Abstract
The introduction of a number of new antipsychotics in the last decade has generated considerable excitement regarding the treatment of schizophrenia and related psychotic conditions. Clinically, it has produced changing expectations regarding treatment outcome, while academically it has encouraged a re-evaluation and expansion of theories of the pathophysiology of schizophrenia and antipsychotic activity. In this review, the development of antipsychotics is traced, beginning with chlorpromazine's introduction in the early 1950s, and followed to the present. Despite 50 years of use and a plethora of antipsychotics available worldwide, our conceptualization of their major mode of action remains essentially unchanged. It was shortly after their development that attention turned to the importance of dopamine, and in particular the dopamine D2 receptor. Current thinking has elaborated on this model, with serotonin and glutamate receiving the greatest attention most recently, but D2 antagonism remains the sine qua non of antipsychotic activity. Although the notion of “atypical” remains somewhat of a moving target, we do have at our disposal a new generation of antipsychotics that reflect a different clinical profile from their conventional counterparts. The precise degree of these differences and the underlying mechanisms remain unclear, however. The direction new antipsychotic development takes will undoubtedly hinge on answers to these questions.
Medical subject headings: antipsychotic agents; clozapine; dopamine; glutamic acid; psychopharmacology; receptors, dopamine; schizophrenia; serotonin; treatment outcome
Abstract
Au cours de la dernière décennie, l'introduction d'un certain nombre de nouveaux antipsychotiques a -suscité beaucoup d'enthousiasme dans le traitement de la schizophrénie et des troubles psychotiques connexes. Dans le contexte clinique, ces nouveaux médicaments ont entraÎné une évolution des attentes relatives à l'issue du traitement, tandis que dans les milieux universitaires, ils ont favorisé la réévaluation et l'enrichissement des théories de la pathophysiologie de la schizophrénie et de l'action antipsychotique. Cette étude décrit le développement des antipsychotiques de l'introduction de la chlorpromazine, au début des années 1950, jusqu'à aujourd'hui. Bien qu'il y ait pléthore d'antipsychotiques disponibles à l'échelle mondiale et qu'on utilise ces médicaments depuis 50 ans, la façon dont nous concevons leur principal mode d'action demeure essentiellement la même. Peu de temps après le développement des antipsychotiques, on a prêté attention à l'importance de la dopamine, et plus particulièrement au récepteur dopaminergique D2. Les réflexions actuelles s'articulent autour de ce modèle. Si, récemment, on a accordé le plus d'attention à la sérotonine et au glutamate, l'antagonisme du récepteur D2 demeure le préalable absolu de l'activité antipsychotique. Encore que la notion «d'atypique» demeure insaisissable dans une certaine mesure, nous disposons d'une nouvelle génération d'antipsychotiques reflétant un profil clinique différent de celui de leurs équivalents classiques. Ceci dit, le degré de différence exact et les mécanismes sous-jacents ne sont toujours pas clairs. Il ne fait aucun doute que le développement des nouveaux antipsychotiques sera fondé sur les réponses à ces questions.
Historical perspective
Even a decade ago, this type of review would have had a different message. Clozapine was only beginning to -re-enter the clinical market here in North America — what we had at that time was a number of antipsychotics that had evolved out of a model that identified dopamine, and in particular the D2 receptor, as the critical component in psychosis.1,2 There was a simple distinction between compounds as a function of this theory's development; low-potency antipsychotics characterized the early years when the mechanisms underlying their antipsychotic response were unclear, whereas a shift to high-potency agents occurred with our increased understanding regarding dopamine's putative role. There was no compelling evidence to suggest that any one of these compounds demonstrated clinical superiority,3 and differences were really confined to side-effect profiles and issues related to their practical use.4,5
At that time, treatment focused on controlling positive symptoms (e.g., delusions, hallucinations). Our limited success in managing positive symptoms, even with these antipsychotics, encouraged ongoing work in this area, and the notion of pharmacological treatment of other symptoms was really just beginning.6 Depot formulations were developed to contend with poor response related to noncompliance,7 and the use of high-dose strategies gained popularity by the 1980s (e.g., rapid neuroleptization), perhaps more out of frustration than supportive evidence.8,9,10
Clozapine and the second-generation antipsychotics
The 1990s saw the most significant shift in the treatment of schizophrenia since the advent of chlorpromazine some 40 years earlier. After an extended hiatus related to a cluster of deaths in the early 1970s (later linked to agranulocytosis), clozapine was reintroduced in a number of countries for clinical use.11,12 It was already clear at this point, highlighted by several unique attributes, that clozapine was not another “me too” antipsychotic; it showed diminished extrapyramidal side effects (EPS), clinical superiority in the treatment of refractory psychosis and the possibility of a broader spectrum of clinical efficacy (i.e., improvement in negative as well as positive symptoms).13
Why was clozapine atypical? Having a compound with these unique clinical features demanded a review of our thinking regarding the pharmacological mechanisms underlying schizophrenia. An agent that could prove clinically superior to already existing highly selective D2 antagonists called into question the focus on dopamine and the D2 receptor. What pharmacological aspects of clozapine accounted for its clinical benefits when compared with conventional antipsychotics? Clozapine's complex pharmacological profile14 (Table 1) provided a number of possibilities, with at least several garnering particular attention.
