Abstract
Objective
To determine whether nitric oxide production levels differ in patients with deficit and non-deficit forms of schizophrenia.
Methods
We investigated plasma nitrate levels, an index of in vivo nitric oxide production, in patients with deficit syndrome (n = 11) and non-deficit syndrome (n = 14) and healthy controls (n = 12) with a combination of high-performance liquid chromatography and the Griess reaction.
Results
There was no difference found in mean plasma nitrite levels, but plasma nitrate levels of patients with deficit syndrome were significantly lower than those with non-deficit syndrome (28.0 [SEM 2.5] μmol/L v. 44.2 [SEM 5.5] μmol/L, p < 0.05).
Conclusions
A decline in nitric oxide production may be involved in primary negative symptoms in schizophrenia.
Medical subject headings: nitric oxide, nitric-oxide synthase, schizophrenia, signs and symptoms
Abstract
Objectif
Déterminer si les taux de production de monoxyde d'azote des patients aux prises avec une forme de schizophrénie s'accompagnant d'un déficit sont différents de ceux des patients souffrant de schizophrénie sans déficit.
Méthodes
Nous avons examiné les concentrations plasmatiques de nitrate, indice de la production in vivo de monoxyde d'azote, chez des patients atteints d'un syndrome avec déficit (n = 11), chez des patients atteints d'un syndrome sans déficit (n = 14) et chez des témoins en bonne santé (n = 12), au moyen de la chromatographie en phase liquide à haut rendement et de la réaction de Griess.
Résultats
On n'a pas observé de différence au chapitre des concentrations plasmatiques moyennes de nitrite, mais les concentrations plasmatiques de nitrate des patients aux prises avec un syndrome avec déficit étaient très inférieures à celles des patients atteints d'un syndrome sans déficit (28,0 [ETM 2,5] μmol/L c. 44,2 [ETM 5,5] μmol/L, p < 0,05).
Conclusions
Une diminution de la production de monoxyde d'azote pourrait contribuer aux principaux symptômes négatifs de la schizophrénie.
Introduction
Since being identified as an endothelium-derived relaxing factor, nitric oxide (NO), which is biosynthesized in neurons as well as endothelium from L-arginine (L-Arg) by nitric oxide synthase (NOS), has been focused on as a physiologically active substance.1 At the cellular level, NO can modulate hormone secretion in the neuroendocrine system,2 as well as the release and reuptake of monoaminergic neurotransmitters,3,4,5,6,7 and is also involved in the plasticity of neurons. In animal experiments, there is some evidence that NO is involved in memory, behaviour and emotion.8,9,10
As evidence supporting the relation between NO and central nervous system function continues to accumulate, evidence of a relation between NO and schizophrenia is also increasing. In 1993, Akbarian et al11,12 reported a distorted distribution of nicotineamide-adenine dinucleotide phosphate-diaphorase (NADPH-d) neurons in various areas of postmortem brains of people who had schizophrenia.11,12 Given that NADPH-d coexists with NOS, their discovery suggests that there may be an NO production abnormality in the brains of patients with schizophrenia. Karson et al13 later showed that the NOS concentration is increased in the cerebellar vermis of postmortem brains of those who had schizophrenia. By means of ex vivo experiments, Das et al14 showed NOS activity to be significantly elevated in the platelets of drug-naive patients with schizophrenia compared with controls, drug-treated patients with schizophrenia and patients with panic disorder, and Herken et al15 reported a remarkable increase in nitrite plus nitrate levels in red blood cells of patients with schizophrenia compared with control subjects. These findings suggest that there is excess NO production in the brains of individuals with schizophrenia. Excess NO is thought to be neurotoxic owing to the formation of peroxinitrite, a very reactive anion with protonation, which is formed from the reaction of NO with superoxide.16,17
Strauss and colleagues18 proposed that positive and negative symptoms in schizophrenia be studied separately, and in 1980, Crow19 suggested that schizophrenia be divided into 2 types — Type I, which is characterized by positive symptoms, and Type II, which is characterized by negative symptoms and constant symptoms. Later, patients with Type II schizophrenia were reported to have decreased intelligence and enlarged cerebral ventricles, suggesting that neuron loss might underlie the pathogenesis of Type II schizophrenia.20,21 Carpenter and colleages22 further classified negative symptoms into 2 types — primary negative symptoms, which are enduring, and secondary negative symptoms, which are more transient and occur secondary to other factors (e.g., adverse effects of neuroleptics, anxiety, suspiciousness, depression and motor retardation). They termed the subtype of schizophrenia that is characterized by primary negative symptoms “deficit syndrome” and established the criteria for its diagnosis.23 Similarly, Keefe et al24,25 attempted to distinguish Kraepelinian from non-Kraepelinian schizophrenia on the basis of symptom outcome and degree of deterioration.
