Abstract
Two isomers of retinoic acid (RA) may be necessary as ligands for retinoid signaling: all-trans-RA for RA receptors (RARs) and 9-cis-RA for retinoid X receptors (RXRs). This was explored by using retinaldehyde dehydrogenase (Raldh)2-/- mouse embryos lacking mesodermal RA synthesis that display early growth arrest unless rescued by all-trans-RA administration. Because isomerization of all-trans-RA to 9-cis-RA can occur, it is unclear whether both ligands are needed for rescue. We show here that an RAR-specific ligand can rescue Raldh2-/- embryos as efficiently as all-trans-RA, whereas an RXR-specific ligand has no effect. Further, whereas all-trans-RA was detected in embryos, 9-cis-RA was undetectable unless a supraphysiological dose of all-trans-RA was administered, revealing that 9-cis-RA is of pharmacological but not physiological significance. Because 9-cis-RA is undetectable and unnecessary for Raldh2-/- rescue, and others have shown that 4-oxo-RA is unnecessary for mouse development, all-trans-RA emerges as the only ligand clearly necessary for retinoid receptor signaling.
The question of whether physiological retinoid signaling requires binding of 9-cis-retinoic acid (RA) to retinoid X receptors (RXRs) in addition to binding of all-trans-RA to RA receptors (RARs) has not been resolved (1). The initial discovery that RXR activates transcription in cell lines after the addition of high levels of all-trans-RA (2), whereas RAR responds to low levels of all-trans-RA (3, 4), led to the proposal that a second pathway of retinoid action exists. Later, 9-cis-RA was identified as a ligand for RXR (5–7), and isomerization of all-trans-RA to 9-cis-RA was demonstrated (8), thus explaining RXR activation with pharmacological doses of all-trans-RA. Although RAR/RXR heterodimers were shown to transmit the retinoid signal (9–12), it was found that 9-cis-RA could stimulate formation of active RXR homodimers (13), suggesting two mechanisms to transmit a retinoid signal. It was also reported that endogenous 9-cis-RA exists in normal mouse tissues, leading to the hypothesis that 9-cis-RA is needed physiologically as an RXR ligand (6). However, RXR forms heterodimers not only with RAR but with other ligand-dependent members of the nuclear receptor family including thyroid hormone and vitamin D receptors (9–12), which demonstrates that the role of RXR is not limited to retinoid signaling and complicates the issue of how the RXR ligand 9-cis-RA might function. Even though a targeted mutation of the RXRα AF-2 ligand-binding domain shows that this protein domain is important for mouse development (14), there is no evidence that AF-2 needs to bind 9-cis-RA to perform its function. Also, although all-trans-RA is easily detectable in many mammalian tissues (15–18), detection of 9-cis-RA as originally reported (6) has not been confirmed. Nevertheless, in vitro studies pursuing the mechanism of retinoid signaling suggest that transcriptional activation by RXR homodimers depends on 9-cis-RA (13), and that RAR/RXR heterodimers may in some contexts depend solely on binding of all-trans-RA to the RAR partner, so-called RXR subordination (19); but in other contexts RXR subordination is overcome, and binding of 9-cis-RA to RXR is involved in the transcriptional mechanism (20).
We now examine whether 9-cis-RA plays a physiologically relevant role in retinoid signaling. Biochemical studies indicate that both isomers of RA can be generated in vitro from vitamin A (retinol) by cytosolic alcohol dehydrogenases (21) and microsomal short-chain dehydrogenases (22, 23), which each catalyze oxidation of all-trans-retinol and 9-cis-retinol to the corresponding retinaldehyde derivatives, followed by cytosolic retinaldehyde dehydrogenase (Raldh), which catalyzes further oxidation of both all-trans-retinaldehyde and 9-cis-retinaldehyde to produce all-trans-RA or 9-cis-RA (24, 25). Mouse genetic studies support a role in RA synthesis for alcohol dehydrogenase genes Adh1, Adh3, and Adh4 (26, 27) as well as Raldh genes Raldh2 (Aldh1a2) (28, 29) and Raldh1 (Aldh1a1) (30). Thus, 9-cis-RA can potentially be synthesized by presently known enzymes, or it can be derived from isomerization of all-trans-RA (8). To determine whether 9-cis-RA binding to RXR is needed in vivo during mouse embryonic development we have examined mice deficient in RA synthesis. Null mutations of Raldh2 have demonstrated that RA is essential for mouse development, because Raldh2-/- embryos lack mesodermal RA (both all-trans-RA and 9-cis-RA) and fail to develop beyond embryonic day (E)8.5 (28, 29). Here we examine the relative ability of all-trans-RA, 9-cis-RA, and receptor-specific synthetic retinoids to rescue the Raldh2-/- embryonic lethal phenotype. Our findings provide evidence that 9-cis-RA is not required for physiological retinoid signaling.
Materials and Methods
Generation and Analysis of Retinoid-Rescued Embryos. All-trans-RA and 9-cis-RA were purchased from Sigma. Heteroarotinoids SHet100, OHet72, and SHetA2 were obtained from K. Darrell Berlin (Oklahoma State University, Stillwater) and synthesized according to published methods (31–33). Generation of Raldh2-/- embryos (from matings of heterozygous parents) and treatment with retinoids and heteroarotinoids were performed as described for all-trans-RA, which eliminates the block in growth at E8.5 observed in untreated mutants (29). Briefly, retinoids were dissolved in corn oil and administered orally to timed-pregnant Raldh2-/+ mice at 12-h intervals on E7.25, E7.75, and E8.25. Dosages varied from 0.025 to 25 mg/kg. At E10.25, embryos were analyzed morphologically to determine whether Raldh2-/- embryos were rescued or whether wild-type embryos had suffered teratogenesis.
Detection of embryonic RA was performed in situ in embryos carrying the RARE-lacZ RA-reporter gene by staining for β-galactosidase activity for 10 h (34).
