Abstract
This study identifies monocyte chemoattractant protein 1 (MCP-1) as an insulin-responsive gene. It also shows that insulin induces substantial expression and secretion of MCP-1 both in vitro in insulin-resistant (IR) 3T3-L1 adipocytes and in vivo in IR obese mice (ob/ob). Thus, MCP-1 resembles other previously described genes (e.g., PAI-1 and SREBP-1c) that remain sensitive to insulin in IR states. The hyperinsulinemia that frequently accompanies obesity and insulin resistance may therefore contribute to the altered expression of these and other genes in insulin target tissues. In vivo studies also demonstrate that MCP-1 is overexpressed in obese mice compared with their lean controls, and that white adipose tissue is a major source of MCP-1. The elevated MCP-1 may alter adipocyte function because addition of MCP-1 to differentiated adipocytes in vitro decreases insulin-stimulated glucose uptake and the expression of several adipogenic genes (LpL, adipsin, GLUT-4, aP2, β3-adrenergic receptor, and peroxisome proliferator-activated receptor γ). These results suggest that elevated MCP-1 may induce adipocyte dedifferentiation and contribute to pathologies associated with hyperinsulinemia and obesity, including type II diabetes.
Obesity poses a serious health hazard and contributes to the increased morbidity and mortality in Western societies where it has reached epidemical proportions (1). Despite the magnitude and cost of this problem, the molecular changes in obesity that lead to poor health remain to be defined. Obesity frequently is accompanied by related metabolic perturbations such as dyslipidemia, hypertension, insulin resistance, hyperinsulinemia, and the development of a procoagulant state, and these changes contribute to an increased risk for cardiovascular diseases (2). Importantly, insulin resistance is also central to the pathophysiology of type II diabetes (3). Although the molecular mechanisms leading to development of insulin resistance also are not fully understood, there appears to be an association between insulin resistance and both the accumulation of abdominal visceral fat (4) and the presence of specific genetic components (5).
Insulin affects a number of biological processes including glucose transport, glucose/lipid metabolism, cell growth, protein synthesis, and gene expression (6). Although insulin resistance, by definition, describes an impaired biological responsiveness to insulin, it is frequently used to describe a defect in insulin-stimulated glucose uptake by muscle and adipocytes and/or a decrease in gluconeogenesis by the liver. The degree to which the other actions of insulin (e.g., gene expression) remain normal or become resistant in type II diabetes is not clear.
In addition to their role in fat storage, adipocytes synthesize and secrete a variety of bioactive proteins (7). During the development of obesity and type II diabetes these cells increase in size and number and their metabolic activity is dramatically altered. It is conceivable that some of the adipocyte-derived factors could underlie the association of insulin resistance with a prothrombotic state and the increased risk for coronary heart disease (8). In this regard, plasminogen activator inhibitor 1 (PAI-1) is elevated in human obesity and type II diabetes (9, 10). Tumor necrosis factor α (TNF-α) is also elevated in obesity and may contribute to many aspects of adipose tissue biology including development of insulin resistance and abnormalities in lipid metabolism (11).
Studies of genetically obese mice and cultured adipocytes demonstrate that insulin and TNF-α are two mediators that regulate PAI-1 expression in the adipocyte in vivo (12). Interestingly, these studies also reveal that insulin-resistant (IR) adipocytes and mice remained sensitive to insulin in terms of PAI-1 gene expression, possibly because glucose homeostasis and PAI-1 gene expression are regulated by different insulin signaling pathways (13). Consistent with this hypothesis, Shimomura and colleagues (14) demonstrated that in murine leptin-deficient states insulin signaling in the liver also diverges along two pathways and they showed that the transcription factor SREBP-1c is another gene that remains sensitive to insulin in these IR mice. Similar selective insulin resistance also has been described in human and rodent muscle (15, 16). Collectively, these observations raise the possibility that in the situation of metabolic insulin resistance accompanied by hyperinsulinemia, the expression of certain insulin-responding genes may dramatically increase in insulin target tissues. Characterization of such genes could provide valuable information about the molecular basis for the association of insulin resistance and type II diabetes with cardiovascular disease.
To begin to define the cluster of genes that remain responsive to insulin in metabolic IR states, microarray analysis was performed. By comparing gene expression profiles between normal and IR 3T3-L1 adipocytes treated with exogenous insulin, we have identified a number of genes that continue to respond normally to insulin in cultured IR adipocytes (unpublished work). One such gene is monocyte chemoattractant protein 1 (MCP-1), a member of the chemokine family. It has been studied in a number of pathological conditions characterized by monocyte infiltration (17) and is expressed by a variety of activated cells (e.g., endothelial cells, monocytes, and smooth muscle cells) exposed to inflammatory stimuli (18). However, information about the role of MCP-1 in obesity and the development of insulin resistance is lacking. This chemokine was recently detected in cultured human adipocytes (19).
In the present study, we show that MCP-1 mRNA is overexpressed in the adipose tissue of genetically obese mice compared with WT littermates and that it continues to respond to exogenous insulin in IR adipocytes and mice. Additional in vitro studies suggest that MCP-1 may contribute to the development of insulin resistance and that it also induces adipocyte dedifferentiation.
