Abstract
Cryo-electron microscopy was exploited to reveal and study the influence of pyruvate dehydrogenase (E1) occupancy on the conformational states of the Saccharomyces cerevisiae pyruvate dehydrogenase complex (PDC). Structures representative of PDC preparations with ≈40% and full E1 occupancy were determined after the electron microscopy images from each preparation were classified according to their sizes. The reconstructions derived from two size groups showed that the deposition of the E1 molecules associated with the larger complex is, unexpectedly, not icosahedrally arranged, whereas in the smaller complex the E1 molecules have an arrangement and architecture similar to their more ordered deposition in the WT bovine kidney PDC. This study also shows that the linker of dihydrolipamide acetyltransferase (E2) that tethers E1 to the E2 core increases in length from ≈50 to 75 Å, accounting largely for the size difference of the smaller and larger structures, respectively. Extensive E1 occupancy of its 60 E2 binding sites favors the extended conformation of the linker associated with the larger complex and appears to be related to the loss of icosahedral symmetry of the E1 molecules. However, the presence of a significant fraction of larger molecules also in the WT PDC preparation with low E1 occupancy indicates that the conformational variability of the linker contributes to the overall protein dynamics of the PDC and the variable deposition of E1. The flexibility of the complex may enhance the catalytic proficiency of this macromolecular machine by promoting the channeling of the intermediates of catalysis between the active sites.
A central feature of eukaryotic pyruvate dehydrogenase complexes (PDCs) is a 60-mer core with the morphology of a pentagonal dodecahedron (1–4). The dihydrolipoamide acetyl-transferase (E2) component serves as a scaffold to which the pyruvate dehydrogenase (E1) and dihydrolipoamide dehydrogenase (E3) components are attached. The E2 dodecahedron consists of sets of three tightly bound subunits at each of its 20 vertices. The trimers are interconnected by 30 tenuous and flexible bridges that enable the core to breathe as evidenced by its size variability of ≈17% (240–280 Å in diameter) at room temperature (5).
3D reconstructions of subcomplexes of the Saccharomyces cerevisiae E2 core have revealed that 12 E3 components are attached by a binding protein (BP) inside the 12 pentagonal openings of the cage-like E2 (6), whereas the E1 components form a shell that surrounds the underlying E2 core (7–9). The reconstructions show that the 60 E2 subunits are organized in 20 trimers. Each E2 subunit contains an ≈50-Å-long linker that anchors an E1 tetramer. Three of these linkers emanate from the outside edges of the triangular-shaped base of the E2 trimer and form a cage around its base that may shelter the lipoyl domains of E2 and the E1 and E2 active sites. We proposed that the lipoyl domain and its tether (swinging arm) rotates about the E1-binding domain of E2, which is centrally located ≈50 Å from the neighboring E1, E2, and E3 active sites (7).
The WT bovine kidney PDC preparation used in the study to determine the deposition of E1 about the core was shown to have about one-third of its E1-binding sites occupied (7). Consequently, this component in the reconstruction was significantly down-weighted in the 3D map. To minimize this effect and potentially further stabilize the E1 components through their self-association about the core, we have determined the structure of an S. cerevisiae PDC preparation containing ≈60 E1s. A priori, this arrangement was expected to promote higher symmetry and significantly improve the resolution of the E1 molecules that form the outer shell of the complex. To our surprise and disappointment, near saturation of the E1-binding sites resulted in an altered arrangement of E1 with loss of their icosahedral symmetry found in the bovine kidney PDC (7) and, therefore, a reconstruction of much lower resolution in the outer shell resulted. Thus, the electron microscopy (EM) map representative of the E1 components is difficult to interpret. Even though the E1 arrangement was altered in >90% of the molecules containing 60 E1s, a small subset of PDC images was shown to have an orderly E1 arrangement very similar to that of the WT bovine kidney PDC (7). Careful analysis of the molecules in this data set revealed yet another aspect of the protein dynamics of this extraordinary molecule; the arm-like extension of the inner linker of E2 may vary in length from ≈50 to 75 Å, resulting in an increase in the gap between the inner core and outer shell and a significant disruption of the E1 icosahedral arrangement. Comparison of the S. cerevisiae structures with ≈24 and 60 E1s bound to the E2 core shows that this conformational change of the inner linker is influenced by the extent of E1 binding to the core and that PDC molecules with the lower E1 occupancy also exhibit a variation in size. Thus, the breathing E2 core and the flexing of its inner-linker arm apparently contribute to the global protein dynamics of the PDC.