Quantitation of RA in Embryos, Placentas, and Serum. All-trans-RA and 9-cis-RA were quantitated by HPLC analysis of embryos, placentas, and maternal serum from untreated or retinoid-treated pregnant mice. For pregnant mice treated by oral gavage, all-trans-RA or 9-cis-RA were dissolved in corn oil, a single dose was administered on E10.5 ranging from 2.5 to 50 mg/kg, and tissues were collected 2 h after administration (or 1 h where indicated). For dietary RA treatment, all-trans-RA was dissolved in corn oil and mixed with powdered food at 0.1 mg/g of food for treatment on E7.5 and at 0.25 mg/g of food for treatment on E8.5-E10.5 as described (35), and tissues were collected at E10.5.
For each wild-type pregnant mouse examined, three samples were prepared at stage E10.5: (i) all embryos were pooled (≈8–10); (ii) all placentas were pooled (≈8–10); and (iii) 0.2 ml of maternal serum was collected.
All extraction and analytical procedures were carried out in a darkened room to protect the retinoids from exposure to light. Serum (0.2 ml) was mixed with 0.2 ml of 0.25 M ammonium acetate (pH 4.0) and 0.6 ml of acetonitrile. Embryo and placenta samples (0.1–0.2 g) were mixed with 0.3 ml of 0.25 M ammonium acetate (pH 4.0) and 0.6 ml of acetonitrile and homogenized on ice. After centrifugation (10,000 × g for 10 min at 4°C) the supernatant was transferred to a new tube, and 0.4 ml of water was added. The resulting mixture was loaded onto a precolumn (Pelliguard LC-18; Supelco) and then into the analytical column as follows. Reversed-phase HPLC analysis was performed on a Waters 2695 HPLC system by using a SUPLEX pkB-100 analytical column (250 × 4.6 mm) (Supelco) at a flow rate of 1 ml/min and column temperature of 35°C. Mobile phase consisted of 2% ammonium acetate/glacial acetic acid/acetonitrile/methanol (16:3:79:2). Detection of retinoids was performed by using a photodiode array detector (Waters model 2996), which collected spectra between 200 and 450 nM. Standard solutions of retinoids (all-trans-retinol, 9-cis-RA, and all-trans-RA) were used to obtain the calibration curves. Characteristic peak spectra and retention times were used to identify each retinoid, and peak areas at λmax used for quantitation were calculated by using MILLENNIUM CHROMATOGRAPHY MANAGER software (Waters).
Quantitation of RA in Adult Liver. Mouse liver (1.0 g) was homogenized in 2 ml of PBS (0.01 M, pH 7.4), and then 3 ml of methanol was added and mixed by vortex. This mixture was extracted twice with 5 ml of hexane. Hexane layers were collected, combined, and evaporated under vacuum. The residue was dissolved in 0.15 ml of mobile phase, and 0.1 ml was analyzed by using the HPLC/photodiode array detector as described above (without precolumn) to identify and quantitate all-trans-RA and 9-cis-RA.
Results
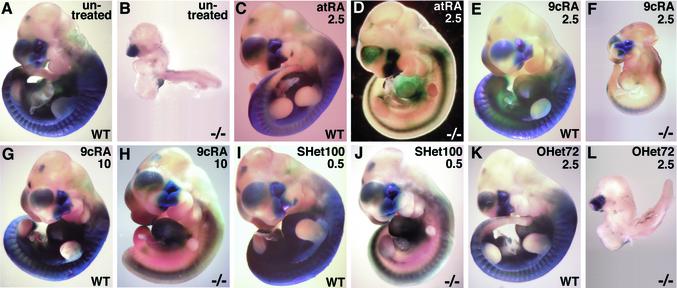
RAR-Specific Ligand Rescues Raldh2-/- Lethality. We have shown previously that Raldh2-/- embryos are efficiently rescued to stage E10.5 by limited maternal treatment on E7.25–8.25 with 2.5 mg/kg all-trans-RA (29). However, if some all-trans-RA has been isomerized to 9-cis-RA (8), rescue may depend on binding of retinoid ligands to both RAR and RXR. Here we compared the rescue efficiency of Raldh2-/- embryos treated maternally with all-trans-RA, 9-cis-RA, and three synthetic heteroarotinoids that differ in their abilities to bind and transactivate RARs or RXR; i.e., SHet100, which is RAR-specific (31), OHet72, which is RXR-specific (32), and SHetA2, which binds neither RAR nor RXR (33) (Fig. 1). Embryos also carried the RARE-lacZ transgene (34), which allowed in situ detection of either all-trans-RA or 9-cis-RA, both of which induce the transgene. The results of embryos examined at E10.25 after the various treatments are summarized in Table 1. Whereas Raldh2-/- embryos from untreated mice have failed to undergo axial rotation and suffer a massive growth defect (Fig. 2 A and B), those treated with 2.5 mg/kg all-trans-RA appear relatively normal in size and morphology with the exception of growth-retarded forelimb buds (Fig. 2 C and D) as reported (29). Treatment with 2.5 mg/kg 9-cis-RA resulted in a partial rescue with Raldh2-/- embryos undergoing axial turning, but growth was restricted severely compared with wild-type (Fig. 2 E and F). However, treatment with 10 mg/kg 9-cis-RA resulted in the typical rescue phenotype observed with all-trans-RA, complete with the same RARE-lacZ expression phenotype (Fig. 2 G and H). It was expected that 9-cis-RA would perform the rescue because of its ability to activate RARs as well as RXRs (5–7). However, it seems that rescue with 9-cis-RA requires an ≈4-fold higher dose than rescue with all-trans-RA (Table 1).
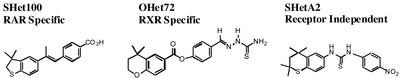
Fig. 1.
