Abstract
The human larynx descends during infancy and the early juvenile periods, and this greatly contributes to the morphological foundations of speech development. This developmental phenomenon is believed to be unique to humans. This concept has formed a basis for paleoanthropological studies on the origin and evolution of human speech. We used magnetic resonance imaging to study the development of three living chimpanzees and found that their larynges also descend during infancy, as in human infants. This descent was completed primarily through the rapid descent of the laryngeal skeleton relative to the hyoid, but it was not accompanied by the descent of the hyoid itself. The descent is possibly associated with developmental changes of the swallowing mechanism. Moreover, it contributes physically to an increased independence between the processes of phonation and articulation for vocalization. Thus, the descent of the larynx and the morphological foundations for speech production must have evolved in part during hominoid evolution, and not in a single shift during hominid evolution.
In the human neonate, the hyoid bone and larynx are positioned as high as in other mammals (1–3). However, they descend gradually during postnatal life (1–6). This descent is completed through the descent of the laryngeal skeleton relative to the hyoid and the descent of the hyoid relative to the mandible and cranial base (4–6). Thus, the human supralaryngeal vocal tract (SVT) develops to form a double resonator system with equally long horizontal [SVTH, from the posterior oropharyngeal wall (POW) to the lips] and vertical (SVTV, from the vocal folds to the velum) components (2, 6). Acoustically, such a configuration, in combination with the tongue's mobility, enables humans to produce complex speech sounds (7, 8).
It is commonly assumed that this developmental descent evolved as an adaptation for speech in a single shift in the human lineage, in combination with decreased prognathism and flexure of the cranial base (2, 9, 10). This concept has formed a basis for paleoanthropological studies on the origin and evolution of human speech, in which the “unique” morphological features related to speech have been examined through comparisons with extant primates (2, 11–16). Thus, the evolution of the morphological basis for human speech has been regarded as synonymous with the evolution of the developmental descent of the larynx. Nonetheless, there are few comparative studies on the developmental changes of humans and non-human mammals (17–19), and it is unclear how and when the unique features of the speech apparatus of adult humans appear and develop during growth.
Subjects and Methods
Magnetic Resonance Imaging (MRI) Procedures. We used MRI technology to examine the developmental changes of the SVT shape in three living chimpanzee infants, named Ayumu (male), Cleo (female), and Pal (female). They were born in 2000 and were reared by their biological mothers in the Kyoto University Primate Research Institute (KUPRI) (20, 21). Care and use of the chimpanzees adhered to the guidelines of the KUPRI, and the protocol for the MRI examination was approved by the Ethics Panel of the KUPRI (22, 23). Sagittal tomographic images of chimpanzee heads and necks were taken with a General Electric Signa Profile MRI scanner (0.2 tesla; General Electric) at KUPRI, with the extremity or head receiving coil at scheduled intervals. The subjects were anesthetized intramuscularly by using a solution of 3.5 mg of ketamine hydrochloride (Sankyo, Tokyo) and 0.035 mg of medetomidine hydrochloride (Meiji Seika Kaisha, Tokyo) per kilogram of body weight. They were placed supine with their heads fixed to the coil with belts. All imaging sequences were sagittal spin echo series with time-to-echo delays ranging from 17 to 32 ms, time-to-repeat of 500 ms, fields of view ranging from 18 to 22 cm, with 2.7- or 3.0-mm slice thickness and 0.8- or 0.5-mm gaps between slices and an acquisition matrix of 192 × 192 with two excitations. These parameters were chosen based on subject size. The matrix of all magnetic resonance images was 256 × 256 pixels, and their image resolution ranged from 0.74 × 0.74 to 0.86 × 0.86 mm per pixel.