Methods
Cell Culture. Murine 3T3-L1 fibroblasts were purchased from the American Type Culture Collection and differentiated in vitro into mature adipocytes as described (13). IR cells were prepared by incubating mature adipocytes for 3 days in the presence of 3 ng/ml murine TNF-α (R & D Systems) (13). In this model, the old media are replaced with fresh media every 24 h over a 3-day period. Normal control adipocytes were treated in the same way but without TNF-α. For experiments with insulin, normal and IR adipocytes were incubated overnight in serum-free medium supplemented with 0.2% BSA. The next day, the cells were incubated with or without 1,000 nM bovine insulin (Sigma) for 3 h. In some experiments, differentiated adipocytes were incubated with mouse MCP-1 (R & D Systems) instead of TNF-α. The conditioned media were collected, and total cell protein was extracted into 1% SDS and stored at -80°C until further processing.
Glucose Uptake. 3H-2-deoxyglucose transport was measured in 3T3-L1 adipocytes as described (13). Values were normalized to total cell protein determined by using the BCA protein assay (Pierce).
Animal Experiments. All animal studies were reviewed and approved by our Institutional Animal Care and Use Committee and the Animal Research Committee, in accordance with Public Health Policy regarding the use and care of laboratory animals. Female obese mice (strain C57BL/6J-ob/ob B6.V-Lep<ob>), 8–11 weeks old, were obtained from The Jackson Laboratory. Age- and gender-matched WT C57BL/6J mice were from the rodent breeding colony at The Scripps Research Institute. For in vivo insulin experiments, WT and ob/ob mice were injected i.p. with 10 or 20 units, respectively, of human insulin (Humulin, Eli Lilly) or an equivalent volume of saline as control. The ob/ob mice are approximately twice the size of the WT mice. Hence, the difference in amount of insulin injected in the two groups corrects for the difference in weight between the animals and results in a comparable dose of insulin per kg of body weight. Plasma was collected at different time points, and the mice were killed by overdose inhalation of Metofane (Schering-Plough) and cervical dislocation. Tissues were rapidly removed and immediately snap-frozen in liquid nitrogen for subsequent RNA extraction. Glucose levels were monitored with a Glucometer Elite Blood Glucose Meter (Bayer, Elkhart, IN).
RNA Isolation. Frozen tissues were powdered in liquid nitrogen, and total RNA was extracted by using TRIzol reagent (GIBCO/ BRL) and a subsequent clean-up RNeasy protocol (Qiagen, Valencia, CA). Total RNA from 3T3-L1 adipocytes was prepared by using similar extraction protocols.
Real-Time RT-PCR. For real-time PCR, cDNA was prepared from 1 μg of total RNA by using random hexamers and Moloney murine leukemia virus reverse transcriptase (Perkin–Elmer) in a final reaction volume of 20 μl. Real-time PCR amplifications were performed from 2.5 μl of cDNA diluted 1:2 by using gene-specific primer sets (Invitrogen) (see Table 1, which is published as supporting information on the PNAS web site, www.pnas.orgg). Each primer set was used at 150 nM in a final volume of 25 μl by using the SYBR Green Master Mix (Perkin–Elmer). All PCRs were performed in an iCycler (Bio-Rad). Quantification of a given gene, expressed as relative mRNA level compared with a control, was calculated after normalization to 18S rRNA and using the ΔΔCT formula as described by Perkin–Elmer. Individual CT values are means of duplicate measurements. Separate control experiments demonstrated that the efficiencies of target and reference amplification were equal. The specificity of the PCR amplification was verified by melt-curve analysis of the final products directly in the iCycler and by agarose-gel electrophoresis.
MCP-1 ELISA. Concentrations of MCP-1 in plasma and conditioned cell culture medium were determined by using an ELISA kit (BioSource International, Camarillo, CA).
Statistical Analysis. All calculations were performed by using PRISM 3.02 software (GraphPad, San Diego). Statistical significance between two groups was determined by using the Student's t test. Comparisons among several groups were performed by ANOVA, and statistical significance was calculated by using Dunnett's multiple comparison test.
Results
Adipocytes Express and Secrete MCP-1 in Vitro. In initial experiments to examine MCP-1 synthesis in vitro, we studied normal 3T3-L1 adipocytes and 3T3-L1 adipocytes made IR by treatment with TNF-α. To evaluate the degree of insulin resistance in the cells, insulin-stimulated 3H-2-deoxyglucose uptake was measured in both cell types. As shown in Fig. 1A Inset, TNF-α pretreatment of the adipocytes resulted in a significantly decreased (≈60%) rate of insulin-stimulated glucose uptake by these cells. However, no change was observed in the basal rate of glucose uptake (not shown). In addition, we detected lower expression levels of a number of genes in the IR cells compared with the normal adipocytes by using microarray technology (data not shown). These genes include GLUT-4, hexokinase II, GLUT-1, IRS-2, phosphatidylinositol 3-kinase p85β subunit, and PKC-ε, all of which have been shown to be involved in adipocyte glucose transport and metabolism (20). The decreased expression of these genes may help to explain the lower rate of insulin-stimulated glucose uptake in these cells. Importantly, TNF-α did not appear to dedifferentiate the mature adipocytes because the expression of a number of adipocyte markers (e.g., adipsin, lipoprotein lipase, and fatty acid synthetase) was not decreased by this treatment.
