Abstract
Borrelia burgdorferi, the agent of Lyme disease, expresses several adhesion molecules that are probably required for initial establishment of infection in mammalian hosts, and for colonization of various tissues within the host. The B. burgdorferi outer membrane protein P66 was previously identified as a ligand for β3-chain integrins by using a variety of biochemical approaches. Although the earlier data suggested that P66 is an adhesin that mediates B. burgdorferi attachment to β3-chain integrins, lack of genetic systems in B. burgdorferi precluded definitive demonstration of a role for P66 in β3 integrin attachment by intact borreliae. Recent advances in the genetic manipulation of B. burgdorferi have now made possible the targeted disruption of the p66 gene. Mutants in p66 show dramatically reduced attachment to integrin αvβ3. This is, to our knowledge, the first description of the targeted disruption of a candidate B. burgdorferi virulence factor with a known biochemical function that can be quantified, and demonstrates the importance of B. burgdorferi P66 in the attachment of this pathogenic spirochete to a human cell-surface receptor.
Molecules that allow bacteria to adhere to host tissues play important roles in infection and disease. Borrelia burgdorferi, the agent of Lyme disease, expresses multiple proteins (adhesins) that mediate attachment to different components of host cells and tissue matrices (1–9). These adhesins are likely to be key to the ability of B. burgdorferi to cause infection. The mammalian receptors for B. burgdorferi that have been most thoroughly studied, and for which candidate bacterial ligands have been identified, are decorin, fibronectin, glycosaminoglycans, and β3-chain integrins (3, 5, 8, 9).
Whether these adhesion pathways participate in the ability of B. burgdorferi to cause infection remains largely unknown, as each has been studied by using mammalian cell cultures, purified host receptor molecules, and recombinant B. burgdorferi adhesins. It is also unclear whether the proteins that have been identified as B. burgdorferi adhesins are the sole mediators of attachment to their respective receptors. Before the work reported here, no targeted disruptions of the genes encoding the B. burgdorferi adhesins have been described, primarily because genetic tools for the manipulation of the B. burgdorferi genome have only recently been developed (10–14). The difficulty in generating targeted mutants in B. burgdorferi is illustrated by the use of mutant mice, rather than mutant bacteria, to investigate the importance of decorin-binding activity in infection (15). In that study, it was found that B. burgdorferi disseminates somewhat less efficiently and causes less severe arthritis in decorin-deficient mice than in wild-type mice (15). The ability of B. burgdorferi to establish disseminated infection in decorin-deficient mice supports the hypothesis that additional adhesion mechanisms participate in the virulence of this organism.
The B. burgdorferi outer membrane protein P66 was identified as a candidate ligand for β3-chain integrins by using a phage display library of total genomic B. burgdorferi DNA (3). The structure of P66 as expressed in the B. burgdorferi outer membrane has not been conclusively determined, but the protein does contain a C-terminal domain that is particularly antigenic and is accessible to digestion when intact bacteria are exposed to proteases (16, 17). The phage clones selected for binding specifically to integrin αIIbβ3 corresponded to approximately the central third of the P66 (amino acids 142–384). When this portion of the protein is expressed in recombinant form, P66 binds specifically to both β3-chain integrins (αIIbβ3 and αvβ3) and competes with B. burgdorferi cells for attachment to the same receptors. A synthetic peptide corresponding to P66 amino acids 203–209, which lie within the region selected from the phage library, inhibited B. burgdorferi attachment to integrin αIIbβ3 with a dose-response curve similar to that generated by using a synthetic Arg-Gly-Asp (RGD) peptide (18). RGD peptides are frequently used as antagonists of certain integrins, including the β3-chain integrins, because they inhibit integrin interaction with ligands, several of which contain RGD motifs (1–3, 19). Interestingly, as is true for invasin of Yersinia, which is a β1 integrin ligand (20), P66 does not contain an RGD motif. P66 expressed on the surface of Escherichia coli increases attachment of the E. coli to mammalian cells that express αvβ3, but not to the parental cell line that expresses no β3-chain integrins (3). Although these data strongly suggest that P66 is an adhesin that mediates attachment to β3-chain integrins, it remained possible that, as expressed by B. burgdorferi, P66 would have no role in attachment. To directly address the question of whether P66 mediates the attachment of B. burgdorferi to β3-chain integrins, we have generated targeted mutants in B. burgdorferi that do not express the integrin-binding domain of P66, and demonstrated that the mutants are deficient in binding to αvβ3.
