Abstract
Primary hyperoxaluria type 1 (PH1) is an inborn error of metabolism resulting from a deficiency of alanine:glyoxylate aminotransferase (AGXT; EC 2.6.1.44). Most of the PH1 alleles detected in the Canary Islands carry the Ile-244 → Thr (I244T) mutation in the AGXT gene, with 14 of 16 patients homozygous for this mutation. Four polymorphisms within AGXT and regional microsatellites also were shared in their haplotypes (AGXT*LTM), consistent with a founder effect. The consequences of these amino acid changes were investigated. Although I244T alone did not affect AGXT activity or subcellular localization, when present in the same protein molecule as Leu-11 → Pro (L11P), it resulted in loss of enzymatic activity in soluble cell extracts. Like its normal counterpart, the AGXT*LTM protein was present in the peroxisomes but it was insoluble in detergent-free buffers. The polymorphism L11P behaved as an intragenic modifier of the I244T mutation, with the resulting protein undergoing stable interaction with molecular chaperones and aggregation. This aggregation was temperature-sensitive. AGXT*LTM expressed in Escherichia coli, as a GST-fusion protein, and in insect cells could be purified and retained enzymatic activity. Among various chemical chaperones tested in cell culture, betaine substantially improved the solubility of the mutant protein and the enzymatic activity in cell lysates. In summary, I244T, the second most common mutation responsible for PH1, is a protein conformational disease that may benefit from new therapies with pharmacological chaperones or small molecules to minimize protein aggregation.
Primary hyperoxaluria type I (PH1; Online Mendelian Inheritance in Man 259900) is a rare autosomal recessive disease caused by mutations in the alanine:glyoxylate aminotransferase gene (AGXT). AGXT (EC 2.6.1.44) is an important enzyme in the detoxification of glyoxylate. In humans, insufficient AGXT activity in peroxisomes leads to increased conversion of glyoxylate to oxalate, a toxic compound normally cleared by the kidney. PH1 patients show progressive deterioration of renal function from calcium oxalate deposition in both the parenchyma and the pyelocaliceal system. Once the oxalate-clearance capacity of the kidneys is exceeded, calcium oxalate deposition becomes wide-spread and life-threatening unless liver and kidney transplantation is performed.
Human AGXT cDNA was cloned (1, 2), and its genomic structure is known (3). A number of mutations at the AGXT gene have been found in PH1 patients (www.uwcm.ac.uk/uwcm/mg/hgmd0.html). PH1 is very heterogeneous at the clinical and molecular levels. About one-third of PH1 patients carry a mistargeting mutation that results in most of the AGXT being localized to the mitochondria instead of the peroxisome (4). We have studied 16 PH1 patients from the Canary Islands and detected the Ile-244 → Thr (I244T) mutation in most of the pathologic alleles. We found that PH1 resulting from I244T, in combination with the common polymorphism Pro-11 → Leu (P11L), is an example of a protein conformational disease that could be amenable to pharmacological intervention.
Materials and Methods
Patients. With informed consent, blood DNA was obtained from 16 patients and their relatives, distributed among 12 families. The parents of one patient were cousins. Two hundred anonymous DNA samples were used to estimate allele frequencies in the Canary Island community.
DNA Analysis. Screening of AGXT gene mutations was performed as described (5). Direct sequencing of PCR products and fragment analysis for D2S125 and D2S140 markers were performed with a CEQ2000XL (Beckman Coulter). PCR-restriction fragment length polymorphism reactions were used to confirm the nucleotide changes and extend the analysis to other family members.
Expression Constructs and Site-Directed Mutagenesis. AGXT coding cDNA was amplified from normal human liver mRNA, cloned, and sequenced by using standard protocols (6). Site-directed mutagenesis was performed (6) to introduce the following changes: P11L (AGXT*L), I244T (AGXT*T), Ile-340 → Met (I340M) (AGXT*M), both P11L and I244T (AGXT*LT), and all three (AGXT*LTM). The various cDNAs were subcloned in the following expression vectors: pGEX-KG (J. Dixon, Purdue University, West Lafayette, IN), pBTM116 (S. Fields, University of Washington, Seattle), pGAD (CLONTECH), pFastBacHis (Invitrogen), pCIneo (Promega), pCIF (pCIneo with Nt Flag epitope), pSG5 (Stratagene), and pcDNA-EF6-V5 (Invitrogen).
In Vitro Transcription and Translation. Rabbit reticulocyte lysates (TnT; Promega) were used for in vitro synthesis of AGXT. After 2 h at 30°C, translation products were analyzed by PAGE/fluorography. Sulfo-MBS (Pierce) was used for cross-linking tests. Immunoprecipitation experiments that used anti-Hsc70 and anti-Hsp90 antibodies (StressGen Biotechnologies, Victoria, BC, Canada) and magnetic beads (Pierce) were performed as described (7). Synthesis was stopped after 30 min with 100 μg/ml cycloheximide and kept at 30°C for 2–6 h [in some controls, 11 μg/ml geldanamycin (Calbiochem) was included at this point]. Limiting proteolysis was carried out as described (8).
