Abstract
The N-terminal domain of the E3L protein of vaccinia virus has sequence similarity to a family of Z-DNA binding proteins of defined three-dimensional structure and it is necessary for pathogenicity in mice. When other Z-DNA-binding domains are substituted for the similar E3L domain, the virus retains its lethality after intracranial inoculation. Mutations decreasing Z-DNA binding in the chimera correlate with decreases in viral pathogenicity, as do analogous mutations in wild-type E3L. A chimeric virus incorporating a related protein that does not bind Z-DNA is not pathogenic, but a mutation that creates Z-DNA binding makes a lethal virus. The ability to bind the Z conformation is thus essential to E3L activity. This finding may allow the design of a class of antiviral agents, including agents against variola (smallpox), which has an almost identical E3L.
Vaccinia is a large, DNA-containing poxvirus with >200 genes, which replicates in the cytoplasm of infected cells (1). When an adapted strain of vaccinia is given to a mouse, the mouse dies in ≈1 week, depending on the dosage and route of infection (2). The viral E3L gene product has been extensively characterized and is essential for virulence (2). E3L encodes a 25-kDa protein that has two essential domains (3); an N-terminal domain with sequence similarity to the vertebrate Zα family of Z-DNA-binding domains (4), and a C-terminal domain that has a typical double-stranded RNA binding motif (refs. 5–7; Fig. 1). Although the N-terminal domain is not required for replication or IFN resistance in cells in culture (6, 7), it is required for full pathogenesis in mice (2, 8, 9). The E3L molecule is a conserved common feature of orthopox viruses. In fact, when comparing vaccinia and variola, the agent of smallpox, the N-terminal (Fig. 1b) and C-terminal (not shown) E3L domains are both nearly identical.
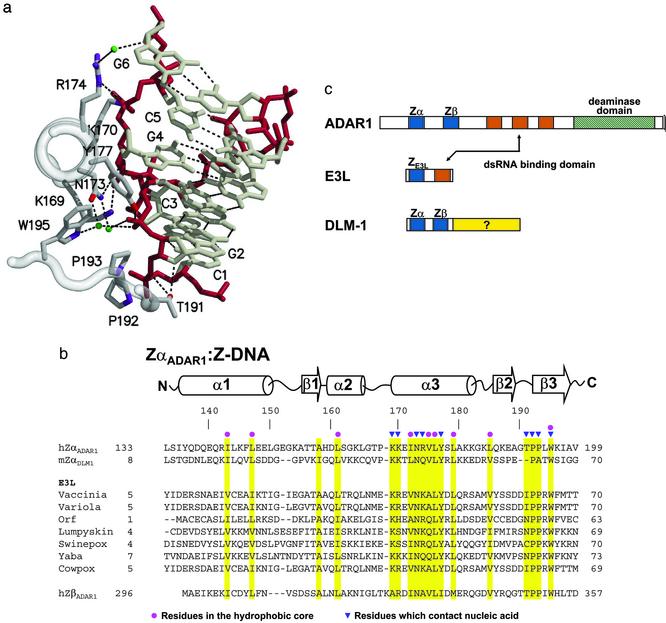
Fig. 1.
A family of Z-DNA-binding proteins. (a) DNA–protein contacts. A portion of the Z-DNA-binding domain of the ADAR1 editing enzyme (ZαADAR1) is shown complexed to a fragment of Z-DNA, as revealed in the structure of the cocrystal (10). The view is down the recognition helix of the protein (left), and a number of amino acids are shown that interact with left-handed Z-DNA stabilized by electrostatic and van der Waals interactions. The Z-DNA backbone is red and water molecules are green. There is an edge-to-face van der Waals interaction between guanine 4 and tyrosine 177. Note the van der Waals interactions between prolines 192, 193, and the Z-DNA backbone. (b) The amino acid sequences of Z-DNA binding and related proteins are shown underneath the secondary structure diagram, as revealed in the cocrystal structures of human ZαADAR1 (10) and mouse ZαDLM1 (11). A number of poxvirus E3L sequences are listed, as is the related sequence of human ZβADAR1. Yellow bars indicate residues important for the protein fold (red dots) and for Z-DNA recognition (blue triangles). The amino acid numbering at the beginning and the end of each sequence is indicated. The GenBank accession numbers for the various sequences are as follows: double-stranded RNA adenosine deaminase, AAB06697.1 (Homo sapiens); Z-DNA-binding protein 1; tumor stroma and activated macrophage protein DLM-1 (Mus musculus) NP_067369; the E3L proteins, AAA02759 (vaccinia virus); NP_042088 (variola virus); AAC08018 (orf virus); AAK84995 (lumpy skin disease virus); NP_570192 (Swinepox); NP_073419 (yaba-like disease virus); and CAC42100 (cowpox virus). (c) Three different families of proteins are illustrated, with domains shown as colored boxes. The Zα and Zβ domains of both ADAR1 and DLM1 are blue, as is the related domain of E3L. Vaccinia E3L protein and the editing enzyme ADAR1 both have domains that bind to double-stranded RNA, shown in red.
