use of a 100-fold molar excess (1 μg) of the unlabeled LexA1 DNA fragment, indicating that binding of MC-1 LexA to its promoter region is indeed specific (Fig. 4B). To establish the position of the MC-1 LexA binding site in its promoter, serial deletions of DIG-labeled fragments from this region were analyzed in EMSA experiments. Whereas probes LexA1, LexA2, and LexA3 showed a shifted band in the presence of pure LexA protein, LexA4 did not shift its electrophoretic mobility (Fig. 4C), suggesting that the LexA binding target, or at least part of it, is located between positions −100 and −86 relative to the putative translational start codon of the lexA gene (Fig. 4A). A visual study of this region revealed the presence of an inverted repeat, ACCTTATTAAAATAAGGT (ACCTTN8AAGGT), 75 bp upstream of the ATG start codon. The ACCT sequence was changed to GGGG in the LexA3 probe by using the JC16 primer (Fig. 4A). The mobility was not changed in the presence of LexA (Fig. 4C), indicating that these four nucleotides of the inverted repeat are part of the specific recognition site for the MC-1 LexA protein. In other studies, the introduction of additional nucleotides in the linker region of the inverted repeat resulted in the abolishment of the binding ability of LexA for its target sequence (12). To determine whether this was also true for our motif, one to three adenines were introduced at position −84 relative to the ATG start codon by using primers JC22 to JC24, thereby lengthening the N10 region between the ACCT and AGGT motifs (Fig. 4A). When either one or two adenines were introduced into the LexA3 probe, some residual binding of LexA was observed. However, when three adenines were introduced into this linker region, the LexA binding activity disappeared completely, providing further corroboration that the ACCTN10AGGT motif is involved in the MC-1 LexA repressor binding.
FIG. 4.
(A) Sequence of the MC-1 lexA gene and promoter. The sequence of the lexA promoter, the first 7 amino acid residues of LexA, and the putative initiation and stop codons are shown. The start points of each fragment of the lexA promoter (LexA1, LexA2, LexA3, and LexA4) used in EMSA experiments are indicated by arrows, and the relative distances to the ATG are given in parentheses. (B) Study of the specific binding of the MC-1 LexA protein. EMSA experiments were performed using different DNAs as competitors. The LexA1 fragment was incubated in the absence (−) and in the presence (+) of pure LexA protein (see Materials and Methods for details). To demonstrate the specificity of LexA binding, the LexA1 fragment was incubated with pure MC-1 LexA protein and several unlabeled DNA competitors in the same reaction mixture containing 3 μg of pBSK, 3 μg of pGADT7, or a 100-fold molar excess (1 μg) of the unlabeled LexA1 DNA fragment (PlexA). (C) Setting the bounds of the MC-1 LexA binding site. LexA1, LexA2, LexA3, LexA4, and a derivative of the LexA3 fragment in which the ACCT tetranucleotide was changed to GGGG (lanes marked “GGGG” above the gel) were incubated in the absence (−) or presence (+) of purified LexA from MC-1. (D) EMSA of LexA3-derived fragments where one (+1), two (+2), or three (+3) adenine residues were inserted at position −84 with respect to the ATG. Different probes were incubated in the presence of the LexA protein of MC-1 and then loaded onto a native Tris-glycine polyacrylamide (5%) gel, as described in Materials and Methods. The wild-type LexA3 fragment incubated in the presence of LexA (+) acted as the positive control.
To study the individual importance of each of the 18 bases of this inverted repeat in LexA binding activity, single-nucleotide changes were introduced by PCR using primers JC25 to JC43 in the LexA3 probe (Fig. 5). EMSA experiments with each single change revealed that bases belonging to the main motifs of the inverted repeat, CCT and AGG, are important in the recognition of LexA protein. More precisely, the most dramatic changes affecting LexA binding activity are located at positions −91 (C), −79 (A), and −78 (G) relative to the ATG start codon, respectively (Fig. 5). When these nucleotides were changed, no DNA-LexA complex was detected, indicating their key importance in LexA binding. Quantification of the retarded band using ImageQuant (version 1.2) Macintosh software revealed that other nucleotides also play a significant role in the recognition of MC-1 LexA repressor. This is the case for A at positions −88, −85, −84, and −80, C at position −92, T at positions −90 and −81, and G at position −77 (relative to the putative translational start codon), since a single-nucleotide change resulted in a >50% loss of LexA binding activity (Fig. 5). These results strongly indicate that CCTTATTAAAATAAGG is the specific binding site recognized by the MC-1 LexA protein in the lexA promoter.
