Abstract
Host cell binding is an essential step in colonization by many bacterial pathogens, and the Lyme disease agent, Borrelia burgdorferi, which colonizes multiple tissues, is capable of attachment to diverse cell types. Glycosaminoglycans (GAGs) are ubiquitously expressed on mammalian cells and are recognized by multiple B. burgdorferi surface proteins. We previously showed that B. burgdorferi strains differ in the particular spectrum of GAGs that they recognize, leading to differences in the cultured mammalian cell types that they efficiently bind. The molecular basis of these binding specificities remains undefined, due to the difficulty of analyzing multiple, potentially redundant cell attachment pathways and to the paucity of genetic tools for this pathogen. In the current study, we show that the expression of decorin-binding protein (Dbp) A and/or DbpB, two B. burgdorferi surface proteins that bind GAGs, is sufficient to convert a high-passage nonadherent B. burgdorferi strain into one that efficiently binds 293 epithelial cells. Epithelial cell attachment was mediated by dermatan sulfate, and, consistent with this GAG-binding specificity, these recombinant strains did not bind EA-Hy926 endothelial cells. The GAG-binding properties of bacteria expressing DbpB or DbpA were distinguishable, and DbpB but not DbpA promoted spirochetal attachment to C6 glial cells. Thus, DbpA and DbpB may each play central but distinct roles in cell type-specific binding by Lyme disease spirochetes. This study illustrates that transformation of high-passage B. burgdorferi strains may provide a relatively simple genetic approach to analyze virulence-associated phenotypes conferred by multiple bacterial factors.
Keywords: cell adhesion, proteoglycans, glycosaminoglycans
Borrelia burgdorferi sensu lato, the causative agent of Lyme disease, is a spirochete that causes a multisystemic infection in mammalian hosts (1). Spirochetes are deposited in the skin during feeding by an infected Ixodes tick and establish a localized infection, from where they may disseminate to multiple secondary tissues, including joints, heart, and central nervous system, leading to the diverse clinical manifestations of Lyme disease. Individual B. burgdorferi strains differ in their ability to cause invasive disease (2–4), and the three species of Lyme disease-associated Borrelia apparently differ in their tissue tropism (5).
Attachment of bacteria to host cells is thought to be a critical step leading to colonization of a particular tissue, and bacterial pathogens typically express adhesins, i.e., bacterial surface proteins that promote host cell attachment (6, 7). Consistent with the ability of B. burgdorferi to cause multisystemic infection, the spirochete can attach to a variety of cell types (8–13). Proteoglycans, a class of ubiquitously expressed host cell molecules, are among the mammalian cell components that are recognized by this pathogen (14–16). Proteoglycans consist of a core protein covalently linked to one or more glycosaminoglycan (GAG) chains (for review, see ref. 17). GAGs are long, linear, and highly sulfated heteropolymers of hexosamine moieties alternating with another sugar, often a uronic acid. Different classes of GAGs, such as heparan sulfate, dermatan sulfate (also known as chondroitin sulfate B), or chondroitin-6-sulfate, differ in the identity of the hexosamine, epimerization of the glycan chain, the extent and location of sulfate modification, and their different sensitivities to cleavage by specific lyases (17).
We previously showed that different classes of GAGs mediate B. burgdorferi attachment to different cell types in vitro (16, 18). For example, the infectious B. burgdorferi strain N40, which recognizes heparan sulfate and dermatan sulfate, binds to both cultured epithelial and endothelial cells, whereas the noninfectious high-passage HB19 strain, which recognizes only dermatan sulfate, binds selectively to epithelial cells (19). Interestingly, GAG binding may influence tissue tropism of a relapsing fever spirochete, Borrelia turicatae: the expression of the GAG-binding adhesin Vsp2 (previously termed VspB) is associated with high-level growth in the blood of infected mice, whereas expression of a related protein, Vsp1 (VspA), which does not efficiently bind GAGs, is associated with infection of the brain (20). These findings suggest that strain-specific differences in GAG binding by B. burgdorferi might influence the specificity of host cell attachment and tissue tropism in vivo. However, the molecular basis of GAG-binding preference by this pathogen remains unclear. Strains that recognize multiple classes of GAGs might encode a single GAG-binding adhesin with broad specificity, or might encode multiple GAG-binding adhesins, each with a distinct spectrum of GAG recognition.