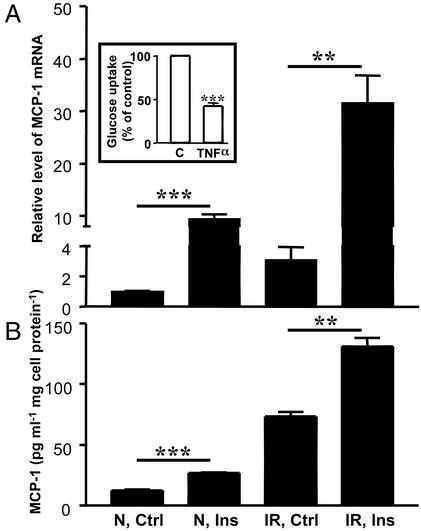
Fig. 1.
Effect of insulin on MCP-1 gene expression in vitro. Mature adipocytes were incubated without insulin (Ctrl) or with 1,000 nM insulin (Ins) for 3 h. (A) MCP-1 mRNA levels were determined in normal (N) and IR 3T3-L1 adipocytes by using real-time RT-PCR and normalized to the expression of 18S rRNA. The data are expressed as relative mRNA level compared with the average expression level in normal cells incubated without insulin (=1). (Inset) The effect of TNF-α on insulin-stimulated glucose uptake was analyzed in mature 3T3-L1 adipocytes incubated without (C) or with 3 ng/ml TNF-α for 3 days. Insulin-stimulated 3H-2-deoxyglucose uptake was determined, and the data are expressed as percentage of the control (i.e., cells incubated without TNF-α). The error bars represent the SE (n = 5); ***, P < 0.0001 relative to control cells. (B) Secretion of MCP-1 protein from normal (N) and IR 3T3-L1 adipocytes was determined in conditioned media by using ELISA. Differentiated adipocytes were treated as described in A. The data were normalized to total cell protein. The error bars represent the SE (n = 3); **, P < 0.01 and ***, P < 0.001 relative to control cells.
As already mentioned, initial microarray gene expression profiling experiments identified MCP-1 as one of several genes that respond similarly to exogenous insulin in normal and metabolically IR 3T3-L1 adipocytes. This observation was confirmed by using real-time RT-PCR (Fig. 1 A). The relative level of MCP-1 expression in normal control adipocytes was 1.0 (by definition) and increased to 9.5 upon insulin treatment. In the IR adipocytes, the relative level of MCP-1 expression in the untreated control cells was 3.1, which increased to 31.6 after insulin treatment. Thus, treatment of the adipocytes for 3 h with 1,000 nM insulin in vitro leads to an ≈10-fold induction in MCP-1 mRNA levels, irrespective of whether the adipocytes are IR or not. Notably, basal expression of MCP-1 was ≈3-fold higher in IR adipocytes compared with normal cells, a result also obtained with microarray analysis (data not shown). Dose–response experiments using normal adipocytes showed half-maximal induction of MCP-1 mRNA at 100 nM insulin, with maximal induction observed at 1,000 nM (data not shown). Recent studies suggest that insulin may induce MCP-1 by binding to both the insulin receptor and the insulin-like growth factor-1 receptor (34). Insulin treatment also led to an increase in MCP-1 secretion from both normal and IR adipocytes (Fig. 1B). Again, the IR adipocytes appeared to secrete more MCP-1 under basal conditions than the normal cells. Thus, similar effects of insulin were observed in both normal and IR cells. Taken together, the results in Fig. 1 suggest that insulin stimulates MCP-1 gene expression and protein secretion in 3T3-L1 adipocytes via signaling pathways that do not become IR.
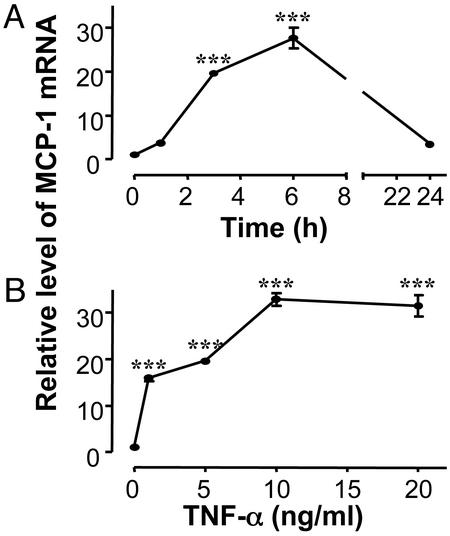
In addition to hyperinsulinemia, obesity is associated with increased TNF-α expression in adipose tissue (11). Experiments were performed to investigate whether TNF-α could induce MCP-1 gene expression in adipocytes in vitro (Fig. 2). Treatment of differentiated 3T3-L1 adipocytes with TNF-α led to a dramatic increase in MCP-1 mRNA with the maximal effect (≈30-fold induction) observed after 6 h of incubation (Fig. 2 A). By 24 h, the level of induction had decreased to 3.4-fold, the level in IR adipocytes (Fig. 1 A). Dose–response experiments (Fig. 2B) demonstrated that the maximal effect was obtained at 10 ng/ml TNF-α, and that the expression of the MCP-1 receptor (i.e., CCR2 mRNA) was not significantly altered by TNF-α (data not shown).