Given these lines of evidence, we hypothesized that excess NO production may lead to neurodegeneration and the formation of enduring negative symptoms in the brains of patients with the deficit form of schizophrenia. To support this working hypothesis, we investigated the levels of nitrate, a marker of in vivo NO production,26 in plasma from patients with deficit and non-deficit forms of schizophrenia and healthy controls.
Methods
This study was carried out in accordance with the latest version of the Declaration of Helsinki, and the design was approved by the Ethics Committee of the Department of Neuropsychiatry, Keio University. Informed consent was obtained from each patient upon provision of a thorough explanation of the procedures.
The subjects consisted of 25 outpatients (14 females) who met the DSM-IV criteria for schizophrenia on their first visit to our clinics and 12 (6 females) healthy volunteers (controls). Each patient was diagnosed using the Japanese version of the Schedule for the Deficit Syndrome27 (SDS) after at least 1 year of follow-up by an attending psychiatrist. Inter-rater and test–retest reliabilities of the differential diagnosis between the deficit and non-deficit forms of schizophrenia in our group were good (kappa = 0.86, and kappa = 0.93, respectively).27
None of the patients had taken antipsychotics for at least 2 weeks before blood collection, but 6 patients had taken 1 or 2 types of benzodiazepine. Patients with conditions possibly affecting their plasma nitrite and nitrate levels, such as drug abuse and certain diseases (blood, neurological, hepatic, cardiovascular or renal), were excluded from this study. Moreover, none of the subjects had any history of these conditions.
Blood was collected between 1000 and 1200 hours. Plasma nitrite and nitrate concentrations were measured using a high-performance liquid chromatography (ENO-20, Eicom, Kyoto, Japan). The lower detection limit in our laboratory was 0.1 mmol/L.
Hepatic and renal functions and cell counts were determined at the time of blood sampling to confirm that these values were within normal limits. There were 2 athletes and 12 smokers among our subjects. In addition to a general analysis of all subject data, we also conducted an analysis excluding data from the patients who took benzodiazepines, the smokers and the athletes. Exclusion of these subjects did not substantially change the overall findings.
Data are expressed as means (and standard error of the mean [SEM]) and were analyzed with 1-way analysis of variance (ANOVA). Post-hoc analysis was performed with Scheffé's test.
Results
The mean age of deficit patients was 27.8 (SEM 1.4) years, non-deficit patients was 31.9 (SEM 1.5) years and controls was 32.7 (SEM 1.6) years. Mean plasma nitrite levels of deficit patients, non-deficit patients and controls were 4.64 (SEM 0.70) μmol/L, 4.87 (SEM 0.55) μmol/L and 4.61 (SEM 0.83) μmol/L, respectively (F = 0.046, p = 0.95). Mean plasma nitrate levels of deficit patients, non-deficit patients and controls were 28.0 (SEM 2.5) μmol/L, 44.2 (SEM 5.5) μmol/L and 36.8 (SEM 2.8) μmol/L, respectively (Fig. 1). Plasma nitrate levels of deficit patients were significantly lower than those of non-deficit patients (F = 3.87, p = 0.03).