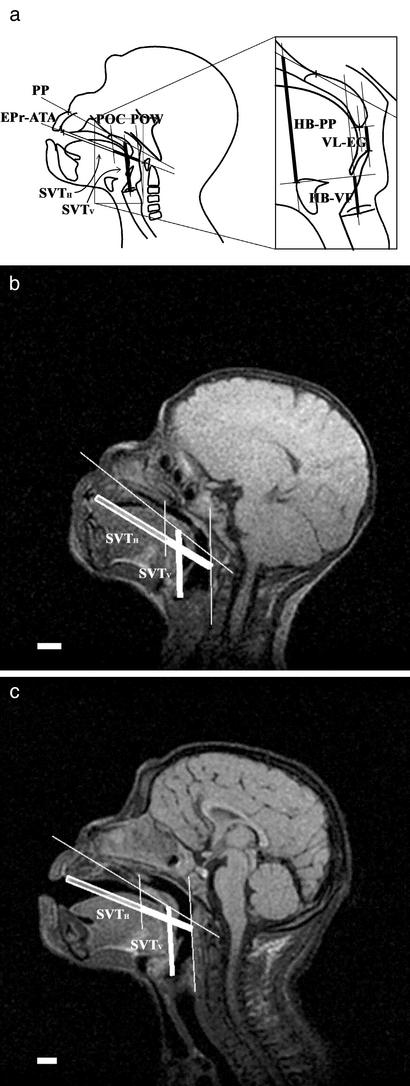
Measurements. We ascertained the proportional changes of the SVT during infancy in the chimpanzees to compare them with those reported for humans (6) using the same anatomic landmarks and dimensions. The coordinate values of the measurement points for linear dimensions were measured three times with IMAGES software (ver. Beta 4.0.2; Scion, Frederick, MD) from magnetic resonance images imported to a personal computer. If the values did not agree, they were remeasured. Standard planes and measurement points on the mid-sagittal plane included the following: PP (palatal plane), the plane from the point at the root of the nasal septum to the posterior nasal spine; POW; POC (posterior margin of the oral cavity), the plane from the posterior nasal spine parallel to the POW; EPr (endoprosthion), the most anterior inferior point of the lingual surface of the maxillary alveolar; ATA, the superior–inferior midpoint of the anterior tubercle of the atlas; HB, the most superior point of the insertion of the geniohyoid muscle onto the hyoid bone; VF, the anterior–posterior midpoint of the vocal fold; VL, the most posterior inferior point of the palatine velum, excluding the uvula; and EG, the most superior point of the epiglottis (see Fig. 1). Measurements included SVTH length, along the EPr–ATA line from the EPr to the POW; the length of the oropharyngeal component of the SVTH, along the EPr–ATA line from the POC to the POW; the length of the oral component of the SVTH, along the EPr–ATA line from the EPr to the POC; SVTV length, parallel to the POW from the VF to the PP; VL–EG length, parallel to the POW from the EG to the VL; and HB–PP and HB–VF lengths, parallel to the POW, from the HB to the PP and the VF, respectively (see Fig. 1). These definitions correspond to those in ref. 6, except for the VL, EG, and VL–EG lengths.
Fig. 1.
SVT growth in chimpanzee infants. (a Left) Mid-sagittal diagram of SVTH and SVTV lengths. The SVTH outlined in bold represents the oral component and the filled SVTH is the oropharyngeal component. (Right) Diagram of VL–EG, HB–PP, and HB–VF lengths. (b and c) Lateral magnetic resonance images of female chimpanzee infant (Pal) at 127 days of age (b) and 734 days of age (c). (Scale bar, 1.0 cm.)
Results
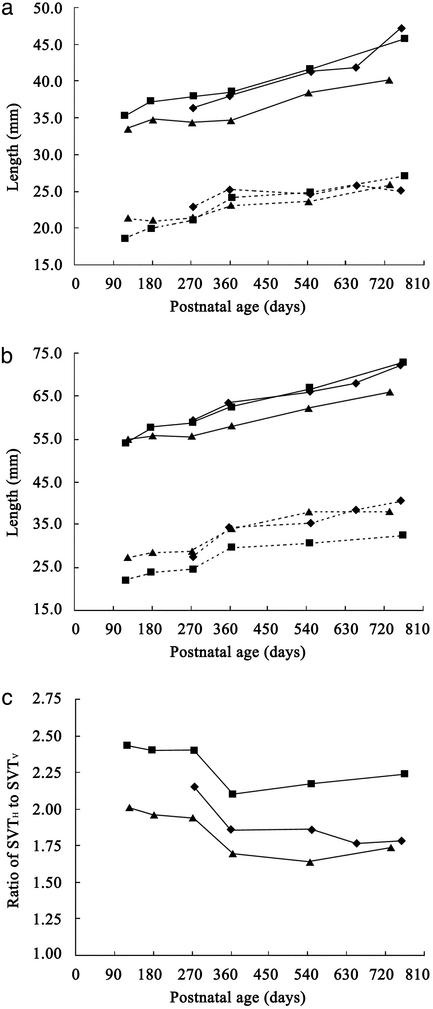
SVT Growth in the Infant Chimpanzees. The growth of the infant chimpanzee SVTH differed from that of the SVTV. The oropharyngeal component of the SVTH was ≈2.0 cm long at 4 months of age (Fig. 2). It grew to ≈2.4 cm during the first year of life, after which it grew negligibly or only slightly. In contrast, the oral component of the SVTH was initially ≈3.4 cm long and increased linearly with age to ≈4.4 cm at 2 years of age (Fig. 2). As a result, the total SVTH