Experimental Procedures
Enzyme Preparations. S. cerevisiae PDC was purified to near homogeneity from baker's yeast by modification of a published procedure (10). Highly purified E1 was obtained by resolution of PDC with 2 M NaCl at pH 7.3 (11) followed by FPLC on a Superdex 200 column. The weight-average molecular weight of the PDC was determined by light scattering measurement to be ≈8 × 106. On the basis of the known molecular weight of the complex and its component enzymes and the experimentally determined polypeptide chain ratios of E2/BP/E3 (12), we estimated that the subunit composition of the S. cerevisiae PDC is ≈24 E1 tetramers, 60 E2 monomers, 12 BP monomers, and 8 E3 dimers. Sufficient E1 was added to a sample of the PDC preparation to increase the molar ratio of E1/E2 core to 60:1. This product is designated larger PDC or ≈60 E1/E2 core PDC.
Cryo-EM. A 3-μl sample of each PDC preparation (≈0.35 mg/ml containing 20 μg/ml bacitracin) was deposited, blotted, and quick-frozen in liquid ethane on a glow-discharged carbon-coated holey grid. The vitrified samples were recorded at ≈1 μm under focus at ≈10 e/Å2 dose for image processing. A second exposure of ≈2–3 μm under focus was recorded and used as an aid in analyzing the images with the focal pair method (13). The images were recorded on Kodak SO 163 film at a nominal magnification of ×50,000 in a JEOL JEM 1200 electron microscope operated at 100 kV.
Data Processing. The focal pair method (13, 14) of orientation determination, refinement, and 3D reconstruction as implemented in the IMIRS software package (15) was used except that an additional step of particle-size evaluation was performed in the current reconstruction as described (5). Data sets consisting of 1,500 and 690 particle images of PDC with a molar ratio of 60 E1/E2 core and ≈24 E1/E2 core, respectively, were processed.
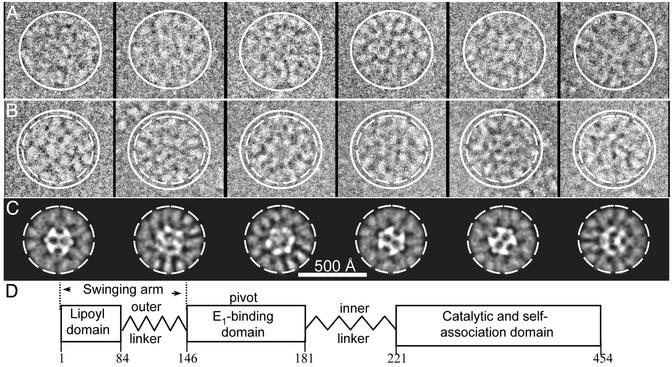
For both data sets, an iterative procedure was implemented to classify the particles according to their sizes by using the SIZEDIFF program (5) with contrast transfer function correction incorporated. A preliminary 3D reconstruction was calculated by combining all of the particles, and this “average” reconstruction was used to classify the images as described (5) into a 1.0 size group comprising a 3% size variation of the images. For the PDC with ≈60 E1/E2 core, the converged structure from 128 images in the 1.0 size group, which was similar to the larger structure shown in Fig. 2 A, was then used as a model to classify 45 and 80 images in the 0.95 and 1.05 size groups, respectively. For the WT PDC preparation (24 E1/E2 core) the converged structure from 80 images in the 1.0 size group (see Fig. 3) was used as model to classify 46 and 53 images in the 0.95 and 1.05 size groups, respectively. The image size distribution appears bell-shaped and is consistent with a more extensive data set of the human PDC (Y.G., Z.H.Z., Y. Hiromasa, H. Bao, X. Yan, T. E. Roche, and J.K.S., unpublished results). The finding that 1.0 size groups consist of the larger and smaller reconstructions in the PDC preparations according to their greater or lesser degree of E1 occupancy, respectively, indicates that the extent of E1 binding is related to the variable size of the molecules.
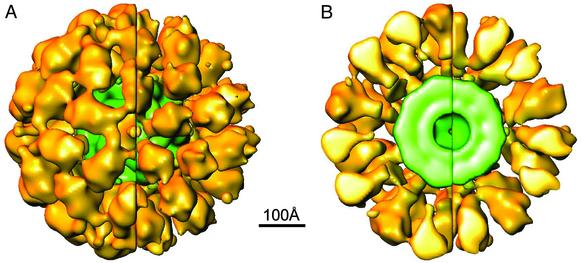
Fig. 2.