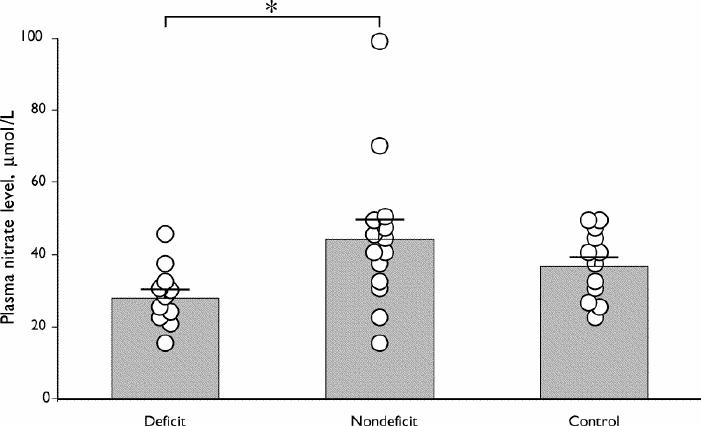
Fig. 1: Individual (circles) and mean (and standard error of the mean) (bars) plasma nitrate levels in patients with deficit and non-deficit schizophrenia and controls. Plasma nitrate levels were significantly lower in the deficit than in the non-deficit form (Scheffé's test, *p < 0.05).
Discussion
It is generally accepted that schizophrenia constitutes a wide spectrum of diseases. Among the many proposals to divide schizophrenia into subgroups,22,24,25 the deficit and non-deficit classification is one of the most heavily investigated. Previous investigations have revealed differences in intelligence, cognitive function, brain structure and brain function between the 2 forms of schizophrenia.28,29,30,31,32,33 The findings of this study (i.e., different plasma nitrate levels) may further support the heterogeneity between deficit and non-deficit forms of schizophrenia.
Contrary to our working hypothesis, data obtained in this study suggest NO production is diminished in patients with deficit syndrome compared with non-deficit syndrome patients. It remains unclear whether reduced NO production is related to the pathogenesis of deficit syndrome (negative symptoms), but interesting possibilities have been raised in the literature. We previously showed that symptoms of deficit syndrome do not respond to bromperidol, a dopamine D2 receptor antagonist, whereas those of non-deficit syndrome do.34 Amphetamines and related substances, dopamine releaser and dopamine reuptake inhibitors induce only positive symptom-like conditions in animals and humans.35 These findings suggest that factors other than the central dopaminergic system underlie the pathogenesis of deficit syndrome.
On the other hand, the phencyclidine (PCP) model of schizophrenia mimics not only positive symptoms but also negative symptoms,36 suggesting N-methyl-D-asparate (NMDA) receptors may be related to negative symptoms35 (because PCP is a NMDA receptor antagonist). The 3 subtypes of NOS are neuronal (NOS1), inducible (NOS2) and endothelial (NOS3).37 Production of neuronal NO is catalyzed by NOS1, is Ca2+-dependent and is stimulated by activation of NMDA receptors that allow for the influx of Ca2+.38 Moreover, PCP itself was shown to be a suicide inhibitor of NOS in the brain.39 Bird et al40 showed PCP-induced behavioural effects and neuronal activity to be reduced in mice treated with NOS1 antisense oligonucleotides and in NOS1 knockout mice, suggesting the NO system in the brain to be necessary for PCP-induced effects. In addition, nitric oxide donors reportedly block PCP-induced behaviour,41 and inhibitors of NOS have been shown to potentiate PCP-induced behavioural effects and neuronal activity.42 These data suggest that reduction of NMDA receptor-mediated NOS activity might contribute to the pathogenesis of negative symptoms and that the NMDA receptor–NO system might be a target for novel drugs designed to treat the negative symptoms of schizophrenia.
This study is limited in that the source of diminished NO production could not be precisely localized. However, Srivastava et al43 showed reduced nitrite levels in polymorphonuclear leukocytes, in which only NOS1 is constitutively expressed, in patients with schizophrenia. Shinkai et al44 showed the NOS1 gene polymorphism to confer increased susceptibility to schizophrenia. These data suggest that NOS activity in the brain is attenuated in some forms of schizophrenia.