Cell Culture and Transfections. BL21 (RIL) Escherichia coli (Stratagene) were grown in LB for GST-fused expression and induced with 0.4 mM isopropyl β-D-thiogalactoside (IPTG) during 4 h at 25°C. Sf9 insect cells were infected with recombinant baculovirus and grown in SFM-II (Invitrogen) at 27°C. COS7, HeLa, and HEK293 cells were grown in DMEM with 5% FBS at either 30°C or 37°C. COS7 and HeLa cells were transfected by electroporation, whereas HEK293 cells were transfected by calcium phosphate (6). Transfection experiments were designed to minimize the variability introduced by transfection efficiency, which was controlled by cotransfection with lacZ-pcDNA, and only transfections with variability <10% were used. To ascertain the effect of various culture conditions in gene expression, all cells were transfected in a single pool and then split. Chemical chaperones were added at the following concentrations: 75–150 mM betaine, 5–10% (vol/vol) glycerol, 100 mM DMSO, 75–150 mM trimethylamine oxide, and 5–10 mM phenylbutyric acid. Pyridoxal phosphate (80 μM) and 2.5 mM aminooxyacetic acid also were tested. For metabolic labeling, COS7 cells were transfected and starved 24 h later in cysteine/methionine-free medium (Invitrogen) for 30 min, pulse-labeled with 40 μCi (1 μCi = 37 kBq) of 35S-labeled methionine/cysteine (Tran35S-label; ICN) for another 30 min, washed, and cultured in complete DMEM for chase periods of 30 min, 12 h, 24 h, 48 h, and 72 h.
AGXT Enzymatic Assay. AGXT activity was determined as described (9). Km for glyoxylate was determined in the substrate range 0–2.5 mM, keeping the alanine concentration at 150 mM. Km for alanine was determined in the substrate range of 0–150 mM, keeping the glyoxylate concentration at 10 mM. Fitting to the Michaelis–Menten equation was performed by nonlinear regression analysis with PRISM software (GraphPad, San Diego).
Western Blots. Cell pellets from 60-mm dishes were sonicated in 400 μl of buffer [50 mM Tris·HCl, pH 7.4/150 mM NaCl/1× protease inhibitors (Complete; Roche, Gipf-Oberfrick, Switzerland) and 0.05% Triton X-100, 0.5% SDS, 1% SDS, 6 M urea, or 6 M guanidine hydrochloride]. Protein concentration was measured with bicinchoninic acid, and equal amounts of protein (20 μg) were analyzed by immunoblotting (6) with anti-Flag M2 (1:10,000) (Sigma), anti-V5 (1:5,000) (Invitrogen), anti-AGXT (1:2,000) (rabbit, raised against recombinant AGXT), anti-Ku70 (1:5,000) (S. Jackson, Wellcome/Cancer Research Campaign, Cambridge, U.K.), or anti-actin (1:10,000) (Sigma). Peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (Jackson ImmunoResearch) was used as secondary antibodies, and chemiluminescence substrate was from Pierce.
Filter Retardation Assay (FRA). Cell extracts were prepared by using lysis buffers with or without SDS, and 20 μg per slot was filtered through 0.2-μm pore cellulose acetate as described (10).
Immunoprecipitation. Cell pellets obtained from 35-mm dishes were sonicated in 200 μl of RIPA buffer (50 mM Tris·HCl, pH 7.4/150 mM NaCl/1% Nonidet P-40/0.5% deoxycholate/0.1% SDS) with protease inhibitors. For 35S-labeled cells, 50 μg of total protein was diluted 10-fold with 10 mM Tris·HCl, pH 7.4/150 mM NaCl/0.1% Triton X-100, immunoprecipitated with anti-Flag agarose, and analyzed by PAGE/fluorography.
Yeast Two-Hybrid Assay. Coding cDNAs were fused to Gal4-AD and lexA-BD and expressed in Saccharomyces cerevisiae L40 (6). In addition to full-length AGXT, the following fragments were tested as lexA-BD fusion proteins: residues 1–105, 106–280, and 278–392.
Results and Discussion
I244T Is the Most Prevalent PH1 Mutation in the Canary Islands. The screening for AGXT mutations among our PH1 patients revealed that 22 of the 24 independent chromosomes studied (91.6%) had a T/C change at nucleotide 853 (exon 7), corresponding to a change of Ile to Thr at residue 244 in the AGXT protein. The other two PH1 chromosomes had a G/A change at nucleotide 630, a common mutation (11). Additional polymorphisms were detected in exon 1 (P11L), intron 1 (74-bp duplication), exon 2 (synonymous C/T change at nucleotide 386), and exon 10 (I340M). The 22 PH1 chromosomes shared not only the I244T mutation but also all of the other intragenic polymorphisms and D2S125 and D2S140 alleles. The haplotype defined by Leu-11, 74-bp duplication, 386T, and Met-340, also known as the minor allele (12), has been found in ≈20% of the population, which is similar to the 19% found in our Canary Islands sample. I244T has been reported in 9% of PH1 chromosomes (11). The high frequency of this mutation among our patients, in a haplotype that shares intragenic and local microsatellites, is suggestive of a founder effect. A North African origin could be speculated, because various patients homozygous for I244T have been found in that region,∥ and natives of the Canary Islands, before European colonization, are thought to originate from Northwest Africa (13).
