Abstract
Steroid hormone action during brain development exerts profound effects on reproductive physiology and behavior that last into adulthood. A variety of in vitro studies indicate that steroid receptors require nuclear receptor coactivators for efficient transcriptional activity. To determine the functional significance of the nuclear receptor coactivator SRC-1 in developing brain, we investigated the consequence of reducing SRC-1 protein during sexual differentiation of the brain. We report that reducing SRC-1 protein interferes with the defeminizing actions of estrogen in neonatal rat brain. Our data indicate that SRC-1 protein expression is critically involved in the hormone-dependent development of normal male reproductive behavior and brain morphology.
Intracellular receptors for steroid hormones constitute a superfamily of transcription factors that includes receptors for estrogens, progestins, androgens, glucocorticoids, thyroid hormone, retinoic acid, and 9-cis retinoic acid along with numerous orphan receptors with as-yet unidentified ligands (1). Spontaneously occurring mutations in steroid receptors or disruptions of normal steroid hormone levels during development have profound and devastating effects on adult reproductive physiology (2–4). However, not all endocrine-related disorders can be explained by disruptions in steroid receptors or hormone levels, and a potential role for an additional class of proteins, nuclear receptor coregulators, has been implicated (5).
Steroid hormones act in the brain by binding to intracellular receptors located predominantly in neurons. On ligand binding, the steroid–receptor complex binds to a hormone response element located on DNA (6, 7) where it regulates gene transcription and ultimately neuronal function (7, 8). Recent studies reveal that steroid receptors interact with other proteins, nuclear receptor coactivators or corepressors, that increase or decrease their binding and action at the hormone response element, respectively. The first nuclear receptor coactivator to be characterized is steroid receptor coactivator-1 (SRC-1; refs. 9 and 10). The majority of studies to date have used transfection assays in cell culture systems in which large quantities of steroid receptors are expressed to elucidate interactions with coactivators and corepressors. Although this approach is valid, it does not allow for assessment of the in vivo role of nuclear receptor coactivators and their physiological significance, which remain largely unknown at this time. A recent study indicates that peripheral steroid target organs of mice containing a targeted disruption of the SRC-1 gene have a decreased response to steroid hormones (11); however, it is not known whether SRC-1 protein mediates steroid hormone action within the developing brain.
To determine the functional significance of SRC-1, we examined the influence of SRC-1 on sexual differentiation of the rodent brain. The developing brain is an exquisitely sensitive target organ for steroid hormones, which differentiate the neural substrate and thereby exert enduring effects on adult physiology and behavior. This steroid-mediated “sexual differentiation” of the brain is determined by the secretion of gonadal hormones during the perinatal period. At birth, male rats are exposed to high levels of testicularly derived testosterone, resulting in behavioral masculinization, defined as increased male-typical behaviors, and behavioral defeminization, defined as decreased female-typical behaviors. The absence of the testosterone surge in females results in the feminization of rat brain. Neonatally castrated male rats grown to adulthood exhibit decreased male and increased female sexual behavior under the appropriate hormonal conditions (12). Likewise, females administered testosterone neonatally do not display normal female sexual behavior as adults (13, 14) but will display male-typical behaviors if treated with exogenous testosterone.