By sequence, the N-terminal half of E3L is a member of the Zα family of Z-DNA-binding domains (Fig. 1b). The crystal structures of two Zα protein domains, ZαADAR1 (Fig. 1a; ref. 10) and ZαDLM1 (11), have been determined complexed to Z-DNA. ZαADAR1 is found at the N terminus of the IFN-inducible (12) RNA editing enzyme double-stranded RNA adenosine deaminase (ADAR1) (Fig. 1c; ref. 4). ZαDLM1 is from the tumor-related DLM-1 protein, which is also IFN induced (13). Both of these domains consist of a helix–turn–helix motif with an additional β-sheet. The structures of ZαADAR1 (10) and ZαDLM1 (11) complexed to Z-DNA are very similar, but the amino acid sequence is not conserved, except for those residues that contact DNA (Fig. 1b). In this article, we ask whether the sequence similarity between the N-terminal domain of the vaccinia E3L protein and the known Z-DNA-binding proteins is reflected in biological activity. By exchanging known Z-DNA-binding domains for the N terminus of viral E3L, we find that these domains are functionally interchangeable. Further insight is provided by exploring mutations in wild-type and chimeric E3L; the choice of mutations was guided by the crystal structures of Z-DNA binding proteins complexed to Z-DNA. The results show that binding to the Z conformation is necessary for E3L biological activity.
Materials and Methods
Cell Culture, Construction, and Amplification of Chimeric Viruses. The construction of chimeric viruses, including the construction of chimeric E3L genes, the introduction of point mutations, and the insertion of these genes into the virus by in vivo recombination are described in detail in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org. Standard methods of cell culture were used as well as methods for virus amplification. These are described in detail in Supporting Text. All animal experiments were approved by the Arizona State University Institutional Animal Care and Use Committee.
Circular Dichroism (CD). The conversion of d(CG)6 from right-handed B-DNA to left-handed Z-DNA is monitored by measuring changes in CD in the UV (14). The rate and extent to which the ZαADAR1 protein converts d(CG)6 from B-DNA to Z-DNA was measured (15). The proteins used in the CD studies were prepared and purified as described (16).
Results
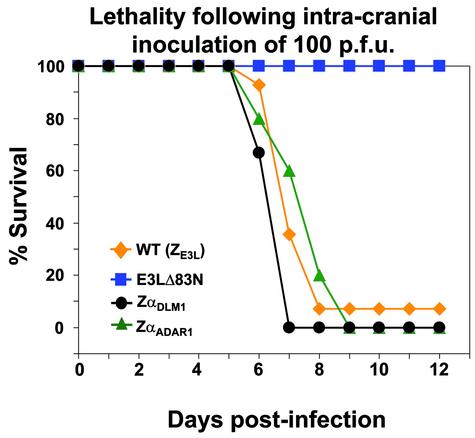
Chimeras with ZαADAR1 or ZαDLM1 Exchanged for the N Terminus of E3L Are Virulent. The N-terminal residues of E3L are essential for virulence. If the first 83 residues are deleted, leaving only the C terminus of E3L (E3LC), there is dramatically decreased pathogenicity in mice (ref. 2; Fig. 2). These 83 residues encompass the Zα motif (Fig. 1b). To examine the importance of Z-DNA binding, a domain swap was carried out in which 67 N-terminal amino acids of E3L were replaced with 64 residues of ZαADAR1 (Fig. 1 b and c). In this replacement, ≈50 residues were changed. The remaining 14 identical residues include those that contact Z-DNA in the ZαADAR1 crystal structure (Fig. 1 a and b). The chimeric virus (ZαADAR1-E3LC) retains the wild-type C-terminal E3L domain. This construct was tested by intracranial injection into mice to assay pathogenicity, as described (2). As shown in Fig. 2, the ZαADAR1-E3LC chimeric virus is as pathogenic as the wild-type virus. A similar domain swap was carried out exchanging 67 amino acids from ZαDLM1 (Fig. 1 b and c) for 67 amino acids at the N terminus of E3L. In this case, 12 residues remain unchanged, including those that bind to Z-DNA (11). Again, the ZαDLM1-E3LC chimera is fully pathogenic (Fig. 2). Thus, only a small number of essential residues within the N-terminal domain of E3L are sufficient for biological activity, as measured by lethality.