Fig. 2.
Effect of TNF-α on MCP-1 gene expression in vitro. Differentiated normal 3T3-L1 adipocytes were cultured as described in Methods, and then incubated in the presence or absence of 5 ng/ml TNF-α for different times (A) or incubated for 3 h with different concentrations of TNF-α (B). MCP-1 gene expression was analyzed by using real-time RT-PCR and normalized to expression of 18S rRNA. The data are expressed as relative mRNA level compared with the average expression in cells incubated without TNF-α (=1). The error bars represent the SE (n = 3); ***, P < 0.001 relative to cells incubated without TNF-α.
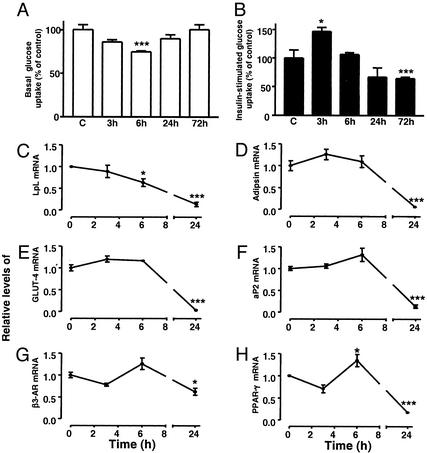
Effect of MCP-1 on Adipocyte Function in Vitro. In an attempt to investigate the influence of MCP-1 on adipocyte function, we explored the possibility that MCP-1 (like TNF-α; see Fig. 1 A Inset) could render adipocytes relatively resistant to the effect of insulin on glucose transport. This possibility was investigated by using two experimental protocols designed to analyze the acute and chronic effects of MCP-1. In the first, differentiated 3T3-L1 adipocytes were incubated in the presence of low concentrations of MCP-1 (1 ng/ml) for various times up to 24 h, and then directly analyzed for changes in basal and insulin-stimulated glucose uptake (acute effect). Fig. 3A shows that after 6 h there was a significant reduction (≈25%) in basal glucose uptake in cells treated with MCP-1, and that this effect was transient because glucose uptake approached control levels at 24 h. Interestingly, MCP-1 increased the rate of insulin-stimulated glucose uptake by ≈50% at 3 h (Fig. 3B). However, this effect was again transient, and prolonged incubation (24 h) with MCP-1 resulted in inhibition of insulin-stimulated glucose uptake. To further address these findings, a second experiment was performed in which we incubated differentiated 3T3-L1 adipocytes with MCP-1 for 72 h (chronic effect), and then analyzed the basal and insulin-stimulated rates of glucose uptake (this protocol is similar to the protocol that was used when treating the cells with TNF-α). As shown in Fig. 3A, the basal rate of glucose uptake in the cells incubated with MCP-1 for 72 h was similar to the control, but the rate of insulin-stimulated glucose uptake was significantly inhibited (≈30%) (Fig. 3B).
Fig. 3.
Effect of MCP-1 on glucose uptake and gene expression in differentiated 3T3-L1 adipocytes. Basal (A) and insulin-stimulated (B) 3H-2-deoxyglucose uptake in differentiated 3T3-L1 adipocytes treated with MCP-1 (1 ng/ml) was measured as described in Methods. The data are expressed as percentage of control (i.e., cells incubated without MCP-1). Changes in expression of adipogenic genes in differentiated 3T3-L1 adipocytes treated with MCP-1 (5 ng/ml) were analyzed by using gene-specific primers and real-time RT-PCR. Genes analyzed include LpL (C), adipsin (D), GLUT-4 (E), aP2 (F), β3-adrenergic receptor (G), and PPAR-γ (H). The results were normalized to expression of 18S rRNA and are expressed as relative mRNA level compared with the average expression in cells incubated without MCP-1 (=1). The error bars represent the SE (n = 3–8); *, P < 0.05 and ***, P < 0.001 relative to control cells.
MCP-1 appears to alter lipid accumulation and differentiation of cultured adipocytes (19). To further investigate the molecular basis for this observation, expression of a number of adipogenic genes was analyzed in differentiated 3T3-L1 adipocytes incubated with or without MCP-1. As shown in Fig. 3 C–H, a substantial reduction in mRNA levels for LpL, adipsin, GLUT-4, aP2, β3-adrenergic receptor, and peroxisome proliferator-activated receptor γ (PPAR-γ) was detected after 24 h of incubation with MCP-1. Taken together, these results indicate that MCP-1 may cause adipocyte dedifferentiation. We did not detect any changes in the morphology of the adipocytes and there was no apparent toxicity induced by MCP-1. No significant changes in total protein were detected even after incubation of differentiated 3T3-L1 adipocytes in the presence of 20 ng/ml MCP-1 for up to 3 days (data not shown). Furthermore, the concentrations used in this study are lower than those used by Gerhardt and colleagues (19) without any reported cytotoxicity.