FIG. 5.
Determination of the specific LexA binding target in the MC-1 lexA promoter. PCR-directed mutagenesis was employed to change single nucleotides in the region from −93 to −76 (boldfaced) relative to the start codon of the MC-1 lexA gene (italicized). EMSA experiments allowed determination of the affinity of the purified LexA protein for the resulting DIG-labeled probes. Arrows point to the nucleotide used to replace the native nucleotide, and the relative position of the nucleotide with respect to the translation initiation codon is given in parentheses. The LexA3 fragment with no change introduced is shown as a positive control (W). The percentage of LexA binding activity remaining after each change was assessed using ImageQuant 1.2 software (Binding activity). Key nucleotides mutations of which caused a decrease of >50% in LexA binding activity (relative to that of the wild-type control) are shown at the bottom (Bases).
umuD and umuC are part of the LexA regulon in strain MC-1.
In E. coli, a minimum of 40 genes are directly regulated by the LexA repressor (6, 11, 17). This is, thus far, the bacterial species for which the largest number of LexA-regulated genes have been described. Hence, these genes were used as a query in a TBLASN search using the unfinished MC-1 genome sequence. Homologues of most of the E. coli SOS genes were identified in the MC-1 chromosome. However, other SOS gene homologues such as dinI, dinG, polB, sbmC, yebG, ydjM, yjiW, and yfbE were not found in the genome of this microorganism, although we cannot be completely sure that these genes are absent from MC-1, since the genome sequence is unfinished. A deep analysis of the promoter regions of each homologue and the whole of the MC-1 genome sequence that is available, using the EditSeq (version 4.05) program of the DNAStar package, revealed the presence of the sequence ACCTAATATTTATTAGGT 30 bp upstream of the umuDC operon in MC-1. Similar motifs were not found in the remainder of the E. coli SOS homologues that have been identified.
To determine if the 18-bp sequence, ACCTAATATTTATTAGGT, is actually recognized by the LexA protein, EMSA experiments were performed by using PCR-amplified DNA fragments from promoters of umuDC, recA, uvrA, and recN as competitors and using DIG-labeled LexA3 as a probe. recA and uvrA, together with ssb, are known LexA-regulated genes in the alpha subclass of Proteobacteria (12, 34). The promoter region of recN, another classical SOS gene, was also used in these experiments. The presence of a 100-fold molar excess of the recA, uvrA, or recN promoter did not eliminate the DNA-LexA complex. However, the retarded band disappeared completely when the umuDC promoter region was included in the reaction mixture, strongly suggesting that the motif CCTAATATTTATTAGG is recognized by the MC-1 LexA repressor and that DNA polymerase V is also regulated by LexA in this bacterium (Fig. 6).
FIG. 6.
(A) Upstream operator sequences of MC-1 lexA and umuDC genes presenting potential LexA binding sites. LexA binding sites are depicted, the putative translation initiation codons are boldfaced, and the distances between them are given. (B). EMSA experiments using the LexA3 fragment incubated with purified MC-1 LexA in the presence of different DNA competitors. The LexA3 fragment was incubated in the absence (−) or presence (+) of pure LexA protein. At the same time, a DIG-labeled LexA3 fragment was incubated with MC-1 LexA protein and one of five unlabeled DNA competitors (100-fold molar excess) containing the promoter regions and potential LexA binding sites from the lexA (PlexA) and umuDC (PumuDC) genes. Similarly, promoter regions of previously characterized LexA-regulated genes in the alpha subclass of the Proteobacteria were used as competitors: recA (PrecA), uvrA (PuvrA), and recN (PrecN).