We found no significant difference in plasma nitrite or nitrate levels between patients with schizophrenia in general (deficit plus non-deficit) and controls. This is contrary to previous studies that suggest NO production is increased in brains of individuals with schizophrenia.11,12,13,15 However, most subjects in previous studies were taking antipsychotics; ours were drug naive. Given the finding that long-term treatment with antipsychotics induces NOS2 mRNA in various regions of the brain, there is a possibility that antipsychotic treatment increased NOS activity in the previous research subjects.45 However, Das et al14 showed increased NOS activity in the platelets of drug-free patients with schizophrenia, and later reported46 decreased plasma nitrate levels of drug-free patients with schizophrenia. Consistent with our results, Srivastava et al43 found no significant change in the plasma total nitrite and nitrate content between drug-free patients and controls. We speculate that the discrepancies reflect the heterogeneous psychotic condition of subjects.
Deficit syndrome is characterized by restricted affect, diminished emotional range, poverty of speech, curbing of interests, diminished sense of purpose and diminished social drive.22,23 Externally, these symptoms are similar to those of depression. However, since we have documented increased plasma nitrate levels in depressed patients compared with those not in a non-depressed state,47 we speculate that the amount of NO production may differ between deficit syndrome and depression.
In conclusion, plasma nitrate levels in patients with deficit-type schizophrenia were significantly lower than those in patients with non-deficit type. These results suggest there is a difference in NO synthesis in patients with deficit and non-deficit types of schizophrenia.
Acknowledgments
We thank Dr. Gohei Yagi and Dr. Masahiro Asai for their introduction of patients and useful discussions.
Footnotes
This study was supported by a Grant-in-Aid for Scientific Research (C) (No. 13671031) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Competing interests: None declared for Dr. Nakamura. Dr. Suzuki has received speaker fees from Eisai Pharmaceutical Co. Dr. Nakaki has received speaker fees from Chugai Pharmaceutical Co. Dr. Miyaoka has received speaker fees from Sankyo Pharmaceutical Co., Sumitomo Pharmaceutical Co. and Fujisawa Pharmaceutical Co.
Correspondence to: Dr. Eiji Suzuki, Department of Psychiatry, Kitasato University School of Medicine, 2-1-1 Asamizodai, Sagamihara, Kanagawa, 228-8520, Japan; fax 81 42 765 3570; e-suzuki@kitasato-u.ac.jp
Submitted Aug. 28, 2002 Revised Dec. 18, 2002 Accepted Jan. 20, 2003
References
- 1.Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci 1991;14:60-7. [DOI] [PubMed]
- 2.Schmidt HH, Walter U. NO at work. Cell 1994;78:919-25. [DOI] [PubMed]
- 3.Zhu XZ, Luo LG. Effect of nitroprusside (nitric oxide) on endogenous dopamine release from rat striatal slices. J Neurochem 1992;59:932-5. [DOI] [PubMed]
- 4.Lorrain DS, Hull EM. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport 1993; 5: 87-9. [DOI] [PubMed]
- 5.Pögün S, Baumann MH, Kuhar NJ. Nitric oxide inhibits [3H]dopamine uptake. Brain Res 1994;641:83-91. [DOI] [PubMed]
- 6.Pögün S, Kuhar MJ. Regulation of neurotransmitter reuptake by nitric oxide. Ann N Y Acad Sci 1994;17:305-15. [DOI] [PubMed]
- 7.Seilicovich A, Lasaga M, Befumo M, Duvilanski BH, del Carman Diaz M, Rettori V, et al. Nitric oxide inhibits the release of norepinephrine and dopamine from the medial hypothalamus of the rat. Proc Natl Acad Sci U S A 1995;92:11299-302. [DOI] [PMC free article] [PubMed]