Synergistic Effect of P11L Polymorphism with I244T Is Crucial for Loss of Function. We expressed the wild-type AGXT cDNA and AGXT*T cDNA, carrying the change I244T in COS7 cells, by using expression vectors with cytomegalovirus promoter and Flag tag (pCIF) and under the simian virus 40 promoter without tag (pSG5). AGXT or AGXT*T resulted in similar levels of AGXT enzymatic activity (138 ± 16 vs. 124 ± 42 μmol/h per mg for pCIF vectors and 262 ± 34 vs. 225 ± 102 μmol/h per mg for pSG5 vectors, respectively; values from three experiments). Western blots also revealed similar AGXT levels in cells transfected with AGXT or AGXT*T by using either our anti-AGXT antibody or anti-Flag antibody. Immunofluorescence analysis of cells expressing either AGXT or AGXT*T resulted in similar patterns, colocalizing with the peroxisomal marker PMP70 (not shown).
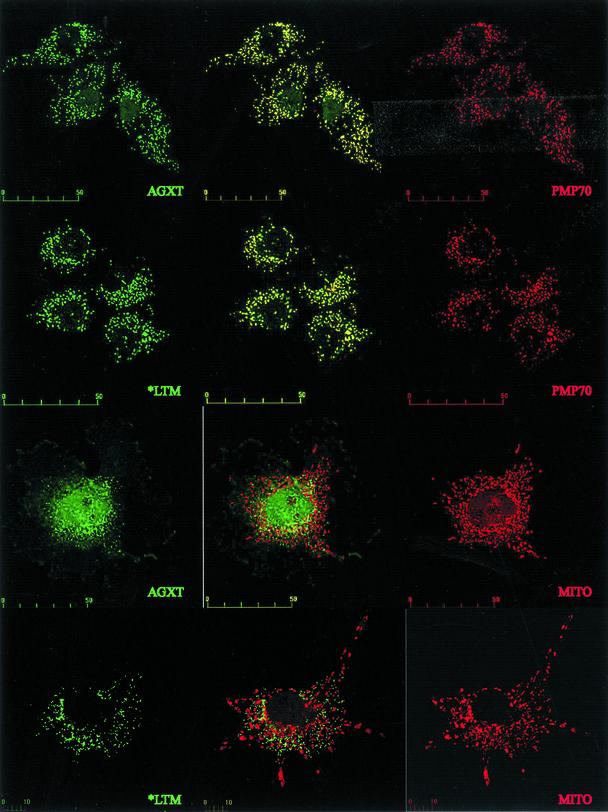
P11L and I340M, linked to I244T in our patients, were tested independently and in combination with I244T (Table 1). P11L had to be present in the same construct as I244T to result in lack of enzymatic activity. A recent study (14) also has shown a synergistic effect between P11L and both Gly170Arg and I244T in E. coli. The interaction between P11L and Gly170Arg is central to the mistargeting of AGXT to the mitochondria (15). However, the synergy between P11L and I244T has a different consequence, because AGXT immunofluorescence of cells transfected with AGXT*LTM colocalized with PMP70 and not with a mitochondrial marker (Fig. 1).
Table 1. AGXT enzymatic activities of transfected COS cells.
| AGXT activity, μmol/h per mg
|
||
|---|---|---|
| Construct | pSG5 vector | pCIF vector |
| AGXT | 262±34 | 138±16 |
| AGXT*L | 187±22 | 106±19 |
| AGXT*T | 225±102 | 124±42 |
| AGXT*M | 406±105 | 208±58 |
| AGXT*LT | Undetectable | Undetectable |
| AGXT*LTM | 22±7 | 37±2 |
| No cDNA | Undetectable | Undetectable |
| No transfection | Undetectable | Undetectable |
COS7 cells were transfected with plasmid pSG5 (simian virus 40 promoter, no epitope tag) or pCIF (cytomegalovirus promoter, N-terminal Flag epitope) containing wild-type AGXT cDNA, as well as cDNAs with the amino acid changes P11L (AGXT*L), I244T (AGXT*T), 1340M (AGXT*M), P11L + 1244T (AGXT*LT), and all three changes (AGXT*LTM). “Undetectable” indicates values similar to or below those obtained without cell lysate. Enzymatic activity is expressed as μmol/h per mg of total protein. Values are expressed as mean ± SD from three experiments
Fig. 1.