Materials and Methods
Reagents. The function-blocking mAb anti-αvβ3 (LM609) was purchased from Chemicon. Anti-α5β1 blocking mAb VD1 (21) was a gift from Ralph Isberg (Tufts University). The peptides GRGDSPK and GRGESPK, and all oligonucleotides, were synthesized at the Tufts Protein Chemistry Core Facility. The plasmid pTAkanA (10) was a generous gift from James Bono and Patricia Rosa (Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, Hamilton, MT).
Integrin αvβ3 was purified from human placenta by affinity chromatography on RGD-Sepharose, as described (1, 2, 22). The αvβ3 preparation consisted primarily of the αv and β3 polypeptides (by gel electrophoresis), but the integrin subunits αIIb, β1, and β5 were also detectable in immunoblots.
Mammalian Cell Culture. The epithelial cell line used here was derived from the human cell line HEK 293 by transfection of the genes encoding the αv and β3 integrin subunits (23). Cells were cultured under 7% CO2 in DMEM/F12 nutrient mix, with 10% FBS, and 400 μg/ml antibiotic G418.
Bacterial Strains and Growth Conditions. E. coli K-12 strain JM109 was grown in standard laboratory media, supplemented with ampicillin to 100 μg/ml or kanamycin to 50 μg/ml where appropriate (24). The noninfectious B. burgdorferi strain HB19 clone 1, as well as culture and storage conditions, have been described (1, 2, 25). Borrelia were cultured at 33–34°C with Expre35S35S labeling mix (New England Nuclear; refs. 1 and 2), and stored at -70°C. For each experiment, bacteria were thawed, pelleted, and resuspended in HBS buffer (25 mM Hepes, pH 7.8/150 mM NaCl/1 mM MnCl2/1 mM MgCl2/0.25 mM CaCl2) supplemented with BSA to 1% wt/vol and dextrose to 0.1% wt/vol (HBSBD buffer) at a concentration of 2.5 × 107 bacteria per ml. The concentration was determined, and viability confirmed, by dark-field microscopy. Virtually all of the bacteria were motile at this stage of the experiments.
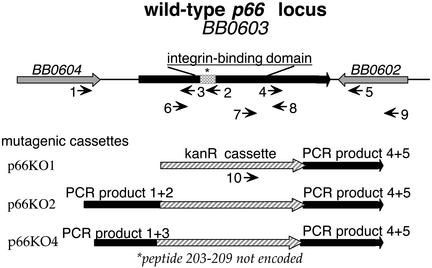
Construction of Mutagenic Plasmids and Transformation of B. burgdorferi. The plasmid pTAkanA (10), which encodes a kanamycin-resistance gene driven by the B. burgdorferi flaB promoter, was used as the backbone for the construction of p66KO1. The PCR product generated from B. burgdorferi genomic DNA using oligonucleotides 4 and 5 (which amplify a 1,265-bp fragment containing the 3′ end of p66 and flanking DNA; see Fig. 1 and Table 1, which is published as supporting information on the PNAS web site, www.pnas.org) was digested with the restriction enzymes BamHI and KpnI and cloned into pTAkanA digested with the same enzymes. To generate p66KO2, the PCR product of oligonucleotides 1 and 2 (1,255 bp) was digested with SalI and NheI and ligated into p66KO1 DNA that had been digested with XhoI and XbaI. The same strategy was used to generate p66KO4, with the exception that oligonucleotide 3 was used in place of oligonucleotide 2 (generating a PCR product of 927 bp). The fragment encompassing the 5′ end of the p66 gene contained in p66KO2 includes the promoter region and encodes amino acids 1–260, whereas in p66KO4 the protein would be truncated at amino acid 150, and does not contain any sequences involved in integrin recognition. The orientation of the kanamycin-resistance cassette would not have polar effects on downstream genes, as the gene immediately downstream is transcribed in the opposite orientation (Fig. 1). Plasmids were electroporated into E. coli strain JM109, then produced on a large scale and purified by cesium chloride density-gradient centrifugation before electroporation into B. burgdorferi.