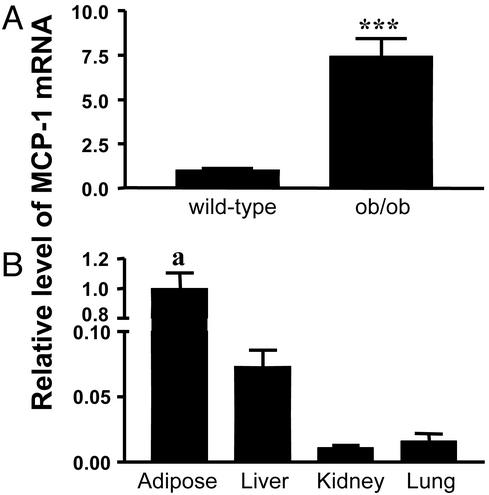
MCP-1 Expression in Vivo. We analyzed MCP-1 gene expression in vivo by using real-time RT-PCR and RNA extracted from s.c. adipose tissues from age- and gender-matched WT and ob/ob mice. The basal level of expression of MCP-1 in the adipose tissue was significantly elevated (i.e., 7-fold) in ob/ob mice compared with WT (Fig. 4A). Fig. 4B shows that the highest level of expression was in s.c. adipose tissue, with the liver having <10% of that detected in adipose tissue, and the kidneys and lungs having only 1–2%. Thus, of the tissues examined in these mice, the adipose tissue appears to be the major source of MCP-1 biosynthesis.
Fig. 4.
MCP-1 gene expression in vivo.(A) Basal levels of MCP-1 mRNA were determined in s.c. adipose tissue from sex- and age-matched WT and ob/ob mice by using real-time RT-PCR. The data are normalized to the expression of 18S rRNA and are expressed as relative mRNA level compared with the average expression in WT mice (=1). The error bars represent the SE (n = 6); ***, P < 0.0001. (B) Basal levels of MCP-1 mRNA were determined in different tissues from ob/ob mice by using real-time RT-PCR. The data are normalized to the expression of 18S rRNA and are expressed as relative mRNA levels compared with the average expression in s.c. adipose tissue (=1). The error bars represent the SE (n = 4); a, P < 0.001 in adipose tissue vs. liver, kidney, and lung.
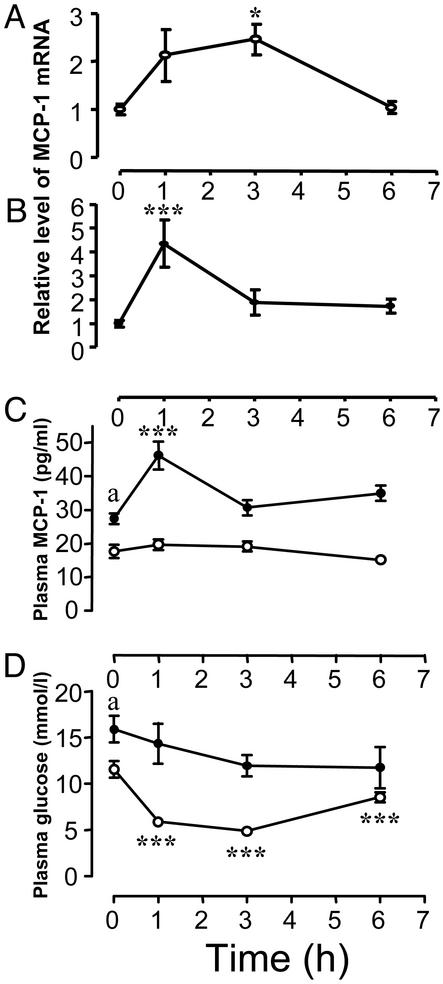
Fig. 5 demonstrates that MCP-1 gene expression in both WT (A) and ob/ob (B) mice is significantly increased after insulin treatment. However, the relative change was higher in the ob/ob mice (4-fold change in ob/ob vs. 2.5-fold in WT) and it peaked at an earlier time (1 h in ob/ob vs. 3 h in WT). Baseline plasma concentrations of MCP-1 also were significantly higher in ob/ob mice compared with WT (Fig. 5C). Moreover, insulin treatment of the ob/ob mice led to a significant (≈2-fold) increase in the plasma concentration of MCP-1 compared with saline-treated control animals. However, no significant change in the plasma concentration of MCP-1 was detected when the WT mice were treated with insulin. Insulin lowers plasma glucose levels in WT mice by ≈50% (Fig. 5D), indicating that these mice are sensitive to exogenous insulin. As expected, ob/ob mice were slightly hyperglycemic and relatively resistant to insulin because they only showed a minor trend toward lower glucose levels in response to insulin. This decrease was not statistically significant. Thus, although the ob/ob mice are relatively resistant to the effects of insulin on glucose transport (i.e., they are metabolically IR), they remain sensitive to insulin in terms of adipose tissue MCP-1 mRNA and plasma MCP-1 levels.