Fig. 2.
The N terminus of the vaccinia E3L protein can be functionally replaced by Z-DNA-binding domains from ADAR1 and DLM1. Chimeric viruses were created with either ZαADAR1 or ZαDLM1 at the E3L N terminus and wild-type E3L sequences at the C terminus. In these and subsequent experiments, groups of four to six C57BL/6 mice (4–6 weeks old) were infected intracranially with the indicated number of pfu of vaccinia virus constructs in 10 μl unless otherwise specified. The method has been described (2). One hundred pfu were used in this experiment and the mice were monitored for mortality for 2 weeks. Data for wild-type vaccinia virus are a composite of four different experiments, each with four mice. Percent survival is plotted against days postinfection. WT, wild-type E3L; E3LΔ83N has 83 amino acids deleted from the N terminus, and ZαADAR1 and ZαDLM1 are chimeric viruses ZαADAR1-E3LC and ZαDLM1-E3LC, respectively.
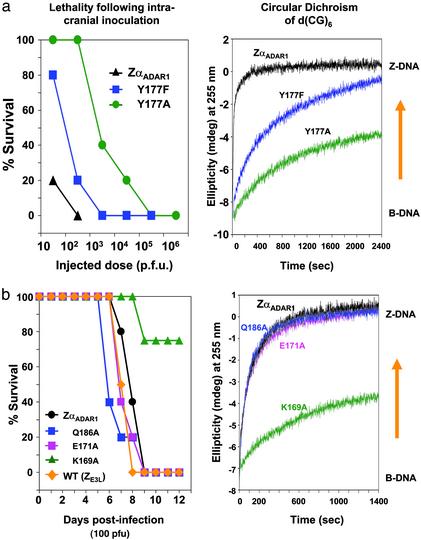
Mutations in the ZαADAR1-E3LC Chimeric Virus. We next assayed point mutations in the ZαADAR1 chimeric construct of E3L and compared the effect of these mutations on pathogenicity. We also measured the effect of ZαADAR1 protein and its mutants on Z-DNA binding by using CD (14). Residues were selected that are known to be important for Z-DNA binding, as seen in the cocrystal structures (10, 11). As shown in Fig. 1a, tyrosine 177 forms an important interaction in recognizing Z-DNA, making an edge-to-face contact between the C8 atom of guanine 4 in the syn-conformation and the planar face of the tyrosine. In addition, the tyrosine hydroxyl group forms a hydrogen bond with a Z-DNA phosphate group. Two different mutations in tyrosine 177 were made in the ZαADAR1-E3LC chimeric vaccinia virus and in the ZαADAR1 protein domain.
Fig. 3a shows viral pathogenicity and the CD changes associated with these mutations. The change in the CD spectrum reflects the stabilization of the oligomer in the Z conformation, requiring conformationally specific binding of the protein to Z-DNA. When tyrosine 177 is mutated to phenylalanine (Y177F), the binding affinity is weakened by the loss of the hydroxyl group, and the CD spectral change is slower than that seen for the wild-type sequence. A tyrosine-to-alanine mutation (Y177A) has an even more dramatic effect on the spectrum, with a much slower and less complete conversion corresponding to weaker binding of the protein. We have also measured the dissociation constant (Kd) of the interaction with Z-DNA of all three constructs by using surface plasmon resonance, as described (4). For this measurement, poly[(dG-dC)] is partially brominated to stabilize it in the Z-DNA conformation. The Kd of ZαADAR1 protein for Z-DNA is 40 nM, Y177F reduces the Kd to 350 nM, and Y177A reduces the Kd further to 700 nM.