Fig. 1.
Schematic representation of the plasmids and oligonucleotides used to generate and analyze mutants in B. burgdorferi (not drawn to scale). (Upper) The B. burgdorferi p66 locus (BB0603 in the Institute for Genomic Research (TIGR) sequence of strain B31). Amino acids 203–209, implicated as being important in integrin-binding activity, are denoted by the gray stippled region. Although the strain HB19 locus has not been sequenced in its entirety, the PCR primers shown amplify fragments of the sizes that are expected from the B31 sequence. The structures of the mutagenic cassettes generated in the pTAkanA vector are depicted below. The amino-terminal portion of P66 is truncated at amino acid 260 in p66KO2 and at amino acid 150 in p66KO4. Numbered arrows depict oligonucleotides. Corresponding sequences, which were based on the strain B31 sequence, are given in Table 1.
B. burgdorferi was prepared for electroporation as described (12). Competent cells plus DNA were placed in a 1-mm cuvette and pulsed at 1.8 kV in a model 2510 electroporator (Eppendorf). The cells were then cultured without antibiotic for 24 h. Transformants were selected in medium supplemented with 200 μg/ml kanamycin, either by limiting dilution in 96-well plates, or by plating in medium supplemented with agarose to 0.75% wt/vol. Plates were then incubated at 33°C in a humidified atmosphere with 4% CO2 for 2–3 weeks. Single-well isolated colonies or positive wells were inoculated into 10 ml of fresh medium containing kanamycin and the cultures were allowed to reach a density of 0.5–1 × 108 bacteria per ml. Clones obtained by using either method were included in our analyses. Aliquots were then prepared for analysis of DNA and protein content. Similar attempts to generate p66 mutants in an infectious B. burgdorferi background have not yet been successful, but are inherently more problematic (26).
Screening of Candidate B. burgdorferi Mutants. All candidates were initially screened for the desired genomic structure by PCR by using oligonucleotides described in Table 1 and Fig. 1. The p66KO1 transformants should retain an intact copy of p66, as well as pTAkanA integrated into the chromosome (PCR products with oligonucleotides 7 and 9 and the vector primers oPTA1 and oPTA2). The p66KO2 and p66KO4 transformants should yield a p66 gene disrupted by the kanamycin-resistance cassette (PCR product with oligonucleotides 9 and 10) and no pTAkanA sequences (no PCR product with oPTA1 and oPTA2). In addition, the sizes of the PCR products generated with oligonucleotides 6 and 8 would be larger in the p66KO2 and p66KO4 transformants than in the p66KO1 transformants or the wild type.
Candidate mutants were also screened for appropriate protein expression. Total protein contents of each candidate were separated by SDS/PAGE under reducing conditions (27) and stained with Coomassie blue. Duplicate gels were transferred to poly(vinylidene difluoride) (PVDF) membranes and probed with a rabbit polyclonal antiserum raised against the integrin-binding domain of P66 (3) according to standard protocols (28, 29). Only those candidates that showed the correct genomic structure, no obvious changes in the total protein profiles, and appropriate reactivity with the anti-P66 serum, were analyzed further. Two-dimensional gel electrophoresis was performed on selected clones (30, 31).