Decorin-binding proteins (Dbp) A and B are related B. burgdorferi surface lipoproteins of 20 and 18 kDa, respectively, that bind decorin (14, 21), a proteoglycan that “decorates” collagen fibers (22–24), and to dermatan sulfate (25). Decorin-binding activity may promote tissue colonization during infection of the mammalian host, because the joints of decorin-deficient mice are not as efficiently colonized by B. burgdorferi as joints of wild-type mice (26). In addition, dbpA and dbpB, which comprise a single operon on the linear plasmid lp54 (54), are expressed by B. burgdorferi in mammalian tissues, and the surface expression of both DbpA and DbpB is enhanced after host adaptation (25, 27).
Despite the evidence that DbpA and DbpB may play an important role in colonization of host tissues by B. burgdorferi, their contribution to mammalian cell attachment in vitro is not yet well characterized, due to a combination of factors. First, the traditional approach of testing antibodies directed against bacterial surface molecules for the ability to block attachment activity is problematic because many such antibodies, including some directed against DbpA, are lethal to B. burgdorferi (28–30). Second, B. burgdorferi encodes a number of potential adhesins that could contribute to cell attachment, including another GAG-binding protein, Bgp, which binds to dermatan sulfate and heparin (31), the integrin-binding protein p66 (32), and the fibronectin-binding protein BBK032 (33). Third, although a reasonable approach to a multiplicity of binding mechanisms is the analysis of purified recombinant proteins, the binding activities of such recombinant derivatives do not always reflect their activities when expressed in their native environments on the bacterial cell surface (34, 35). Finally, tools for genetic manipulation of B. burgdorferi have been developed only recently (36). Even now, it is difficult to generate defined mutations in low-passage strains, a significant limitation because in vitro passage of B. burgdorferi results in dramatic changes in phenotype, including the loss of virulence (37), infectivity (38–43), and cell attachment activity (44).
In this study, we have used a shuttle vector developed by Rosa and coworkers (36) to express DbpA and/or DbpB in a nonadherent high-passage strain of B. burgdorferi strain that lacks the dbpAB operon. These studies provide definitive genetic evidence that DbpA and DbpB indeed mediate host cell attachment by B. burgdorferi. Furthermore, DbpA and -B conferred distinct cell type-specific attachment, because they promoted bacterial attachment to different subsets of mammalian cells. Thus, complementation of high-passage B. burgdorferi strains provides a genetic approach for the analysis of virulence-associated phenotypes conferred by multiple B. burgdorferi factors.
Materials and Methods
Bacterial Strains and Cell Lines. All strains of B. burgdorferi and Borrelia transformants were cultured in BSK-H complete medium (Sigma) at either 33°C or 37°C. The high-passage B314 strain of B. burgdorferi (45) was a generous gift of T. Schwan (Rocky Mountain Laboratories, Hamilton, MT). 293 (human kidney epithelial) cells, C6 (rat glioma), and EA-hy926 (human endothelial) cells were cultured as described (19).
Plasmids and Cloning. The shuttle vector pBSV2 was generously provided by P. Rosa (Rocky Mountain Laboratories). For construction of the pDbpAB plasmid, the Dbp operon was amplified from the infectious N40 Borrelia strain by using PCR using dbpB primer 5′-AAAACTGCAGAAAATCACAAGCCAGATTGCATAGC-3′ and dbpA primer 5′-CGCGGATCCTATGTCTTGATTATCGGGCGAAGAG-3′. The PCR product was cloned in pBSV2 vector by using PstI and BamHI enzymes to generate pDbpAB. To generate pDbpB, pDbpAB was digested with ScaI and AfeI, which cleave within DbpA. The 8-kb fragment was isolated and recircularized by using blunt-end ligation with T4 DNA ligase. To generate pDbpA, pDbpAB was digested with AgeI, which cleaves within DbpB, and the 5′ overhangs were then filled in with T4 DNA polymerase to cause a 4-bp insertion within DbpB.