had lengthened from ≈5.4 to 7.0 cm at 2 years of age (Fig. 2). By contrast, the SVTV length increased rapidly by ≈0.6 cm between 9 and 12 months of age, although it increased slowly and linearly with age during other periods (Fig. 2). As a result, SVTV length increased by ≈1.2 cm, from 2.5 to 3.7 cm, in the first 2 years of life (Fig. 2). Therefore, the ratio of the SVTH to SVTV lengths decreased greatly by ≈0.35 between 9 and 12 months of age, from 2.20 to 1.85 on average (Fig. 2). During other periods, this ratio changed negligibly in all subjects. This finding means that the descent of the larynx relative to the palatal plane is more rapid than the growth of the oral cavity during the first year of life in chimpanzees.
Fig. 2.
SVT growth in the three chimpanzee infants: Ayumu (male, ⋄), Cleo (female, ▴), and Pal (female, ▪). (a) Growth of the oral (solid line) and oropharyngeal (dotted line) components of the SVTH.(b) Growth of the SVTH (solid line) and SVTV (dotted line). (c) Age-related changes in the ratio of SVTH to SVTV lengths.
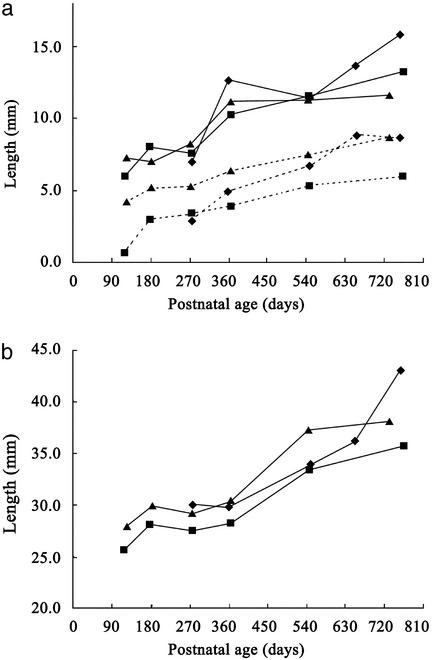
We examined the contributions made by changes in the spatial relations among the velum, epiglottis, hyoid bone, and laryngeal skeleton to the lengthening of the SVTV in chimpanzee infants. The epiglottis, which is attached to the thyroid cartilage of the laryngeal skeleton, descended relative to the velum during the first year of life. It completely lost contact with the velum by at least 6 months of age, and the distance between them (the VL–EG length) increased during early infancy in all subjects (Fig. 3). In contrast, the distance between the hyoid and the palatal plane (the HB–PP length) did not increase in the first year of life, even though the hyoid is tethered to the thyroid cartilage with muscles, ligaments, and membranes (Fig. 3). The laryngeal skeleton, including the vocal folds, thus descended relative to the hyoid throughout that period. This finding is demonstrated by the increase in the distance from the hyoid to the vocal fold (the HB–VF length) from 6.6 to 11.4 mm (Fig. 3). The spatial relationship between the hyoid and laryngeal skeleton was then maintained at the level observed at 1 year of age (Fig. 3). In contrast, the HB–PP length increased from 29.5 to 39.0 mm in the second year of life (Fig. 3). However, because this increase corresponded to the SVTH growth trajectory during that period, the SVTH to SVTV ratio remained virtually unchanged (Fig. 2). These measurements demonstrate that the laryngeal descent observed in chimpanzees during the first year of life is caused primarily by the descent of the laryngeal skeleton relative to the hyoid bone along the neck, and that this is not accompanied by a descent of the hyoid relative to the palatal plane.
Fig. 3.