Quantification of Borrelia Binding to Purified Integrins and to Mammalian Cells. Assessment of B. burgdorferi binding to cultured mammalian cells and to purified integrins has been described (2). Plates containing immobilized integrin αvβ3 or cultured cells were blocked by incubation for 1 h at room temperature with 200 μl per well HBSBD, then incubated with B. burgdorferi cells in the same buffer. When peptides or antibodies were tested for inhibition, the wells were preincubated in the presence of the appropriate reagent before the addition of spirochetes. The plates were then incubated for 1 h at room temperature. Unbound bacteria were removed by washing, and bound spirochetes were quantified by scintillation counting (1, 2). Statistical analyses were performed using the two-tailed t test. Where results are noted as significantly different in the text, P ≤ 0.05.
Results and Discussion
Generation of B. burgdorferi Mutants That Do Not Express P66. P66 was identified as a candidate β3-chain integrin ligand encoded by B. burgdorferi by several criteria (3), but the role of P66 in B. burgdorferi attachment to integrins had not been directly demonstrated. To achieve this objective, recently developed genetic methods were used to generate B. burgdorferi clones that do not express the integrin-binding domain of P66 (3, 10, 18). The constructs used to disrupt p66, and the oligonucleotide primers used to generate the plasmids and to screen transformants by PCR, are diagrammed in Fig. 1. The mutants were generated in a noninfectious clone of strain HB19 (1, 25). B. burgdorferi transformants obtained after selection in the presence of kanamycin were screened by PCR for the desired genomic arrangement (see Fig. 6, which is published as supporting information on the PNAS web site). Transformants obtained with p66KO1 (designated KO1) are kanamycin resistant because of a single crossover event, resulting in integration of the entire plasmid into the B. burgdorferi chromosome. These clones retain an intact copy of p66 plus plasmid sequences and a copy of the 3′ end of the p66 gene (encoding amino acids 396–618). The KO1 transformants serve as a control for the manipulations required to generate the mutants that do not retain an intact copy of p66. The KO2 and KO4 transformants are kanamycin resistant because of double crossovers, resulting in replacement of internal p66 sequences with the drug-resistance cassette, and do not retain pTAkanA plasmid sequences.
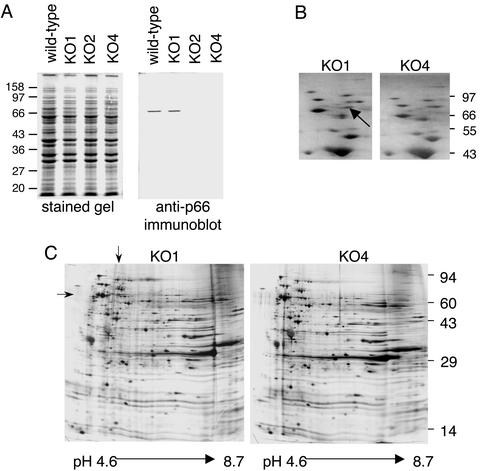
Analyses of Protein Expression Profiles of Mutants. To assess the possibility that the mutants might have lost genetic material because of transformation or selection, and consequently, display altered protein expression, each clone that appeared to have the desired genetic structure in the PCR-based screening was analyzed for overall protein expression and for P66 expression. The total protein profiles of representative clones are shown in Fig. 2A. By one-dimensional gel electrophoresis, all of the transformants had total protein contents similar to that of wild type. When duplicate samples were transferred to a membrane support and probed with a rabbit polyclonal antiserum directed against the integrin-binding domain of P66 (3), the wild-type and KO1 mutant both displayed the expected band, whereas the KO2 and KO4 mutants had no detectable P66 protein (Fig. 2 A). Similar results were obtained with all of the candidate mutants that showed the appropriate genetic structure in the PCR analyses. Two-dimensional gels of the KO1 and KO4 mutants showed no discernable differences, with the exception being that a protein of ≈66 kDa and pI of ≈5.6 was absent from the KO4 protein profile (Fig. 2 B and C). Both the molecular weight and pI values are consistent with the missing protein being P66.