A central aspect of steroid-mediated differentiation of the brain is that, although testosterone secreted by the testis is the primary hormonal signal, once in the brain, it is metabolized into two principle ligands: dihydrotestosterone by 5α-reductase or estradiol by aromatase. The subsequent activation of either androgen or estrogen receptors mediates distinct aspects of the differentiation process in Sprague–Dawley rats. For example, increased estrogen receptor activation is responsible for defeminization (12, 15, 16), whereas increased androgen receptor activation seems to be responsible for masculinization (12, 17–19). Blocking the aromatization of testosterone into estradiol interferes with defeminization but not masculinization in male rats (20), because androgen receptors are still being activated. Similarly, treatment of neonatal female rats with a nonaromatizable androgen, dihydrotestosterone, increases male sexual behavior (18) but does not suppress female sexual behavior (21, 22), indicating that androgen receptor activation masculinizes but does not defeminize sexual behavior. One neural substrate that is markedly influenced by estrogen action is the sexually dimorphic nucleus (SDN) of the preoptic area, which is three to four times larger in males than in females (23). This dimorphism depends strictly on activation of estrogen receptors via the normal aromatization of testosterone into estradiol. Thus, the select involvement of either the androgen receptor or estrogen receptor in distinct behavioral responses and brain morphology makes this system ideal for investigating the specificity of a highly promiscuous steroid receptor coactivator, such as SRC-1, in vivo.
Materials and Methods
Antisense Oligodeoxynucleotide Infusions.
We bilaterally infused antisense oligonucleotides, scrambled oligonucleotides, or vehicle into the hypothalamus on the day of birth [postnatal day 0 (PN0)], PN1, and PN2 with a modified stereotaxic apparatus and a 1-μl Hamilton syringe. Pups were cryoanesthetized before each infusion. Bregma is visible through the skin under bright light and was used as a landmark for the hypothalamus. Bilateral injections [1 μg of oligodeoxynucleotides (ODNs) dissolved in 1 μl of 0.9% saline] were aimed at the mediobasal hypothalamus (1.0 mm anterior and 0.8 mm lateral to bregma; 5.0 mm below the skull). Dye injections indicate that the spread of injection is focused but not limited to the hypothalamus. The sequence for the 21-mer antisense oligonucleotide spanning the putative start codon of SRC-1 is 5′-CTG-TCC-CCA-AGG-CCA-CTC-ATG-3′, and the 21-mer scrambled control oligonucleotide is 5′-CCC-TAG-CAG-CTA-CAG-CTT-CGC-3′. Chimeric ODNs were obtained from Oligos Etc. (Wilsonville, OR). These second-generation chimeric ODNs contain limited phosphorothioate linkages allowing for greater resistance to nuclease cleavage and increased activation of RNase H, an enzyme that cleaves the RNA strand of DNA/RNA hybrids.
SDN Volume.
Newborn Sprague–Dawley female rats were cryoanesthetized and placed in a modified stereotaxic apparatus with a 1-μl Hamilton syringe. Pups were then bilaterally infused into the hypothalamus with 1 μl of saline containing 1 μg of either SRC-1 antisense ODNs (n = 6) or scramble ODNs (n = 5) on the day of birth, PN1, and PN2. On PN1, rats were injected with 100 μg of testosterone propionate, a dose known to masculinize behavior and increase SDN volume. On PN13, rats were perfused with 4% paraformaldehyde, and brains were removed and placed into 30% sucrose overnight. Sections (50-μm-thick) through the preoptic area were mounted onto glass slides and stained with cresyl violet. We measured the area of the SDN in each section that it appeared, which ranged from 4 to 7 sections per rat. Measurements of SDN volume were made twice by two different experimenters blind to the treatment groups, and the results were essentially identical for the two readings. Volumetric reconstruction of the SDN was accomplished via camera lucida drawings that were then analyzed on a light box with a Zeiss microscope fitted with a MTI charge-coupled device 72 camera (Dage–MTI, Michigan City, IN) connected to a Macintosh computer. The software used for image analysis was the public domain National Institutes of Health image program (http://rsb.info.nih.gov/nih-image/). Nuclear volume was determined according to the procedure of Bloch and Gorski (ref. 24; total area times section thickness and corrected for magnification).
Female Sexual Behavior.