- 8.O'Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, et al. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science 1994; 265: 542-6. [DOI] [PubMed]
- 9.Salemme E, Diano S, Maharajan P, Maharajan V. Nitric oxide, a neuronal messenger. Its role in the hippocampus neuronal plasticity. Riv Biol 1996;89:87-107. [PubMed]
- 10.Kleppisch T, Pfeifer A, Klatt P, Montowski A, Fassler R, Hofmann F. Long-term potentiation in the hippocampal CA1 region of mice lacking cGMP-dependent kinases is normal and susceptible to inhibition of nitric oxide synthase. J Neurosci 1999; 19: 48-55. [DOI] [PMC free article] [PubMed]
- 11.Akbarian S, Bunney WE Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA, et al. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbance of cortical development. Arch Gen Psychiatry 1993;50:169-77. [DOI] [PubMed]
- 12.Akbarian S, Viñuela A, Kim JJ, Potkin SG, Bunney WE Jr, Jones EG. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry 1993;50:178-87. [DOI] [PubMed]
- 13.Karson CN, Griffin WST, Mark RE, Husain M, Dawson TM, Snyder SH, et al. Nitric oxide synthase (NOS) in schizophrenia: increases in cerebellar vermis. Mol Chem Neuropathol 1996; 27: 275-84. [DOI] [PubMed]
- 14.Das I, Khan NS, Puri BK, Sooranna SR, de Belleroche J, Hirsch SR. Elevated platelet calcium mobilization and nitric oxide synthase activity may reflect abnormalities in schizophrenic brain. Biochem Biophys Res Commun 1995;212:375-80. [DOI] [PubMed]
- 15.Herken H, Uz E, Özyurt H, Akyol Ö. Red blood cell nitric oxide levels in patients with schizophrenia. Schizophr Res 2001; 52: 289-90. [DOI] [PubMed]
- 16.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HSV, Sucher NJ, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 1993;364:626-32. [DOI] [PubMed]
- 17.Nakaki T, Mishima A, Fujii T, Suzuki E, Shintani F. Nitric oxide and neurodegenerative diseases. Curr Top Pulm Pharmacol Toxicol 1997;3:157-63.
- 18.Strauss JS, Carpenter WT, Bartko JJ. An approach to the diagnosis and understanding of schizophrenia: speculations on the processes that underlie schizophrenic symptoms and signs. Schizophr Bull 1974;1:61-9. [DOI] [PubMed]
- 19.Crow TJ. Molecular pathology of schizophrenia: more than one dimension of pathology? BMJ 1980;280:66-8. [DOI] [PMC free article] [PubMed]
- 20.Owens DGC, Johnstone EC. The disabilities of schizophrenia: their nature and the factors contributing to their development. Br J Psychiatry 1980;136:384-95. [DOI] [PubMed]
- 21.Owens DGC, Johnstone EC, Crow TJ, Firth CD, Jagoe JR, Kreel L. Cerebral ventricular enlargement in schizophrenia: relationship to the disease process and its clinical correlates. Psychol Med 1985;15:27-41. [DOI] [PubMed]
- 22.Carpenter WT, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578-83. [DOI] [PubMed]
- 23.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT. The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989;30:119-23. [DOI] [PubMed]
- 24.Keefe RSE, Mohs RC, Davidson M, Losonczy MF, Silverman JM, Lesser JC, et al. Kraepelinian schizophrenia: a subgroup of schizophrenia? Psychopharmacol Bull 1988;24:56-61. [PubMed]
- 25.Keefe RSE, Lobel DS, Mohs RC, Silverman JM, Harvey PD, Davidson M, et al. Diagnostic issues in chronic schizophrenia: Kraepelinian schizophrenia, undifferentiated schizophrenia, and state-independent negative symptoms. Schizophr Res 1991;4:71-9. [DOI] [PubMed]
- 26.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002-12. [DOI] [PubMed]
- 27.Suzuki E, Kanba S, Nibuya M, Inada T, Sekiya U, Ashikari I, et al. The schedule for the deficit syndrome and reliability of the Japanese version [In Japanese]. Seishin Igaku Kenkyusho Gyosekishu 1993;35:1097-103.