Structures and receptor specificities of heteroarotinoids. Heteroarotinoids are a class of retinoids that exhibit decreased toxicity in animal models due to the insertion of a heteroatom in the cyclic ring of the arotinoid structure. Specific structural alterations of the heteroarotinoid structure have been shown to alter the receptor specificities of the compounds. The synthesis and receptor specificity of each heteroarotinoid indicated here was described previously as follows. SHet100 (previously compound 18; ref. 31) activates all RARs in the nanomolar range but does not activate RXRs up to 10 μM. OHet72 (previously compound 6; ref. 32) activates RXRs in the nanomolar range but is inactive with RARs up to 10 μM. SHetA2 does not activate either RARs or RXRs (33).
Table 1. Rescue of E10.25 Raldh2-/- embryos by various retinoids.
| Retinoid | Dose, mg/kg | Total no. of embryos | No. of +/+ or -/+ | No. of -/- unrescued | No. of -/- rescued | Teratogenesis* |
|---|---|---|---|---|---|---|
| All-trans-RA | 1.0 | 19 | 12 | 7 | 0 | 0 |
| All-trans-RA | 2.5 | 41 | 27 | 1 | 13 | 0 |
| 9-cis-RA | 2.5 | 30 | 20 | 8 | 2(partial) | 0 |
| 9-cis-RA | 10 | 30 | 23 | 1 | 6 | 0 |
| SHet100 | 0.025 | 11 | 9 | 2 | 0 | 0 |
| SHet100 | 0.25 | 10 | 8 | 0 | 2(partial) | 0 |
| SHet100 | 0.5 | 20 | 13 | 1 | 6 | 0 |
| SHet100 | 2.5 | 11 | — | — | — | 11 |
| OHet72 | 2.5 | 8 | 4 | 4 | 0 | 0 |
| OHet72 | 10 | 19 | 16 | 3 | 0 | 0 |
| OHet72 | 25 | 17 | 12 | 5 | 0 | 0 |
| SHetA2 | 2.5 | 8 | 7 | 1 | 0 | 0 |
| SHetA2 | 10 | 18 | 13 | 5 | 0 | 0 |
| SHetA2 | 25 | 7 | 6 | 1 | 0 | 0 |
Embryos indicated as +/+ (wild-type) or -/+ (Raldh2-heterozygous) were normal in appearance with RARE-lacZ expression throughout trunk mesoderm, whereas -/- (Raldh2-homozygous) unrescued embryos were much smaller and failed to undergo axial rotation, and -/- rescued embryos were normal in size but with growth-retarded forelimb buds and no RARE-lacZ expression in somites or lateral plate mesoderm
Teratogenesis was reported as positive after observation of an obvious growth defect or malformation in +/+ or -/+ embryos. In the single instance where teratogenesis is indicated, all embryos in that litter failed to undergo axial rotation and were stalled between stages E8.5 and E9.0
Fig. 2.
Efficiency of Raldh2-/- embryonic rescue with natural and synthetic retinoids. E10.5 wild-type (WT) and Raldh2-/- embryos are shown from pregnant mice that were untreated (A and B) or treated on E7.25–8.25 with 2.5 mg/kg all-trans-RA (atRA, C and D), 2.5 mg/kg 9-cis-RA (9cRA, E and F), 10 mg/kg 9-cis-RA (G and H), 0.5 mg/kg RAR-specific SHet100 (I and J), and 2.5 mg/kg RXR-specific OHet72 (K and L). Also, the extent of RARE-lacZ transgene expression is compared for each embryo as an indicator of where retinoid signaling is occurring. It can be seen that treatment with 2.5 mg/kg all-trans-RA, 10 mg/kg 9-cis-RA, and 0.5 mg/kg SHet100 results in a very similar rescued phenotype. However, it should be stressed that, whereas rescued Raldh2-/- embryos appear grossly normal, forelimb outgrowth is severely retarded (see D, H, and J), and other developmental abnormalities such as those reported for rhombomere segmentation (43) and cardiac development (44) may still exist.
The above findings do not reveal whether binding of 9-cis-RA to both RAR and RXR is needed for the rescue. Interestingly, treatment with 0.5 mg/kg RAR-specific SHet100 also resulted in the typical rescue phenotype observed with all-trans-RA plus the same RARE-lacZ expression phenotype (Fig. 2 I and J). Treatment with 0.25 mg/kg SHet100 resulted in a partial rescue of Raldh2-/- embryos, similar to that observed with 2.5 mg/kg 9-cis-RA, and 2.5 mg/kg SHet100 was teratogenic for all embryos in a litter (Table 1). Treatment with 2.5 mg/kg of either RXR-specific OHet72 (Fig. 2 K and L) or non-receptor-binding SHetA2 (Table 1) resulted in no rescue, with Raldh2-/- embryos developing the same as untreated embryos and having the RARE-lacZ expression pattern typical of nonrescued mutants. Increasing the dosage of OHet72 and SHetA2 to 10 or 25 mg/kg also resulted in no rescue, and no teratogenesis was observed in litters treated with OHet72 or SHetA2 at any dosage (Table 1).
Our results indicate that an RAR-specific ligand can provide the same rescue phenotype as all-trans-RA, whereas an RXR-specific ligand cannot provide any degree of rescue. These findings provide evidence that binding of 9-cis-RA to RXR is not required for rescue. Also, it can be concluded that all-trans-RA does not need to be isomerized to 9-cis-RA in order to rescue the Raldh2-/- phenotype.