Table 1

D1 receptor
Clozapine demonstrates greater affinity for the D1 than the D2 receptor.14 This feature gains importance when viewed in the context of evidence indicating that D1 receptors are predominant in the prefrontal cortex, an area critical to cognitive tasks,19 implicated in negative symptoms20 and hypothesized to play a key role in a feedback loop mediating more caudal structures associated with the positive symptoms.21 D1 and D2 receptors interact at a cellular level, suggesting that D1 antagonism might at the very least play a role through modulating D2 activity.22,23,24
Although this latter point cannot be ruled out on the basis of current evidence, clinical trials with selective D1 antagonists failed to support their role as effective antipsychotics per se.25,26,27
D4 receptor
Clozapine's profile of greater D4 versus D2 affinity,28,29 in combination with evidence that D4 receptors were elevated in the brains of individuals with schizophrenia,30 gave rise to an interest in the D4 receptor. The initial enthusiasm has been tempered, however, by challenges to the latter finding,31,32 as well as clinical trials with selective D4 antagonists that did not indicate effective antipsychotic activity.33
Dopamine and serotonin
One component of clozapine's pharmacological profile that garnered a great deal of attention was its greater 5-HT2 versus D2 binding, both in vitro and in vivo.34,35,36 Meltzer and colleagues proposed that atypicality might be predicted by this particular feature,34,37 and it soon became an attribute sought in the development of emerging antipsychotics. At present, most atypical antipsychotics available in North America (clozapine, olanzapine, quetiapine, risperidone, ziprasidone), in addition to sertindole (withdrawn from the market for reasons related to cardiac side effects) and zotepine, share this particular feature.38 Amisulpiride, a selective D2/D3 antagonist,39,40 is an exception to this rule, as is aripiprazole.
Re-visiting the dopamine-serotonin model
Dopamine's role in psychosis has been well established, but what do we know about serotonin and, specifically, the 5-HT2 receptor?
First, evidence that selective serotonin antagonists are effective antipsychotics has not been forthcoming.41,42 Furthermore, positron emission tomographic (PET) data have demonstrated that 5-HT2 occupancy approaches saturation even at very low doses of atypicals such as clozapine, risperidone and olanzapine,43 but the clinical reality is that higher doses are required to be effective as antipsychotics. Of course, neither of these findings rule out the possibility of a primary effect on other clinical dimensions, such as affect or cognition, or modulation of systems more directly involved in psychosis (i.e., dopamine).
The work of Meltzer et al34,37 did not, in fact, suggest serotonin antagonists in and of themselves would be effective antipsychotics. The model was premised on greater 5-HT2 than D2 activity, and went so far as to suggest that a 5-HT2/D2 ratio of 1.12 and above (based on pKi values for cortical 5-HT2 and striatal D2 binding sites) characterized atypical antipsychotics.
Indirect support for the importance of greater 5-HT2 versus D2 activity comes from PET in vivo data. Loxapine has equal 5-HT2 and D2 binding44 and takes on a typical profile in the clinical setting. Similarly, compounds such as olanzapine and risperidone lose this profile in a dose-dependent fashion. That is, occupancy of 5-HT2 receptors approximates saturation even at lower therapeutic doses, but as the dose is increased, D2 occupancy rises and ultimately overrides the differential that existed at lower doses.36,45,46 In both cases, this results in an increased risk of EPS, albeit to a greater extent in risperidone, which lacks any inherent anticholinergic activity.47
That virtually all atypicals currently available for clinical use (including clozapine, olanzapine, quetiapine, risperidone, zotepine and ziprasidone) share this profile of greater 5-HT2 versus D2 antagonism, offers further indirect support for this model. However, amisulpiride, a selective D2/D3 antagonist, also claims atypical status,38,48 as did remoxipride, a selective D2 antagonist,49 before its withdrawal because of an associated risk of aplastic anemia. Thus, a combined dopamine–serotonin profile as outlined may provide atypicality, but it cannot be considered necessary or sufficient in this regard.
Fast off the D2 receptor: an alternative model
A feature that all currently available antipsychotics, typical as well as atypical, share is that of D2 antagonism. In contrast to selective 5-HT2 antagonists, selective D2 antagonists have proven to be effective antipsychotics; taken together, the evidence would suggest that D2 blockade is the sine qua non of antipsychotic activity. At the same time, it cannot be sufficient, as we can see refractory forms of psychosis even in the face of significant D2 antagonism.50,51
An interesting twist has arisen more recently with the notion of a differential blockade of D2 receptors,52,53,54,55 on both the molecular and systemic levels. Regarding the former, in vitro studies have demonstrated that antipsychotics dissociate from the D2 receptor at very different rates, expressed as a koff value. As a group, the atypicals have higher koff values, that is faster dissociation rates, than the conventionals, but they differ among themselves on this dimension as well (e.g., quetiapine > clozapine > olanzapine).53,54
Systemically, the principle is similar, but other variables must be factored into the equation as well (e.g., half-life and koff). In vivo PET data have demonstrated that plasma kinetics do not mirror CNS kinetics. For example, after a single dose of risperidone, the mean plasma half life for risperidone plus 9-OH-risperidone is 10.3 hours, but the mean half life for striatal D2 occupancy is 66.6 hours.56
What are the implications of this model? It is well known that dopamine is required for a number of functions (e.g., movement, affect, cognition),57 and it is possible that its role in normal functioning may be less disturbed by compounds that do not cause sustained D2 blockade. At a molecular level, antipsychotics with higher koff values are better able to decrease their occupancy in response to increased dopamine surges required for task-related activities. At a systems level, the net result includes only transient prolactin elevation and a diminished D2 up-regulation with continued administration.52,53 Clinically, it can be argued that many of the benefits ascribed to the second-generation antipsychotics reflect this profile of transient rather than sustained D2 antagonism. This would include decreased EPS (and therefore secondary negative symptoms), in addition to decreased affective and cognitive disturbances.