Fig. 3.
Z-DNA binding by mutants of ZαADAR1 protein correlates with pathogenicity of vaccinia viruses containing the corresponding mutations in chimeric ZαADAR1-E3LC genes. (a) Mice were infected with vaccinia viruses containing ZαADAR1-E3LC chimeric genes, including the indicated mutations, as described in Fig. 2. The mice were monitored for mortality for 2 weeks. (Left) Dose–response curve in which percent survival is plotted against dose of viruses administered. (Right) The ability of ZαADAR1 protein and two of its mutant proteins to convert d(CG)6 from the right-handed B-form to the left-handed Z-form is shown. The ellipticity at 255 nm is monitored as a function of time, using 90 μM (base pair) d(CG)6 and 30 μM protein, as described (26). ZαADAR1 protein converts the oligonucleotide to the Z-DNA conformation quite rapidly, and the mutants Y177F and Y177A affect the conversion at increasingly slower rates, corresponding to the weakened pathogenicity of the relevant viral constructs. (b) The lethality after intracranial inoculation of 100 pfu is shown for wild-type and a number of chimeric E3L vaccinia viruses, with the indicated mutations. Wild-type virus, chimeric ZαADAR1-E3LC virus, and chimeric virus with Q186A or E171A mutations are all equally lethal. In contrast, the K169A mutant shows decreased virulence with considerable survival. The CD is shown (Right). ZαADAR1 protein and the Q186A and E171A mutants rapidly convert d(CG)6 to the Z-DNA form. The K169A mutant achieves only partial conversion and at a slower rate.
The effect of mutating tyrosine 177 in ZαADAR1 of the chimeric E3L virus is presented in Fig. 3a. For example, at a viral dose of 100 plaque-forming units (pfu), 100% of the animals infected with vaccinia virus containing the ZαADAR1-E3LC chimera have died, consistent with the results shown in Fig. 2. However, the chimera in which tyrosine 177 was changed to alanine (Y177A) was ≈1,000 times or 3 log10s less pathogenic than the unmutated ZαADAR1 chimeric virus. The mutation to phenylalanine is ≈10 times, or 1 log10 less pathogenic. The results support a strong dependence of pathogenicity on Z-DNA binding.
When comparing the Kd values, the CD results, and the pathogenicity of these three constructs, it should be noted that each assay measures a somewhat different thing. Kd measurements using surface plasmon resonance reflect the affinity of the Z-DNA-binding protein for preformed Z-DNA in a brominated substrate (4). The CD assay requires a conversion of the right-handed B-DNA into left-handed Z-DNA followed by binding (14–16). In vivo binding may also reflect other factors such as substrate accessibility, which affects Z-DNA-binding kinetics; therefore, it is not surprising that the results are not numerically proportional. Nevertheless, there is a strong correlation between the trends in pathogenicity, Kd, and the ability to effect a B-DNA to Z-DNA conversion as monitored by CD for these mutants.
We then compared the importance of amino acid side chains that interact with Z-DNA in the cocrystal structure and those that do not (Fig. 3b). As shown in Fig. 1a, lysine 169 (K169) forms a hydrogen bond to a phosphate group (10), but the second residue along the recognition helix, glutamic acid 171 (E171, not shown in Fig. 1a) does not interact with Z-DNA. Glutamine 186 (Q186) on the β-sheet also does not interact with Z-DNA. These residues were each mutated to alanine with strikingly different results. Mutant K169A ZαADAR1 protein converts d(CG)6 from B-DNA to Z-DNA more slowly and less completely, compared with unmutated ZαADAR1 protein, whereas the rates of conversion of both E171A and Q186A mutant ZαADAR1 proteins are comparable to that observed with the unmutated ZαADAR1 (Fig. 3b). Fig. 3b also shows that chimeric virus with ZαADAR1 substituted for the N-terminal domain is indistinguishable in lethality from viruses with the mutations Q186A and E171A. In contrast, the mutation K169A in ZαADAR1 protein has decreased ability to bind to Z-DNA as illustrated in the CD spectrum, and it also has markedly reduced pathogenicity: all animals live longer, and 75% of them survive in the 12-day experiment. Once again, mutations that weaken Z-DNA binding yield weakened virulence, whereas those that do not influence Z-DNA binding do not affect virulence. The virulence results for individual mutations of non-Z-DNA-binding residues are compatible with those for the numerous collective changes in >50 amino acids occurring in the substitution of ZαADAR1 or ZαDLM1 for ZE3L, as described in Fig. 2.