Increases in the HB–VF, VL–EG, and HB–PP lengths in the three chimpanzee infants. (a) Increases in the lengths of HB–VF (solid line) and VL–EG (dotted line). (b) Increases in the length of HB–PP. The symbols for individual subjects are as in Fig. 2.
Comparisons of SVT Growth Patterns Between Chimpanzees and Humans. A proportional change similar to that of the SVT of chimpanzees is observed in human infants (2, 6, 24). Thus, the two components of the SVTH in humans (6) grow similarly to those in chimpanzees during the first 2 years of life, although the length of the oral component is ≈1.0 cm shorter in humans than in chimpanzee infants over that period. The SVTV in humans (6) also increases in length by an increment similar to that observed in chimpanzees during infancy, although it is ≈1.0 cm longer than that of the chimpanzees. The period of accelerated growth that occurs in chimpanzees is also detected in longitudinal studies of human infants (24). The human ratio of SVTH to SVTV is ≈1.5 at birth and decreases by ≈0.2 within the first year of life (6), similar to that seen in chimpanzees. The ratio in humans also decreases to ≈1.0 between 5 and 8 years of age (6). Consequently, the descent of the human larynx is more rapid than the growth of the oral cavity, during both early infancy and the early juvenile period.
The HB–PP length in humans (6) increases by an increment similar to that observed in chimpanzees during infancy, although it is ≈3 mm longer than that in chimpanzees. The HB–VF length in humans (6) also increases by an increment similar to that observed in chimpanzees during infancy. However, it is unclear how these growth patterns individually contribute to the proportional changes in the SVT in humans. The descent of the human larynx is achieved not only by the descent of the laryngeal skeleton relative to the hyoid bone, but also by the descent of the hyoid relative to the mandible and cranial base, even in infancy (4–6). Thus, the laryngeal skeleton descends relative to the hyoid in both chimpanzees and humans, and this descent contributes to the descent of the larynx during early infancy. By contrast, hyoid descent per se contributes to the descent of the larynx only in humans and not in chimpanzees.
Discussion
The present study supports the hypothesis (25) that the descent of the human larynx evolved in two steps, not one. Briefly, the first step was the descent of the laryngeal skeleton relative to the hyoid, at least in the common ancestor of humans and chimpanzees, and the second was the descent of the hyoid relative to the mandible and the cranial base in the human lineage. Moreover, this study suggests that the first phase of descent would have developed primarily during infancy.
The positional changes of the larynx in descending the neck are considered to interact with a variety of related functions, such as breathing, swallowing, and locomotion, as well as vocalization (1–4, 6, 8, 26–29). Therefore, no single selective advantage can account for this evolutionary trait. It is possible that the first phase of descent may have been associated with developmental changes in the swallowing mechanism (25). In humans, the epiglottis loses contact with the velum in early infancy, between 4 and 6 months of age, and this accompanies laryngeal descent (26, 27). However, this conformational change is thought to increase the risk of accidental aspiration during swallowing (1–3, 26, 27). To reduce this risk, the adult mode of swallowing develops in human infants, wherein the hyoid ascends, the laryngeal skeleton approximates the hyoid, the epiglottis bends, and the laryngeal orifice closes (26–28, 30–32). The approximation of the laryngeal skeleton to the hyoid contributes to the latter two movements (30, 31, 33, 34). The first descent not only allows the epiglottis to lose its contact with the velum but also weakens the physical linkages between the laryngeal skeleton and hyoid. This descent enables the larynx to move independently of the hyoid (25). Thus, it possibly confers an advantage for the development of the movements observed in adult swallowing. If this suggestion is correct, then the first descent evolved through a developmental alternation of the swallowing mechanism as an adaptation to changes in hominoid diet and increases in body size (35).
The first phase of descent also helps explain the evolution of the physical foundations of phonation and articulation. Independent controls of these functions are prerequisites for speech production (7, 36). The laryngeal skeleton, including the vocal folds, is the physical foundation for phonation, and the hyoid provides the basis for the tongue movements that participate in articulation of the vocal tract. The first descent ensured that the physical linkages between the laryngeal skeleton and the hyoid were weakened in the ancestors of both humans and chimpanzees. This weakened physical linkage may have conferred an advantage for the control of articulation independently to phonation in physical terms. Therefore, chimpanzee vocalizations may also reflect an increased independence between the activities of phonation and articulation. However, this remains unclear, because there is little information on the bioacoustics of phonation and articulation in the vocalizations of non-human hominoids.