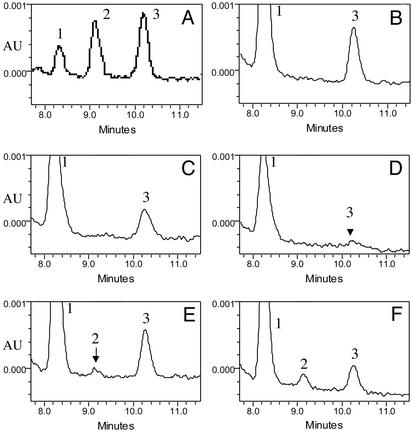
Is 9-cis-RA Normally Present in Embryos at a Concentration High Enough to Transactivate RXRs? We performed HPLC analysis of pooled E10.5 wild-type mouse embryos and observed a clear peak of all-trans-RA (7.5 ± 1.3 ng/g, or 25 pmol/g ≈ 25 nM), but no detectable 9-cis-RA (detection limit of 0.2 ng/g, or 0.7 pmol/g ≈ 0.7 nM) (Fig. 3 A and B; also see Table 2, untreated mice). Because RXRs exhibit binding constants for 9-cis-RA ranging from 14 to 18 nM plus EC50 values for 9-cis-RA transactivation ranging from 7 to 20 nM (7), our finding of a value <0.7 nM for 9-cis-RA indicates that there is no evidence that 9-cis-RA is present in embryos at a concentration high enough to regulate RXR. Other studies that have detected all-trans-RA in mouse embryos have also mentioned that 9-cis-RA is undetectable (15, 17). Whereas it remains possible that a small population of cells in the embryo may contain sufficient 9-cis-RA to activate RXR, no evidence for this has been reported.
Fig. 3.
Detection of all-trans-RA and 9-cis-RA in E10.5 wild-type and Raldh2-/- embryos. HPLC chromatograms at 355 nm show the characteristic retention times for 1 ng each of all-trans-retinol (peak 1), 9-cis-RA (peak 2), and all-trans-RA (peak 3) (A) as well as detection of these retinoids in 10 pooled wild-type embryos untreated (B); 9 pooled wild-type embryos (C) and 9 pooled Raldh2-/- embryos (D) collected on E10.5 from four litters of mice subjected only to the RA-rescue protocol (three doses of all-trans-RA from E7.25–8.25); and 10 pooled wild-type embryos (E) and 10 pooled Raldh2-/- embryos (F) collected on E10.5 from four litters of mice subjected to the RA-rescue protocol, then administered 10 mg/kg 9-cis-RA on E10.5 2 h before collection.
Table 2. Quantitation of all-trans-RA and 9-cis-RA in wild-type E10.5 embryos, placenta, and maternal serum of untreated or retinoid-treated mice.
| Embryo, ng/g
|
Placenta, ng/g
|
Maternal serum, ng/ml
|
||||
|---|---|---|---|---|---|---|
| Treatment | All-trans-RA | 9-cis-RA | All-trans-RA | 9-cis-RA | All-trans-RA | 9-cis-RA |
| None | 7.5 ± 1.3 | ND | ND | ND | ND | ND |
| All-trans-RA (2.5 mg/kg) | 53.2 ± 5.7 | ND | 54.2 ± 5.4 | ND | 30.0 ± 5.6 | ND |
| All-trans-RA (10 mg/kg) | 376.7 ± 86.4 | 3.8 ± 0.3 | 432.7 ± 125.0 | 7.0 ± 0.6 | 371.7 ± 11.7 | 3.3 ± 0.7 |
| All-trans-RA (50 mg/kg) | 2,213 ± 374 | 14.7 ± 0.9 | 3,247 ± 1,009 | 55.1 ± 20.2 | 4,004 ± 822 | 27.6 ± 5.7 |
| All-trans-RA (diet) | 9.3 ± 0.6 | ND | 3.6 ± 1.8 | ND | 1.8 ± 0.8 | ND |
| 9-cis-RA (10 mg/kg), 1 h | 12.2 ± 0.4 | 7.8 ± 4.6 | 5.7 ± 1.3 | 11.2 ± 0.2 | 4.0 ± 1.9 | 13.1 ± 0.5 |
| 9-cis-RA (10 mg/kg), 2 h | 8.1 ± 1.0 | 1.4 ± 0.7 | 4.0 ± 0.8 | 10.2 ± 3.3 | ND | 10.2 ± 3.3 |
Values are mean ± SE (n = 3 litters). ND, not detectable (<0.2 ng/g)
RA Levels in Rescued Raldh2-/- Embryos. We next determined the effect of the RA rescue treatment (three maternal doses of 2.5 mg/kg all-trans-RA on E7.25–8.25) on RA levels ≈2 days later in wild-type and Raldh2-/- E10.5 embryos. Because only two to three Raldh2-/- embryos are obtained per litter, mutants from four litters were pooled to obtain sufficient material for analysis. Under rescue conditions, E10.5 wild-type embryos had nearly the same values for all-trans-RA (≈6.9 ng/g) as untreated wild-type embryos (≈7.5 ng/g), but 9-cis-RA was undetectable in both cases (<0.2 ng/g) (Fig. 3 B and C). This indicates that the all-trans-RA administered between E7.25 and E8.25 had been totally cleared after 2 days. Rescued E10.5 Raldh2-/- embryos had much lower all-trans-RA (≈1.5 ng/g) than wild-type littermates and no detection of 9-cis-RA (Fig. 3D). This indicates that Raldh2 accounts for ≈80% of the RA synthesis at E10.5, with the remaining 20% evidently synthesized by other enzymes. A comparison of these findings with the corresponding RARE-lacZ data (Fig. 2 C and D) shows that rescued E10.5 Raldh2-/- embryos have much less RARE-lacZ detection in the trunk than wild-type embryos due to a lack of Raldh2 expression in mesoderm (29), which thus is consistent with the lower all-trans-RA value detected by HPLC. The RARE-lacZ expression still detected in E10.5 rescued Raldh2-/- embryos is due to other RA-synthesizing genes including Raldh1 (Aldh1a1) and Raldh3 (Aldh1a3) expressed in eye and olfactory pit plus an additional activity in the neural tube and heart (29).