To establish exactly the important bases in LexA recognition in the umuDC operator and to establish a consensus sequence for the LexA target in MC-1, single-nucleotide changes were introduced into the ACCTAATATTTATTAGGT motif by PCR amplification using primers JC57 to JC74 and EMSA experiments were performed with the resulting DIG-labeled probes, as with the lexA operator. The results showed that there are several key nucleotides involved in LexA binding. More precisely, changes introduced in C at position −46, A at −34, and G at −33 with respect to the putative ATG start codon completely abolished the LexA-DNA complex (Fig. 7). Again, analysis of the LexA-DNA-complex band intensity using ImageQuant 1.2 Macintosh software showed that almost every nucleotide base in the ACCTAATATTTATTAGGT motif played a significant role in the recognition of the LexA repressor (Fig. 7). It should be emphasized that the nucleotides found to be key in the binding of LexA to the lexA promoter are also crucial in LexA binding at the upstream umuDC region. Hence, comparison of our experimental results demonstrating the involvement of individual nucleotide bases in LexA binding to both the lexA and umuDC operators allows us to conclude that the consensus motif CCTN10AGG is indeed the specific recognition binding site for the MC-1 LexA protein.
FIG. 7.
Determination of the specific LexA binding target in the MC-1 umuDC promoter. PCR-directed mutagenesis was employed to change single nucleotides in the region from −48 to −31 (boldfaced) relative to the start codon of the MC-1 umuD gene (italicized). EMSA experiments allowed determination of the affinity of the purified LexA protein for the resulting DIG-labeled probes. Arrows point to the nucleotides used to replace the native nucleotides, and the relative positions of nucleotides with respect to the translation initiation codon are given in parentheses. The probe fragment with no change introduced is shown as a positive control (W). The percentage of LexA binding activity remaining after each change was assessed using ImageQuant 1.2 software (Binding). Key nucleotides mutations of which caused >50% decreases in LexA binding activity (compared to that of the wild-type control) are given at the bottom (Bases).
Composition of the LexA regulon.
The number of LexA-regulated genes in bacteria is extremely variable and is dependent on the species. E. coli is currently the bacterium containing the largest number of such genes (more than 40) (6, 11, 17). Other species, such as X. fastidiosa (5) and strain MC-1, appear to contain only a few. So far, by use of computational searches, only three LexA-regulated transcriptional units have been characterized in the entire genomes of these two microorganisms. This is also the case for D. ethenogenes, in whose entire genome only two LexA-regulated genes have been identified (10). In fact, most of the E. coli LexA-regulated genes found in the X. fastidiosa, D. ethenogenes, and MC-1 genomes are not under the control of their LexA repressor (5, 6, 10, 11, 17). A possible explanation for this wide variability in the number of LexA-regulated genes in different species of bacteria might be related to the environment which these microorganisms inhabit. Bacterial species subjected to a permanent stress in their natural habitat should constitutively express many DNA repair genes and thus be not sensitive to the fine-tuning of LexA. It should be mentioned that the lack of a LexA binding site in the recA promoter of strain MC-1 indicates that this classical SOS gene is not directly regulated by LexA in this organism. This situation is not unique and has been proposed to occur in Deinococcus radiodurans and D. ethenogenes also (10, 28). In the case of D. radiodurans, the recA gene is induced by DNA damage but in a LexA-independent manner (2, 28), although it has been reported that D. radiodurans contains a second copy of the lexA gene whose role in recA regulation has not been determined (28).
As discussed earlier, strain MC-1 is phylogenetically affiliated with the alpha subclass of the Proteobacteria, which contains several other magnetotactic species including Magnetospirillum magnetotacticum (7, 32). In this phylogenetic group, recA, uvrA, and ssb have been described as LexA-regulated genes (12, 34). In this study, we demonstrated that the LexA repressor in strain MC-1 does not bind to the promoter regions of such genes. In the case of ssb, we could not completely confirm this, since ssb is present in a very short contig (contig 471) lacking its promoter region (the genome has not yet been annotated and completely mapped). We have shown that the MC-1 LexA binding site is different from the direct repeat typically found in the alpha subclass of the Proteobacteria. Thus, our findings do not correlate with the current phylogenetic affiliation of strain MC-1 based on the sequence of the 16S rRNA gene. A dendrogram performed from CLUSTAL W alignment of 48 N-terminal DNA binding domains of different bacterial LexA proteins belonging to diverse phylogenetic groups demonstrates this difference and shows that the MC-1 LexA protein clearly diverges very early from the main branch of the alpha subclass of the Proteobacteria (Fig. 8). Despite the fact that MC-1 LexA shares its highest level of identity with the LexA proteins of gram-positive bacteria, the MC-1 LexA protein appears to form an independent branch separate from the gram-positive bacteria that is relatively closer to gram-negative representatives of the alpha subclass of the Proteobacteria (Fig. 8). Another possibility is that the MC-1 lexA gene is of viral origin, because the amino acid residues involved in the autocleavage of both the LexA protein and the lytic repressors of bacteriophage as well as their hydrolysis mechanisms are the same (36). In fact, it has recently been hypothesized that the second lexA gene of D. radiodurans might be of viral origin, since this gene is at the left end of a defective temperate bacteriophage inserted in chromosome I of this organism (27). However, there are no experimental data that confirm this supposition. Moreover, it seems unlikely that this is the case for strain MC-1, since there appear to be no bacteriophage-related genes surrounding the lexA gene or the umuDC genes, which, as shown in this work, belong to its LexA network (http://www.jgi.doe.gov/JGI_microbial/html/magnetococcus/magneto_homepage.html).