Confocal microscopy of COS7 cells transfected with Flag-tagged plasmids expressing either the wild-type AGXT or the AGXT*LTM cDNA. Mouse anti-Flag antibodies and FITC-labeled anti-mouse IgG were used to detect the expressed AGXT protein. The peroxisomes were identified in the same cells with rabbit anti-PMP70 serum and rhodamine-labeled anti-rabbit IgG. The mitochondria were labeled with red fluorescent Mitotracker. Both wild-type AGXT and AGXT*LTM proteins show a peroxisomal distribution, with the merged images (Center) producing yellow fluorescence because of the colocalization of AGXT and PMP70. No mitochondrial mistargeting of AGXT*LTM is observed, because the protein shows a localization different from the Mitotracker labeling and similar to the pattern obtained with wild-type AGXT. (Scale bar units are micrometers.)
AGXT*LTM Represents a Conformational Mutation. All variants tested (wild-type AGXT, AGXT*L, AGXT*T, AGXT*M, AGXT*LT, and AGXT*LTM) were synthesized efficiently in vitro by transcription-coupled translation, producing a main band of around 43 kDa. Cross-linking of TnT products of all variants tested resulted in ≈90-kDa bands, consistent with functional dimerization. Once synthesized, all protein variants were similarly stable to temperature (up to 65°C, 30 min; data not shown).
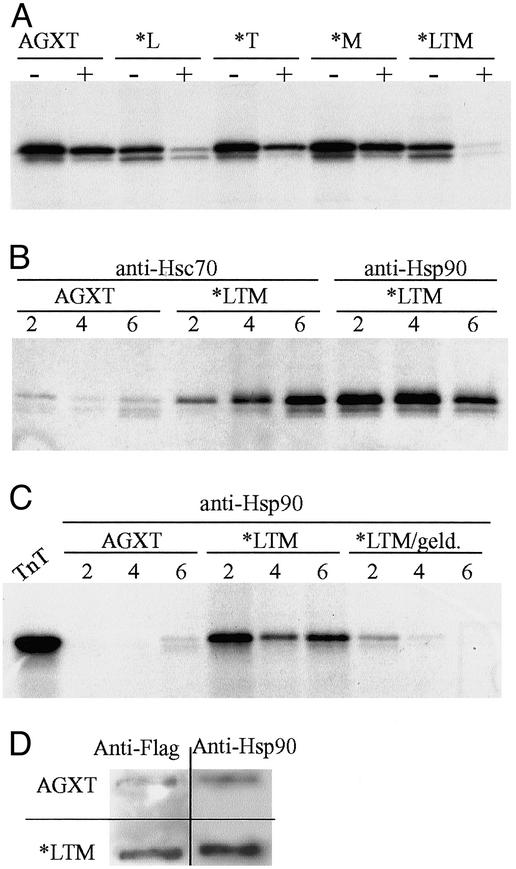
Treatment of the various AGXT variants with 12 μg/ml trypsin showed a significantly higher proteolytic sensitivity for variants carrying the P11L polymorphism, which became extremely sensitive when both P11L and I244T changes were present in the same molecule (Fig. 2A). Limited proteolysis has been proposed as an efficient procedure to detect global changes in protein conformation (11). Our findings indicate that the common polymorphism P11L has a significant impact on protein conformation and that this impact is synergistic with the I244T mutation.
Fig. 2.
(A) SDS/PAGE and fluorography of in vitro-synthesized AGXT proteins subject to 12 μg/ml trypsin for 10 min show significantly higher proteolytic sensitivity for variants carrying the P11L polymorphism, which is potentiated by the coexistence of the I244T mutation. (B) Immunoprecipitation of in vitro-synthesized wild-type AGXT and AGXT*LTM after 2–6 h of incubation at 30°C and reaction with either anti-Hsc70 or anti-Hsp90 antibodies; the mutant allele shows a much more stable association with both molecular chaperones than the wild type, and the association with Hsp90 can be inhibited specifically with 11 μg/ml geldanamycin. (C) The lane TnT represents half the input used for each immunoprecipitation. (D) FRA of nondenaturing lysates from COS7 cells transfected with plasmids containing either the wild-type AGXT or AGXT*LTM cDNA. Duplicate filters probed with either anti-Flag or anti-Hsp90 antibodies show that cells transfected with AGXT*LTM cDNA contain both AGXT and Hsp90 in aggregates retained in the 0.2-μm-pore membrane.
Peroxisomal matrix proteins are synthesized on free cytoplasmic ribosomes and imported into the organelle as partly or fully folded and oligomeric proteins (16). The involvement of molecular chaperones in the cytosolic phase of peroxisomal proteins has been demonstrated in model organisms (17). Immunoprecipitation with antibodies to major cytosolic molecular chaperones (Hsc70 and Hsp90) present in reticulocyte lysates (11) was used to ascertain AGXT–chaperone interactions. Whereas the interaction of Hsc70 and Hsp90 with wild-type AGXT is transient, mutant AGXT*LTM showed stable associations with both molecular chaperones (Fig. 2B). The specificity of the interaction detected with Hsp90 could be confirmed by adding geldanamycin, which is known to interact with Hsp90 (Fig. 2C). In vitro-synthesized AGXT*LTM might not acquire the correct structure, making the interaction with molecular chaperones longer-lasting. Expression in COS7 cells was consistent with this, because AGXT*LTM was present mainly in aggregates detected by FRA, and these aggregates also contained Hsp90 (Fig. 2D).