Although administration of all-trans-RA by oral gavage from E7.25–8.25 is quite effective in rescuing Raldh2-/- embryonic development to E10.5 (29), forelimb bud development is not rescued even if treatments are extended to E10.25 when forelimb buds should be growing (28, 29). However, forelimb bud development can be rescued if all-trans-RA is administered continuously in the maternal diet from E7.5 to E10.5 (35). We examined tissues in wild-type mice at E10.5 after such dietary RA treatment (Table 2). Under these conditions all-trans-RA in wild-type embryos (9.3 ng/g) was slightly increased relative to controls (7.5 ng/g), and all-trans-RA was now detectable in placenta and maternal serum, whereas it was undetectable in controls. 9-cis-RA was undetectable in embryos, placentas, and maternal serum. Thus, the more complete rescue of Raldh2-/- embryos previously observed by dietary RA treatment (35) does not depend on production of 9-cis-RA; it is more likely due to the continuous nature of this treatment being a superior delivery method for all-trans-RA, because it gives rise to a stable moderate increase in systemic all-trans-RA, whereas punctuated oral gavages result in wide fluctuations in the level of all-trans-RA.
Because 9-cis-RA was undetectable in E10.5 wild-type or rescued mutant embryos, we examined embryos subjected to the maternal rescue protocol on E7.25–8.25, followed by maternal treatment on E10.5 with 10 mg/kg 9-cis-RA 2 h before collection to determine whether 9-cis-RA gained access to embryos. Under these conditions 9-cis-RA was observed in wild-type (≈1.3 ng/g) and Raldh2-/- (≈3.0 ng/g) embryos (Fig. 3 E and F). All-trans-RA was essentially unchanged in wild-type 9-cis-RA-treated embryos (≈7.2 ng/g) compared with those not treated with 9-cis-RA (≈6.9 ng/g) (Fig. 3 C and E), but all-trans-RA was significantly increased in Raldh2-/- 9-cis-RA-treated embryos (≈4.1 ng/g) compared with those not treated with 9-cis-RA (≈1.5 ng/g) (Fig. 3 D and F). Thus, administered 9-cis-RA can gain access to embryos and remain intact, plus it can increase the level of all-trans-RA in Raldh2-/- embryos, possibly through isomerization (8). As to why an increase of all-trans-RA was observed in 9-cis-RA-treated mutants but not wild type, it is possible that an upper limit of all-trans-RA may be set by the rate of all-trans-RA degradation to oxidized derivatives (36).
9-cis-RA Is Undetectable in Mouse Liver. The original study reporting that 9-cis-RA is present endogenously in adult mouse liver at 4 ng/g (6) was a crucial factor leading to the current hypotheses proposing that 9-cis-RA could have a physiological function in retinoid signaling through binding to RXR (13, 20). We also examined retinoids in adult mouse liver by HPLC and detected all-trans-RA at 1.5 ± 0.2 ng/g (n = 6), but 9-cis-RA was undetectable in all samples analyzed (<0.2 ng/g detection limit; n = 6). A previous study also failed to detect 9-cis-RA in adult rat liver but detected all-trans-RA (3.4 ± 1.4 ng/g) (16). Our findings thus compare favorably with the studies on rat liver, suggesting that the original study that detected 9-cis-RA in mouse liver is in error (6). Also, the previous study of rat adult tissues indicated that 9-cis-RA is undetectable in liver, brain, kidney, fat, seminal vesicle, spleen, epididymis, pancreas, testis, and plasma, whereas all-trans-RA was detected in all these tissues ranging from 0.5 to 8.8 ng/g (16). A further study that followed RA production in adult rats after a physiological dose of radioactively labeled all-trans-retinol found significant synthesis of all-trans-RA in various tissues but no synthesis of 9-cis-RA (18). Thus, we conclude that there is no evidence that 9-cis-RA is present in mammalian embryos or adult tissues at a concentration high enough to regulate RXR activity.
Detection of 9-cis-RA Under Pharmacological Conditions. We next asked to what extent maternal oral treatment with increasing doses of all-trans-RA resulted in transport of all-trans-RA to embryos, placentas, and maternal serum examined 2 h after treatment and whether this treatment could generate 9-cis-RA in these tissues (Table 2). After treatment of pregnant wild-type mice on E10.5 with 2.5 mg/kg all-trans-RA, the level of all-trans-RA observed 2 h later in embryos was increased 7-fold (53 ng/g) compared with untreated controls, and all-trans-RA was now detectable in maternal serum (30 ng/g) and placenta (54 ng/g), whereas without treatment it was undetectable in both these tissues. 9-cis-RA remained undetectable in all tissues examined after treatment with 2.5 mg/kg all-trans-RA. However, treatment with 10 mg/kg all-trans-RA did result in detection of 9-cis-RA in embryos (3.8 ng/g), placentas (7 ng/g), and maternal serum (3.3 ng/g) at ≈1% the value of all-trans-RA, which was increased greatly in all these tissues. Treatment with 50 mg/kg all-trans-RA further increased the levels of both 9-cis-RA and all-trans-RA in embryos, placentas, and maternal serum with 9-cis-RA still present at a level ≈1% that of all-trans-RA (Table 2). These results clearly show that 9-cis-RA is not a significant retinoid ligand under normal physiological conditions, but that under pharmacological conditions it can appear and be measured.