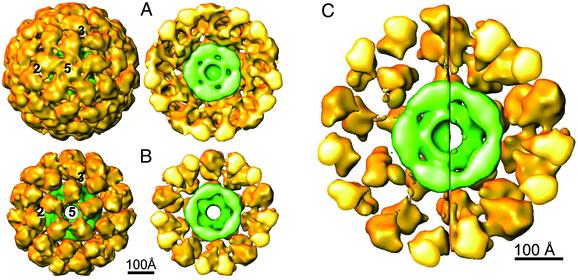
3D reconstructions representative of the larger (A) and smaller (B) molecules associated with the PDC preparation with the 60 E1/E2 core. The larger structure (≈550 Å in diameter) has significant protein density in the 5-fold opening of the outer shell, and its cutaway structure shows that heart-shaped architecture of the E1 component is distorted. The smaller structure in B (≈500 Å in diameter) has no protein density in the 5-fold opening of the outer shell, and its cutaway structure shows that the architecture of the E1 component is similar to the x-ray structure of E1 (Fig. 5). (C) The side-by-side comparison of these cutaway structures shows that the most significant difference between the size of the two molecules is related to the difference in the diameters of the outer shells. Presumably, the inner linker (Fig. 1D) is more extended in the larger structure and this conformation is related to the loss of resolution in the outer shell (70 Å in A).
Fig. 3.
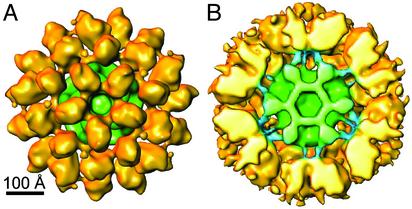
Solid-shaded view of the 3D reconstruction representative of images in the 1.0 size group from WT PDC preparation with 24 E1/E2 core (A). At a reduced threshold its cutaway structure reveals the inner linker that binds E1 to the core (B). The linkers have a similar elbow-bend shape and configuration about the E2 trimer shown in the bovine kidney PDC (7).
3D Visualization and Fitting of Atomic Structures. The 3D visualization was performed by using IRIS EXPLORER (NAG, Downers Grove, IL) with custom-designed modules (16). The maps were displayed at a threshold that could just accommodate the size and shape of the x-ray structure of the E1 tetramer unless otherwise indicated. In the figures of the WT PDC, the threshold of the reconstruction corresponds to ≈0.66 σ (SD) above the mean density of the map, whereas in the figures of the ≈60 E1/E2 core PDC, it corresponds to ≈1.0 σ. The atomic coordinates of the PDC components were downloaded directly from the Protein Data Bank for fitting into our cryo-EM structures as described (7).
Results
Image Classification. We have used size variation analyses (5) to classify images recorded from preparations of the WT S. cerevisiae PDC to which sufficient E1 was added to occupy its 60 binding sites (Fig. 1 A and B) and the same preparation with about one-third of the E1 binding sites occupied. Two 3D reconstructions representative of images that vary in size by 10–12% (≈50 Å in diameter) from these preparations were computed to document the E1 organization about the core and the length of its inner linkers (Fig. 1D). In this regard, our previous structure of the WT bovine kidney PDC in which ≈22 E1s were bound indicated that the outer shell could readily accommodate 60 molecules of E1 without significant crowding. Surprisingly, this study shows that extensive E1 binding favors a more extended inner linker and an altered arrangement of E1 about the core.
Fig. 1.
Representative images of the S. cerevisiae PDC molecules with ≈60 E1/E2 core (A and B) and a diagram of the domain organization of E2 showing its E1-binding site (D). The solid circle around the images in A and B corresponds to the approximate diameter of the large molecules in A (≈550 Å in diameter) and serves as an aid in determining the relative size of the smaller images (≈500 Å in diameter) in B. The images in A and B are representative of those used for the 3D reconstructions shown in Fig. 2 A and B, respectively. The broken circle corresponds to the size of the smaller images in B and shows that the projections (C) of the reconstruction in Fig. 2B are representative of the same orientation of molecules in B. These images have a similar size and protein density distribution in the E1 outer shell. The E1 molecules are bound to the E2 central core by its E1-binding domain in the inner linker as illustrated in D.