Electroporation of B. burgdorferi B314. B314 was transformed with 30 μg of plasmid DNA and cultured in BSK-H complete medium at 37°C for 24 h as described (46). The entire transformation mix was added to 1.7% analytical grade agarose (Bio-Rad) and plated onto a BSK-H/agarose bottom layer in sterilized 100 × 20-mm tissue culture dishes (Falcon) in the presence of kanamycin (200 μg/ml), and plates were incubated at 37°C in a 5% CO2 atmosphere for 2 wk. Colonies were picked and cultured at 37°C to mid-log phase density in liquid BSK-H complete medium containing kanamycin (200 μg/ml).
PCR, Silver Stain, and Western Blot. To detect the presence of pDbpAB and pBSV2 in transformants, bacterial pellets from 10 ml of 37°C-grown cultures were lysed by heating to 95°C for 10 min and subjected to 35 cycles of PCR by using dbp operon primers as given above. Maintenance of pBSV2 was confirmed by PCR with 5′-kanR primer, 5′-CCTTAATTAAAATCATGAGCCGGC-3′, and 3′-kanR primer, 5′-CCTTAATTAAGGCGAATGAGCTA-3′. To detect DbpA and/or DbpB proteins, lysates from 2.5 × 107 bacteria were separated by 12% SDS/PAGE and visualized with a Silver Stain Plus detection kit (Bio-Rad). DbpA, DbpB, or flagellin were identified by immunoblotting using appropriate polyclonal mouse antibodies against DbpA or DbpB (diluted 1:5,000; ref. 25) and monoclonal antibody CB1 (a gift from J. Benach, Stony Brook University, Stony Brook, NY) against flagellin (diluted 1:200) by using protocols as described (31).
Indirect Immunofluorescence Assays. Immunofluorescent staining was performed as described (31). DAPI (4′,6-diamidino-2-phenylindole) and FITC epifluorescence staining were observed with a fluorescence microscope, and photographs were taken with a Nikon inverted photomicroscope.
Radiolabeling of B. burgdorferi and Transformants. Radiolabeled bacteria were prepared by growing at 33°C in BSK-H complete medium supplemented with 60 μCi (1 Ci = 37 GBq) of [35S]methionine per ml. When cultures achieved mid-log phase (≈5 × 107 bacteria per ml), bacteria were centrifuged at 10,000 × g, and pellets were washed three times with 0.2% BSA in PBS and stored as aliquots at -80°C in BSK-H containing 20% glycerol.
Attachment of Radiolabeled Bacteria to Mammalian Cells and Purified GAGs. Assays to determine attachment to purified GAGs and mammalian cells have been described in detail (19).
Results
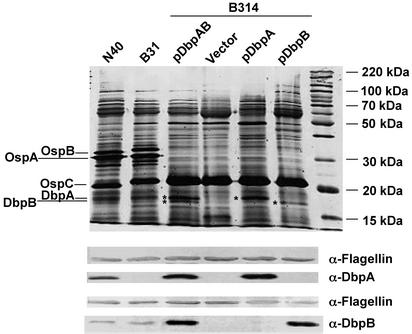
Expression of DbpA and DbpB Is Sufficient To Convert a Nonadherent B. burgdorferi Strain into One That Is Capable of Binding to Purified GAGs. B314, a high-passage derivative of the B. burgdorferi B31 strain, has lost several plasmids including lp54, which encodes the dbp operon (28, 47), and preliminary experiments showed that B314 spirochetes do not adhere to cultured mammalian cells (see Fig. 4 below). Thus, despite the multiplicity of cell attachment pathways encoded by B. burgdorferi, strain B314 does not express any adhesin that is functional in this experimental system. To determine whether the expression of DbpA and DbpB in B314 might provide this strain with a functional attachment pathway, strain B314 was transformed with a shuttle vector harboring dbpA and dbpB and their 5′-flanking sequence, including their predicted promoter sequence (27), from the infectious strain N40 D10/E9. The presence of this plasmid, pDbpAB, in transformants was confirmed by PCR amplification with dbp-specific and kanR-specific primers (see Materials and Methods; data not shown). Silver staining and immunoblotting of bacterial lysates separated by SDS/PAGE revealed that DbpA and DbpB were not only expressed in transformants, but expressed at levels higher than in the low-passage strains N40 or B31 (Fig. 1).