Fig. 5.
Effect of exogenous insulin on MCP-1 gene expression in vivo. The effect of insulin on MCP-1 gene expression in s.c. adipose tissue from WT (A) and ob/ob (B) mice was determined. Female mice were injected i.p. with 10 units (WT) or 20 units (ob/ob) of human insulin or an equivalent volume of saline as control (shown at the 0-h time point). The mice were killed at different times, and MCP-1 gene expression was determined in the s.c. adipose tissue by using real-time RT-PCR. *, P < 0.05 and **, P < 0.01 relative to saline-treated mice. Plasma was collected from the same mice and analyzed for MCP-1 antigen (C) and glucose (D). ○, results from WT mice; •, ob/ob mice. a, P < 0.01 for WT vs. ob/ob mice at baseline (C), and a, P < 0.05 for WT vs. ob/ob mice at baseline (D); ***, P < 0.001 relative to saline-treated mice. The error bars indicate SE (n = 6).
Discussion
MCP-1 is a monomeric polypeptide produced by a variety of cells including human adipocytes (18, 19), frequently in response to inflammatory stimuli such as IL-1, IL-4, or TNF-α (18). It is a well-studied member of the CC chemokine family, which to date comprises >50 members, and plays a crucial role in the recruitment of monocytes and memory T lymphocytes into tissues (21). However, many chemokines also have physiological activities that extend beyond cell recruitment (22). The main receptor in vivo for MCP-1 is CCR2, although other receptors capable of interacting with MCP-1 have been identified (23). Several different cell types (18) including adipocytes (19) have been reported to express CCR2. Importantly, long-term incubation of adipocytes with exogenous chemokines suppressed lipid accumulation and PPAR-γ expression, and chemokines acutely increased leptin secretion from mature adipocytes (19). These observations suggest that chemokines may have important biological effects on adipocytes.
In this article, we demonstrate that MCP-1 also is expressed by murine adipocytes (Fig. 1 A) and show that the expression of MCP-1 is up-regulated in white adipose tissue (Fig. 4A) and the plasma (Fig. 5C) of obese mice compared with their lean counterparts. The adipose tissue from obese mice expressed 10- to 100-fold more MCP-1 mRNA than the liver, kidneys, and lungs (Fig. 4B), suggesting that the adipose tissue may be a major source of the increased plasma levels of MCP-1 observed in these animals. In situ hybridization analysis of adipose tissue from ob/ob mice detected MCP-1 mRNA in cells that morphologically resemble adipocytes (see Fig. 6, which is published as supporting information on the PNAS web site). However, the hybridization signal was relatively weak, and it is possible that other cells in the fat may also express MCP-1 mRNA. The fact that cultured murine adipocytes (Fig. 1) and adipocytes isolated from human fat depots (19) synthesize and secrete MCP-1 is consistent with the conclusion that the adipocyte is a primary source of MCP-1 in fat.
Our studies not only revealed that insulin induces MCP-1 expression in normal adipocytes (Fig. 1), but also that it continues to induce expression in IR adipocytes and mice. Increased MCP-1 protein synthesis accompanied the increases in MCP-1 mRNA in vitro and in vivo. Although a direct relationship between the increase in MCP-1 mRNA (Fig. 1 A) and secreted MCP-1 protein (Fig. 1B) was not always observed, this was not surprising because we know little about the relative half-life and translational efficiency of MCP-1 mRNA in normal vs. IR adipocytes. We did not detect increased plasma levels of MCP-1 in WT mice after insulin treatment. It is possible that the relatively low levels of MCP-1 produced in the WT mice may be sequestered in the extracellular and pericellular matrix (24) and thus not reach the circulation. In any case, our data indicate that MCP-1 can be included in the growing family of genes [e.g., PAI-1 (13) and SREBP-1c (14)] that continue to respond to insulin in IR states. The inappropriate induction of these genes in hyperinsulinemic states such as obesity, type II diabetes, and polycystic ovary disease (25) may contribute to the increased risk for atherothrombotic disease in these conditions.
The data presented in this study and that of others (19) suggest that MCP-1 may alter adipocyte function and metabolism and may contribute to development of insulin resistance and adipocyte dedifferentiation. For example, based on the observations that MCP-1 is overproduced in the IR adipocytes and the adipose tissue of IR obese mice, we hypothesized that MCP-1 itself could affect insulin sensitivity in adipocytes. To test this possibility, we incubated fully differentiated 3T3-L1 adipocytes in vitro with low concentrations of MCP-1. We observed that insulin-stimulated glucose uptake was severely blunted by MCP-1 (Fig. 3B), even at very low concentrations (i.e., 100 pg/ml; not shown). These observations suggest that MCP-1 may promote insulin resistance in differentiated adipocytes in the situation of prolonged exposure to MCP-1. This effect may have in vivo relevance because MCP-1 protein and mRNA levels are increased in obese mice (Figs. 4 and 5). Therefore, MCP-1 should be added to the list of agents that may contribute to the initiation and progression of insulin resistance.