Comparison of the 3D Reconstructions Representative of the Smaller and Larger Images from the PDC Preparation with Near Full E1 Occupancy. The 5-fold view of the larger structure and its cutaway presentation (Fig. 2A) shows that the 5-fold axes are occupied by the E1 molecules and the shape of the E1 component is significantly distorted from that of the E1 tetramer. Even though the resolution of the reconstruction is ≈30 Å, the resolution value of the outer shell is significantly greater (70 Å). Thus, the deterioration of the resolution and the perturbation of the E1 morphology in the EM map is apparently related to a significant lack of icosahedral arrangement of the E1. Even though the map of the E1 component is distorted, its shape indicates that the apex of the E1 tetramer comprising the binding domain for E2 is oriented toward the core in a manner similar to its orientation in the smaller structure (see below). Of interest, the apex is not revealed in this map at an increased threshold of 1.5 σ, and the outer shell becomes thinner (compare Fig. 2 A and C), thus better corresponding to this component in the Bacillus stearothermophilus PDC (9). The thin outer shell led to the proposal that the 2-fold axis (long axis) of E1 is approximately tangent to the outer shell, and consequently, its E1 binding domain (see Fig. 5B) is 90 Å from the core (9) (see below).
Fig. 5.
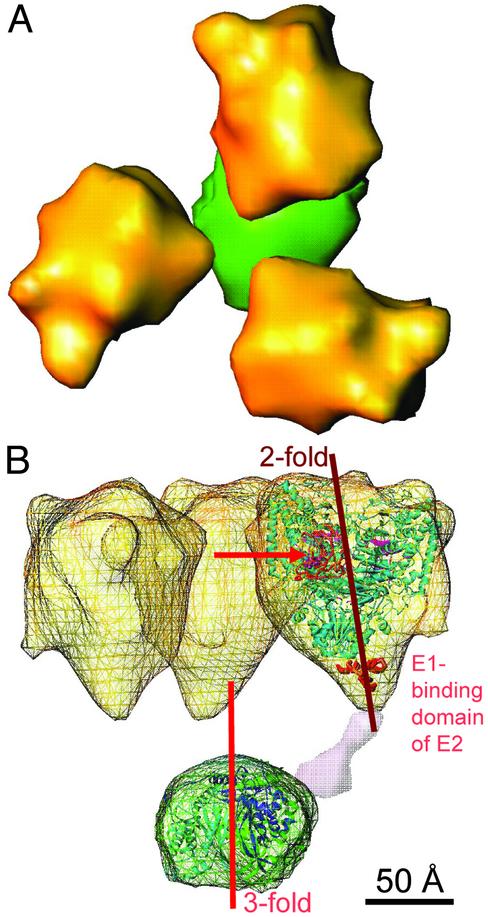
Cutaway representation of the structural unit from the smaller structure (Fig. 2B) of the 60 E1/E2 core S. cerevisiae PDC. (A) Top view comprising the E2 trimer and its associated E1 tetramers. (B) Side view of the wire frame presentation of the EM envelope in which the atomic structure of Pseudomonas putida E1 (light blue) (33), the B. stearothermophilus truncated E2 (27), lipoyl domain (orange, denoted by the arrow) (34), and the putative E1-binding domain of E2 (bold red) (35) are docked. The E1 tetramer was docked in the cryo-EM envelope so that the E1-binding domain of E2 and its inner linker are close to the 2-fold axis of E1 where the C-terminal domains of the β subunits meet (33). One of the three inner linkers (purple) is inserted according to its deposition in the EM map of Fig. 3B. The structural organization of this functional unit is very similar to the corresponding component of the bovine kidney PDC (7).
The size variation analysis revealed a subset of smaller molecules in this PDC preparation that represents ≈5% of the data set (compare Fig. 1 A and B). In contrast to the structure in Fig. 2 A, the E1 components associated with the ≈10% smaller reconstruction have a morphology more representative of the E1 molecule and lack the deposition of the E1 component on the 5-fold opening of the complex (compare Fig. 2 A and B). The size (≈500 Å) and architecture of this structure are also very similar to those of the bovine kidney PDC (7).
The side-by-side comparison of the cutaway structures representative of the size groups (Fig. 2C) shows the striking difference in the architectures of their E1 components and reveals that the major difference in size of these PDCs is primarily related to the difference in the diameters of their outer shells (550 versus 500 Å). Because their inner E2 cores are of similar size and architecture it appears that the size difference is primarily related to an increase in length of the inner linker from ≈50 to 75 Å (Fig. 2C). We do not attribute the size difference to a variable extent of E1 binding to the E2 core because the binding interaction between E1 and the E1-binding domain of E2 is very tight (Kd = 0.3 nM) (17) and the E1-binding sites were saturated. Moreover, the averaged protein density in the outer shell of the smaller structure (Fig. 2B) is comparable to the density of the 60-subunit inner E2 core. Interestingly, the larger structure exhibits significantly more BP and E3 bound inside the E2 core than is associated with the smaller structure. Our size variation analysis of BP and E3 bound to truncated S. cerevisiae core shows that their extent of binding is more prominent in the larger core (18).