FIG. 8.
Dendrogram constructed with CLUSTAL W software (Mac Vector, version 6.5) using 48 N-terminal LexA binding domains from different phylogenetic groups (from the first residue to the Ala-Gly cleavage site). The matrix employed was BLOSUM30. Clusters are identified by shades of gray grouping, in each case, the different bacterial species showing a determined LexA binding site found in their lexA promoter. Organisms that do not show any of the five LexA boxes characterized to date in their lexA promoters are underlined. Phylogenetic groups are shown in parentheses. α, β, γ, and δ, the alpha, beta, gamma, and delta subclasses of the Proteobacteria, respectively; Gp, gram-positive; Gn, green nonsulfur bacteria; T, Thermotogales; T/D, Thermus/Deinococcus group.
The CLUSTAL W alignment is a useful tool for assigning a new LexA protein to a determined group on the basis of the target sequence that this protein recognizes. As seen in Fig. 8, with CLUSTAL W, different bacterial species can be clustered by using the LexA binding sites. Species belonging to different phylogenetic groups, such as Burkholderia fungorum and Bordetella pertussis (beta subclass of the Proteobacteria), are grouped together with the members of the gamma subclass that recognize the same LexA box, CTGN10CAG (Fig. 8). The LexA protein of the green nonsulfur bacterium D. ethenogenes recognizes the DinR box, and as a consequence, this organism is clustered with the gram-positive bacteria (Fig. 8). Species of the genera Xylella and Xanthomonas, despite being affiliated with the gamma subclass of the Proteobacteria, group independently of the gamma subclass (Fig. 8), since their LexA repressor recognizes a different target, TTAN6TACTA, as has been recently demonstrated (5).
This characterization of the MC-1 LexA binding site raises the total number of such motifs in the domain Bacteria to five. However, this number will likely increase, since other bacterial species including Desulfovibrio desulfuricans (delta subclass of the Proteobacteria) and Thermotoga maritima (Thermotogales) do not contain any of these five motifs but have lexA-like genes in their genomes (http://igweb.integratedgenomics.com/GOLD/index.cgi?want=Prokaryotic+Ongoing+Genomes), suggesting that they utilize other, undescribed sequences as LexA binding sites. Identification of new targets for LexA in these species will provide valuable information about the evolution of the LexA repressor and the gene network regulated by this damage-inducible protein.
Acknowledgments
This work was funded by grants BMC2001-2065 from the Ministerio de Ciencia y Tecnología (MCyT) de España and 2001SGR-206 from the Departament d'Universitats, Recerca i Societat de la Informació (DURSI) de la Generalitat de Catalunya. A. R. Fernández de Henestrosa is the recipient of a postdoctoral reincorporation contract, and Gerard Mazón and Jordi Cuñé are recipients of a predoctoral fellowship from the MCyT. D. A. Bazylinski is supported by grant NAG 9-1115 from the U.S. National Aeronautics and Space Administration (NASA) Johnson Space Center.
We acknowledge Pilar Cortés and Joan Ruiz for excellent technical assistance.