Fig. 4.
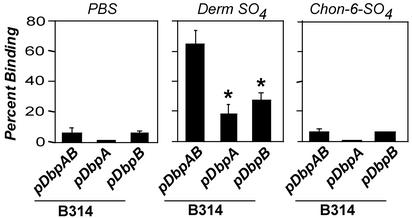
DbpA and -B promote spirochetal attachment to dermatan sulfate present on the surface of epithelial cells. (A) Radiolabeled B. burgdorferi N40, B31, or B314 strains (see Fig. 3 legend) were added to 293 epithelial cell monolayers, and the percentage of stably bound bacteria was determined. (B) Radiolabeled B314 transformants, indicated below the graph, were added to empty wells (No Cell) or to wells containing 293 epithelial cell monolayers that had been untreated (Untreated) or treated with buffer (Mock) or with the indicated lyase (Hep, heparinase; Hpt, heparitinase; Chon ABC, chondroitinase ABC) at a concentration of 0.5 units/ml for 1 h, and the percentage of stably bound spirochetes was determined (see ref. 19). Each bar represents the average ± SD of four determinations. Asterisks indicate significant inhibition of binding of bacteria by chondroitinase ABC-treatment of cells compared with mock-treated cells (P < 0.01). (C) Radiolabeled B314 harboring pDbpAB was preincubated with PBS alone, dermatan sulfate, or chondroitin-6-sulfate, and the percentage of stably bound spirochetes to 293 epithelial cells was determined. Each point represents the average ± SD of four determinations. Asterisks indicate that attachment to 293 cells was significantly inhibited by dermatan sulfate (P < 0.01) and chondroitin-6-sulfate (P = 0.01) compared with that by PBS.
Fig. 1.
Expression of DbpA and DbpB in a high passage B. burgdorferi strain by using a shuttle plasmid carrying the dbpAB operon. Total cell lysates from B. burgdorferi strains N40, B31, or high passage B314 strain harboring the indicated plasmid were subjected to electrophoresis on a 12% SDS-polyacrylamide gel. Proteins were detected by silver stain (Upper) or immunoblotting with the indicated antibody (Lower). Molecular mass markers are indicated at right, and various lipoproteins are indicated at left. DbpA and DbpB in B314 transformants are indicated by asterisks. (The apparent difference in the protein profiles of B314 transformants in the 50- to 100-kDa range is not a consistent finding.)
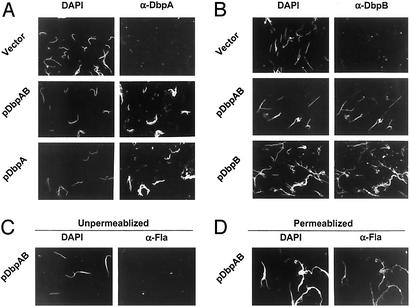
To test whether plasmid-encoded DbpA and DbpB are expressed on the bacterial surface, B314/pDbpAB was subjected to indirect immunofluorescent staining by using anti-DbpA or anti-DbpB antibodies. Antibodies to DbpA or DbpB showed that neither protein is expressed on the surface of B314 harboring the parental shuttle vector pBSV2, but both proteins were detected on the surface of B314/pDbpAB (Fig. 2 A and B), and apparently at levels somewhat higher than on the positive control strain N40 (data not shown). Parallel experiments by using anti-flagellin antibody detected the periplasmic flagellin protein only after bacterial membrane permeabilization with methanol (Fig. 2 C and D), indicating that the anti-DbpA and anti-DbpB staining described above specifically detected surface antigen.
Fig. 2.
DbpA and DbpB expressed from shuttle plasmids are present on the surface of B. burgdorferi transformants. Transformants harboring plasmids containing dbpA-dbpB operon (pDbpAB), dbpA only (pDbpA), dbpB only (pDbpB), or pBSV2 vector alone (Vector) were subjected to indirect immunofluorescent surface staining with α-DbpA (A) or α-DbpB (B; see ref. 19 and Materials and Methods). All bacteria in a given field were identified by DAPI staining. B314/pDbpAB did not stain with antibody directed against the periplasmic flagellin protein (C) unless membranes were permeabilized with methanol before staining (D), indicating the maintenance of outer membrane integrity during this experiment.