Fig. 3 C–H shows that MCP-1 also down-regulates expression of LpL and a number of other adipogenic genes in mature adipocytes. This observation may begin to explain why lipid accumulation in adipocytes is inhibited by the presence of MCP-1 (19). Similar effects have been reported for other proinflammatory cytokines such as IL-6 and TNF-α (26, 27). Taken together, these observations indicate that MCP-1 may have important functions in regulating the maturation and differentiation state of adipocytes. Interestingly, by using microarray analysis Nadler et al. (28) recently showed that the expression of adipogenic genes was decreased in adipose tissue from obese and type II diabetic mice. Our data raise the possibility that MCP-1 may be involved in initiating and sustaining these abnormalities.
Although various functions of MCP-1 have been well studied, considerably less is known about the transcriptional regulation of MCP-1 gene expression. The promoter regions that control MCP-1 gene expression are separated by >2 kb of DNA, suggesting that regulation of MCP-1 expression reflects complex and extensive interactions between transcription factors and promoter elements (29). In this regard, there are several mechanisms that may underlie the observation that MCP-1 is overexpressed in obese adipose tissue. The activation of NF-κB is probably involved because of the increased levels of TNF-α (11, 29), but the murine MCP-1 promoter also contains AP-1 and SP-1 binding sites as well as a hypermethylation-sensitive site together with two orphan sites, which all appear to contribute to TNF-α-induced MCP-1 expression (29). Obviously, further studies are necessary to elucidate the signaling pathways that govern insulin-mediated MCP-1 expression in normal and IR adipocytes.
The biological/pathological role of MCP-1 expression in adipocytes and adipose tissue is not understood, and there are many potential effects of MCP-1 on adipose tissue biology. For example, our findings support the notion that immunological and inflammatory processes are important features of the adipose tissue in the obese state (30), and that these processes may affect development of insulin resistance. MCP-1 plays an important role in the recruitment of monocytes, and as such may contribute to the initiation and maintenance of inflammatory reactions in the adipose tissue. MCP-1, in concert with other proinflammatory cytokines expressed by adipocytes, such as TNF-α and IL-6, may also influence glucose and lipid metabolism (7, 11). MCP-1 also has a direct angiogenic effect on endothelial cells (31) and thus may be involved in the expansion and remodeling of the adipose tissue during development of obesity. In this regard, it was recently observed that MCP-1 accelerates wound healing, a process that depends on blood vessel growth (32). These findings indicate that MCP-1 may have broader roles in adipocyte physiology than inflammatory cell recruitment.
In conclusion, we have demonstrated that MCP-1 is overexpressed in obesity and that MCP-1 can be included in the family of genes that continue to respond to exogenous insulin in IR mice and adipocytes. Furthermore, in vitro studies showed that MCP-1 itself might contribute to the development of insulin resistance and cause adipocyte dedifferentiation. We hypothesize that the regulation of MCP-1 gene expression in adipose tissue could play an important role in altering adipocyte function and metabolism, especially in the transition from the lean to the obese state. Finally, it should be noted that although our studies were performed with murine experimental models, it was recently reported that the serum levels of MCP-1 also were significantly increased in patients with type II diabetes compared with a matched control group (33). These observations suggest that similar mechanisms may be of relevance in humans.
Supplementary Material
Acknowledgments
We thank Marcia McRae for preparing the manuscript. This work was supported in part by a grant from Novartis Pharmaceuticals and National Institutes of Health Grant HL59459 (to D.J.L.). P.S. was supported by a postdoctoral fellowship from the Henning and Johan Throne-Holst Foundation. This is The Scripps Research Institute manuscript no. 14956-CB.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PAI-1, plasminogen activator inhibitor 1; TNF-α, tumor necrosis factor α; IR, insulin-resistant; MCP-1, monocyte chemoattractant protein 1; PPAR, peroxisome proliferator-activated receptor.