A. R. Fernández de Henestrosa and Jordi Cuñé contributed equally to this work.
REFERENCES
- 1.Bazylinski, D. A., A. J. Garratt-Reed, and R. B. Frankel. 1993. Electron microscopic studies of magnetosomes in magnetotactic bacteria. Microsc. Res. Tech. 27:389-401. [DOI] [PubMed] [Google Scholar]
- 2.Bonacossa De Almeida, C., G. Coste, S. Sommer, and A. Bailone. 2002. Quantification of RecA protein in Deinococcus radiodurans reveals involvement of RecA, but not LexA, in its regulation. Mol. Genet. Genomics 268:28-41. [DOI] [PubMed] [Google Scholar]
- 3.Brent, R., and M. Ptashne. 1981. Mechanism of action of the lexA gene product. Proc. Natl. Acad. Sci. USA 78:4204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks, P. C., F. Movahedzadeh, and E. O. Davis. 2001. Identification of some DNA damage-inducible genes of Mycobacterium tuberculosis: apparent lack of correlation with LexA binding. J. Bacteriol. 183:4459-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campoy, S., G. Mazon, A. R. Fernández de Henestrosa, M. Llagostera, R. B. Monteiro, and J. Barbé. 2002. A new regulatory DNA motif of the gamma subclass Proteobacteria: identification of the LexA binding site of the plant pathogen Xylella fastidiosa. Microbiology 148:3583-3597. [DOI] [PubMed] [Google Scholar]
- 6.Courcelle, J., A. Khodursky, B. Peter, P. O. Brown, and P. C. Hanawalt. 2001. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158:41-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLong, E. F., R. B. Frankel, and D. A. Bazylinski. 1993. Multiple evolutionary origins of magnetotaxis in bacteria. Science 259:803-806. [DOI] [PubMed] [Google Scholar]
- 8.del Rey, A., J. Diestra, A. R. Fernández de Henestrosa, and J. Barbé. 1999. Determination of the Paracoccus denitrificans SOS box. Microbiology 145:577-584. [DOI] [PubMed] [Google Scholar]
- 9.Eisen, J. A., and P. C. Hanawalt. 1999. A phylogenomic study of DNA repair genes, proteins, and processes. Mutat. Res. 435:171-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández de Henestrosa, A. R., J. Cune, I. Erill, J. K. Magnuson, and J. Barbé. 2002. A green nonsulfur bacterium, Dehalococcoides ethenogenes, with the LexA binding sequence found in gram-positive organisms. J. Bacteriol. 184:6073-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández de Henestrosa, A. R., T. Ogi, S. Aoyagi, D. Chafin, J. J. Hayes, H. Ohmori, and R. Woodgate. 2000. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 35:1560-1572. [DOI] [PubMed] [Google Scholar]
- 12.Fernández de Henestrosa, A. R., E. Rivera, A. Tapias, and J. Barbé. 1998. Identification of the Rhodobacter sphaeroides SOS box. Mol. Microbiol. 28:991-1003. [DOI] [PubMed] [Google Scholar]
- 13.Fogh, R. H., G. Ottleben, H. Ruterjans, M. Schnarr, R. Boelens, and R. Kaptein. 1994. Solution structure of the LexA repressor DNA binding domain determined by 1H NMR spectroscopy. EMBO J. 13:3936-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel, R. B., D. A. Bazylinski, M. S. Johnson, and B. L. Taylor. 1997. Magneto-aerotaxis in marine coccoid bacteria. Biophys. J. 73:994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. American Society for Microbiology, Washington, D.C.
- 16.Garriga, X., S. Calero, and J. Barbé. 1992. Nucleotide sequence analysis and comparison of the lexA genes from Salmonella typhimurium, Erwinia carotovora, Pseudomonas aeruginosa and Pseudomonas putida. Mol. Gen. Genet. 236:125-134. [DOI] [PubMed] [Google Scholar]
- 17.Khil, P. P., and R. D. Camerini-Otero. 2002. Over 1000 genes are involved in the DNA damage response of Escherichia coli. Mol. Microbiol. 44:89-105. [DOI] [PubMed] [Google Scholar]
- 18.Kimble, L. K., L. Mandelco, C. R. Woese, and M. T. Madigan. 1995. Heliobacterium modesticaldum, sp. nov., a thermophilic heliobacterium of hot springs and volcanic soils. Arch. Microbiol. 163:259-267. [Google Scholar]
- 19.Koch, W. H., and R. Woodgate. 1998. The SOS response, p. 107-134. In J. A. Nickoloff and M. F. Hoekstra (ed.), DNA damage and repair: DNA repair in prokaryotes and lower eukaryotes, 1st ed. Humana Press, Totowa, N.J.