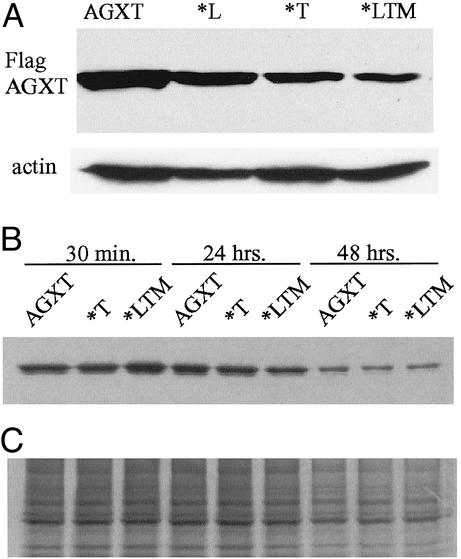
Mutant AGXT*LTM Is Expressed in Mammalian Cells as Stable Protein Aggregates. All variants tested (AGXT, AGXT*L, AGXT*T, AGXT*M, and AGXT*LTM) could be expressed in COS7, HeLa, and HEK293 cells, without major differences, by Western blotting at 48 h after transfection by using denaturing lysis solutions. Extracted AGXT*LTM levels were relatively lower at 72 h (Fig. 3A). Misfolded proteins frequently are targeted to the proteasome for degradation (18), but AGXT*LTM Western bands were not consistent with major in vivo degradation. Pulse-chase studies failed to detect differences in stability of the protein variants (Fig. 3B).
Fig. 3.
(A) Western blot of COS7 cells transfected with plasmids containing either the wild-type AGXT cDNA or cDNAs carrying the P11L polymorphism alone (*L), the I244T mutation alone (*T), or the haplotype found in our patients (*LTM); anti-Flag immunostaining was used to detect the AGXT variants and anti-actin antibody was used as a control in a replicate gel run in parallel. (B) Anti-Flag immunoprecipitation of 50 μg of total protein from 35S-Met-labeled cells expressing AGXT, *T, or *LTM after chase times of 30 min, 24 h, and 48 h; 1/20th of the labeled lysate used for immunoprecipitation was run in parallel as a control. (C) 35S-autoradiogram of total lysate. No significant differences are observed in the stability of the protein containing the I244T mutation alone or in combination with the P11L and I340M polymorphisms, relative to the wild-type protein.
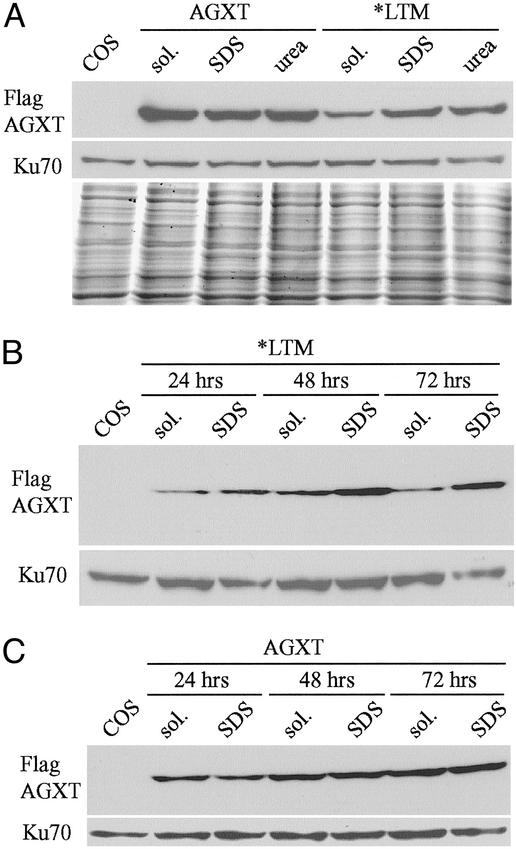
Partially misfolded proteins can form increasingly larger aggregates that might be stable but functionally inactive (19). Differences in lysis buffers used for enzymatic activity assay (detergent-free, nondenaturant solution) and Western blots (with nonionic detergents) prompted us to investigate the solubility of AGXT*LTM protein. AGXT could be extracted largely with nondenaturing solutions, whereas AGXT*LTM was poorly soluble in nondenaturing buffers but soluble with increasing concentrations of ionic detergent (SDS) and chaotropic salts (urea and guanidine·HCl) (Fig. 4A). The aggregation of AGXT*LTM protein was progressive, more evident at 72 h than at 24 h after transfection (Fig. 4B).
Fig. 4.