Fig. 2.
Comparison of protein expression by B. burgdorferi wild type and mutants. (A) The total protein contents of ≈2.5 × 107 B. burgdorferi cells were solubilized and fractionated by electrophoresis through replicate 12.5% polyacrylamide gels under denaturing conditions. One gel was stained with Coomassie brilliant blue (Left) and the second was transferred to a poly(vinylidene difluoride) (PVDF) membrane and probed with a polyclonal rabbit antiserum directed against the integrin-binding domain of P66 (Right). Wild-type B. burgdorferi is shown in comparison to a KO1 mutant, a KO2 mutant, and a KO4 mutant. Positions of markers are shown in kilodaltons. (B) Silver-stained two-dimensional gels of the KO1 and KO4 mutants with the first dimension isoelectric focusing (IEF) gels run by using ampholines (pH 5–7). The images were cropped to focus on the region expected to contain P66. The pH gradient (left to right for each panel) is ≈5.0–5.9. (C) Silver-stained two-dimensional gels of the KO1 and KO4 mutants, with the IEF first dimension performed by using ampholines (pH 3.5–10); the second-dimension gels were 10% polyacrylamide. The entire gels are shown. Arrows indicate the position of P66.
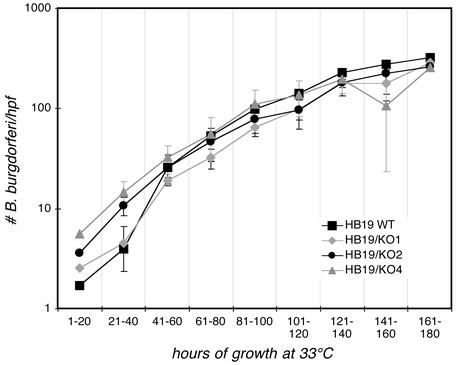
In Vitro Growth Kinetics of the P66-Deficient Mutants. P66 has been identified as a B. burgdorferi porin (32). It was therefore possible that, in the nutritionally demanding B. burgdorferi background, the growth of the P66-deficient cells might be compromised. We noted no discernable differences in the KO1, KO2, and KO4 mutants regarding the time required for colony formation, and there were no obvious differences in the motility of bacteria in liquid cultures (data not shown). None of the mutants (KO1, KO2, or KO4) differed significantly from the wild-type strain in growth in liquid medium (Fig. 3). These results indicate that the lack of P66 expression does not grossly affect B. burgdorferi physiology in laboratory culture medium.
Fig. 3.
Growth of wild-type and mutant B. burgdorferi in vitro. B. burgdorferi cultures at densities of 300–400 bacteria per high-power field (hpf) (late-logarithmic phase) in MKP medium were diluted into fresh medium to a density of ≈1 per hpf and the cultures were placed at 33°C. Growth was followed by dark-field microscopy through the time course indicated on the x axis. Shown are the means and standard deviations of four independent growth experiments for each strain. Several fields were counted for each sample, and at high spirochete densities, quarter fields were counted. The clones used for this experiment were the same as those analyzed in Fig. 2.
Attachment of B. burgdorferi P66-Deficient Mutants to Integrin αvβ3. To determine the effect of deletion of P66 sequences involved in β3-chain integrin recognition on B. burgdorferi attachment to integrins, binding to integrin αvβ3 was assessed. αvβ3 was used because the parental B. burgdorferi strain, HB19, binds most efficiently to this integrin (2). Several independent clones from each transformation were analyzed. As shown in Fig. 4, the wild-type strain and the KO1 mutants displayed similar binding to integrin αvβ3, both in purified form and when expressed by an epithelial cell line transfected with the genes encoding αv and β3. In contrast, the KO2 and KO4 mutants displayed significantly reduced attachment to both purified αvβ3 and to the epithelial cells (Fig. 4). All of the KO2 and KO4 mutants displayed very similar binding efficiencies, suggesting that the decreased attachment does not occur because of random secondary mutations.