Other models
In the last decade, much of our attention regarding “atypicality” has been focused on dopamine and serotonin, and they continue to be the subject of investigation. Work on serotonin has largely focused on the 5-HT2A receptor, but more recent attention has turned to other serotonergic receptors in terms of both clinical response and side effects.47,58,59,60,61 For example, the 5-HT1A receptor has been implicated in anxiety, depression and negative symptoms, whereas the 5-HT2C receptor has been linked to weight gain and improvement in EPS. In terms of dopamine, the role of the D3 receptor, if for no other reason than its location in limbic regions, remains intriguing but poorly understood.62,63,64 As discussed earlier, the predominance of D1 compared with D2 receptors in the prefrontal cortex, their apparent interactive roles and the prominent D1-binding properties of clozapine, all contribute to an ongoing interest in this particular receptor.
Aripiprazole, recently approved for clinical use in the United States, offers an interesting variation on the dopamine and serotonin story. What makes it particularly unique among the newer antipsychotics is its partial dopamine agonist properties.65 It demonstrates both 5-HT1A agonism and 5-HT2 antagonism, although its affinity for the D2 receptors exceeds that for serotonin by an order of magnitude. Thus, it does not conform to the standard 5-HT2/D2 model. The drug has a very high affinity for the D2 receptor and hence is unlikely to be fast koff. Similarly, it has a long half-life and is therefore unlikely to be transient at a systemic level. Initial occupancy studies indicate that although it occupies more than 90% of dopamine receptors, aripiprazole does not cause EPS, suggesting that its inherent agonism may provide a mechanism that protects against excessive blockade of the D2 system.66 Although clearly not fast off the D2 receptor, a parallel can be drawn in that both mechanisms of action provide appropriate modulation of D2 transmission at the receptor level.
There is abundant reason to look beyond dopamine and serotonin, but such an expansive review is beyond the scope of this article; for this, the reader is referred to other sources.67,68,69 The one system warranting comment on the basis of the amount of attention it is currently receiving is glutamate.65,70,71,72 A strong argument for a glutamate model arises from the fact that phencyclidine (PCP), a psychotomimetic street drug, noncompetitively blocks the ion channel of the N-methyl-D-aspartate (NMDA) subtype of the glutamate receptor. This model does not contradict a role for dopamine; for example, one action of dopamine is to inhibit glutamate release. Thus, a state of dopaminergic hyperactivity could lead to NMDA receptor hypofunction, which in turn could produce various symptoms linked with psychosis. To date, work with compounds acting at the level of the NMDA receptor (e.g., D-cycloserine, glycine), have reported modest benefits in the treatment of positive symptoms, with more compelling evidence favouring effectiveness in treating negative and cognitive symptoms.73,74,75,76,77,78
Second-generation antipsychotics: the clinical evidence
To better evaluate the clinical evidence, schizophrenia must be seen as more than a unitary disease entity. Individuals undergoing a first psychotic episode are not the same as those in later stages of the illness. For example, they are more susceptible to certain side effects, such as acute dystonic reactions, but at the same time demonstrate a high rate of clinical response.79,80,81 At this time in first-episode psychosis, there is a lack of evidence to suggest that atypicals are superior to their conventional counterparts, although this conclusion is drawn in the face of several caveats. With a response rate as high as 80%,80 there is a kind of “ceiling effect” in place, making it difficult to distinguish differences between treatments. In addition, historically clinical trials have focused on response as measured along a small number of dimensions. For many years, the focus was confined to positive symptoms, before attention turned to a positive–negative symptom dichotomy. There is now evidence that a variety of other dimensions warrant investigation, including clinical measures (e.g., cognition, affect, quality of life) and side effects (e.g., weight gain, diabetes).82 We are now only beginning to systematically evaluate some of these areas, and it is quite possible that as this evidence accumulates we shall see differences between the typicals and atypicals uncovered. Of course, it is important to qualify that the advantage could work in both directions.
Using response as a means of classification, it is also useful to distinguish between “partial responders” and those with “refractory” (also referred to as treatment-resistant) schizophrenia.82,83,84,85 Partial responders best typify the population sampled for the pivotal clinical trials used for registration, that is individuals who have already received antipsychotic therapy, frequently with at least several agents, but who continue to demonstrate a sub-optimal response. Several large meta-analyses have been published evaluating these types of trials, and their findings are similar. Geddes and associates86 concluded that atypicals offer modest clinical benefits compared with conventionals (usually represented by haloperidol or chlorpromazine in the trials). However, these benefits were lost when a distinction was made between those who received doses of the comparator below 12-mg haloperidol equivalents (in fact, it could be argued that the comparator doses were still too high). Evidence from a variety of sources has established that much lower doses of conventional antipsychotics than what have been used in the past are often sufficient to achieve clinical response. Moreover, these lower doses are associated with fewer side effects and better tolerability.8,10,87,88,89,90 Having said this, data indicate that even at low doses the conventional antipsychotics can demonstrate a notable liability for EPS.91,92,93
The meta-analysis of Leucht et al94 did not make this same distinction on the basis of dose, but it too concluded that the benefits of the atypicals in terms of total, positive and negative symptoms were modest at best. The most robust evidence favouring the atypicals was with respect to EPS, using antiparkinsonian drug use as a proxy. Again though, this meta-analysis did not attempt to address dose in the same fashion as Geddes et al.86 Both meta-analyses acknowledged the lack of data currently available to evaluate the other clinical dimensions now receiving more attention.