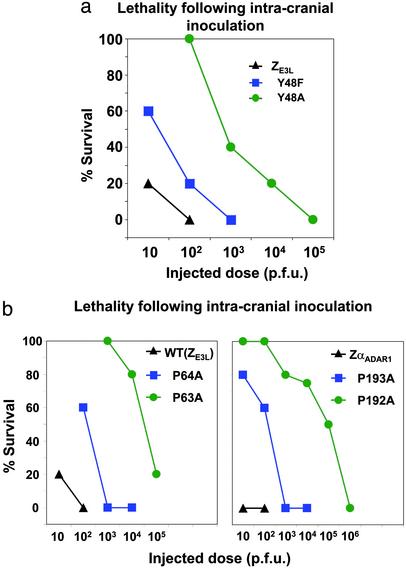
Comparison of Mutations in ZE3L and ZαADAR1. The in vivo effects of mutations in ZαADAR1-E3LC chimeric viruses were then compared with mutations of the analogous positions of wild-type vaccinia virus E3L (ZE3L). Tyrosine 48 (Y48) of E3L, which is analogous to tyrosine 177 (Y177) of ZαADAR1 (Fig. 1b), was mutated to phenylalanine and alanine. The results, shown in Fig. 4a, can be compared with the results for chimeric ZαADAR-E3LC in Fig. 3a. In each case, the tyrosine-to-phenylalanine mutation results in an ≈1 log10 loss of pathogenicity compared with wild-type virus, whereas the alanine mutation causes an ≈3log10 change. The biological effects are thus similar for these mutations in the chimeric virus (ZαADAR1-E3LC) and in the wild-type virus.
Fig. 4.
A comparison of the in vivo pathogenic effects of analogous mutations in the Z-DNA-binding domains of ZαADAR1-E3LC chimeric and wild-type E3L vaccinia virus. (a) Experiments analogous to Fig. 3a were carried out on the E3L gene of wild-type vaccinia virus. Dose–response curves are shown of the lethality after intracranial inoculation, monitored for 2 weeks, for different doses of the virus. Results are shown for virus containing wild-type E3L and for virus containing Y48F or Y48A mutations. The results are similar to those shown in Fig. 3a for the ZαADAR1-E3Lc chimeric virus. (b) Comparison of the effect of mutations in analogous proline residues of the E3L gene in wild-type vaccinia virus, as well as the ZαADAR1-E3LC chimeric virus. (Left) Mice were infected intracranially with the indicated doses of wild-type vaccinia virus [WT (ZE3L)], or with vaccinia virus containing P63A or P64A mutations. (Right) Mice were infected with virus containing chimeric (ZαADAR1-E3LC) or the chimeric virus containing P192A or P193A mutations. The P64A mutation in the wild-type E3L gene produces decreased virulence in a manner similar to that seen with the P193A mutation of ZαADAR1-E3LC chimeric virus, with an LD50 of ≈102 pfu. The P63A mutation in wild-type E3L or the P192A mutations in ZαADAR1-E3LC chimeric virus results in further weakening of the virus, with an LD50 of 104 to 105 pfu.
A pair of prolines (P192, P193) are found in the β turn of ZαADAR1 and make van der Waals contacts at the periphery of the Z-DNA-binding surface, as shown in Fig. 1a. These residues are essential for Z-DNA binding, especially P192, as shown in a Southwestern assay (17). Proline 192 of ZαADAR1 is in the cis conformation and makes extensive van der Waals contact with a phosphate group (10). Fig. 4b shows that the P193A mutation of ZαADAR1-E3LC decreases lethality by 1–2 log10s. The P64A mutation in the analogous position of wild-type virus also decreases lethality by 1–2 log10s. Mutating proline 192 to alanine has a far more deleterious effect on pathogenicity, yielding a virus that is ≈4 log10s less virulent than the unmutated chimeric virus E3L. Likewise, P63A yields a virus that is 4–5 log10s less virulent. In another experiment, mutation K40A in wild-type virus, analogous to K169A in ZαADAR1 (Fig. 3b), also produces a less virulent phenotype (results not shown). All of these experiments reinforce the correlation of Z-DNA binding of E3L with pathogenicity in vaccinia virus.