References
- 1.Zimmet, P., Alberti, K. G. & Shaw, J. (2001) Nature 414, 782-787. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg, H. N. (2000) J. Clin. Invest. 106, 453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratley, R. E. & Weyer, C. (2001) Diabetologia 44, 929-945. [DOI] [PubMed] [Google Scholar]
- 4.Bjorntorp, P. (1999) Diabetes Metab. Res. Rev. 15, 427-441. [DOI] [PubMed] [Google Scholar]
- 5.Haffner, S. M., Stern, M. P., Hazuda, H. P., Mitchell, B. D. & Patterson, J. K. (1988) N. Engl. J. Med. 319, 1297-1301. [DOI] [PubMed] [Google Scholar]
- 6.Ferrannini, E., Galvan, A. Q., Gastaldelli, A., Camastra, S., Sironi, A. M., Toschi, E., Baldi, S., Frascerra, S., Monzani, F., Antonelli, A., et al. (1999) Eur. J. Clin. Invest. 29, 842-852. [DOI] [PubMed] [Google Scholar]
- 7.Loskutoff, D. J. & Samad, F. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1-6. [DOI] [PubMed] [Google Scholar]
- 8.Juhan-Vague, I., Alessi, M. C. & Morange, P. E. (2000) Ann. Med. 32, 78-84. [PubMed] [Google Scholar]
- 9.Juhan-Vague, I., Roul, C., Alessi, M. C., Ardissone, J. P., Heim, M. & Vague, P. (1989) Thromb. Haemostasis 61, 370-373. [PubMed] [Google Scholar]
- 10.McGill, J. B., Schneider, D. J., Arfken, C. L., Lucore, C. L. & Sobel, B. E. (1994) Diabetes 43, 104-109. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. (1993) Science 259, 87-91. [DOI] [PubMed] [Google Scholar]
- 12.Samad, F., Uysal, K. T., Wiesbrock, S. M., Pandey, M., Hotamisligil, G. S. & Loskutoff, D. J. (1999) Proc. Natl. Acad. Sci. USA 96, 6902-6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samad, F., Pandey, M., Bell, P. A. & Loskutoff, D. J. (2000) Mol. Med. 6, 680-692. [PMC free article] [PubMed] [Google Scholar]
- 14.Shimomura, I., Matsuda, M., Hammer, R. E., Bashmakov, Y., Brown, M. S. & Goldstein, J. L. (2000) Mol. Cell 6, 77-86. [PubMed] [Google Scholar]
- 15.Cusi, K., Maezono, K., Osman, A., Pendergrass, M., Patti, M. E., Pratipanawatr, T., DeFronzo, R. A., Kahn, C. R. & Mandarino, L. J. (2000) J. Clin. Invest. 105, 311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiahg, Z. Y., Lin, Y.-W., Clemont, A., Feener, E. P., Hein, K. D., Igarashi, M., Yamauchi, T., White, M. F. & King, G. L. (1999) J. Clin. Invest. 104, 447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerard, C. & Rollins, B. J. (2001) Nat. Immunol. 2, 108-115. [DOI] [PubMed] [Google Scholar]
- 18.Rollins, B. J. (1997) Blood 90, 909-928. [PubMed] [Google Scholar]
- 19.Gerhardt, C. C., Romero, I. A., Cancello, R., Camoin, L. & Strosberg, A. D. (2001) Mol. Cell. Endocrinol. 175, 81-92. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd, P. R. & Kahn, B. B. (1999) N. Engl. J. Med. 341, 248-257. [DOI] [PubMed] [Google Scholar]
- 21.Baggiolini, M. (1998) Nature 392, 565-568. [DOI] [PubMed] [Google Scholar]
- 22.Mackay, C. R. (2001) Nat. Immunol. 2, 95-101. [DOI] [PubMed] [Google Scholar]
- 23.Kunkel, S. L. (1999) J. Clin. Invest. 104, 1333-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuschert, G. S., Coulin, F., Power, C. A., Proudfoot, A. E., Hubbard, R. E., Hoogewerf, A. J. & Wells, T. N. (1999) Biochemistry 38, 12959-12968. [DOI] [PubMed] [Google Scholar]
- 25.Ehrmann, D. A., Schneider, D. J., Sobel, B. E., Cavaghan, M. K., Imperial, J., Rosenfield, R. L. & Polonsky, K. S. (1997) J. Clin. Endocrinol. Metab. 82, 2108-2116. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg, A. S., Nordan, R. P., McIntosh, J., Calvo, J. C., Scow, R. O. & Jablons, D. (1992) Cancer Res. 52, 4113-4116. [PubMed] [Google Scholar]
- 27.Zechner, R., Newman, T. C., Sherry, B., Cerami, A. & Breslow, J. L. (1988) Mol. Cell. Biol. 8, 2394-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadler, S. T., Stoehr, J. P., Schueler, K. L., Tanimoto, G., Yandell, B. S. & Attie, A. D. (2000) Proc. Natl. Acad. Sci. USA 97, 11371-11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ping, D., Jones, P. L. & Boss, J. M. (1996) Immunity 4, 455-469. [DOI] [PubMed] [Google Scholar]
- 30.Frohlich, M., Imhof, A., Berg, G., Hutchinson, W. L., Pepys, M. B., Boeing, H., Muche, R., Brenner, H. & Koenig, W. (2000) Diabetes Care 23, 1835-1839. [DOI] [PubMed] [Google Scholar]
- 31.Salcedo, R., Ponce, M. L., Young, H. A., Wasserman, K., Ward, J. M., Kleinman, H. K., Oppenheim, J. J. & Murphy, W. J. (2000) Blood 96, 34-40. [PubMed] [Google Scholar]
- 32.Low, Q. E., Drugea, I. A., Duffner, L. A., Quinn, D. G., Cook, D. N., Rollins, B. J., Kovacs, E. J. & DiPietro, L. A. (2001) Am. J. Pathol. 159, 457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura, S., Shouzu, A., Omoto, S., Nishikawa, M. & Fukuhara, S. (2000) Clin. Exp. Immunol. 121, 437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulligan, C., Rochford, J., Denyer, G., Stephens, R., Yeo, G., Freeman, T., Siddle, K. & O'Rahilly, S. (2002) J. Biol. Chem. 277, 42480-42487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.