The refractory or treatment-resistant population presents somewhat different findings. Dating back to the work of Kane et al13 evaluating clozapine's efficacy in this sample, more rigid criteria have been used to distinguish these individuals from those who are better described as partial responders.13 The existing evidence suggests modest benefits favouring the atypicals, with the additional caveat that clozapine seems superior even to the other atypicals in this population.95,96,97,98
Again, it must be emphasized that these conclusions are limited by the available data. We are only beginning to more objectively evaluate differences on other dimensions (e.g., cognition, suicide, subjective response, quality of life), and it is possible that further advantages may be claimed on some of these other measures (Table 2). Conversely, we are also aware that the newer agents have problems that were less of an issue with the conventionals, and in any comparison these cannot be overlooked. Here, in particular, reference is made to the mounting evidence regarding increased risk of weight gain, diabetes and possible secondary cardiovascular complications.99,100,101,102,103
Table 2
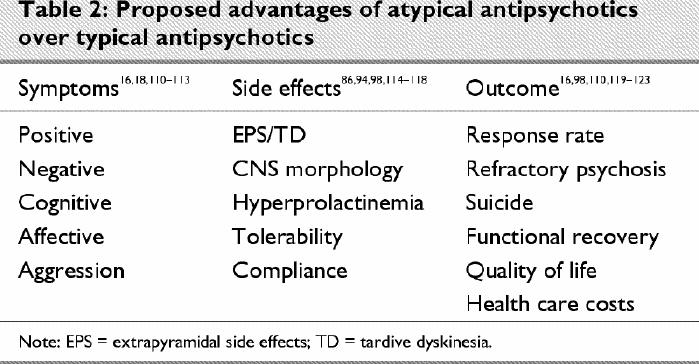
With so many dimensions, in terms of efficacy as well as side effects, the concept of “atypicality” is called into question. Should we expect that an atypical antipsychotic be superior on all dimensions to warrant this classification? Clozapine, the prototype of atypical antipsychotics, was initially identified as unique because of its lack of D2-related adverse events (i.e., EPS, hyperprolactinemia), features that characterized existing antipsychotics at the time. Only later did attention turn to its possible benefits along other clinical dimensions. Even using this more restrictive definition related to EPS and elevated prolactin, there are problems. Risperidone, for example, is routinely classified as atypical, although it has been clearly associated with elevated prolactin and increased risk of EPS in a dose-related fashion.104,105,106,107 It is not so surprising then that recent discussions have turned to the topic of correct terminology to describe these newer agents,108 or that the suggestion has been made that these compounds be best viewed in a dimensional rather than dichotomous fashion strictly tied to a typical–atypical classification.109
Future directions
It is somewhat ironic that in the face of so much excitement during recent years about gains in our understanding of schizophrenia and its treatment, we return dopamine to centre stage. Less than 10 years ago, clozapine was being held forth as evidence that a compound with relatively low D2 occupancy could be an effective antipsychotic, calling into question the integral role of D2 antagonism in the action of antipsychotics.
A closer examination of clozapine, particularly through the benefits of in vivo imaging, has clarified this issue, refining rather than dismissing existing notions regarding the importance of D2 activity. Clozapine's D2-binding profile does not negate data suggesting a relation between D2 thresholds, clinical response and side effects. Rather, it has demonstrated that sustained D2 occupancy is not necessary for antipsychotic effectiveness. Moreover, compounds that dissociate from the D2 receptor quickly may bestow benefits along a number of clinical dimensions (e.g., cognitive, affective and negative symptoms), in addition to a lack of D2-related side effects.
A critic of this explanation might argue that we can still not be sure that clozapine's D2 activity plays a role in its antipsychotic action. However, to date there is no evidence indicating that a compound without some degree of D2 binding can act as an effective antipsychotic. There is, in fact, pre-clinical data indicating that altering clozapine's D2 property transforms it into a standard conventional antipsychotic.124
Our understanding of clozapine is incomplete though, for it remains unclear why it is superior in refractory psychosis, even when compared with other atypicals. That it relates to its D2 profile of rapid dissociation would gain support from evidence that a drug such as quetiapine shares the same clinical advantage. Evidence of this sort with quetiapine has not been forthcoming however, although it must be acknowledged that there exists a paucity of data involving quetiapine's use in this population. Clozapine's pharmacological profile has been closely scrutinized in an effort to unlock this secret, and at one time or another hopes have been pinned on 5-HT2, D1 and D4 activity. Selective antagonists for each of these receptors have simply confirmed that at least individually they are not responsible for clozapine's unique clinical profile. This does not rule out their involvement — it merely suggests that the net effect of clozapine may be dependent on some complex interplay between its different pharmacological actions.
In a sense it can be argued that we are back to square one. In truth, we have no better understanding of clozapine's superiority in refractory psychosis than we did a decade ago, and we have come full circle to the realization that D2 blockade is essential for antipsychotic activity. In the ongoing search, though, we have learned a great deal. Consolidating the existing evidence, we can state the following:
· dopamine antagonism is a necessary, but not sufficient, requirement for antipsychotic efficacy
· sustained D2 occupancy is not necessary to achieve this
· there is no evidence that other systems can produce a primary antipsychotic action
· other systems may influence antipsychotic activity through modulating dopamine activity
· other systems may play a primary role on other clinical dimensions related to the illness (e.g., affect, cognition), although at present this remains conjecture
Our excitement regarding clozapine was magnified by evidence that improvement occurred across numerous clinical dimensions — not only did it not cause EPS or sustained hyperprolactinemia, it improved positive, negative, cognitive and even affective symptoms. It is not so surprising then that efforts to develop other effective antipsychotics were accompanied by the expectation that an antipsychotic must simultaneously do many things beyond just treating psychosis. This, in turn, has fostered the development of compounds that also affect numerous receptors and systems; however, it is time to question this position.
I close with a rather contrary position regarding clozapine. Yes, it appears superior in refractory psychosis (though recently the degree and domains of this superiority have been challenged), and we should continue our search to understand whatever gains it gives us. Efforts so far to establish a unitary explanation have failed, and it is easy to imagine that the answer lies in some interaction of its rich pharmacology. Continuing to develop such pharmacologically complex compounds, however, makes it extremely difficult to tease apart the critical feature(s) underlying its benefit.