Newborn Sprague–Dawley rats were bilaterally infused with SRC-1 antisense ODNs, scrambled ODNs, or saline vehicle into the hypothalamus on the day of birth, PN1, and PN2, as described above. On PN1, one group of females was injected with 100 μg of testosterone propionate in sesame oil, and one group of females was injected with control vehicle. Males were injected with control vehicle. All of the rats were grown to adulthood and gonadectomized around PN45 under ketamine/acepromazine anesthesia. At 2 weeks after surgery, rats were steroid primed with 10 μg of estradiol benzoate followed 48 h later by 500 μg of progesterone, a paradigm that reliably induces female sexual behavior. At 4 h after progesterone treatment, rats were placed in a glass arena and allowed to acclimate. Behavioral testing occurred during the dark phase of the light cycle under dim red light. The flanks and perineum were stimulated manually by the experimenter, and lordosis quotients and ratings were scored. Lordosis quotients indicate the number of responses/number of attempts multiplied by 100. The lordosis rating indicates a lordosis intensity range based on a 0–3 scale. The experimenter scoring the lordosis response was blind to the treatment group.
Male Sexual Behavior.
The same males and androgenized females from above were implanted with testosterone-filled capsules after testing for female sexual behavior. Neonatally oil-injected females were not tested, because they consistently show no or very low levels of male sexual behavior. At 3 weeks after surgery, rats were placed in a glass arena and allowed to acclimate. A sexually receptive female was then placed into the arena, and the total number of mounts, intromissions, and latency to first mount were recorded over a 15-min test period. Rats were tested twice for male sexual behavior over a 2-week period. Behavioral testing occurred during the dark phase of the light cycle under dim red light.
Western Immunoblots for SRC-1 Protein.
Newborn male rats were hypothalamically infused with either SRC-1 antisense ODNs or scramble ODNs on the day of birth. After 24 h, hypothalamic brain tissue was excised, placed on a chilled surface, snap frozen in isopentane on dry ice, and stored frozen until homogenization with ice-cold lysis buffer consisting of 50 mM Tris⋅HCL, 1% Na-deoxycholate, 0.25% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, and protease inhibitors (1 μg/ml of aprotinin, leupeptin, and pepstatin; 1 mM PMSF). After tissue homogenization, samples were centrifuged at 2,000 × g for 15 min at 4°C to sediment cellular debris and nuclei. The supernatant fraction was collected, and protein concentration was determined by a Bradford assay. Total protein (20 μg) from each rat was gel electrophoresed by using a precast SDS/PAGE [8–16% Tris glycine] and transferred to a poly(vinylidene difluoride) membrane. Membrane was washed briefly in 0.1 M TBS and blocked for 1 h in 0.1 M TBS containing 5% nonfat dry milk with constant agitation at room temperature. The membrane was then incubated with SRC-1 antibody (mouse monoclonal; 1:1,000; a generous gift of Bert W. O'Malley, Baylor College of Medicine, Houston) in TBS containing 2% nonfat dry milk for 3 h at room temperature with agitation and washed three times for 5 min each in TBS containing 0.05% Tween 20. After washes, the poly(vinylidene difluoride) membrane was incubated in a goat anti-mouse horseradish peroxidase-linked secondary antibody for 1 h at room temperature with agitation and then washed three times for 5 min each in TBS containing 0.05% Tween 20. Immunoreactive bands were detected with an enhanced chemiluminescence kit (ECL; New England Biolabs), and membrane was exposed to film (Hyperfilm-ECL, Amersham Pharmacia). Membrane was then stripped of antibodies and reblotted with a mouse monoclonal antibody against the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (1:200,000, Chemicon). Films were placed on a light box and analyzed by using the public domain National Institutes of Health image program after calibration with known standard densities.
Statistical Analysis.
Behavioral data were analyzed with a one-way ANOVA with the statistical software program sigmastat (Jandel, Corta Madera, CA). Post hoc comparisons were done by using the Student–Newman–Keuls method. SDN volume data and Western data were analyzed with Student's t test. Groups were considered statistically significant at P < 0.05.
Results
SDN Volume.