(A) Western blot of COS7 cells transfected with Flag-tagged plasmids containing either the wild-type AGXT or AGXT*LTM cDNA. Two days after transfection, three aliquots of the cells were extracted with lysis buffers without detergents (sol.) or containing either 1% SDS or 6 M urea + 0.5% SDS (urea); mouse anti-Flag immunostaining was used to detect the AGXT variants, whereas rabbit anti-Ku70 antibody was used as a control and a replicate gel was stained with Coomassie blue. COS indicates untransfected cells. When followed over time, the differences in solubility of the expressed AGXT*LTM protein in sol. or containing 0.5% SDS become more evident 48 and 72 h after transfection (B), whereas no differences are found with the wild-type construct (C).
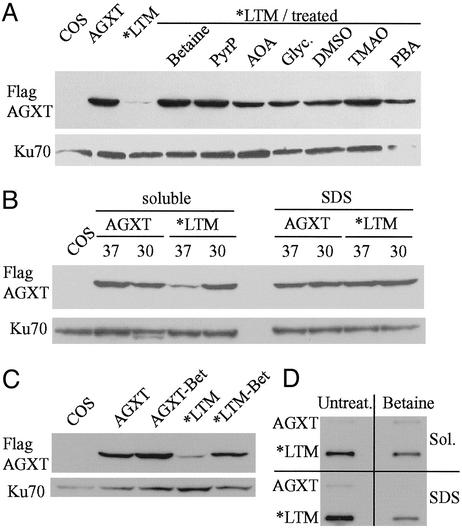
Effect of Chemical Chaperones and Temperature on Solubility of AGXT*LTM. Small-molecule osmolytes (chemical chaperones) have been shown to favor protein folding and stability (20–22). Treatment with common chemical chaperones resulted in modest increases in AGXT*LTM solubility in nondenaturing buffer (Fig. 5A). In three independent experiments, betaine showed the strongest and most consistent effect on AGXT*LTM solubility. These compounds did not have an effect on the total protein content, assessed by either PAGE or total trichloroacetic acid-precipitable protein. Neither compound showed a consistent effect on wild-type AGXT, which is readily soluble in nondenaturing buffers.
Fig. 5.
(A) Effect of various chemical chaperones on AGXT*LTM solubility. COS7 cells were transfected with Flag-tagged AGXT*LTM cDNA, divided into aliquots, and grown for 3 days in medium with 75 mM betaine, 80 μM pyridoxal phosphate (PyrP), 2.5 mM aminooxyacetic acid (AOA), 5% glycerol (Glyc.), 100 mM DMSO, 75 mM trimethylamine oxide (TMAO), or 10 mM phenylbutyric acid (PBA). Cell lysates were prepared with buffer without detergent, and 20 μg of protein was analyzed by Western blotting with anti-Flag antibody. The left lanes contain lysates of nontransfected COS7 cells or cells transfected with either wild-type AGXT or AGXT*LTM cDNA and grown in medium without additives, respectively. Anti-Ku70 antibody was used as a control. (B) Effect of temperature on AGXT*LTM solubility. COS7 cells were transfected with Flag-tagged plasmids containing either the wild-type AGXT or AGXT*LTM cDNA, divided into aliquots and incubated at either 37°C or 30°C. Four days after transfection, aliquots of the cells were extracted with lysis buffers containing either no detergent (soluble) or 0.5% SDS. Anti-Ku70 antibody was used as a control. Native lysates of cells expressing either AGXT or AGXT*LTM also were analyzed in parallel by Western blotting (C) and FRA (D) to ascertain the effect of betaine treatment on the solubility of the expressed protein. Betaine treatment consistently increased the AGXT*LTM Western blot signal detected in cell lysates without detergent. The same lysates (Sol.) showed, by FRA, a parallel reduction in the amount of insoluble aggregates after betaine treatment. This reduction of retained AGXT*LTM aggregates also is detected in cell extracts containing 1% SDS.
Pyridoxal phosphate, a cofactor for AGXT, has been shown to improve the course of the disease in some PH1 patients (23). Small molecules that bind to proteins have been shown to assist in the correct folding of mutant proteins (24). We tested the effect of 80 μM pyridoxal phosphate and 1 mM aminooxyacetic acid, an AGXT inhibitor, in AGXT*LTM solubility. The presence of pyridoxal phosphate significantly improved AGXT*LTM solubility, whereas the effect of aminooxyacetic acid was negligible (Fig. 5A).
Some of the mutant proteins with improved native folding in the presence of molecular chaperones are also temperature-sensitive. Culture at 30°C improved the solubility of AGXT*LTM expressed by COS7 cells (Fig. 5B).
AGXT*LTM is produced largely as insoluble aggregates detectable by FRA (Fig. 5 C and D). The same native extracts were analyzed by Western blotting and FRA to demonstrate that the increase in soluble AGXT*LTM observed with betaine was accompanied by a decrease in the amount of insoluble protein.