The second phase of descent was possibly related to skeletal developments that evolved during hominid evolution, such as the ventral flexion of the cranial base during infant and early juvenile development (2, 11, 12, 37), reduced prognathism caused by sphenoidal shortening (9), and increases in mandibular height (6). Furthermore, the shorter oral component of the SVTH in humans also contributes to a decrease in the ratio of SVTH to SVTV. These modifications in the development of the facial–mandibular skeleton would have contributed to the evolution of the double resonator system of the SVT, together with improvements in tongue mobility. In contrast, the second descent possibly increased the risk of accidental aspiration of food or liquids. The first descent may thus have been an adaptation to avoid such risks, but it was also a preadaptive or secondarily advantageous prerequisite for the evolution of speech in the human lineage.
Thus, these separate descent processes facilitate speech as a whole, but may have evolved under different selection pressures, possibly originally directed toward advantages unrelated to speech. If this hypothesis is correct, the mosaic evolution of the anatomy of the SVT may have been a preadaptive set of functional modifications leading to the evolution of spoken language in humans.
Acknowledgments
We are grateful to A. Kato, K. Kumazaki, N. Maeda, S. Goto, C. Hashimoto, K. Matsubayashi, M. Tomonaga, M. Tanaka, and other staff of KUPRI for daily veterinary care for and help with the MRI examinations of three chimpanzee infants, and to K. Honda, H. Takemoto, Y. Shimada, I. Fujimoto, S. Masaki of the Advanced Telecommunications Research Institute (Kyoto) for advice on operating the MRI scanner and processing magnetic resonance images. We thank Y. Hamada, M. Tomonaga, and M. A. Huffman for support and comments on the manuscript, and D. E. Lieberman for kindly supplying information (6). This work was supported in part by grants-in-aid for Center of Excellence Research (Grant 10CE2005 to O. Takenaka), for 21st Century Center of Excellence Program (A2 to Kyoto University), and for Specially Promoted Research (Grant 12002009 to T.M.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SVT, supralaryngeal vocal tract; SVTH, horizontal component of the SVT; SVTV, vertical component of the SVT; KUPRI, Kyoto University Primate Research Institute; PP, the plane from the point at the root of nasal septum to the posterior nasal spine; POW, posterior oropharyngeal wall; EPr, the most anterior inferior point of the lingual surface of the maxillary alveolar; ATA, the superior–inferior midpoint of the anterior tubercle of the atlas; HB, the most superior point of the insertion of the geniohyoid muscle onto the hyoid bone; VF, vocal fold; VL, most posterior inferior point of the palatine velum; EG, the most superior point of the epiglottis.
References
- 1.Negus, V. E. (1949) The Comparative Anatomy and Physiology of the Larynx (William Heinemann Medical Books, London).
- 2.Lieberman, P. (1984) The Biology and Evolution of Language (Harvard Univ. Press, Cambridge, MA).
- 3.Harrison, D. F. N. (1995) The Anatomy and Physiology of the Mammalian Larynx (Cambridge Univ. Press, Cambridge, U.K.).
- 4.Crelin, E. S. (1973) Functional Anatomy of the Newborn (Yale Univ. Press, New Haven, CT).
- 5.Westhorpe, R. N. (1987) Anaesth. Intens. Care 15, 384–388. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman, D. E., McCarthy, R. C., Hiiemae, K. M. & Palmer, J. B. (2001) Arch. Oral Biol. 46, 117–128. [DOI] [PubMed] [Google Scholar]
- 7.Fant, G. (1960) Acoustic Theory of Speech Production (Mouton, The Hague, The Netherlands).