Gain-of-Function Mutation for Z-DNA Binding Increases Pathogenicity. The experiments described in Figs. 3 and 4 show mutations that weaken Z-DNA binding also decrease pathogenicity or virulence of the virus. Those changes produce a loss of function. Here we start with an inactive chimera, containing an N-terminal domain that does not bind Z-DNA and mutate it to simultaneously create both Z-DNA binding and biological activity. Human ZβADAR1 is a domain of ADAR1 with many sequence features of the Zα family; however, it lacks several residues important for Z-DNA binding (Fig. 1b). Furthermore, it does not bind Z-DNA (ref. 16; Y.-G.K. and A.R., unpublished work), and it does not alter the CD spectrum of d(CG)6 (Fig. 5). Fourteen residues are conserved in the ZβADAR1-E3LC chimeric virus, as compared with wild-type E3L. This result is comparable to the number conserved in the ZαADAR1-E3LC construct, but the results are entirely different. The ZβADAR1-E3LC chimera is not pathogenic to mice, even when 106 pfu are inoculated intracranially (Fig. 5). This finding further supports the correlation between lack of Z-DNA binding and lack of pathogenicity.
Fig. 5.
Gain of biological function. Mutation of ZβADAR1-E3LC chimeric virus leads to Z-DNA binding and to pathogenesis in mice. Mice were infected intracranially with 106 pfu of either a ZβADAR1-E3LC chimeric virus or the chimeric virus with a I335Y mutation. The ZβADAR1-E3LC chimeric virus has no lethality after intracerebral inoculation of 106 pfu, and the ZβADAR1 protein is unable to change the CD of d(CG)6, as shown on the right. Substituting tyrosine for isoleucine at position 335 of ZβADAR1 in ZβADAR1-E3LC chimeric virus leads to significant mortality. The same mutation in ZβADAR1 protein converts d(CG)6 from the B-DNA to the Z-DNA form.
It is possible to mutate ZβADAR1 to produce a Z-DNA binding protein. Human ZβADAR1 lacks the tyrosine residue in the binding interface analogous to Y177 in hZαADAR1 (Fig. 1b). When isoleucine 335 is changed to tyrosine, the resulting protein, ZβADAR1I335Y binds Z-DNA. Fig. 5 shows the conversion of B-DNA to Z-DNA in the presence of ZβADAR1I335Y protein as monitored by CD. The ZβADAR1I335Y-E3LC chimeric virus shows significant pathogenicity when inoculated into mice, although less than wild-type virus or the ZαADAR1-E3LC chimera. The gain of Z-DNA binding in the mutated protein domain correlates well with the gain of biological function in the mutated chimeric virus.
Discussion
It is likely that Z-DNA binding is a common feature of E3L gene products in poxviruses. The N-terminal E3L domains have been expressed from vaccinia, orf, lumpyskin, swinepox, and yaba-like disease viruses (Fig. 1b). All of them show specificity for binding to Z-DNA in vitro, but with varying affinities (Y.-G.K. and A.R., unpublished work). In vivo Z-DNA binding can be assayed by using a yeast one-hybrid system. The reporter gene, β-galactosidase, is expressed when ZαADAR1 fused to an activation domain is transfected into a cell containing a Z-DNA-forming sequence upstream of the reporter gene (18). Using this system, ZE3L shows comparable Z-DNA-binding activity as ZαADAR1 or ZαDLM1 (Y.-G.K. and A.R., unpublished work).
Although the experiments presented here focused on Z-DNA binding, that is clearly an oversimplification. Vaccinia E3L has been implicated in at least four possible roles, which may be important for viral pathogenesis: Z-DNA binding, nuclear localization, IFN-blocking activities, and higher-order oligomerization (2). Likewise, a nuclear export signal has been localized in the ZαADAR1 domain, which is important for nuclear-cytoplasmic shuttling of ADAR1 (19, 20). This signal, which does not involve the Z-DNA-binding residues, could result in a lower nuclear concentration of chimeric ZαADAR1-E3LC protein. Any or all of these functions may modulate the biological activity of the N terminus of E3L in both wild-type and chimeric viruses. However, the experiments described here have focused on the essential contribution of Z-DNA binding.