Because placental transfer of 9-cis-RA is reportedly less than that of all-trans-RA and is affected by high turnover including conversion to all-trans-RA (37), we examined tissues at 1 or 2 h after treatment with 9-cis-RA (Table 2). Treatment of pregnant wild-type mice on E10.5 with 10 mg/kg 9-cis-RA resulted in detection of higher levels of embryonic 9-cis-RA at 1 h (7.8 ng/g) compared with 2 h (1.4 ng/g) and higher levels of 9-cis-RA in placenta and maternal serum at 1 h compared with 2 h. However, the amount of placental transfer of 9-cis-RA to embryos is only ≈2% the amount of placental transfer of all-trans-RA to embryos after treatment with a comparable 10 mg/kg dose of all-trans-RA (Table 2). Further, with 9-cis-RA treatment the amount of embryonic all-trans-RA was increased at 1 h (12.2 ng/g) but was nearly back to control levels at 2 h (8.1 ng/g). Also, all-trans-RA normally was undetectable in placenta and maternal serum, but treatment with 10 mg/kg 9-cis-RA resulted in all-trans-RA now being detectable in placenta at 1 h (5.7 ng/g) and 2 h (4 ng/g), plus in maternal serum at 1 h (4.0 ng/g) but not 2 h. These findings provide evidence that maternal 9-cis-RA can gain access to embryos, although less efficiently than all-trans-RA, and can be converted to all-trans-RA in some tissues leading to increased all-trans-RA in embryos. This observation explains the ability of 9-cis-RA to perform rescue of Raldh2-/- embryos as either 9-cis-RA or all-trans-RA produced from it can activate RARs with equally high affinity (7). The lower placental transfer or higher turnover of 9-cis-RA relative to all-trans-RA is consistent with our observation that rescue of Raldh2-/- embryos requires higher doses of 9-cis-RA than all-trans-RA (Table 1).
Discussion
Whereas current hypotheses on the mechanism of retinoid signaling emphasize the importance of 9-cis-RA binding to RXR (5, 6, 13, 19, 20), our demonstration that 9-cis-RA is unnecessary for rescue of Raldh2-/- embryos provides good reason to doubt the importance of 9-cis-RA. The studies we report here indicate that a synthetic retinoid ligand able to bind RARs but not RXRs is sufficient to correct the deficiency in RA synthesis created by mutation of Raldh2, a deficiency that is widespread throughout the embryo as indicated by our HPLC studies showing that Raldh2 is responsible for 80% of the RA detectable in E10.5 embryos. Analysis of nonrescued and conditionally rescued Raldh2-/- embryos carrying the RARE-lacZ reporter (which detects the presence of both all-trans-RA and 9-cis-RA) shows that Raldh2 is responsible for all RA synthesis in several embryonic tissues, i.e., paraxial mesoderm, somitic mesoderm, lateral plate mesoderm, forelimb buds, dorsal spinal cord, foregut, maxillary process, and allantois (29). Thus, one cannot argue that there is another enzyme in these tissues producing 9-cis-RA and that the defect of Raldh2-/- embryos is limited to synthesis of all-trans-RA. Also, although Raldh2 itself can potentially catalyze synthesis of both all-trans-RA and 9-cis-RA (24), we have demonstrated that 9-cis-RA is not normally detectable in wild-type embryos or rescued Raldh2-/- embryos, thus it is not present in the nanomolar range necessary to activate RXRs (7). This absence may be due to a lack of 9-cis-retinoid substrates, a low amount of isomerization (we demonstrate only 1% here), or a high turnover rate as demonstrated here. Although our Raldh2-/- rescue studies address only the early stage of mouse embryogenesis, the inability of this laboratory and others to detect 9-cis-RA in normal tissues at any developmental stage should nullify the hypothesis that 9-cis-RA has a physiological function. Unless future studies demonstrate that 9-cis-RA normally exists in some tissue at a concentration high enough to regulate RXR and is needed in that tissue for a physiological process, there is no reason to believe that it plays anything other than a pharmacological role.
What is the function of the RXR ligand-binding domain if it does not bind 9-cis-RA? Deletion of the RXRα ligand-binding domain (AF-2) is lethal during mouse development (14), suggesting that it plays an important role. The RXR AF-2 domain may bind the nonretinoid RXR ligand docosahexenoic acid found in adult brain (38), although it has not been demonstrated whether docosahexenoic acid is needed for retinoid signaling or any other nonretinoid signaling pathway in which RXR may be involved. On the other hand, it has been pointed out that the importance of the AF-2 domain for RXR function may be independent of ligand binding, because this domain may be a site of posttranslational modification such as phosphorylation (14). With this in mind, a binary reporter assay that detected RXR activity in mouse embryonic spinal cord may not have detected a tissue containing an RXR ligand as hypothesized (39) but rather a tissue in which RXR modification occurs. RXR is unique among all the nuclear receptors in that it interacts as a heterodimer partner for a diverse array of other nuclear receptors including RAR (40). Because many of these nuclear receptors have ligands, one might suspect that RXR functions ligand-independently so as not to alter the ligand specificity of the heterodimer complexes it forms (thus not placing them under control of retinoid signaling), and in fact RXR subordination has been observed experimentally (19). However, in the case of RAR/RXR heterodimers, the ligand for both partners would be a retinoid if RXR binds 9-cis-RA. With this in mind, in vitro studies suggest that in some cell types containing certain types of corepressors and coactivators, transcription may be synergistically activated when RAR/RXR heterodimers bind 9-cis-RA to the RXR partner as well as all-trans-RA to the RAR partner (20). Also, it has been suggested that RXR homodimers activated by 9-cis-RA might have a function in retinoid signaling (13). Our data suggest that 9-cis-RA does not perform these functions under normal physiological conditions, at least not in the numerous embryonic tissues in which Raldh2 is the sole enzyme producing RA. Also, although one could argue that Raldh2-/- rescue may still depend on binding of a nonretinoid ligand to RXR in addition to binding of all-trans-RA to RAR, there is no evidence that such a ligand exists in mouse embryos. Thus, RXR may possess a vestigial retinoid-binding domain that does not provide physiological retinoid function, because 9-cis-RA is normally not present in a sufficient amount. In this case, RXR may function solely as a scaffold that correctly positions RAR and transcriptional coregulators.