These studies suggest that near saturation of the E1-binding sites of the S. cerevisiae PDC results in a predominance of molecules with the extended inner linker. The WT bovine kidney PDC with ≈22 E1s bound did not have this prominence of larger molecules (7). Accordingly, we have investigated the effect of the extent of E1 binding on its deposition about the E2 core by determining the structures of the WT S. cerevisiae PDC with ≈24 E1 bound. It is interesting to note that WT bovine kidney and heart PDCs include 20–22 and ≈30 E1 molecules about the E2 core, respectively, and the WT S. cerevisiae PDC has ≈24 E1 molecules associated with its complex.
Size Variation Analyses of the WT S. cerevisiae PDC. In contrast to the prominence of larger molecules associated with extensive E1 binding, smaller molecules with a diameter of ≈500 Å dominate this data set in this preparation (≈24 E1/E2 core) (see below). The architecture and deposition of the E1 molecules is similar to the reconstruction shown in Fig. 2B and to that of the bovine kidney PDC (≈22 E1/E2 core) (Fig. 3). Furthermore, at a reduced threshold, the inner linker that anchors three E1 molecules to the underlying E2 trimer of the core has a similar arrangement and the elbow bend configuration (Fig. 3B) revealed in the bovine kidney PDC (7). Presumably the inner linker was not revealed in the reconstruction in Fig. 2B because of the more prominent Fresnel fringe effect (19) associated with images recorded at greater defocus (2–3 μm). A subset of images representing ≈10% of the molecules in the data set is similar to the ≈550-Å size of the larger complex (Fig. 4), and the E1 component has the distorted morphology similar to that associated with the larger structure (Fig. 2 A) containing ≈60 E1s. Thus, these comparisons show that both PDC preparations consist of molecules with an inner linker of variable length and that extensive E1 binding to the core favors molecules with the more extended linker.
Fig. 4.
Side-by-side comparison of the 3D reconstruction representative of the larger (≈550 Å in diameter) and smaller (≈500 Å in diameter) derived from images of the WT PDC preparation with a E1/E2 core ratio of 24:1. These structures were obtained from images classified in the smaller and larger size groups. The outside (A) and cutaway (B) views show that the architecture of the E1 component is distorted in the larger structure whereas the morphology of this component associated with the smaller structure is more representative of the shape of the x-ray structure (Fig. 5B). The difference in the size of these structures appears primarily related to variation in the length of the inner linker as seen in Fig. 2C. The identification of the two size groups in the WT PDC preparation indicates that variable deposition of E1 about the core has in vivo relevance.
Discussion
Relationship Between the Length of the Inner Linker and E1 Occupancy in the PDC. The E2 component of S. cerevisiae PDC is organized with three major domains: the C-terminal catalytic and self-association domain, the E1-binding domain, and the N-terminal lipoyl domain (Fig. 1D). These domains are connected by flexible linkers, and the length of the inner linker that connects the E1 binding domain to the core is variable (Figs. 2C and 4).
A possible explanation for the relationship between E1 occupancy and the length of the inner linker is the crowding of the E1 molecules in the outer shell. To relieve the crowding, the inner linker of E2 and its E1-binding domain extend further from the core, thus increasing the radius of the outer shell (Fig. 2C). Moreover, the E1 molecule may reside on the 5-fold axis, further reducing the crowding (compare Fig. 2 A and C). Although attractive, this explanation is discounted by a comparison of WT S. cerevisiae PDC structures of different sizes in which only ≈24 E1s are bound (Fig. 4). The side-by-side cutaway presentation representative of the smaller and larger molecules shows that an extended inner linker contributes significantly to the size difference of the complexes under a circumstance in which only about one-third of the E1-binding sites are occupied. Furthermore, the presence of a significant number of smaller molecules (5%) in which nearly all of the E1-binding sites are occupied (Fig. 1 A and B) is not consistent with an explanation involving steric crowding.
The possibility has been considered that the inner linker may have a conformation that varies between taut (elbow-bend shape) and relaxed (extended shape). The more extensive the E1 binding appears to favor the more extended state of the linker, which is also associated with the lack of apparent icosahedral arrangement of the E1 molecules (Fig. 2 A and B) about the core. Perhaps the interaction between adjacent E1 molecules is more favorable with an extended, flexible inner linker, and this conformation is also favored by more extensive E1 binding. In any event, it is known that extent of ligand binding to oligomeric proteins may influence the conformation state of the complex (20).