Given that DbpA and DbpB bind to purified GAGs and to decorin in vitro, we tested whether expression of DbpA and DbpB on the surface of B314 was sufficient to mediate spirochete attachment to immobilized GAGs. Microtiter wells were mock-coated or coated with purified dermatan sulfate (chondroitin sulfate B) or chondroitin-6-sulfate (chondroitin sulfate C). B314/pDbpAB efficiently bound purified dermatan sulfate but not chondroitin-6-sulfate, consistent with the observation that recombinant DbpA and B bind dermatan sulfate (Fig. 3; ref. 25).
Fig. 3.
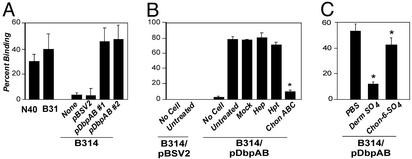
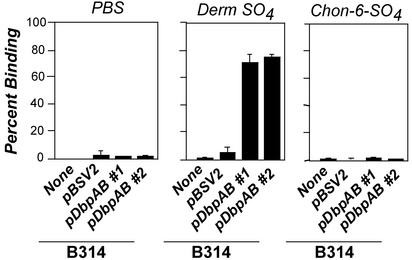
DbpA and -B promote spirochetal attachment to purified dermatan sulfate. Radiolabeled B. burgdorferi B314 harboring no plasmid (None), pBSV2 vector alone (pBSV2), or a plasmid harboring both dbpA and dbpB (pDbpAB#1 or pDbpAB#2, representing two independently isolated transformants) was added to microtiter wells coated with PBS, dermatan sulfate, or chondroitin-6-sulfate, and the percentage of stably bound spirochetes was determined by scintillation counting (see ref. 19). Each bar represents the average ± SD of four determinations.
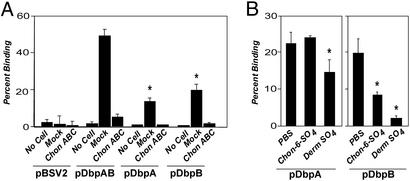
Dermatan Sulfate GAGs Mediate Attachment of B314/pDbpAB to Epithelial Cells. Because DbpA and -B expressed on the spirochete surface could promote efficient attachment to GAGs, we tested whether DbpA and -B promoted attachment to cultured mammalian cells. In fact, B314/pDbpAB bound to 293 kidney embryonal epithelial cells, and this binding was more efficient than binding by strain N40 (Fig. 4A). As expected, the parental B314 strain and the B314/pBSV2 control strain did not adhere to epithelial cells. Attachment of B. burgdorferi N40 to 293 epithelial cells is mediated by dermatan sulfate GAGs (18), so we tested whether attachment of B314/pDbpAB to these cells required GAGs, by enzymatically removing different classes of GAGs from the surface of 293 epithelial cells before incubation with bacteria. Treatment of epithelial cells with heparinase or heparitinase, which cleaves heparin/heparan sulfate-related GAGs, respectively, did not reduce the level of bacterial attachment conferred by DbpA and DbpB. In contrast, removal of chondroitin and dermatan sulfate GAGs reduced attachment of B314/pDbpAB by almost 90% (Fig. 4B). Thus, bacterial attachment to epithelial cells mediated by DbpA and DbpB requires chondroitin and/or dermatan sulfate.
To confirm that B314/pDbpAB recognizes dermatan sulfate on 293 epithelial cells, we examined whether exogenous dermatan sulfate could inhibit attachment to epithelial cells. Indeed, exogenous dermatan sulfate dramatically inhibited attachment of B314/pDbpAB to epithelial cells, whereas chondroitin-6-sulfate inhibited attachment only slightly (Fig. 4C). Thus, DbpA and DbpB likely promote bacterial attachment to dermatan sulfate present on the surface of epithelial cells.