9-cis-RA is interesting from a pharmacological perspective exactly because it can generate active RXR homodimers when provided at supraphysiological levels (13) and affect the activity of other nuclear receptors with which it forms heterodimers (19, 20). This capability has led to the development of synthetic rexinoid ligands specific for various RXRs (32, 41) and related compounds that bind neither RARs nor RXRs (33), which may be of therapeutic use against cancer or other diseases because of their higher specificity for certain targets leading to improved therapeutic ratios (efficacy/toxicity). In our studies, the inability of RXR-specific OHet72 and receptor-independent SHetA2 to rescue the Raldh2-/- phenotype and their lack of teratogenicity compared with RAR-specific SHet100 (Table 1) suggests that heteroarotinoids that do not activate RARs may not be teratogenic when administered to humans. The potential long-term administration of these compounds as cancer-chemoprevention agents warrants that they should not induce significant toxicity or teratogenicity. Thus, 9-cis-RA and its derivatives are clearly of pharmacological interest, but our findings indicate that it is incorrect to refer to 9-cis-RA as a physiological ligand for RXR. Also, whereas oxidative derivatives of RA such as 4-oxo-RA were originally thought to function as RAR ligands (42), recent findings have demonstrated that they are not required for physiological retinoid signaling (36). In conclusion, there is no evidence that the physiological mechanism of retinoid signaling involves anything other than all-trans-RA as the ligand. This knowledge will help direct future studies on the mechanism of retinoid action in target tissues.
Acknowledgments
We thank K. Darrell Berlin for kindly providing the heteroarotinoids SHet100, OHet72, and SHetA2, and J. Rossant for providing RARE-lacZ mice. This work was funded by National Institutes of Health Grants GM62848 (to G.D.) and CA77711 (to D.M.B).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RA, retinoic acid; RXR, retinoid X receptor; RAR, RA receptor; Raldh, retinaldehyde dehydrogenase; En, embryonic day n.
References
- 1.Clagett-Dame, M. & DeLuca, H. F. (2002) Annu. Rev. Nutr. 22, 347-381. [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf, D. J., Ong, E. S., Dyck, J. A. & Evans, R. M. (1990) Nature 345, 224-229. [DOI] [PubMed] [Google Scholar]
- 3.Petkovich, M., Brand, N. J., Krust, A. & Chambon, P. (1987) Nature 330, 444-450. [DOI] [PubMed] [Google Scholar]
- 4.Giguère, V., Ong, E. S., Segui, P. & Evans, R. M. (1987) Nature 330, 624-629. [DOI] [PubMed] [Google Scholar]
- 5.Levin, A. A., Sturzenbecker, L. J., Kazmer, S., Bosakowski, T., Huselton, C., Allenby, G., Speck, J., Kratzeisen, C., Rosenberger, M., Lovey, A. & Grippo, J. F. (1992) Nature 355, 359-361. [DOI] [PubMed] [Google Scholar]
- 6.Heyman, R. A., Mangelsdorf, D. J., Dyck, J. A., Stein, R. B., Eichele, G., Evans, R. M. & Thaller, C. (1992) Cell 68, 397-406. [DOI] [PubMed] [Google Scholar]
- 7.Allenby, G., Bocquel, M.-T., Saunders, M., Kazmer, S., Speck, J., Rosenberger, M., Lovey, A., Kastner, P., Grippo, J. F., Chambon, P. & Levin, A. A. (1993) Proc. Natl. Acad. Sci. USA 90, 30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urbach, J. & Rando, R. R. (1994) Biochem. J. 299, 459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu, V. C., Delsert, C., Andersen, B., Holloway, J. M., Devary, O. V., Näär, A. M., Kim, S. Y., Boutin, J.-M., Glass, C. K. & Rosenfeld, M. G. (1991) Cell 67, 1251-1266. [DOI] [PubMed] [Google Scholar]
- 10.Leid, M., Kastner, P., Lyons, R., Nakshatri, H., Saunders, M., Zacharewski, T., Chen, J.-Y., Staub, A., Garnier, J.-M., Mader, S. & Chambon, P. (1992) Cell 68, 377-395. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, X., Hoffmann, B., Tran, P. B.-V., Graupner, G. & Pfahl, M. (1992) Nature 355, 441-446. [DOI] [PubMed] [Google Scholar]
- 12.Kliewer, S. A., Umesono, K., Mangelsdorf, D. J. & Evans, R. M. (1992) Nature 355, 446-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang, X., Lehmann, J., Hoffmann, B., Dawson, M. I., Cameron, J., Graupner, G., Hermann, T., Tran, P. & Pfahl, M. (1992) Nature 358, 587-591. [DOI] [PubMed] [Google Scholar]
- 14.Mascrez, B., Mark, M., Dierich, A., Ghyselinck, N. B., Kastner, P. & Chambon, P. (1998) Development (Cambridge, U.K.) 125, 4691-4707. [DOI] [PubMed] [Google Scholar]
- 15.Horton, C. & Maden, M. (1995) Dev. Dyn. 202, 312-323. [DOI] [PubMed] [Google Scholar]
- 16.Kurlandsky, S. B., Gamble, M. V., Ramakrishnan, R. & Blaner, W. S. (1995) J. Biol. Chem. 270, 17850-17857. [DOI] [PubMed] [Google Scholar]