The E3L molecule has two theaters of operation. In the cytoplasm of the cell where the virus replicates, it plays an important role in inhibiting the activity of proteins involved in the IFN response. The IFN-induced protein kinase, PKR, binds to double-stranded RNA and acts to phosphorylate, and thereby inactivate the protein synthesis initiation factor eIF2α. The E3L proteins are potent inhibitors of PKR (21), requiring both domains for full inhibition (refs. 7 and 22; J. O. Langland and B.L.J., unpublished work). Likewise, E3L blocks the activation of the 2′-5′ oligoadenylate synthase (23, 24). E3L has recently been shown to inhibit induction of IFN-β by blocking phosphorylation of the transcription factor IRF-3 (8, 9). Thus, E3L acts in the cytoplasm to broadly block the IFN system.
It is known that E3L also accumulates in the nucleus (6, 25), but little is known about its activities there. For instance, we do not know the target of E3L in the nucleus. It is likely that the N terminus of E3L is binding to Z-DNA in the nucleus, but we cannot rule out binding to Z-form double-stranded RNA (26). Z-DNA is a left-handed conformation of the double helix stabilized by negative supercoiling (14), and many human genes have sequences favoring Z-DNA formation near the transcription start site (27). Movement of RNA polymerase along DNA generates negative supercoiling behind it, which can be sufficient to stabilize the left-handed Z-conformation, then reverting to the right-handed B-conformation when transcription ceases (28). Z-DNA is thus a transient conformational change associated with actively transcribing genes (29).
The knowledge that E3L consists of two domains, a Z-DNA-binding domain at the N terminus and a double-stranded RNA-binding domain at the C terminus, offers a possible clue to its activity in the nucleus. Both of these domains bind with conformational specificity, but not sequence specificity, suggesting that E3L acts at multiple sites. Z-DNA-forming sequences found near the transcription start site (27) can flip into the Z-conformation in some actively transcribing genes (28, 29), and E3L may bind to the Z-DNA segment of such genes. The double-stranded RNA-binding motif might then be available to bind to a foldback or transient hairpin structure of the pre-mRNA. This action could have the effect of decreasing the extent to which selected genes in the host cell can respond to the viral infection, either by impairing transcription or by interfering with the splicing apparatus. Such a mechanism would represent yet another example of the way in which the virus uses features of the host cell to prevent it from maintaining an antiviral defense.
The structures of ZαADAR1 and ZαDLM1 bound to Z-DNA are known, and by analogy we can infer something about the conformation of the Z-DNA-binding pocket in the N-terminal domain of the vaccinia E3L molecule. Future structural studies are needed to confirm the nature of the binding pocket. The yeast one hybrid system (18) requires Z-DNA binding to activate the reporter gene. This could be the basis of an assay system to develop a molecule that would attach selectively to the binding pocket. Treating a mouse with such a molecule could interfere with E3L Z-DNA binding and prevent the lethality associated with vaccinia infection. Likewise, some people have adverse reactions to vaccination with vaccinia virus. A molecule-blocking E3L binding may halt the progress of the virus and play a useful role in minimizing these reactions. Because this Z-DNA-binding pocket occurs rarely in host cell proteins, interfering with Z-DNA binding might have only minimal adverse effects.
Comparison of the sequence of the E3L molecule of variola, the agent of smallpox, with that of vaccinia reveals that the molecules are essentially identical (Fig. 1b). None of the residues that differ are found near the Z-DNA-binding site. A small molecule binding to variola E3L may prevent its pathology and be effective in treating smallpox.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health and the Dana Foundation.
Abbreviations: pfu, plaque-forming units; ADAR, adenosine deaminase acting on RNA.