What if, instead of its rich pharmacology, much of clozapine's benefits in the other realms simply reflect what it doesn't do? Using Parkinson's disease as a model, we know that sub-optimal dopamine functioning can adversely influence not only movements, but other features clozapine is seen to be superior in “treating,” including cognition, affective disturbances and negative symptoms. It is easy to ascribe these benefits to different pharmacological features of clozapine, when they may simply reflect its lack of sustained D2 occupancy. The decreasing superiority of atypicals over typicals when more appropriate doses of the latter are being used for comparison purposes supports this position, as does the evidence that amisulpride, a selective D2/D3 blocker, emulates almost all the features in Table 2. Of note is the more recent finding that amisulpride shares in common with clozapine rapid dissociation from the D2 receptor.53
If clozapine's benefits along these other clinical dimensions can be explained by its dopamine-sparing properties, we may be misguided in (a) looking for a single pill to address the complex symptom profile of schizophrenia when we all agree that the illness is heterogeneous and multidimensional; (b) trying to link the different benefits noted with clozapine to different aspects of its diverse pharmacology, and in so doing (c) continuing the practice of synthesizing complex molecules to mirror clozapine on these different clinical measures.
Perhaps we should focus on finding a better antipsychotic, leaving it to others to continue the search for better antidepressants, anxiolytics, cognitive enhancers and so on. It will then be the clinician's job to use the different agents flexibly and appropriately in the service of a particular patient's illness.
Acknowledgments
I thank Dr. Shitij Kapur for his ongoing collaboration, which is reflected in numerous aspects of this manuscript.
Footnotes
Competing interests: Dr. Remington is a consultant for Janssen, Eli Lilly, Pfizer, AstraZeneca, Novartis and Bristol-Myers Squibb; has received honoraria for PET research from Janssen, Eli Lilly, Pfizer and AstraZeneca; and has received speaker fees from Janssen, Eli Lilly, AstraZeneca, Pfizer and Novartis.
Correspondence to: Dr. Gary Remington, Centre for Addiction and Mental Health, 250 College St., Toronto ON M5T 1R8; fax 416 979-6849; gary_remington@camh.net
Submitted Sept. 9, 2002 Revised Jan. 15, 2003 Accepted Jan. 22, 2003
References
- 1.Wiesel FA. Neuroleptic treatment of patients with schizophrenia: mechanisms of action and clinical significance. Br J Psychiatry 1994;164(Suppl 23):65-70. [PubMed]
- 2.Deniker P. The neuroleptics: a historical survey. Acta Psychiatr Scand 1990;82(Suppl 358):83-7. [DOI] [PubMed]
- 3.Davis JM, Garver D. Neuroleptics: clinical use in psychiatry. In: Iverson L, Iversen SD, Snyder S, editors. Handbook of psychopharmacology. New York: Plenum Press; 1978. p. 129-64.
- 4.Richelson E. Pharmacology of neuroleptics in use in the United States. J Clin Psychiatry 1985;46(8):8-14. [PubMed]
- 5.Silverstone T. Clinically relevant differences between antipsychotic compounds. Acta Psychiatr Scand 1990;82(Suppl 358):88-91. [DOI] [PubMed]
- 6.Meltzer HY, Sommers AA, Luchins DJ. The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol 1986;6(6):329-38. [PubMed]
- 7.Kane JM. The use of depot neuroleptics: clinical experience in the United States. J Clin Psychiatry 1984;45(5):5-12. [PubMed]
- 8.Bollini P, Pampallona S, Orza MJ, Adams ME, Chalmers TC. Antipsychotic drugs: is more worse? A meta-analysis of the published randomized control trials. Psychol Med 1994;24(2): 307-16. [DOI] [PubMed]
- 9.Krakowski MI, Kunz M, Czobor P, Volavka J. Long-term high-dose neuroleptic treatment: who gets it and why? Hosp Community Psychiatry 1993;44(7):640-4. [DOI] [PubMed]
- 10.Thompson C. The use of high-dose antipsychotic medication. Br J Psychiatry 1994;164(4):448-58. [DOI] [PubMed]
- 11.Baldessarini RJ, Frankenburg FR. Clozapine. A novel antipsychotic agent. N Engl J Med 1991;324(11):746-54. [DOI] [PubMed]
- 12.Hippius H. A historical perspective of clozapine. J Clin Psychiatry 1999;60(Suppl 12):22-3. [PubMed]
- 13.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45(9):789-96. [DOI] [PubMed]
- 14.Coward DM, Imperato A, Urwyler S, White TG. Biochemical and behavioural properties of clozapine. Psychopharmacology 1989; 99(Suppl):S6-12. [DOI] [PubMed]
- 15.Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 1998;18(2):63-101. [DOI] [PubMed]
- 16.Blin O. A comparative review of new antipsychotics. Can J Psychiatry 1999;44(3):235-44. [DOI] [PubMed]
- 17.Reus VI. Olanzapine: a novel atypical neuroleptic agent. Lancet 1997; 349(9061):1264-5. [DOI] [PubMed]
- 18.Stip E. Novel antipsychotics: issues and controversies. Typicality of atypical antipsychotics. J Psychiatry Neurosci 2000;25(2):137-53. [PMC free article] [PubMed]
- 19.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 1991; 251(4996):947-50. [DOI] [PubMed]
- 20.Lynch M. Schizophrenia and the D1 receptor: focus on negative symptoms. Prog Neuropsychopharmacol Biol Psychiatry 1992; 16: 797-832. [DOI] [PubMed]
- 21.Weinberger DR. The biological basis of schizophrenia: new directions. J Clin Psychiatry 1997;58(Suppl 10):22-7. [PubMed]
- 22.Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science 1987;236(4802):719-22. [DOI] [PubMed]
- 23.Waddington JL. Therapeutic potential of selective D-1 dopamine receptor agonists and antagonists in psychiatry and neurology. Gen Pharmacol 1988;19(1):55-60. [DOI] [PubMed]