- 17.Ulven, S. M., Gundersen, T. E., Weedon, M. S., Landaas, V. O., Sakhi, A. K., Fromm, S. H., Geronimo, B. A., Moskaug, J. O. & Blomhoff, R. (2000) Dev. Biol. 220, 379-391. [DOI] [PubMed] [Google Scholar]
- 18.Werner, E. A. & DeLuca, H. F. (2001) Arch. Biochem. Biophys. 393, 262-270. [DOI] [PubMed] [Google Scholar]
- 19.Kurokawa, R., DiRenzo, J., Boehm, M., Sugarman, J., Gloss, B., Rosenfeld, M. G., Heyman, R. A. & Glass, C. K. (1994) Nature 371, 528-531. [DOI] [PubMed] [Google Scholar]
- 20.Germain, P., Iyer, J., Zechel, C. & Gronemeyer, H. (2002) Nature 415, 187-192. [DOI] [PubMed] [Google Scholar]
- 21.Allali-Hassani, A., Peralba, J. M., Martras, S., Farrés, J. & Parés, X. (1998) FEBS Lett. 426, 362-366. [DOI] [PubMed] [Google Scholar]
- 22.Chai, X., Boerman, M. H. E. M., Zhai, Y. & Napoli, J. L. (1995) J. Biol. Chem. 270, 3900-3904. [DOI] [PubMed] [Google Scholar]
- 23.Mertz, J. R., Shang, E. Y., Piantedosi, R., Wei, S. H., Wolgemuth, D. J. & Blaner, W. S. (1997) J. Biol. Chem. 272, 11744-11749. [DOI] [PubMed] [Google Scholar]
- 24.Gagnon, I., Duester, G. & Bhat, P. V. (2002) Biochim. Biophys. Acta 1596, 156-162. [DOI] [PubMed] [Google Scholar]
- 25.Montplaisir, V., Lan, N. C., Guimond, J., Savineau, C., Bhat, P. V. & Mader, S. (2002) J. Biol. Chem. 277, 17486-17492. [DOI] [PubMed] [Google Scholar]
- 26.Molotkov, A., Fan, X., Deltour, L., Foglio, M. H., Martras, S., Farrés, J., Parés, X. & Duester, G. (2002) Proc. Natl. Acad. Sci. USA 99, 5337-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molotkov, A., Deltour, L., Foglio, M. H., Cuenca, A. E. & Duester, G. (2002) J. Biol. Chem. 277, 13804-13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niederreither, K., Subbarayan, V., Dollé, P. & Chambon, P. (1999) Nat. Genet. 21, 444-448. [DOI] [PubMed] [Google Scholar]
- 29.Mic, F. A., Haselbeck, R. J., Cuenca, A. E. & Duester, G. (2002) Development (Cambridge, U.K.) 129, 2271-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan, X., Molotkov, A., Manabe, S.-I., Donmoyer, C. M., Deltour, L., Foglio, M. H., Cuenca, A. E., Blaner, W. S., Lipton, S. A. & Duester, G. (2003) Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 31.Benbrook, D. M., Subramanian, S., Gale, J. B., Liu, S., Brown, C. W., Boehm, M. F. & Berlin, K. D. (1998) J. Med. Chem. 41, 3753-3757. [DOI] [PubMed] [Google Scholar]
- 32.Zacheis, D., Dhar, A., Lu, S., Madler, M. M., Klucik, J., Brown, C. W., Liu, S., Clement, F., Subramanian, S., Weerasekare, G. M., et al. (1999) J. Med. Chem. 42, 4434-4445. [DOI] [PubMed] [Google Scholar]
- 33.Guruswamy, S., Lightfoot, S., Gold, M. A., Hassan, R., Berlin, K. D., Ivey, R. T. & Benbrook, D. M. (2001) J. Natl. Cancer Inst. 93, 516-525. [DOI] [PubMed] [Google Scholar]
- 34.Rossant, J., Zirngibl, R., Cado, D., Shago, M. & Giguère, V. (1991) Genes Dev. 5, 1333-1344. [DOI] [PubMed] [Google Scholar]
- 35.Niederreither, K., Vermot, J., Schuhbaur, B., Chambon, P. & Dollé, P. (2002) Development (Cambridge, U.K.) 129, 3563-3574. [DOI] [PubMed] [Google Scholar]
- 36.Niederreither, K., Abu-Abed, S., Schuhbaur, B., Petkovich, M., Chambon, P. & Dollé, P. (2002) Nat. Genet. 31, 84-88. [DOI] [PubMed] [Google Scholar]
- 37.Kochhar, D. M., Jiang, H., Penner, J. D. & Heyman, R. A. (1995) Teratology 51, 257-265. [DOI] [PubMed] [Google Scholar]
- 38.De Urquiza, A. M., Liu, S. Y., Sjöberg, M., Zetterström, R. H., Griffiths, W., Sjovall, J. & Perlmann, T. (2000) Science 290, 2140-2144. [DOI] [PubMed] [Google Scholar]
- 39.Solomin, L., Johansson, C. B., Zetterström, R. H., Bissonnette, R. P., Heyman, R. A., Olson, L., Lendahl, U., Frisén, J. & Perlmann, T. (1998) Nature 395, 398-402. [DOI] [PubMed] [Google Scholar]
- 40.Chawla, A., Repa, J. J., Evans, R. M. & Mangelsdorf, D. J. (2001) Science 294, 1866-1870. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann, J. M., Jong, L., Fanjul, A., Cameron, J. F., Lu, X. P., Haefner, P., Dawson, M. I. & Pfahl, M. (1992) Science 258, 1944-1946. [DOI] [PubMed] [Google Scholar]
- 42.Pijnappel, W. W. M., Hendriks, H. F. J., Folkers, G. E., Van den Brink, C. E., Dekker, E. J., Edelenbosch, C., Van der Saag, P. T. & Durston, A. J. (1993) Nature 366, 340-344. [DOI] [PubMed] [Google Scholar]
- 43.Niederreither, K., Vermot, J., Schuhbaur, B., Chambon, P. & Dollé, P. (2000) Development (Cambridge, U.K.) 127, 75-85. [DOI] [PubMed] [Google Scholar]
- 44.Niederreither, K., Vermot, J., Messaddeq, N., Schuhbaur, B., Chambon, P. & Dollé, P. (2001) Development (Cambridge, U.K.) 128, 1019-1031. [DOI] [PubMed] [Google Scholar]