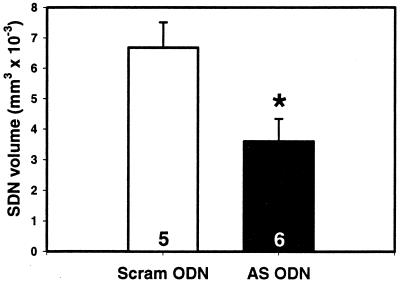
Neonatal infusions of SRC-1 antisense ODNs into the hypothalamus reduced the actions of neonatal testosterone treatment (via reducing the actions of its metabolite, estradiol) on SDN volume. The volume of the SDN in androgenized females treated with scrambled ODN was 6.68 mm3 × 10−3 ± 0.837 SEM, which is comparable to that previously reported for males at PN12 (25). Neonatal infusion of SRC-1 antisense ODNs in androgenized females reduced SDN volume by 46% (3.60 mm3 × 10−3 ± 0.744 SEM; P < 0.05, t test; Fig. 1), which is comparable to the near 50% reduction of SDN volume achieved by estrogen receptor-α antisense ODNs in androgenized female rats (16).
Figure 1.
SRC-1 antisense ODNs reduce SDN volume. Androgenized female rats (PN13) infused neonatally with SRC-1 antisense ODNs (AS ODN) had significantly smaller SDN volume contrasted with scrambled ODN controls (Scram ODN). The numbers in the bars represent the total number of rats per group. *, P < 0.05; t test.
Female Sexual Behavior.
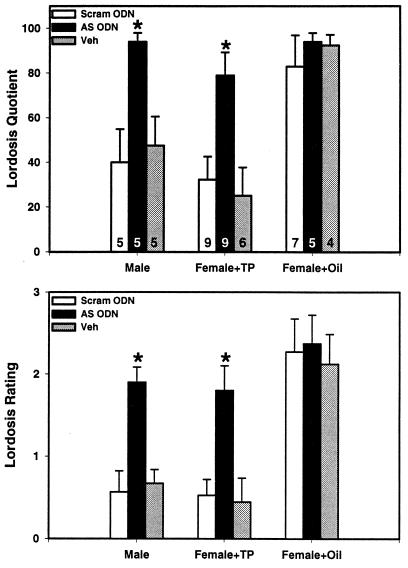
Neonatal infusions of SRC-1 antisense ODNs into the hypothalamus blocked the defeminizing actions of neonatal testosterone treatment on female sexual behavior. Male and androgenized female rats infused neonatally with SRC-1 antisense ODNs displayed significantly higher levels of lordosis contrasted to scrambled ODN- or saline-infused control males or androgenized females (P < 0.05, ANOVA; Fig. 2). As would be expected, normal female rats showed maximum levels of lordosis, and infusion of SRC-1 antisense ODNs, scrambled ODNs, or saline did not alter these responses.
Figure 2.
SRC-1 antisense ODNs block behavioral defeminization of rat brain. Lordosis quotients and lordosis ratings of neonatally oil-treated male (Male), androgen-treated female (Female + TP), and oil-treated female (Females + Oil) rats hypothalamically infused with SRC-1 antisense ODNs (AS ODN), scrambled ODNs (Scram ODN), or saline vehicle control (Veh). The numbers in the bars represent total number of rats per group. Male and androgenized female rats infused neonatally with SRC-1 AS ODNs displayed higher levels of lordosis contrasted to Scram ODN or Veh control males or androgenized females (*, P < 0.05, ANOVA).
Male Sexual Behavior.
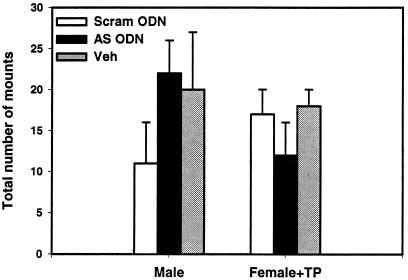
In contrast to the effects on female sexual behavior, neonatal infusions of SRC-1 antisense ODNs into the hypothalamus did not interfere with the masculinizing actions of neonatal testosterone treatment on male sexual behavior. SRC-1 antisense treatment did not alter the total number of mounts, intromissions, or mount latencies in male or androgenized female rats on the first (data not shown) or second test (P > 0.05, ANOVA; Fig. 3; Table 1).