References
- 1.Moss, B. (1996) in Fields Virology, eds. Fields, B., Knipe, D. & Howley, P. (Lippincott-Raven, Philadelphia), Vol. 2, pp. 2637–2671. [Google Scholar]
- 2.Brandt, T. A. & Jacobs, B. L. (2001) J. Virol. 75, 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho, C. K. & Shuman, S. (1996) Virology 217, 272–284. [DOI] [PubMed] [Google Scholar]
- 4.Herbert, A., Alfken, J., Kim, Y.-G., Saira Mian, I., Nishikura, K. & Rich, A. (1997) Proc. Natl. Acad. Sci. USA 94, 8421–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, H. W. & Jacobs, B. L. (1993) Virology 194, 537–547. [DOI] [PubMed] [Google Scholar]
- 6.Chang, H. W., Uribe, L. H. & Jacobs, B. L. (1995) J. Virol. 69, 6605–6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shors, T., Kilber, K. V., Perkins, K. B., Seidler-Wulff, R., Banaszak, M. P. & Jacobs, B. L. (1997) Virology 239, 269–276. [DOI] [PubMed] [Google Scholar]
- 8.Smith, E. J., Marie, I., Prakash, A., Garcia-Sastre, A. & Levy, D. E. (2001) J. Biol. Chem. 276, 8951–8957. [DOI] [PubMed] [Google Scholar]
- 9.Xiang, Y., Condit, R. C., Vijaysri, S., Jacobs, B., Williams, B. R. G. & Silverman, R. H. (2002) J. Virol. 76, 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz, T., Rould, M. A., Lowenhaupt, K., Herbert, A. & Rich, A. (1999) Science 284, 1841–1845. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz, T., Behlke, J., Lowenhaupt, K., Heinemann, U. & Rich, A. (2001) Nat. Struct. Biol. 8, 761–765. [DOI] [PubMed] [Google Scholar]
- 12.Patterson, J. B. & Samuel, C. E. (1995) Mol. Cell. Biol. 15, 5376–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu, Y., Comella, N., Tognazzi, K., Brown, L. F., Dvorak, H. F. & Kocher, O. (1999) Gene 240, 157–163. [DOI] [PubMed] [Google Scholar]
- 14.Rich, A., Nordheim, A. & Wang, A. H. (1984) Annu. Rev. Biochem. 53, 791–846. [DOI] [PubMed] [Google Scholar]
- 15.Berger, I., Winston, W., Manoharan, R., Schwartz, T., Alfken, J., Kim, Y.-G., Lowenhaupt, K., Herbert, A. & Rich, A. (1998) Biochemistry 37, 13313–13321. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz, T., Lowenhaupt, K., Kim, Y.-G., Li, L., Brown, B. A., II, Herbert, A. & Rich, A. (1999) J. Biol. Chem. 274, 2899–2906. [DOI] [PubMed] [Google Scholar]
- 17.Schade, M., Turner, C. J., Lowenhaupt, K., Rich, A. & Herbert, A. (1999) EMBO J. 18, 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh, D. B., Kim, Y.-G. & Rich, A. (2002) Proc. Natl. Acad. Sci. USA 99, 16666–16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulsen, H., Nilsson, J., Damgaard, C. K., Egelbjerg, J. & Kjems, J. (2001) Mol. Cell. Biol. 21, 7862–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strehblow, A., Hallegger, M. & Jantsch, M. F. (2002) Mol. Biol. Cell 13, 3822–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang, H. W., Watson, J. C. & Jacobs, B. L. (1992) Proc. Natl. Acad. Sci. USA 89, 4825–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano, P. R., Zhang, F., Tan, S.-L., Garcia-Barrio, M. T., Katze, M. G., Dever, T. E. & Hinnebusch, A. G. (1998) Mol. Cell. Biol. 18, 7304–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beattie, E., Denzler, K. L., Tartaglia, J., Perkus, M. E., Paoletti, E. & Jacobs, B. L. (1995) J. Virol. 69, 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivas, C., Gil, J., Melkova, Z., Esteban, M. & Diaz-Guerra, M. (1998) Virology 243, 406–414. [DOI] [PubMed] [Google Scholar]
- 25.Yuwen, H., Cox, J. H., Yewdell, J. W., Bennink, J. R. & Moss, B. (1993) Virology 195, 732–744. [DOI] [PubMed] [Google Scholar]
- 26.Brown, B. A., Lowenhaupt, K., Wilbert, C. M., Hanlon, E. B. & Rich, A. (2000) Proc. Natl. Acad. Sci. USA 97, 13532–13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroth, G. P., Chou, P. J. & Ho, P. S. (1992) J. Biol. Chem. 267, 11846–11855. [PubMed] [Google Scholar]
- 28.Wittig, B., Wolfl, S., Dorbic, T., Vahrson, W. & Rich, A. (1992) EMBO J. 11, 4653–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittig, B., Dorbic, T. & Rich, A. (1991) Proc. Natl. Acad. Sci. USA 88, 2259–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.