DbpA and DbpB Each Promote Attachment of Spirochetes to Dermatan Sulfate and Mammalian Cells but Have Distinguishable GAG-Binding Specificities. Recombinant DbpB, but not recombinant DbpA, binds to chondroitin-6-sulfate, although with relatively low efficiency (25), and DbpA, but not DbpB, blocks binding of B. burgdorferi to decorin (21). To determine whether the differences in recombinant versions of these proteins was reflected in differences in bacterial strains that expressed DbpA or DbpB on their surface, we transformed B314 with pBSV2-derived plasmids harboring either dbpA or dbpB (see Materials and Methods). Silver staining and immunoblotting of extracts of B314/pDbpA and B314/pDbpB after SDS/PAGE confirmed that these strains expressed DbpA and DbpB, respectively (Fig. 1), and immunofluorescent staining demonstrated that the respective proteins were expressed on the bacterial surface (Fig. 2 A and B).
DbpB and DbpA can each function independently as a spirochetal adhesin, because B314/pDbpA and B314/pDbpB each attached to purified dermatan sulfate and to 293 epithelial cells significantly better than did the control B314/pBSV2 strain (Fig. 5, data not shown). Dermatan sulfate plays a central role in 293 cell attachment by both B314/pDbpA and B314/pDbpB, because removal of dermatan sulfate with chondroitinase ABC or pretreatment of bacteria with exogenous dermatan sulfate diminished binding by both strains (Fig. 6A). B314/pDbpA and B314/pDbpB were indistinguishable from each other in their efficiency of attachment to epithelial cells (Fig. 6A) or to dermatan sulfate attachment (Fig. 5), and neither strain bound these substrates as efficiently as did B314/pDbpAB, which expresses both DbpA and B. Thus, the contributions of DbpA and DbpB to spirochetal attachment to 293 epithelial cells are apparently equivalent.
Fig. 5.
DbpA and DbpB each contribute to attachment of B. burgdorferi to purified GAGs. Radiolabeled B314 transformants harboring indicated plasmids were added to microtiter wells mock-coated (PBS) or coated with dermatan sulfate or chondroitin-6-sulfate, and the percent of stably bound spirochetes was determined (see ref. 19). Each bar represents the average ± SD of four determinations. Asterisks denote that the binding of B314 expressing DbpA or DbpB alone to dermatan sulfate is significantly less than binding of B314 expressing both DbpA and DbpB (P < 0.01).
Fig. 6.
DbpA and DbpB each contribute to host cell attachment but have distinguishable GAG-binding activities. (A) Radiolabeled B314-harboring plasmids, as indicated (see Fig. 2 legend), were added to empty wells (No Cell) or to wells containing 293 epithelial cell monolayers that had been treated with buffer alone (Mock) or with chondroitinase ABC (Chon ABC) at 0.5 units/ml concentration, and the percentage of stably bound bacteria was determined (see Materials and Methods). Each bar represents the average ± SD of four determinations. Asterisks indicate that B314/pDbpA and B314/pDbpB bind significantly less well to mock-treated 293 cells than does B314/pDbpAB (P < 0.01). (B) Radiolabeled B314 harboring either pDbpA or pDbpB were preincubated with PBS, dermatan sulfate (Derm SO4), or chondroitin-6-sulfate (Chon-6-SO4), and the percentage of stably bound bacteria to 293 cells was determined. Asterisks indicate that preincubation with Derm SO4 or Chon-6-SO4 results in significantly lower bacterial binding compared with preincubation of cells with PBS alone (P < 0.05).
Although B314/pDbpA and B314/pDbpB bound to cells with equivalent efficiency, they were distinguishable by the sensitivity of their cell attachment activity to exogenous GAGs. Chondroitin-6-sulfate partially inhibited cell attachment by B314-expressing DbpB, but had no inhibitory effect on cell attachment by B314-expressing DbpA (Fig. 6B), consistent with the previous finding that recombinant DbpB but not recombinant DbpA bound detectably to purified chondroitin-6-sulfate (25).