Figure 3.
SRC-1 antisense ODNs do not block behavioral masculinization of rat brain. Total number of mounts from either neonatally oil-treated male (Male) or androgen-treated female (Female + TP) rats hypothalamically infused with SRC-1 antisense ODNs (AS ODN), scrambled ODNs (Scram ODN), or saline vehicle control (Veh).
Table 1.
Male reproductive behavior
| Group | Mounts, no. | Intromissions, no. | Latency, s |
|---|---|---|---|
| Male + Scram | 11.0 ± 5.07 | 4.80 ± 2.44 | 493 ± 168.0 |
| Male + AS | 22.3 ± 4.28 | 10.67 ± 2.63 | 205.2 ± 98.9 |
| Male + Veh | 20.5 ± 7.66 | 8.50 ± 4.99 | 413.3 ± 173.7 |
| TP-Female + Scram | 17.7 ± 2.68 | 1.11 ± 0.54 | 154.4 ± 33.3 |
| TP-Female + AS | 12.0 ± 3.87 | 2.50 ± 1.69 | 437.6 ± 138.3 |
| TP-Female + Veh | 18.8 ± 1.99 | 1.17 ± 0.83 | 189.7 ± 43.1 |
There were no statistical differences between treatment groups in the number of mounts (Mounts), the number of intromissions (Intromissions), or the latency to first mount (Latency), which was measured in seconds. Scram, scrambled ODNs; AS, antisense ODNs; Veh, vehicle control; TP, androgen-treated.
Effect of SRC-1 Antisense ODNs on SRC-1 Protein.
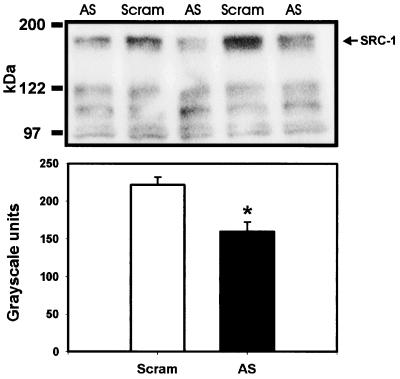
Neonatal infusions of SRC-1 antisense ODNs into the hypothalamus significantly reduced the expression of SRC-1 protein in hypothalamic tissue within 24 h contrasted to scrambled ODN controls when quantified by Western immunoblotting (P < 0.05, t test; Fig. 4). The membrane was then stripped of antibodies and reprobed with antibodies to the housekeeping glyceraldehyde-3-phosphate dehydrogenase. The levels of glyceraldehyde-3-phosphate dehydrogenase expression were not different between rats receiving SRC-1 antisense or scrambled control ODNs (P > 0.05, t test).
Figure 4.
SRC-1 antisense ODN significantly reduces SRC-1 protein in hypothalamic tissue. (Upper) Photomicrograph showing a reduction of SRC-1 protein at 160 kDa by SRC-1 antisense ODNs (AS) contrasted to scrambled ODNs (Scram). (Lower) Histogram of data collected from film analysis expressed as arbitrary gray-scale units. *, P < 0.05; t test.