- 28.Nibuya M, Kanba S, Sekiya U, Suzuki E, Matsuo Y, Kinoshita N, et al. Schizophrenic patients with deficit syndrome have higher plasma homovanillic acid concentrations and ventricular enlargement. Biol Psychiatry 1995;38:50-6. [DOI] [PubMed]
- 29.Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT Jr. Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry 1994;51:804-11. [DOI] [PubMed]
- 30.Buchanan RW, Strauss ME, Breier A, Kirkpatrick B, Carpenter WT Jr. Attentional impairments in deficit and nondeficit forms of schizophrenia. Am J Psychiatry 1997;154:363-70. [DOI] [PubMed]
- 31.Bustillo JR, Thaker G, Buchanan RW, Moran M, Kirkpatrick B, Carpenter WT Jr. Visual information processing impairments in deficit and nondeficit schizophrenia. Am J Psychiatry 1997; 154: 647-54. [DOI] [PubMed]
- 32.Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F, et al. Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 1993;150:59-65. [DOI] [PubMed]
- 33.Tamminga CA, Thaler GK, Buchanan RW, Kirkpatrick B, Alphs LD, Chase TN, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992;49:522-30. [DOI] [PubMed]
- 34.Suzuki E, Kanba S, Koshikawa H, Nibuya M, Yagi G, Asai M. Negative symptoms in nondeficit syndrome respond to neuroleptic treatment with changes in plasma homovanillic acid concentrations. J Psychiatry Neurosci 1996;21:167-71. [PMC free article] [PubMed]
- 35.Gainetdinov RP, Mohn AR, Caron MG. Genetic models: focus on schizophrenia. Trends Neurosci 2001;24:527-33. [DOI] [PubMed]
- 36.Sams-Dodd F. Effects of continuous d-amphetamine and phencyclidine administration on social behavior, stereotyped behavior, and locomotor activity in rats. Neuropsychoharmacology 1998;19:18-25. [DOI] [PubMed]
- 37.Föstermann U, Kleinert H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn Schmiedebergs Arch Pharmacol 1995;352:351-64. [DOI] [PubMed]
- 38.Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol 1989;172:413-6. [DOI] [PubMed]
- 39.Osawa Y, Davila JC. Phencyclidine, a psychotomimetic agent and drug abuse, is a suicide inhibitor of brain nitric oxide synthase. Biochem Biophys Res Commun 1993;194:1435-9. [DOI] [PubMed]
- 40.Bird DC, Bujas-Bobanovic M, Robertson HA, Dursun SM. Lack of phencyclidine-induced effects in mice with reduced neuronal nitric oxide synthase. Psychopharmacology 2001; 155:299-309. [DOI] [PubMed]
- 41.Bujas-Bobanovic M, Bird DC, Robertson HA, Durson SM. Blockade of phencyclidine-induced effects by a nitric oxide donor. Br J Pharmacol 2000;130:1005-12. [DOI] [PMC free article] [PubMed]
- 42.Bujas-Bobanovic M, Robertson HA, Dursun SM. Effects of nitric oxide synthase inhibitor N(G)-nitro-L-arginine methyl ester on phencyclidine-induced effects in rats. Eur J Pharmacol 2000;409:57-65. [DOI] [PubMed]
- 43.Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Srimal RC, et al. Nitrite content and antioxidant enzyme levels in the blood of schizophrenia patients. Psychopharmacology 2001;158:140-5. [DOI] [PubMed]
- 44.Shinkai T, Ohmori O, Hori H, Nakamura J. Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Mol Psychiatry 2002;7:560-3. [DOI] [PubMed]
- 45.Suzuki E, Nakaki T, Shintani F, Kanba S, Miyaoka H. Antipsychotic, antidepressant, anxiolytic, and anticonvulsant drugs induce type II nitric oxide synthase mRNA in rat brain. Neurosci Lett 2002;333:217-9. [DOI] [PubMed]
- 46.Das I, Kahn NS, Puri BK, Hirsch SR. Elevated endogenous nitric oxide synthase inhibitor in schizophrenic plasma may reflect abnormalities in brain nitric oxide production. Neurosci Lett 1996;215:209-11. [DOI] [PubMed]
- 47.Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M. Elevated plasma nitrate levels in depressive states. J Affect Disord 2001;63:221-4. [DOI] [PubMed]