Fig. 4.
Attachment of wild-type and P66-deficient B. burgdorferi to purified αvβ3 and to epithelial cells. Purified αvβ3 was plated at 5 μg/ml; buffer alone was plated as a control. Cells were plated to achieve at least 95% coverage of the well area on the day of the assay; medium alone served as the control. Wells were probed with B. burgdorferi wild-type or mutant clones and attachment was quantified as described in Materials and Methods. For the experiment on the right, antibodies were used at 10 μg/ml and preincubated with the cells for 30 min before the addition of the bacteria. Shown are the means and standard deviations of four replicates. *, P ≤ 0.05 in comparison to wild type.
To determine the extent to which binding to intact cells was specifically mediated by αvβ3, the binding of a KO4 mutant was compared with wild type in the presence of a blocking monoclonal antibody directed against αvβ3. This antibody decreased the attachment of the wild-type bacteria, but not the mutant, to the cells (Fig. 4). The attachment of the KO4 mutant to the cells was not significantly different from the attachment of the wild-type bacteria in the presence of anti-αvβ3. Antibody directed against integrin α5β1 did not significantly alter the attachment of either the mutant or wild-type strain. These data demonstrate that P66-deficient mutants are significantly less efficient in binding to integrin αvβ3, and that the mutants display residual binding activity that is reminiscent of that displayed by the wild-type strain in the presence of anti-αvβ3 blocking antibody. More importantly, these results also suggest that P66 is the only significant B. burgdorferi ligand for αvβ3. The residual cell attachment activity of the P66-deficient mutants is likely to be attributable to other binding pathways (2, 7, 33). Binding to the nontransfected cell line was inefficient for the mutant and wild-type strains and was not significantly affected by anti-αvβ3.
The potential role of additional integrins, as opposed to other receptor types, in the attachment of P66-deficient mutants was addressed by quantification of attachment to the αvβ3 preparation in the presence of an RGD peptide (Fig. 5). The αvβ3 was purified from human placenta by RGD-Sepharose affinity chromatography, and contains trace amounts of other integrin subunits. RGD peptides inhibit B. burgdorferi attachment to both β3-chain integrins, to α5β1 (1, 2), and to other integrins purified on the basis of RGD affinity. RGD peptides, however, interfere with cellular attachment to plates, and so are not appropriate for studies of B. burgdorferi binding to intact cells. As a control, an Arg-Gly-Glu (RGE) peptide, which is not an efficient integrin antagonist (1, 2, 19), was also tested.
Fig. 5.
Binding of wild-type and P66-deficient B. burgdorferi to purified αvβ3 in the presence of RGD and RGE peptides. αvβ3 was plated at 1 μg/ml and attachment was quantified as described in Materials and Methods. The B. burgdorferi clones used for this experiment were the same as those analyzed in Figs. 2 and 3. The RGD and RGE peptides were used at a concentration of 0.1 mg/ml. Shown are the means and standard deviations of four replicates. *, P ≤ 0.05 in comparison to wild type, all in the absence of peptides. In the presence of RGD, but not RGE peptide, binding was significantly different (P < 0.05) from the control without peptide for each strain.
The wild-type strain and KO1 mutant bound to αvβ3 with comparable efficiencies (Fig. 5), consistent with the results shown in Fig. 4. This attachment was inhibited by the RGD, but not the RGE peptide, in both cases. Binding of the KO2 and KO4 mutants was significantly lower than that of the wild-type and KO1 mutant, but was still inhibitable by the RGD peptide (Fig. 5). In combination with the results shown in Fig. 4, these results demonstrate that, although binding to αvβ3 is determined by P66, it is likely that B. burgdorferi express at least one additional integrin ligand that binds to other integrins present in trace amounts in this receptor preparation. B. burgdorferi has been shown to bind to integrin α5β1, but P66 is not a ligand for this integrin (3), suggesting that additional B. burgdorferi integrin ligands await characterization.