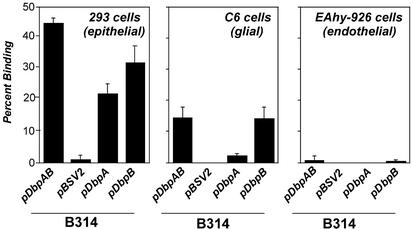
B. burgdorferi Strains Expressing DbpA or DbpB Display Distinct Cell Type-Specific Binding to Mammalian Cells. We previously showed that the spectrum of mammalian cell types that was efficiently recognized by different strains of B. burgdorferi in vitro varies depending on the particular GAG-binding specificity of each strain (18). For example, attachment of B. burgdorferi to endothelial cells required robust recognition of heparan sulfate. Consistent with this finding, B314-expressing DbpA and/or DbpB did not bind to EAhy-926 endothelial cells (Fig. 7), indicating that both DbpA and DbpB provide the bacterium with mechanisms for discrimination among mammalian cell types.
Fig. 7.
DbpA and DbpB confer cell type-specific attachment to epithelial and glial cells, but not endothelial cells. Radiolabeled B314 harboring vector pBSV2 alone, pDbpAB, pDbpA, or pDbpB were incubated with 293 epithelial cell, C6 glial cell, or EAhy-926 endothelial cell monolayers, and the percentage of stably bound spirochetes was determined (see Materials and Methods). Each point represents the average ± SD of four determinations per experiment.
Given that the attachment of B314/pDbpA and B314/pDbpB to 293 cells differed from each other in their sensitivities to exogenous dermatan sulfate and chondroitin sulfate, we postulated that they might also differ in the mammalian cell types they recognize. In fact, B314/pDbpB but not B314/pDbpA bound to C6 glial cells (Fig. 7). B314/pDbpAB also bound C6 cells, with efficiency indistinguishable from B314/pDbpB, consistent with the suggestion that DbpB but not DbpA mediated bacterial attachment to these cells (Fig. 7). C6 cell binding by both B314/pDbpAB and B314/pDbpB was abrogated by enzymatic removal of GAGs (data not shown). We conclude that, through their GAG-recognition capacities, DbpA and DbpB each promote host cell attachment in a cell type-specific manner, and that the subsets of mammalian cell types recognized by these adhesins are distinct from each other.
Discussion
The analysis of cell adhesion by B. burgdorferi has been slowed by the difficulty of genetic manipulation of the organism and by the difficulty in distinguishing individual binding pathways among multiple, potentially redundant cell attachment mechanisms (14, 21, 31–33, 48). As a result, the investigation of individual adhesion pathways has generally been limited by their reliance on the analysis of adhesins (or putative adhesins) in recombinant form, rather than when expressed in their native environment on the spirochetal surface. For example, the surface lipoproteins DbpA and DbpB bind to the proteoglycan decorin (21), but neither DbpA nor DbpB has been demonstrated to block binding of B. burgdorferi to mammalian cells or extracellular matrix, and the precise roles of these proteins in cell attachment has remained unclear.
In the current study, we assessed cell binding by B. burgdorferi strain B314, a high-passage strain that has lost the linear plasmid that encodes DbpA and DbpB (28). Several other (putative) B. burgdorferi adhesins, such as Bgp or p66 could potentially promote cell attachment, and in fact, immunoblot analysis revealed that Bgp is expressed by B314 (N.P., unpublished observation). Nevertheless, this strain did not detectably bind to cultured mammalian cells. It is possible that, in strain B314, the level of expression of Bgp or other putative adhesins, particularly at the spirochetal surface, is insufficient to promote cell attachment in the experimental conditions used in this study.
Regardless, strain B314 provided us with an opportunity to assess the function of exogenously expressed adhesins, and we used a recently developed shuttle vector (36) to express DbpA, DbpB, or both, in this strain. The resulting strains expressed these lipoproteins on the spirochetal surface and acquired the ability to attach efficiently to epithelial cells. Several observations strongly suggest that DbpA and DbpB, rather than alternative adhesins that might for unknown reasons be expressed at higher levels in the dbpA and dbpB transformants, are directly responsible for the cell attachment activity. First, consistent with our previous observation that recombinant DbpA and DbpB bound to purified dermatan sulfate (25), the transformants also bound to purified dermatan sulfate, and bacterial binding to 293 cells was diminished either by exogenous dermatan sulfate or by the removal of dermatan and chondroitin sulfates from the epithelial cell surface. Second, the level of expression of the other candidate B. burgdorferi GAG-binding adhesin, Bgp, by B314 strain and its dbpA or dbpB transformants was indistinguishable when assessed by immunoblotting (J.R.F., unpublished data). Thus, these experiments provide strong genetic evidence of roles for DbpA and DbpB as adhesins.