- 24.Seeman P, Niznik HB, Guan HC, Booth G, Ulpian C. Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc Natl Acad Sci U S A 1989;86(24):10156-60. [DOI] [PMC free article] [PubMed]
- 25.de Beaurepaire R, Labelle A, Naber D, Jones BD, Barnes TR. An open trial of the D1 antagonist SCH 39166 in six cases of acute psychotic states. Psychopharmacology 1995;121(3):323-7. [DOI] [PubMed]
- 26.Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, et al. Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology 1995;121(3):317-22. [DOI] [PubMed]
- 27.Labelle A, de Beaurepaire R, Boulay LJ, Naber D, Jones BD, Barnes TR. A pilot study of the safety and tolerance of SCH 39166 in patients with schizophrenia. J Psychiatry Neurosci 1998; 23(2):93-4. [PMC free article] [PubMed]
- 28.Seeman P. Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology 1992;7(4):261-84. [PubMed]
- 29.Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 1991;350(6319):610-4. [DOI] [PubMed]
- 30.Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature 1993;365(6445):441-5. [DOI] [PubMed]
- 31.Kerwin RW, Collier D. The dopamine D4 receptor in schizophrenia: an update. Psychol Med 1996;26(2):221-7. [DOI] [PubMed]
- 32.Reynolds GP, Mason SL. Are striatal dopamine D4 receptors increased in schizophrenia? J Neurochem 1994;63(4):1576-7. [DOI] [PubMed]
- 33.Kramer MS, Last B, Getson A, Reines SA. The effects of a selective D4 dopamine receptor antagonist (L-745,870) in acutely psychotic inpatients with schizophrenia. Arch Gen Psychiatry 1997; 54(6):567-72. [DOI] [PubMed]
- 34.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin-2 pKi values. J Pharmacol Exp Ther 1989; 251 (1): 238-46. [PubMed]
- 35.Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull 1989;25(3):390-2. [PubMed]
- 36.Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA, et al. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry 1998;155 (7): 921-8. [DOI] [PubMed]
- 37.Stockmeier CA, DiCarlo JJ, Zhang Y, Thompson P, Meltzer HY. Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. J Pharmacol Exp Ther 1993;266(3):1374-84. [PubMed]
- 38.Waddington JL, Scully PJ, O'Callaghan E. The new antipsychotics and their potential for early intervention in schizophrenia. Schizophr Res 1997;28(2-3):207-22. [DOI] [PubMed]
- 39.Chivers JK, Gommeren W, Leysen JE, Jenner P, Marsden CD. Comparison of the in-vitro receptor selectivity of substituted benzamide drugs for brain neurotransmitter receptors. J Pharm Pharmacol 1988;40(6):415-21. [DOI] [PubMed]
- 40.Xiberas X, Martinot JL, Mallet L, Artiges E, Canal M, Loc'h C, et al. In vivo extrastriatal and striatal D2 dopamine receptor blockade by amisulpride in schizophrenia. J Clin Psychopharmacol 2001;21(2):207-14. [DOI] [PubMed]
- 41.Management decisions on priority pipeline products — MDL 100907 [announcement]. Vision Extra 1999;4:2-3.
- 42.Duinkerke SJ, Botter PA, Jansen AA, van Dongen PA, van Haaften AJ, Boom AJ, et al. Ritanserin, a selective 5-HT2/1C antagonist, and negative symptoms in schizophrenia. A placebo-controlled double-blind trial. Br J Psychiatry 1993;163:451-5. [DOI] [PubMed]
- 43.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 1999; 156(2):286-93. [DOI] [PubMed]
- 44.Kapur S, Zipursky R, Remington G, Jones C, McKay G, Houle S. PET evidence that loxapine is an equipotent blocker of 5-HT2 and D2 receptors: implications for the therapeutics of schizophrenia. Am J Psychiatry 1997;154(11):1525-9. [DOI] [PubMed]
- 45.Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry 1996;153(4):466-76. [DOI] [PubMed]
- 46.Kapur S, Remington G, Zipursky RB, Wilson AA, Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci 1995; 57(10):103-7. [DOI] [PubMed]
- 47.Megens AA, Awouters FH, Schotte A, Meert TF, Dugovic C, Niemegeers CJ, et al. Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology 1994;114 (1): 9-23. [DOI] [PubMed]
- 48.Leucht S, Pitschel-Walz G, Engel RR, Kissling W. Amisulpride, an unusual and “atypical” antipsychotic: a meta-analysis of randomized controlled trials. Am J Psychiatry 2002;159(2):180-90. [DOI] [PubMed]
- 49.Lewander T, Westerbergh S-E, Morrison D. Clinical profile of remoxiprode — a comined analysis of a comparative double-blind multicentre trial programme. Acta Psychiatr Scand 1990; 82 (Suppl 358):92-8. [DOI] [PubMed]
- 50.Kapur S, Remington G. Dopamine D2 receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry 2001;50:873-83. [DOI] [PubMed]
- 51.Kapur S. Receptor occupancy by antipsychotics — concepts and findings. In: Lidow MS, editor. Neurotransmitter receptors in actions of antipsychotics. London: CRC Press; 2000. p. 163-76.
- 52.Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am J Psychiatry 2001;158(3):360-9. [DOI] [PubMed]
- 53.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry 2002;47(1):27-38. [PubMed]
- 54.Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: an explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine or quetiapine. Am J Psychiatry 1999;156(6):876-84. [DOI] [PubMed]
- 55.Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry 1998;3(2):123-34. [DOI] [PubMed]
- 56.Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S. Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 2002;7:317-21. [DOI] [PubMed]
- 57.Le Moal M. Mesocortoclimbic dopaminergic neurons: functional and regulatory roles. In: Bloom FE, Kupfer D, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press, Ltd.; 1995. p. 283-94.