The inner linker of E1 consists of ≈40 residues (21–23) and, consequently, its fully extended polypeptide chain ≈140 Å in length (3.5 Å per residue), may readily accommodate the size variation of the outer shells (50 Å in diameter) of the PDCs. Moreover, the inner linker includes three proline residues, and this residue is often associated with a flexible region in polypeptide chains (24).
Comparison of Models Proposed for the Arrangement of E1 About the Core. A model of the architecture of recombinant E1/E2 complex of B. stearothermophilus containing ≈60 E1 molecules per core was proposed recently (9). This composition is similar to that of the S. cerevisiae ≈60 E1/E2 PDC preparation, and the resolutions of the two reconstructions are also similar, ≈28 and 30 Å (Fig. 2 A), respectively. However, we determined the resolution value of the E1 outer shell of the S. cerevisiae PDC reconstruction, which was estimated by generally accepted procedures (25, 26) to be ≈70 Å. This much higher value than the 30 Å for the entire complex indicates that the architecture of this region of the complex is not appropriately rendered in a preparation of larger molecules in which nearly all of the E1-binding sites are occupied. The resolution value of the E1 outer shell of B. stearothermophilus E1/E2 complex was not reported, but it is probable that its outer shell would be of decreased resolution. We did not attempt to dock the E1 x-ray structure in the outer envelope of the larger S. cerevisiae PDC structure because of its loss of icosahedral symmetry (Fig. 2 A). Furthermore, the projections of various orientations of the B. stearothermophilus PDC structure show that the outer ring is poorly delineated and thinner (washed out) with respect to their class average images at corresponding orientations (compare figure 3 B and E in ref. 9), and the reconstruction appears significantly smaller than the images of the molecules and their class averages. In contrast, our comparison of the projections of the smaller structure and the images at the corresponding orientations of the S. cerevisiae PDC molecules show a good correspondence between their size and the width of their outer rings (Fig. 1 B and C). These comparisons provide strong evidence that the EM map for the outer shell of the smaller ≈60 E1/E2 core preparation is reliable and lends strong support for our proposed arrangement of E1 about the core.
Structure–Function Relationships. The central core of the eukaryotic PDCs consists of 60 E2 subunits arranged in sets of three at 20 vertices of a pentagonal dodecahedron. The three E2 subunits exhibit extensive interactions between them (27) that may be important in holding together the much larger (about six times) E1 molecules (Fig. 5). The E2 trimers are cone-shaped with an outward-directed triangular base and are held together by 30 bridges with tenuous, flexible connections. The cutaway structural unit of the S. cerevisiae PDC is very similar to the corresponding unit of the bovine kidney PDC (7) (Fig. 5). Thus, the 2-fold symmetry axis of E1 and the 3-fold symmetry axis of E2 have a similar relationship, and the single lipoyl domain of E2 (Fig. 1D) appears to reside inside the cage that is formed by the three inner linkers, the E1 components, and the E2 trimer that forms the base of the cage (Fig. 5B). The improved fit of the ribbon structures of the lipoyl domain, E1-binding domain of E2, and the E1 tetramer compared with the bovine kidney PDC probably results from the much higher E1 occupancy in the S. cerevisiae PDC preparation of ≈60 E1s bound compared with ≈22 E1s associated with the bovine kidney PDC (7). It was argued that this arrangement of catalytic units cannot easily account for the well-documented insensitivity of the overall catalytic activity to the inactivation of a significant fraction of E1 or to the excision of lipoyl domains from E2 (9). To the contrary, because the E1 component is involved in the rate-limiting step of the PDC reactions, the PDC activity is sensitive to its E1 occupancy (see below). Moreover, this is the basis for the regulation of PDC activity by the phosphorylation and dephosphorylation of E1 by kinases and phosphatases, respectively (28). Furthermore, the insensitivity of the overall reaction to limited excision of lipoyl domains is consistent with the arrangement of Fig. 5B, because the lipoyl domains in the WT PDC preparations exceed the low E1 occupancy by ≈3:1 (S. cerevisiae PDC) or 6:1 (mammalian PDC).
Similar to its arrangement in the bovine kidney PDC, the S. cerevisiae E1-binding domain serves as a pivot or anchoring point for the “swinging arm” comprising the outer linker and the lipoyl domain (7). The anchor appears to be centrally located ≈50 Å from the E1, E2, and E3 active sites. This study shows that the elbow bend configuration of the 50-Å long inner linker (Fig. 3B) may become more extended to ≈75 Å, and that E1 binding to the linker favors the more relaxed or extended state. In the extended conformation, the icosahedral symmetry arrangement of the E1 component is lost (Fig. 2 A). Moreover, in this arrangement the pivot of lipoyl domain is no longer centrally located because it is ≈75 Å from the E2 and E3 active sites located near the outer shell of the core but 50 Å from the E1 active site (Figs. 1D and 4B). Even so, the flexibility of the outer linker (Fig. 1D) appears to effectively accommodate this variability in the distance and the alteration in the arrangement of the E1 molecules because the activity of human recombinant PDC lacking E1 is approximately proportional to E1 added (Y. Hiromasa, J.K.S., and T. E. Roche, unpublished results).