- 20.Kulaeva, O. L., J. C. Wootton, A. S. Levine, and R. Woodgate. 1995. Characterization of the umu-complementing operon from R391. J. Bacteriol. 177:2737-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little, J. W. 1993. LexA cleavage and other self-processing reactions. J. Bacteriol. 175:4943-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little, J. W., S. H. Edmiston, L. Z. Pacelli, and D. W. Mount. 1980. Cleavage of the Escherichia coli LexA protein by the RecA protease. Proc. Natl. Acad. Sci. USA 77:3225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little, J. W., D. W. Mount, and C. R. Yanisch-Perron. 1981. Purified LexA protein is a repressor of the recA and lexA genes. Proc. Natl. Acad. Sci. USA 78:4199-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo, Y., R. A. Pfuetzner, S. Mosimann, M. Paetzel, E. A. Frey, M. Cherney, B. Kim, J. W. Little, and N. C. Strynadka. 2001. Crystal structure of LexA: a conformational switch for regulation of self-cleavage. Cell 106:585-594. [DOI] [PubMed] [Google Scholar]
- 25.Marmur, J. 1961. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 26.Meldrum, F. C., S. Mann, B. R. Heywood, R. B. Frankel, and D. A. Bazylinski. 1993. Electron microscopy study of magnetosomes in a cultured coccoid magnetotactic bacterium. Proc. R. Soc. Lond. B 251:231-236. [Google Scholar]
- 27.Morgan, G. J., G. F. Hatfull, S. Casjens, and R. W. Hendrix. 2002. Bacteriophage Mu genome sequence: analysis and comparison with Mu-like prophages in Haemophilus, Neisseria and Deinococcus. J. Mol. Biol. 317:337-359. [DOI] [PubMed] [Google Scholar]
- 28.Narumi, I., K. Satoh, M. Kikuchi, T. Funayama, T. Yanagisawa, Y. Kobayashi, H. Watanabe, and K. Yamamoto. 2001. The LexA protein from Deinococcus radiodurans is not involved in RecA induction following gamma irradiation. J. Bacteriol. 183:6951-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radman, M. 1974. Phenomenology of an inducible mutagenic DNA repair pathway in Escherichia coli: SOS repair hypothesis, p. 128-142. In L. Prakash, F. Sherman, M. Miller, C. W. Lawrence, and H. W. Tabor (ed.), Molecular and environmental aspects of mutagenesis. Charles C. Thomas, Springfield, Ill.
- 30.Riera, J., and J. Barbe. 1995. Cloning, sequence and regulation of expression of the lexA gene of Aeromonas hydrophila. Gene 154:71-75. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Spring, S., and D. A. Bazylinski. 1 December 2000, posting date. Magnetotactic bacteria. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, release 3.4. [Online.] Springer-Verlag New York, Inc., New York, N.Y. http://141.150.157.117:8080/prokPUB/index.htm.
- 33.Tang, M., X. Shen, E. G. Frank, M. O'Donnell, R. Woodgate, and M. F. Goodman. 1999. UmuD′2C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl. Acad. Sci. USA 96:8919-8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tapias, A., and J. Barbé. 1999. Regulation of divergent transcription from the uvrA-ssb promoters in Sinorhizobium meliloti. Mol. Gen. Genet. 262:121-130. [DOI] [PubMed] [Google Scholar]
- 35.Voloshin, O. N., B. E. Ramirez, A. Bax, and R. D. Camerini-Otero. 2001. A model for the abrogation of the SOS response by an SOS protein: a negatively charged helix in DinI mimics DNA in its interaction with RecA. Genes Dev. 15:415-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker, G. C. 1984. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol. Rev. 48:60-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wertman, K. F., and D. W. Mount. 1985. Nucleotide sequence binding specificity of the LexA repressor of Escherichia coli K-12. J. Bacteriol. 163:376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winterling, K. W., A. S. Levine, R. E. Yasbin, and R. Woodgate. 1997. Characterization of DinR, the Bacillus subtilis SOS repressor. J. Bacteriol. 179:1698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]