- 58.Leonard BE. Serotonin receptors—where are they going? Int Clin Psychopharmacol 1994;9(Suppl 1):7-17. [DOI] [PubMed]
- 59.Lucki I. Serotonin receptor specificity in anxiety disorders. J Clin Psychiatry 1996;57(Suppl 6):5-10. [PubMed]
- 60.Roth BL. Multiple serotonin receptors: clinical and experimental aspects. Ann Clin Psychiatry 1994;6(2):67-78. [DOI] [PubMed]
- 61.Sussman N. The potential benefits of serotonin receptor-specific agents. J Clin Psychiatry 1994;55(Suppl):45-51. [PubMed]
- 62.Griffon N, Sokoloff P, Diaz J, Levesque D, Sautel F, Schwartz JC, et al. The dopamine D3 receptor and schizophrenia: pharmacological, anatomical and genetic approaches. Eur Neuropsychopharmacol 1995;5(Suppl):3-9. [DOI] [PubMed]
- 63.Healy D. D1 and D2 and D3. Br J Psychiatry 1991;159:319-24. [DOI] [PubMed]
- 64.Sokoloff P, Martres MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol 1992;43(4):659-66. [DOI] [PubMed]
- 65.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 1999;20(6):612-27. [DOI] [PubMed]
- 66.Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology 2002;27(2):248-59. [DOI] [PubMed]
- 67.Jones HM, Pilowsky L. New targets for antipsychotics. Expert Rev Neurotherapeutics 2002;2:61-8. [DOI] [PubMed]
- 68.Kilts CD. The changing roles and targets for animal models of schizophrenia. Biol Psychiatry 2001;50(11):845-55. [DOI] [PubMed]
- 69.Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology (Berl) 1996; 124(1-2):2-34. [DOI] [PubMed]
- 70.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991;148(10):1301-8. [DOI] [PubMed]
- 71.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995;52(12):998-1007. [DOI] [PubMed]
- 72.Tamminga CA, Holcomb HH, Gao XM, Lahti AC. Glutamate pharmacology and the treatment of schizophrenia: current status and future directions. Int Clin Psychopharmacol 1995; 10 (Suppl 3):29-37. [PubMed]
- 73.Leiderman E, Zylberman I, Zukin SR, Cooper TB, Javitt DC. Preliminary investigation of high-dose oral glycine on serum levels and negative symptoms in schizophrenia: an open-label trial. Biol Psychiatry 1996;39(3):213-5. [DOI] [PubMed]
- 74.Heresco-Levy U, Javitt DC, Ermilov M, Silipo G, Shimoni J. Double-blind, placebo-controlled, crossover trial of D-cycloserine adjuvant therapy for treatment-resistant schizophrenia. Int J Neuropsychopharmcol 1998;1(2):131-5. [DOI] [PubMed]
- 75.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 1999;56(1):29-36. [DOI] [PubMed]
- 76.Heresco-Levy U. N-Methyl-D-aspartate (NMDA) receptor-based treatment approaches in schizophrenia: the first decade. Int J Neuropsychopahrmacol 2000;3(3):243-58. [DOI] [PubMed]
- 77.Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC. Placebo-controlled trial of D-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry 2002;159(3):480-2. [DOI] [PubMed]
- 78.Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, et al. A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry 1999;56(1):21-7. [DOI] [PubMed]
- 79.Aguilar EJ, Keshavan MS, Martinez-Quiles MD, Hernandez J, Gomez-Beneyto M, Schooler NR. Predictors of acute dystonia in first-episode psychotic patients. Am J Psychiatry 1994; 151 (12): 1819-21. [DOI] [PubMed]
- 80.Lieberman J, Jody D, Geisler S, Alvir J, Loebel A, Szymanski S, et al. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry 1993; 50(5):369-76. [DOI] [PubMed]
- 81.Chakos MH, Mayerhoff DI, Loebel AD, Alvir JM, Lieberman JA. Incidence and correlates of acute extrapyramidal symptoms in first episode of schizophrenia. Psychopharmacol Bull 1992; 28(1):81-6. [PubMed]
- 82.Kapur S, Remington G. Atypical antipsychotics. BMJ 2000; 321 (7273): 1360-1. [DOI] [PMC free article] [PubMed]
- 83.Brenner HD, Dencker SJ, Goldstein MJ, Hubbard JW, Keegan DL, Kruger G, et al. Defining treatment refractoriness in schizophrenia. Schizophr Bull 1990;16(4):551-61. [DOI] [PubMed]
- 84.Kane JM. Treatment-resistant schizophrenic patients. J Clin Psychiatry 1996;57(Suppl 9):35-40. [PubMed]
- 85.Angrist B, Schulz SC. The neuroleptic-nonresponsive patient: characterization and treatment. Washington: American Psychiatric Press, Inc.; 1990.