It has been appreciated for many years that the swinging arms (Fig. 1D) contribute to the protein dynamics of the PDC and function to shuttle the intermediates of catalysis between the numerous catalytic centers (4). Our studies show that the E2 breathing core and the flexible arm of its inner linker, the varying orientations of E1 about the core, and the wobbling E3 within its pentagonal opening also contribute to the global protein dynamics of the PDC and presumably to its catalytic proficiency. In retrospect, this exceptional example of protein dynamics is not unexpected because of the loose and open packing arrangement of its components in the complex (5). An organization in which the E2 trimers are loosely held together in an open scaffold that loosely accommodates the E3 dimer in its pentagonal opening and the E1 tetramers tethered on its extended, flexible linker is well suited to accommodate the protein dynamics of the largest multienzyme complex known. Proteins that exhibit thermally induced flexibility have been aptly named “soft proteins” and neutron scattering and other physicochemical methods have been used to characterize their dynamics (29).
It has been hypothesized that thermally driven enzyme dynamics may be crucial to an enzyme's activity even when the motions are unrelated to its active site (30). Our studies of the PDC (5) show that cryo-EM is well suited to contribute to this aspect of protein dynamics (29, 31) because it offers a snapshot of the images of protein molecules in variable conformational states at room temperature. Classification protocols such as the one used herein (5) make it possible to document the conformational states of macromolecular complexes in 3D and, therefore, to relate the structural changes to function. Consequently, it appears that 3D EM will become one of the major contributors and add impetus to this rising interest in enzyme dynamics (32).
Acknowledgments
We thank Dr. Austin Riggs and Claire Riggs for determining the molecular mass of the S. cerevisiae PDC, Dr. Marvin Hackert and Dr. Thomas Roche for helpful discussion, and Imani Muhammad for secretarial support. This work was supported in part by American Heart Association Grant 0240216N (to Z.H.Z.), U.S. Public Service Grants EB00276 and HL42886 (to J.K.S.), AI46420 and CA94809 (to Z.H.Z.), and GM06590 (to L.J.R.), and the Foundation for Research (to L.J.R.).
Abbreviations: E1, pyruvate dehydrogenase; PDC, E1 complex; E2, dihydrolipamide acetyl-transferase; E3, dihydrolipoamide dehydrogenase; BP, binding protein; EM, electron microscopy.
References
- 1.Reed, L. J. & Hackert, M. L. (1990) J. Biol. Chem. 265, 8971–8974. [PubMed] [Google Scholar]
- 2.Patel, M. S. & Roche, T. E. (1990) FASEB J. 4, 3224–3233. [DOI] [PubMed] [Google Scholar]
- 3.Guest, J. R., Angier, S. J. & Russell, G. C. (1989) Ann. N.Y. Acad. Sci. 573, 76–99. [DOI] [PubMed] [Google Scholar]
- 4.Perham, R. N. (2000) Annu. Rev. Biochem. 69, 961–1004. [DOI] [PubMed] [Google Scholar]
- 5.Zhou, Z. H., Liao, W., Cheng, R. H., Lawson, J. E., McCarthy, D. B., Reed, L. J. & Stoops, J. K. (2001) J. Biol. Chem. 276, 21704–21713. [DOI] [PubMed] [Google Scholar]
- 6.Stoops, J. K., Cheng, R. H., Yazdi, M. A., Maeng, C. Y., Schroeter, J. P., Klueppelberg, U., Kolodziej, S. J., Baker, T. S. & Reed, L. J. (1997) J. Biol. Chem. 272, 5757–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou, Z. H., McCarthy, D. B., O'Connor, C. M., Reed, L. J. & Stoops, J. K. (2001) Proc. Natl. Acad. Sci. USA 98, 14802–14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenknecht, T., Grassucci, R., Radke, G. A. & Roche, T. E. (1991) J. Biol. Chem. 266, 24650–24656. [PubMed] [Google Scholar]