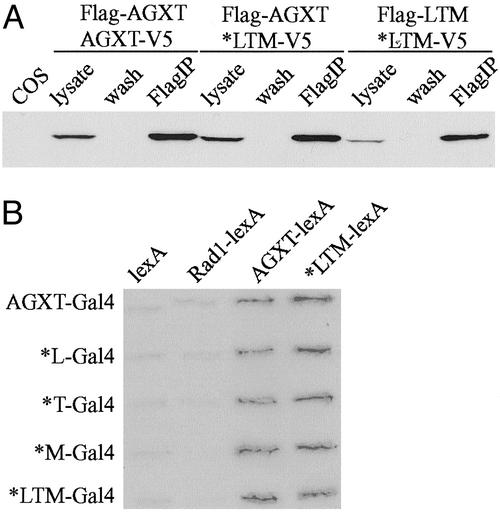
Soluble Mutant AGXT*LTM Protein Forms Dimers and Retains Specific Activity. Active AGXT is a homodimer (25), which can be imported as such into the peroxisomes. We tested whether AGXT*LTM could form homodimers and heterodimers with wild-type AGXT. COS7 cells transfected with Flag- and V5-tagged constructs were grown in the presence of 75 mM betaine, and protein interactions were detected by immunoprecipitation (anti-Flag), followed by anti-V5 Western blotting. AGXT*LTM interacted with both AGXT and itself (Fig. 6A), consistent with a negligible effect of the mutation on dimerization. We also determined the protein interactions by using the yeast two-hybrid method. No differences were found in the interaction of AGXT with itself or AGXT*LTM, which also seemed able to interact with itself (Fig. 6B). Both the N-terminal and C-terminal fragments from either AGXT or AGXT*LTM were able to interact with full-length AGXT, whereas the central portion of the protein did not seem to interact. No significant differences were observed whether the N terminus contained Pro-11 vs. Leu-11 or whether the C terminus contained Ile-340 vs. Met-340 (data not shown).
Fig. 6.
Interaction between wild-type AGXT and the mutant AGXT*LTM proteins. (A) Coimmunoprecipitation of proteins expressed in COS7 cells grown in the presence of 75 mM betaine to enhance the proportion of soluble AGXT*LTM protein. Transfection was performed with combinations of AGXT and AGXT*LTM cDNAs tagged either at the N terminus with Flag epitope or at the C terminus with V5 epitope, as indicated at the top. Two days after transfection, the cells were lysed in 0.5% Triton X-100, and proteins were immunoprecipitated with anti-Flag agarose beads. The autoradiogram represents a Western blot analysis with anti-V5 antibody of untransfected cells (COS) and the various lysates (before immunoprecipitation), wash buffer (wash), and immunoprecipitated protein (FlagIP). The presence of either tag does not seem to affect the predicted dimerization of wild-type AGXT, and similar dimerization can be observed with the mutant AGXT*LTM. Heterodimers between both protein variants also are observed. (B) Yeast two-hybrid interaction between various forms of the AGXT protein. Cotransfection of S. cerevisiae was performed with plasmids encoding Gal4 and lexA fusion proteins. Gal4 fusion constructs were produced with either wild-type AGXT or cDNAs carrying each of the amino acid changes (*L = P11L, *T = I244T, and *M = I340M) as well as a combination of all three (*LTM). lexA fusion constructs were produced with either wild-type AGXT or AGXT*LTM cDNAs. All protein interactions seem possible, as revealed by the positive X-gal staining of the transfectants, compared with negative controls (constructs with lexA cDNA and constructs with an unrelated human cDNA, RAD1).
Expression in insect cells at 27°C and mammalian cells at either 30°C or 37°C with betaine resulted in significant production of soluble AGXT*LTM, which could be tested for enzymatic activity. Lysates of betaine-treated COS7 cells expressing AGXT*LTM contained about half the activity detected in cells expressing AGXT (120 ± 19 vs. 61 ± 1 μmol/h per mg of total lysate protein, mean ± SD, for values from three experiments). His-tagged AGXT purified from insect cells yielded 4,900 ± 1,100 μmol/h per mg of purified protein, whereas AGXT*LTM resulted in 4,100 ± 860 μmol/h per mg (values from two purifications). Km values for purified recombinant AGXT (14.9 ± 5.3 mM for alanine and 0.36 ± 0.1 mM for glyoxylate) were similar to AGXT*LTM (12.9 ± 5.6 mM for alanine and 0.22 ± 0.1 mM for glyoxylate) (P = 0.6 and 0.2, respectively). AGXT*LTM expressed in E. coli fused to GST also allowed its purification under native conditions. Purified wild-type GST-AGXT yielded 6,200 ± 1,500 μmol/h per mg, whereas purified GST-AGXT*LTM resulted in 3,800 ± 1,100 μmol/h per mg (values from five purifications).
In summary, the main form of PH1 seen in the Canary Islands constitutes a protein conformational disease, in which the synergistic effect of a common polymorphism (P11L) and a founder mutation (I244T) results in a misfolded protein that tends to form inactive aggregates. The solubility problem can be reversed partially with chemical chaperones, particularly betaine, and the resulting soluble protein retains significant enzymatic activity. Thus, some important criteria for a potential pharmacological treatment of these patients have been fulfilled. Betaine is well tolerated as an oral medication, and it has been used to reduce homocysteinemia. It is possible that betaine, or similar pharmacological agents, could be used in the chemoprophylaxis of oxalate accumulation in PH1 patients homozygous for I244T. Such conservative treatment would be highly desirable for a disease in which patients currently face the risks of having an otherwise healthy liver replaced by an allograft.