- 86.Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 2000;321(7273): 1371-6. [DOI] [PMC free article] [PubMed]
- 87.Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psychiatry 1988;45(1):79-91. [DOI] [PubMed]
- 88.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 2000;157(4):514-20. [DOI] [PubMed]
- 89.Stone CK, Garver DL, Griffith J, Hirschowitz J, Bennett J. Further evidence of a dose-response threshold for haloperidol in psychosis. Am J Psychiatry 1995;152(8):1210-2. [DOI] [PubMed]
- 90.Volavka J, Cooper TB, Czobor P, Meisner M. Plasma haloperidol levels and clinical effects in schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 1995;52(10):837-45. [DOI] [PubMed]
- 91.Jeste DV, Lacro JP, Palmer B, Rockwell E, Harris MJ, Caligiuri MP. Incidence of tardive dyskinesia in early stages of low-dose treatment with typical neuroleptics in older patients. Am J Psychiatry 1999;156(2):309-11. [DOI] [PubMed]
- 92.McEvoy JP. The neuroleptic threshold as a marker of minimum effective neuroleptic dose. Compr Psychiatry 1986;27(4): 327-35. [DOI] [PubMed]
- 93.Zimbroff DL, Kane JM, Tamminga CA, Daniel DG, Mack RJ, Wozniak PJ, et al. Controlled, dose-response study of sertindole and haloperidol in the treatment of schizophrenia. Am J Psychiatry 1997;154(6):782-91. [DOI] [PubMed]
- 94.Leucht S, Pitschel-Walz G, Abraham D, Kissling W. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res 1999;35(1):51-68. [DOI] [PubMed]
- 95.Azorin JM, Spiegel R, Remington G, Vanelle JM, Pere JJ, Giguere M, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry 2001;158(8):1305-13. [DOI] [PubMed]
- 96.Conley RR, Tamminga CA, Kelly DL, Richardson CM. Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry 1999;46(1):73-7. [DOI] [PubMed]
- 97.Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry 2001;50(11):898-911. [DOI] [PubMed]
- 98.Remington G, Kapur S. Atypical antipsychotics: are some more atypical than others? Psychopharmacology 2000;148(1):3-15. [DOI] [PubMed]
- 99.Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry 2001;62(Suppl 7):22-31. [PubMed]
- 100.Fayek M, Kingsbury SJ, Zada J, Simpson GM. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001;52(5):607-9. [DOI] [PubMed]
- 101.Henderson DC. Atypical antipsychotic-induced diabetes mellitus: how strong is the evidence? CNS Drugs 2002;16(2):77-89. [DOI] [PubMed]
- 102.Mir S, Taylor D. Atypical antipsychotics and hyperglycaemia. Int Clin Psychopharmacol 2001;16(2):63-73. [DOI] [PubMed]
- 103.Welch R, Chue P. Antipsychotic agents and QT changes. J Psychiatry Neurosci 2000;25(2):154-60. [PMC free article] [PubMed]
- 104.Chung YC, Eun HB. Hyperprolactinemia induced by risperidone. Int J Neuropsychopharmacol 1998;1:93-4. [DOI] [PubMed]
- 105.Caracci G, Ananthamoorthy R. Prolactin levels in premenopausal women treated with risperidone compared with those of women treated with typical neuroleptics. J Clin Psychopharmacol 1999;19(2):194-6. [DOI] [PubMed]
- 106.Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol 1993; 13(1):25-40. [PubMed]
- 107.Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994;151(6):825-35. [DOI] [PubMed]
- 108.Fleischhacker WW. Second generation antipsychotics. Psychopharmacology 2002;162(1):90-1. [DOI] [PubMed]
- 109.Waddington JL, O'Callaghan E. What makes an antipsychotic ‘atypical’? CNS Drugs 1997;7(5):341-6.
- 110.Fleischhacker WW, Hummer M. Drug treatment of schizophrenia in the 1990s. Achievements and future possibilities in optimising outcomes. Drugs 1997;53(6):915-29. [DOI] [PubMed]
- 111.Tamminga CA. The promise of new drugs for schizophrenia treatment. Can J Psychiatry 1997;42(3):265-73. [DOI] [PubMed]
- 112.Purdon SE. Measuring neuropsychological change in schizophrenia with novel antipsychotic medications. J Psychiatry Neurosci 2000;25(2):108-16. [PMC free article] [PubMed]
- 113.Buckley PF. The role of typical and atypical antipsychotic medications in the management of agitation and aggression. J Clin Psychiatry 1999;60(Suppl 10):52-60. [PubMed]
- 114.Emsley RA, Roberts MC, Rataemane S, Pretorius J, Oosthuizen PP, Turner J, et al. Ethnicity and treatment response in schizophrenia: a comparison of 3 ethnic groups. J Clin Psychiatry 2002; 63(1):9-14. [DOI] [PubMed]
- 115.Glazer WM. Expected incidence of tardive dyskinesia associated with atypical antipsychotics. J Clin Psychiatry 2000;61 (Suppl 4):21-6. [PubMed]
- 116.Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 2002;159(1):103-8. [DOI] [PubMed]
- 117.Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry 1998;44(8):675-84. [DOI] [PubMed]
- 118.Rosenheck R, Chang S, Choe Y, Cramer J, Xu W, Thomas J, et al. Medication continuation and compliance: a comparison of patients treated with clozapine and haloperidol. J Clin Psychiatry 2000;61(5):382-6. [PubMed]
- 119.Kane JM. Management strategies for the treatment of schizophrenia. J Clin Psychiatry 1999;60(Suppl 12):13-7. [PubMed]
- 120.Keck PE Jr, Strakowski SM, McElroy SL. The efficacy of atypical antipsychotics in the treatment of depressive symptoms, hostility, and suicidality in patients with schizophrenia. J Clin Psychiatry 2000;61(Suppl 3):4-9. [PubMed]
- 121.Hargreaves WA, Shumway M. Pharmacoeconomics of antipsychotic drug therapy. J Clin Psychiatry 1996;57(Suppl 9):66-76. [PubMed]
- 122.Meyer PS, Bond GR, Tunis SL, McCoy ML. Comparison between the effects of atypical and traditional antipsychotics on work status for clients in a psychiatric rehabilitation program. J Clin Psychiatry 2002;63(2):108-16. [DOI] [PubMed]
- 123.Tempier R, Pawliuk N. Influence of novel and conventional antipsychotic medication on subjective quality of life. J Psychiatry Neurosci 2001;26(2):131-6. [PMC free article] [PubMed]
- 124.Kapur S, McClelland RA, VanderSpek SC, Wadenberg ML, Baker G, Nobrega J, et al. Increasing D2 affinity results in the loss of clozapine's atypical antipsychotic action. Neuroreport 2002; 13(6):831-5. [DOI] [PubMed]