- 8.Lieberman, P. H., Klatt, D. H. & Wilson, W. H. (1969) Science 164, 1185–1187. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman, D. E. (1998) Nature 393, 158–162. [DOI] [PubMed] [Google Scholar]
- 10.Fitch, W. T. (2000) Trends Cognit. Sci. 4, 258–267. [DOI] [PubMed] [Google Scholar]
- 11.Laitman, J. T., Heimbuch, R. C. & Crelin, E. S. (1979) Am. J. Phys. Anthropol. 51, 15–34. [Google Scholar]
- 12.Laitman, J. T. & Heimbuch, R. C. (1982) Am. J. Phys. Anthropol. 59, 323–343. [DOI] [PubMed] [Google Scholar]
- 13.Arensburg, B., Tillier, A M., Vandermeersch, B., Duday, H., Schepartz, L A. & Rak, Y. (1989) Nature 338, 758–760. [DOI] [PubMed] [Google Scholar]
- 14.Arensburg, B., Schepartz, L A., Tillier, A M., Vandermeersch, B. & Rak, Y. (1990) Am. J. Phys. Anthropol. 83, 137–146. [DOI] [PubMed] [Google Scholar]
- 15.Kay, R. F., Cartmill, M. & Balow, M. (1998) Proc. Natl. Acad. Sci. USA 95, 5417–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLarnon, A. M. & Hewitt, G. P. (1999) Am. J. Phys. Anthropol. 109, 341–363. [DOI] [PubMed] [Google Scholar]
- 17.Flügel, C. & Rohen, J. W. (1991) Mech. Aging Dev. 61, 65–83. [DOI] [PubMed] [Google Scholar]
- 18.Fitch, W. T. & Reby, D. (2001) Proc. R. Soc. London B Biol. Sci. 268, 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissengruber, G. E., Forstenpointner, G., Peters, G., Kübber-Heiss, A. & Fitch, W. T. (2002) J. Anat. 201, 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzawa, T. (2002) in The Cognitive Animal, eds. Bekoff, M., Allen, G. & Burghardt, G. (MIT Press, Cambridge, MA), pp. 189–195.
- 21.Okamoto, S., Tomonaga, M., Ishii, K., Kawai, N., Tanaka, M. & Matsuzawa, T. (2002) Anim. Cognit. 5, 107–114. [DOI] [PubMed] [Google Scholar]
- 22.Primate Research Institute, Kyoto University (1986) Guide for the Care and Use of Laboratory Primates (Primate Research Institute, Kyoto Univ., Inuyama, Japan).
- 23.Primate Research Institute, Kyoto University (2002) Guide for the Care and Use of Laboratory Primates (Primate Research Institute, Kyoto Univ., Inuyama, Japan), 2nd Ed.
- 24.Vorperian, H. K., Kent, R. D., Gentry, L. R. & Yandell, B. S. (1999) Int. J. Pediatr. Otorhinolaryngol. 49, 197–206. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura, T. (2003) Primates 44, 41–49. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki, C. T., Levine, P. A., Laitman, J. T. & Crelin, E. S. (1977) Arch. Otolaryngol. 103, 169–171. [DOI] [PubMed] [Google Scholar]
- 27.Laitman, J. T. & Crelin, E. S. (1980) Perinatol. Neonatol. 4, 15–22. [Google Scholar]
- 28.Bosma, J. F. (1992) in Advances in Otolaryngology: Head and Neck Surgery, ed. Myers, E. N. (Year Book Medical Publishers, Chicago), Vol. 6, pp. 225–275. [Google Scholar]
- 29.Hayama, S., Honda, K., Oka, H. & Okada, M. (2002) Z. Morphol. Anthropol. 83, 149–159. [PubMed] [Google Scholar]
- 30.Ekberg, O. (1982) Acta Otolaryngol. 93, 123–129. [DOI] [PubMed] [Google Scholar]
- 31.Ekberg, O. & Sigurjónsson, S. V. (1982) Gastrointest. Radiol. 7, 101–107. [DOI] [PubMed] [Google Scholar]
- 32.Ekberg, O. (1986) Invest. Radiol. 21, 408–410. [DOI] [PubMed] [Google Scholar]
- 33.Vandaele, D. J., Perlman, A. L. & Cassell, M. D. (1995) J. Anat. 186, 1–15. [PMC free article] [PubMed] [Google Scholar]
- 34.Reidenbach, M. M. (1997) Eur. Arch. Otorhinolaryngol. 254, 410–412. [DOI] [PubMed] [Google Scholar]
- 35.Fleagle, J. G. (1999) Primate Adaptation and Evolution (Academic, San Diego), 2nd Ed.
- 36.Fitch, W. T. (2003) in Primate Audition: Ethology and Neurobiology, ed. Ghazanfar, A. A. (CRC Press, Boca Raton, FL), pp. 65–137.
- 37.Laitman, J. T., Heimbuch, R. C. & Crelin, E. S. (1978) Am. J. Anat. 152, 467–482. [DOI] [PubMed] [Google Scholar]