- 9.Milne, J. L., Shi, D., Rosenthal, P. B., Sunshine, J. S., Domingo, G. J., Wu, X., Brooks, B. R., Perham, R. N., Henderson, R. & Subramaniam, S. (2002) EMBO J. 21, 5587–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kresze, G. B. & Ronft, H. (1981) Eur. J. Biochem. 119, 573–579. [DOI] [PubMed] [Google Scholar]
- 11.McCartney, R. G., Sanderson, S. J. & Lindsay, J. G. (1997) Biochemistry 36, 6819–6826. [DOI] [PubMed] [Google Scholar]
- 12.Maeng, C. Y., Yazdi, M. A., Niu, X. D., Lee, H. Y. & Reed, L. J. (1994) Biochemistry 33, 13801–13807. [DOI] [PubMed] [Google Scholar]
- 13.Zhou, Z. H., He, J., Jakana, J., Tatman, J. D., Rixon, F. J. & Chiu, W. (1995) Nat. Struct. Biol. 2, 1026–1030. [DOI] [PubMed] [Google Scholar]
- 14.Fuller, S. D. (1987) Cell 48, 923–934. [DOI] [PubMed] [Google Scholar]
- 15.Liang, Y., Ke, E. Y. & Zhou, Z. H. (2002) J. Struct. Biol. 137, 292–304. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty, M. & Chiu, W. (2000) Microsc. Microanal. 6, 282–283. [Google Scholar]
- 17.Lessard, I. A., Fuller, C. & Perham, R. N. (1996) Biochemistry 35, 16863–16870. [DOI] [PubMed] [Google Scholar]
- 18.Zhou, Z. H., Reed, L. J. & Stoops, J. S. (2003) in Thiamine: Catalytic Mechanisms and Role in Normal and Disease States, eds. Jordan, F. & Patel, M. S. (Dekker, New York), in press.
- 19.Hall, C. E. (1966) Introduction to Electron Microscopy (McGraw–Hill, New York).
- 20.Fersht, A., ed. (1984) Enzyme Structure and Mechanism (Freeman, New York), pp. 263–292.
- 21.Lawson, J. E., Behal, R. H. & Reed, L. J. (1991) Biochemistry 30, 2834–2839. [DOI] [PubMed] [Google Scholar]
- 22.Lawson, J. E., Niu, X. D. & Reed, L. J. (1991) Biochemistry 30, 11249–11254. [DOI] [PubMed] [Google Scholar]
- 23.Rahmatullah, M., Gopalakrishnan, S., Radke, G. A. & Roche, T. E. (1989) J. Biol. Chem. 264, 1245–1251. [PubMed] [Google Scholar]
- 24.Branden, C. I. & Tooze, J. (1991) Introduction to Protein Structure (Garland, New York).
- 25.Mancini, E. J., Clarke, M., Gowen, B. E., Rutten, T. & Fuller, S. D. (2000) Mol. Cell 5, 255–266. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, H., Zhang, J., Yu, X., Lu, X., Zhang, Q., Jakana, J., Chen, D. H., Zhang, X. & Zhou, Z. H. (1999) J. Virol. 73, 1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izard, T., Aevarsson, A., Allen, M. D., Westphal, A. H., Perham, R. N., de Kok, A. & Hol, W. G. (1999) Proc. Natl. Acad. Sci. USA 96, 1240–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roche, T. E., Baker, J. C., Yan, X., Hiromasa, Y., Gong, X., Peng, T., Dong, J., Turkan, A. & Kasten, S. A. (2001) Prog. Nucleic Acid Res. Mol. Biol. 70, 33–75. [DOI] [PubMed] [Google Scholar]
- 29.Zaccai, G. (2000) Science 288, 1604–1607. [DOI] [PubMed] [Google Scholar]
- 30.Wilson, E. K. (2000) Chem. Eng. News 78, 42–45. [Google Scholar]
- 31.Schulten, K. (2000) Science 290, 61–62. [DOI] [PubMed] [Google Scholar]
- 32.Balabin, I. A. & Onuchic, J. N. (2000) Science 290, 114–117. [DOI] [PubMed] [Google Scholar]
- 33.Aevarsson, A., Seger, K., Turley, S., Sokatch, J. R. & Hol, W. G. (1999) Nat. Struct. Biol. 6, 785–792. [DOI] [PubMed] [Google Scholar]
- 34.Dardel, F., Davis, A. L., Laue, E. D. & Perham, R. N. (1993) J. Mol. Biol. 229, 1037–1048. [DOI] [PubMed] [Google Scholar]
- 35.Robien, M. A., Clore, G. M., Omichinski, J. G., Perham, R. N., Appella, E., Sakaguchi, K. & Gronenborn, A. M. (1992) Biochemistry 31, 3463–3471. [DOI] [PubMed] [Google Scholar]