Discussion
Our results indicate that SRC-1 protein mediates steroid hormone action within the developing brain. These results are provocative in that they indicate that SRC-1 protein is essential for some of the defeminizing but not the masculinizing actions of testosterone during sexual differentiation of the brain. Sexual differentiation of the SDN is known to be caused by the actions of estradiol. Neonatal exposure to estrogens or aromatizable androgens increases the volume of the SDN to levels that resemble intact males (26), whereas treatment with estrogen receptor antagonists (27) or estrogen receptor antisense ODNs (16) reduces the size of the SDN in androgenized females to resemble that of normal females. We report herein that neonatally androgenized females that received SRC-1 antisense ODNs had significantly smaller SDN volume than those treated with scrambled ODNs. The 46% reduction of SDN volume caused by SRC-1 antisense ODN treatment resembles the percentage of reduction of SDN volume by estrogen receptor-α antisense ODN treatment in neonatally androgenized female rats (16). An examination of the development of sex differences in SDN volume found that males have an SDN volume twice as large as females at PN12 (25). In the current study, SDN volume was assessed at PN13, and the SDN volume in androgenized females infused with scrambled control ODNs was similar to that of males at PN12, whereas SDN volumes from androgenized females infused with SRC-1 antisense ODNs are similar to those of normal females (25). These results suggest that SRC-1 antisense ODN treatment completely blocked the increase in SDN volume caused by testosterone treatment.
An important aspect of sexual differentiation of sexual behavior is that behavioral defeminization results from estrogen action (12, 15, 16). Our current data show that male and androgenized female rats infused with SRC-1 antisense ODNs exhibited higher levels of lordosis contrasted to control males or control androgenized females. This result indicates that reducing SRC-1 protein interfered with estradiol's ability to defeminize or reduce female sexual behavior. SRC-1 antisense ODN treatment did not interfere with the normal development of female sexual behavior in oil-treated females, because female rats infused with SRC-1 antisense ODNs showed similarly high levels of lordosis compared with scrambled ODN- or saline-infused control females. These results indicate a critical role for SRC-1 protein in modulating the actions of estrogen in developing rat brain.
In contrast to estradiol mediating defeminization of sexual behavior, masculinization of sexual behavior in Sprague–Dawley rats is largely the result of androgen action. This duality of hormone action is illustrated in experiments involving either androgen antagonists or 1,4,6-androstatriene-3,17-dione, an aromatase inhibitor that blocks the conversion of testosterone into estradiol. Treatment with an antiandrogen, cyproterone acetate or flutamide, disrupts behavioral masculinization but not behavioral defeminization of sexual behavior (19, 28). More importantly, neonatal 1,4,6-androstatriene-3,17-dione treatment disrupts behavioral defeminization but not behavioral masculinization of sexual behavior. That is, neonatal 1,4,6-androstatriene-3,17-dione treatment results in male rats that show high levels of both male and female sexual behavior under the appropriate hormonal milieu (20). In the current experiments, males and androgenized females infused with SRC-1 antisense ODNs show high levels of both male and female sexual behavior. Therefore, reducing SRC-1 protein disrupts the actions of testosterone in suppressing female sexual behavior but does not interfere with testosterone's effect on male sexual behavior. Our data do not rule out a role for SRC-1 in modulating the development of some components of male sexual behavior or androgen action. Rather, they suggest that SRC-1 may be more important in influencing estrogen receptor action than androgen receptor action. Preferential modulation of steroid receptor action has been reported to occur in coactivators for androgen receptors. The androgen receptor coactivator, ARA 70, seems to be specific for androgen receptors (29); therefore, reducing ARA 70 protein may impact androgen action more than estrogen action. It is also possible that other coactivators for androgen receptors may be able to compensate for the loss of SRC-1. Alternatively, changes in the development of female sexual behavior may be easier to detect than changes in male sexual behavior. Thus, although our data support no critical role for SRC-1 in androgen action, they do not exclude the possibility that SRC-1 is important for androgen receptor action in ways we did not detect.