Acknowledgments
We thank C. Fumero, R. Freire, X. Li, and other colleagues. This work was supported by Spanish Ministry of Science Grant SAF2001-0317 and a grant from the Oxalosis and Hyperoxaluria Foundation.
Abbreviations: PH1, primary hyperoxaluria type 1; AGXT, alanine:glyoxylate aminotransferase; FRA, filter retardation assay.
Footnotes
Basmaison, O., Bozon, D., Rolland, M. O., Koch-Nogueira, P. C., Dumontel, C., Divry, P. & Cochat, P. (2000) Pediatr. Nephrol. 14, C36 (abstr.).
References
- 1.Nishiyama, K., Berstein, G., Oda, T. & Ichiyama, A. (1990) Eur. J. Biochem. 194, 9-18. [DOI] [PubMed] [Google Scholar]
- 2.Takada, Y., Kaneko, N., Esumi, H., Purdue, P. E. & Danpure, C. J. (1990) Biochem. J. 268, 517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purdue, P. E., Lumb, M. J., Fox, M., Griffo, G., Hamon-Benais, C., Povey, S. & Danpure, C. J. (1991) Genomics 10, 34-42. [DOI] [PubMed] [Google Scholar]
- 4.Purdue, P. E., Allsop, J., Isaya, G., Rosenberg, L. E. & Danpure, C. J. (1991) Proc. Natl. Acad. Sci. USA 88, 10900-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Schnakenburg, C. & Rumsby, G. (1997) J. Med. Genet. 34, 489-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sambrook, J. & Russell, D. W. (2001) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 3rd Ed.
- 7.Fuller, W. & Curthbert, A. W. (2000) J. Biol. Chem. 275, 37462-37468. [DOI] [PubMed] [Google Scholar]
- 8.Fedorov, A. & Baldwin, T. O. (1998) Methods Enzymol. 290, 1-17. [DOI] [PubMed] [Google Scholar]
- 9.Rumsby, G., Weir, T. & Samuell, C. T. (1997) Ann. Clin. Biochem. 34, 400-404. [DOI] [PubMed] [Google Scholar]
- 10.Wanker, E. E., Scherzinger, E., Heiser, V., Sittler, A., Eickhoff, H. & Lehrach, H. (1999) Methods Enzymol. 309, 375-386. [DOI] [PubMed] [Google Scholar]
- 11.Tarn, A. C., von Schnakenburg, C. & Rumsby, G. (1997) J. Inherit. Metab. Dis. 20, 689-696. [DOI] [PubMed] [Google Scholar]
- 12.Purdue, P. E., Takada, Y. & Danpure, C. J. (1990) J. Cell Biol. 111, 2341-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores, C., Larruga, J. M., Gonzalez, A. M., Hernandez, M., Pinto, F. M. & Cabrera, V. (2001) Curr. Anthropol. 42, 749-755. [Google Scholar]
- 14.Lumb, M. J. & Danpure, C. J. (2000) J. Biol. Chem. 275, 36415-36422. [DOI] [PubMed] [Google Scholar]
- 15.Motley, A., Lumb, M. J., Oatey, P. B., Jennings, P. R., De Zoysa, P. A., Wanders, R. J. A., Tabak, H. F. & Danpure, C. J. (1995) J. Cell Biol. 131, 95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, M. D. & Schnell, D. J. (2001) Cell 105, 293-296. [DOI] [PubMed] [Google Scholar]
- 17.Feldman, D. E. & Frydman, J. (2000) Curr. Opin. Struct. Biol. 10, 26-33. [DOI] [PubMed] [Google Scholar]
- 18.Bross, P., Corydon, T. J., Andersen, B. S., Jogensen, M. M., Bolund, L. & Gregersen, N. (1999) Hum. Mutat. 14, 186-198. [DOI] [PubMed] [Google Scholar]
- 19.Jaenicke, R. (1998) Biol. Chem. 379, 237-243. [DOI] [PubMed] [Google Scholar]
- 20.Brown, C. R., Hong-Brown, L. Q. & Welch, W. J. (1997) J. Clin. Invest. 99, 1432-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burrows, J. A., Willis, L. K. & Perlmutter, D. H. (2000) Proc. Natl. Acad. Sci. USA 97, 1796-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamarappoo, B. K. & Verkman, A. S. (1998) J. Clin. Invest. 101, 2257-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marangella, M. (1999) Nephrol. Dial. Transplant. 14, 301-303. [DOI] [PubMed] [Google Scholar]
- 24.Fan, J. Q., Ishii, S., Asano, N. & Suzuki, Y. (1999) Nat. Med. 5, 112-115. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi, T. & Takada, Y. (1979) Arch. Biochem. Biophys. 196, 645-647. [DOI] [PubMed] [Google Scholar]