In the experiment shown in Fig. 5, the attachment efficiency of the KO2 mutant was somewhat different from that of the KO4 mutant, a result that has been observed repeatedly when the αvβ3 is plated at low concentrations. One possible explanation for this phenomenon is that the P66 peptide 203–209, which is retained in the KO2 mutants but not in the KO4 mutants, allows some residual recognition of integrins. At higher integrin plating concentrations, B. burgdorferi attachment to additional receptors present in the preparation, such as β1-chain integrins, is more readily detectable, which minimizes the differences between the KO2 and KO4 mutants. The amino-terminal portion of P66 would presumably be expressed in both mutants (although this domain is not detectable with our antibody), and the intact secretion signal is likely to direct the fragment to the outer membrane. How the fragment would be retained at the bacterial surface is unclear, as a predicted transmembrane α-helical anchor is located in the carboxyl-terminal third of P66. The stability of these putative fragments is unknown, but it is possible that interactions with other proteins (34) may retain the aminoterminal portion of P66 at the bacterial surface, allowing amino acids 203–209 to interact with integrins.
Concluding Remarks. The results presented here clearly demonstrate that B. burgdorferi mutants that do not express the integrin-binding domain of P66 are severely deficient in attachment to integrin αvβ3. This conclusion is supported by the reduced integrin-binding activity of several clones each of the KO2 and KO4 mutants. The similar behavior of the different clones, and of the clones derived from different mutagenic constructs, argue against the possibility that the reduced integrin-binding phenotype occurs simply because of random mutations at other sites. The mutants that do not express the integrin-binding domain of P66 are not grossly different from the wild type by several criteria: motility is equivalent (not shown), the overall protein profiles are the same (Fig. 2), and the growth kinetics in vitro are virtually superimposable (Fig. 3). To our knowledge, this study constitutes the first reported targeted mutation of a candidate virulence factor of B. burgdorferi in which a quantifiable change in function was found. Investigation of the potential role of P66 in vivo awaits the generation of a similar set of targeted mutants in an infectious B. burgdorferi strain background. Our work demonstrates that P66, which contains no previously known integrin-binding motifs, is a ligand for β3-chain integrins. These results also demonstrate that our identification of P66 as an integrin ligand by using the phage display library was successful, and that additional B. burgdorferi integrin ligands await discovery.
Supplementary Material
Acknowledgments
We thank J. Leong, L. Hu, J. Mecsas, and C. Thorpe for careful review of the manuscript, and Dr. Patricia Rosa and Dr. James Bono for providing plasmid pTAkanA and for many helpful suggestions. This work was supported by a Biomedical Science Grant from the Arthritis Foundation; by Public Health Service Grants AI-40938 and AI-051407 from the National Institute of Allergy and Infectious Diseases (to J.C.); by the Mathers Foundation; and by the Center for Gastroenterology Research on Absorptive and Secretory Processes at Tufts–New England Medical Center, Public Health Service Grant 1 P30DK39428 awarded by the National Institute of Diabetes and Digestive and Kidney Diseases.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Coburn, J., Leong, J. & Erban, J. (1993) Proc. Natl. Acad. Sci. USA 90, 7058-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coburn, J., Magoun, L., Bodary, S. C. & Leong, J. M. (1998) Infect. Immun. 66, 1946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coburn, J., Chege, W., Magoun, L., Bodary, S. C. & Leong, J. M. (1999) Mol. Microbiol. 34, 926-940. [DOI] [PubMed] [Google Scholar]