SRC-1 interacts with androgen receptor in vitro; however, this interaction is different from that with estrogen receptors. SRC-1 interacts with the ligand-binding domain on estrogen receptors via its LXXLL motif, whereas it interacts with the N terminus on androgen receptors without the requirement of its LXXLL motif (30). These differences may differentially influence ligand-dependent activation of estrogen and androgen receptors such that SRC-1's interaction with estrogen receptors is essential for estrogen action, but its interaction with androgen receptors is not essential for androgen action. In addition, SRC-1 has been reported to either increase or decrease androgen receptor activity in vitro (31, 32). However, our data show no enhancement of masculine sexual behavior by SRC-1 antisense ODN treatment, which would be predicted if SRC-1 inhibited androgen receptor activity.
It is not known whether SRC-1-containing neurons also coexpress estrogen and androgen receptors. However, because estrogen receptors are coexpressed in cells that contain androgen receptors (33) and because data from our lab indicate that SRC-1 protein expression is widely distributed throughout neonatal rat brain (A.P.A. and M.M.M., unpublished data), the possibility of androgen receptor, estrogen receptor, and SRC-1 coexpression is likely. In support of this possibility, many estrogen receptor-containing neurons in the ventromedial hypothalamus, a region that is known to regulate hormone-dependent female reproductive behavior, coexpress SRC-1 (U. M. Imtiaz and M.J.T., unpublished data). Interestingly, SRC-1 is coexpressed with estrogen receptors in mammary stromal cells; however, it is not coexpressed with estrogen receptors in mammary epithelium cells (34). Therefore, it is possible that not all estrogen or androgen receptor-containing cells will coexpress SRC-1. Future studies will have to investigate the potential interactions that can occur within particular populations of cells.
The current data provide functional evidence that SRC-1 protein expression is critically involved in modulating estrogen action within the developing brain. The presence and regulation of nuclear receptor coactivators in the brain provide an additional mechanism by which steroid hormone action can be modulated or disrupted. Investigating the functional roles of steroid receptor coactivators may also contribute to the design of new therapies for endocrine disorders as well as provide information on how estrogen mimetics or other compounds disrupt reproductive physiology and behavior.
Acknowledgments
We thank Melissa Mroziak for her expert technical assistance and Ann Murphy for comments on the manuscript. We would also like to thank the member editor and the referees for constructive comments on the manuscript. Support for this work was provided by National Institutes of Health Grant MH52716 to M.M.M and HD08618 to A.P.A.
Abbreviations
- SDN
sexually dimorphic nucleus
- ODN
oligodeoxynucleotide
- PNn
postnatal day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tsai M J, O'Malley B W. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.Tenbaum S, Baniahmad A. Int J Biochem Cell Biol. 1997;29:1325–1341. doi: 10.1016/s1357-2725(97)00087-3. [DOI] [PubMed] [Google Scholar]
- 3.Brown T R. Prostate Suppl. 1996;6:9–12. [PubMed] [Google Scholar]
- 4.Toppari J, Skakkebaek N E. Baillieres Clin Endocrinol Metab. 1998;12:143–156. doi: 10.1016/s0950-351x(98)80529-6. [DOI] [PubMed] [Google Scholar]
- 5.Hsiao P W, Lin D L, Nakao R, Chang C. J Biol Chem. 1999;274:20229–20234. doi: 10.1074/jbc.274.29.20229. [DOI] [PubMed] [Google Scholar]
- 6.Jensen E V, Suzuki T, Kawashima T, Stumpf W E, Jungblut P W, DeSombre E R. Proc Natl Acad Sci USA. 1968;59:632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walters M R. Endocr Rev. 1985;6:512–543. doi: 10.1210/edrv-6-4-512. [DOI] [PubMed] [Google Scholar]
- 8.Carson-Jurica M A, Schrader W T, O'Malley B W. Endocr Rev. 1990;11:201–219. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- 9.Shibata H, Spencer T E, Onate S A, Jenster G, Tsai S Y, Tsai M J, O'Malley B W. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- 10.Onate S A, Tsai S Y, Tsai M J, O'Malley B W. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Qiu Y, Demayo F J, Tsai S Y, Tsai M J, O'Malley B W. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 12.Whalen R E, Edwards D A. Anat Rec. 1967;157:173–180. doi: 10.1002/ar.1091570208. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J G, Hamilton J B, Young W C. Yale J Biol Med. 1940;13:189–202. [PMC free article] [PubMed] [Google Scholar]
- 14.Feder H H, Phoenix C H, Young W C. J Endocrinol. 1966;34:131–132. doi: 10.1677/joe.0.0340131. [DOI] [PubMed] [Google Scholar]
- 15.Sodersten P. J Endocrinol. 1978;76:241–249. doi: 10.1677/joe.0.0760241. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy M M, Schlenker E H, Pfaff D W. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- 17.van der Schoot P. J Endocrinol. 1980;84:397–407. doi: 10.1677/joe.0.0840397. [DOI] [PubMed] [Google Scholar]
- 18.Tonjes R, Docke F, Dorner G. Exp Clin Endocrinol. 1987;90:257–263. doi: 10.1055/s-0029-1210699. [DOI] [PubMed] [Google Scholar]
- 19.Ward I L, Renz F J. J Comp Physiol Psychol. 1972;78:349–355. doi: 10.1037/h0032375. [DOI] [PubMed] [Google Scholar]
- 20.Vreeburg J T, van der Vaart P D, van der Schoot P. J Endocrinol. 1977;74:375–382. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- 21.Booth J E. J Endocrinol. 1977;72:135–141. doi: 10.1677/joe.0.0720135. [DOI] [PubMed] [Google Scholar]
- 22.Whalen R E, Rezek D L. Horm Behav. 1974;5:125–128. doi: 10.1016/0018-506x(74)90035-x. [DOI] [PubMed] [Google Scholar]
- 23.Gorski R A, Gordon J H, Shryne J E, Southam A M. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 24.Bloch G J, Gorski R A. J Comp Neurol. 1988;275:604–612. doi: 10.1002/cne.902750408. [DOI] [PubMed] [Google Scholar]
- 25.Sickel M J, McCarthy M M. J Neuroendocrinol. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- 26.Dohler K D, Hancke J L, Srivastava S S, Hofmann C, Shryne J E, Gorski R A. Prog Brain Res. 1984;61:99–117. doi: 10.1016/S0079-6123(08)64430-1. [DOI] [PubMed] [Google Scholar]
- 27.Dohler K D, Srivastava S S, Shryne J E, Jarzab B, Sipos A, Gorski R A. Neuoroendocrinology. 1984;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- 28.Brand T, Slob A K. Behav Brain Res. 1991;44:43–51. doi: 10.1016/s0166-4328(05)80238-4. [DOI] [PubMed] [Google Scholar]
- 29.Yeh S, Chang H C, Miyamoto H, Takatera H, Rahman M, Kang H Y, Thin T H, Lin H K, Chang C. Keio J Med. 1999;48:87–92. doi: 10.2302/kjm.48.87. [DOI] [PubMed] [Google Scholar]
- 30.Bevan C L, Hoare S, Claessens F, Heery D M, Parker M G. Mol Cell Biol. 1999;19:8383–8392. doi: 10.1128/mcb.19.12.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikonen T, Palvimo J J, Janne O A. J Biol Chem. 1997;272:29821–29828. doi: 10.1074/jbc.272.47.29821. [DOI] [PubMed] [Google Scholar]
- 32.Takeshita A, Yen P M, Misiti S, Cardona G R, Liu Y, Chin W W. Endocrinology. 1996;137:3594–3597. doi: 10.1210/endo.137.8.8754792. [DOI] [PubMed] [Google Scholar]
- 33.Wood R I, Newman S W. Neuroendocrinology. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- 34.Shim W S, DiRenzo J, DeCaprio J A, Santen R J, Brown M, Jeng M H. Proc Natl Acad Sci USA. 1999;96:208–213. doi: 10.1073/pnas.96.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]