- 4.Guo, B. P., Norris, S. J., Rosenberg, L. C. & Hook, M. (1995) Infect. Immun. 63, 3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo, B. P., Brown, E. L., Dorward, D. W., Rosenberg, L. C. & Hook, M. (1998) Mol. Microbiol. 30, 711-723. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs, R. (1994) J. Clin. Invest. 93, 809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leong, J. M., Morrissey, P. E., Ortega-Barria, E., Pereira, M. E. & Coburn, J. (1995) Infect. Immun. 63, 874-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parveen, N. & Leong, J. M. (2000) Mol. Microbiol. 35, 1220-1234. [DOI] [PubMed] [Google Scholar]
- 9.Probert, W. S. & Johnson, B. J. (1998) Mol. Microbiol. 30, 1003-1015. [DOI] [PubMed] [Google Scholar]
- 10.Bono, J. L., Elias, A. F., Kupko, J. J., 3rd, Stevenson, B., Tilly, K. & Rosa, P. (2000) J. Bacteriol. 182, 2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabello, F. C., Sartakova, M. L. & Dobrikova, E. Y. (2001) Trends Microbiol. 9, 245-248. [DOI] [PubMed] [Google Scholar]
- 12.Samuels, D. S. (1995) Methods Mol. Biol. 47, 253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartakova, M., Dobrikova, E. & Cabello, F. C. (2000) Proc. Natl. Acad. Sci. USA 97, 4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart, P. E., Thalken, R., Bono, J. L. & Rosa, P. (2001) Mol. Microbiol. 39, 714-721. [DOI] [PubMed] [Google Scholar]
- 15.Brown, E. L., Wooten, R. M., Johnson, B. J. B., Iozzo, R. V., Smith, A., Dolan, M. C., Guo, B. P., Weis, J. J. & Hook, M. (2001) J. Clin. Invest. 107, 845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunikis, J., Noppa, L. & Bergstrom, S. (1995) FEMS Microbiol. Lett. 131, 139-145. [DOI] [PubMed] [Google Scholar]
- 17.Probert, W. S., Allsup, K. M. & LeFebvre, R. B. (1995) Infect. Immun. 63, 1933-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Defoe, G. & Coburn, J. (2001) Infect. Immun. 69, 3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes, R. O. (1992) Cell 69, 11-25. [DOI] [PubMed] [Google Scholar]
- 20.Isberg, R. R. & Leong, J. M. (1990) Cell 60, 861-871. [DOI] [PubMed] [Google Scholar]
- 21.Tran Van Nhieu, G. & Isberg, R. R. (1991) J. Biol. Chem. 266, 24367-24375. [PubMed] [Google Scholar]
- 22.Pytela, R., Pierschbacher, M. D., Argraves, S., Suzuki, S. & Ruoslahti, E. (1987) Methods Enzymol. 144, 475-489. [DOI] [PubMed] [Google Scholar]
- 23.Chuntharapai, A., Bodary, S., Horton, M. & Kim, K. J. (1993) Exp. Cell Res. 205, 345-352. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis, T., Fritsch, E. F. & Sambrook, J. (1982) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 25.Coburn, J., Barthold, S. W. & Leong, J. M. (1994) Infect. Immun. 62, 5559-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrenz, M. B., Kawabata, H., Purser, J. E. & Norris, S. J. (2002) Infect. Immun. 70, 4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 28.Harlow, E. & Lane, D. (1988) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 29.Towbin, H., Staeheli, T. & Gordon, J. (1979) Proc. Natl. Acad. Sci. USA 76, 4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ames, G. F. L. & Nikaido, K. (1976) Biochemistry 15, 616-623. [DOI] [PubMed] [Google Scholar]
- 31.O'Farrell, P. H. (1975) J. Biol. Chem. 250, 4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 32.Skare, J. T., Mirzabekov, T. A., Shang, E. S., Blanco, D. R., Erdjument-Bromage, H., Bunikis, J., Bergstrom, S., Tempst, P., Kagan, B. L., Miller, J. N., et al. (1997) Infect. Immun. 65, 3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leong, J. M., Wang, H., Magoun, L., Field, J. A., Morrissey, P. E., Robbins, D., Tatro, J. B., Coburn, J. & Parveen, N. (1998) Infect. Immun. 66, 994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunikis, J. & Barbour, A. G. (1999) Infect. Immun. 67, 2874-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.