We also previously observed that recombinant DbpA and B bound to a commercial preparation of heparin (25), but heparan sulfate did not detectably contribute to 293 cell binding, because enzymatic removal of this class of GAGs did not diminish binding of B314 expressing DbpA and/or DbpB. In addition, the B314 transformants did not bind to cultured endothelial cells, an activity that we previously showed was associated with the recognition of heparan sulfate (49). Heparin is more negatively charged than heparan sulfate, and the ability to bind commercial preparations of heparin does not uniformly correspond to an ability to bind heparan sulfate (15). Nevertheless, it is possible that heparan sulfate GAGs contribute to DbpB-mediated attachment to some cell types, because complete inhibition of C6 glioma cell attachment by the DbpB-expressing recombinant strain required removal of heparan sulfate in addition to chondroitin and dermatan sulfate GAGs (J.R.F., unpublished observation). One explanation for this apparent cell type-specific recognition of heparan sulfate is that species- and tissue-specific structural differences exist even within a given class of GAGs, such that heparan sulfate expressed by one cell line may differ from heparan sulfate expressed by another (17, 50).
Although GAGs clearly mediate attachment of DbpA- or DbpB-expressing bacteria to epithelial cells, the identity of the specific proteoglycan(s) linked to these GAGs is not known. One obvious candidate is decorin, and indeed, B314-expressing DbpA and DbpB bound immobilized decorin (J.R.F., unpublished observation). However, epithelial cell attachment by this strain could not be inhibited by exogenous decorin, and attempts to detect decorin in extracts of 293 cells by immunoblotting was inconclusive due to the poor sensitivity of commercially available anti-decorin antibody (J.R.F., unpublished observation). B. burgdorferi shows only a mild colonization defect in the joints of decorin-deficient mice, and it has been postulated that other proteoglycans, perhaps with structural similarity to decorin, are also recognized by DbpA or DbpB and promote attachment of B. burgdorferi (26).
Evidence for a role of GAG-binding profile as a determinant of tissue tropism has been presented for several microbial pathogens (51–53), including Borrelia turicatae (20), and it is tempting to speculate that the repertoire of GAG-binding adhesins expressed by Lyme disease spirochetes may contribute to observed strain and species differences in tissue tropism (5). Among Lyme disease spirochetes, some strains bind to multiple classes of GAGs and attach to a variety of cell types in vitro, whereas other strains recognize only a specific class of GAGs and demonstrate a corresponding restriction in cell-type specific binding (19). Recognition of multiple classes of GAGs might result from the expression of a single GAG-binding adhesin with promiscuous binding specificity; alternatively, the ability to recognize diverse GAGs might reflect the expression of multiple GAG-binding adhesins, each with a distinct GAG-binding specificity. The current study supports the latter hypothesis, because isogenic strains expressing either DbpA or DbpB displayed distinct GAG and cell attachment activities. Cell attachment mediated by DbpB was more sensitive to exogenous chondroitin-6-sulfate and dermatan sulfate than was attachment mediated by DbpA. Furthermore, whereas the expression of DbpB on the spirochetal surface promoted attachment to 293 epithelial and C6 glial cells, DbpA promoted attachment only to 293 cells. It is not surprising that DbpA and DbpB of strain N40 exhibited different GAG-binding properties, because their amino acid sequences are only 59% similar (ref. 27; and J.R.F., unpublished observation). In particular, of the three lysine residues in DbpA that have been shown to be critical for decorin binding (55), one is not conserved in strain N40 DbpB, and it is possible that the decorin binding and GAG binding are mediated by identical or overlapping domains. Finally, given that DbpA exhibits significant sequence variation among B. burgdorferi strains, an attractive hypothesis is that strain variation in cell attachment phenotypes among different Lyme disease strains may reflect not only differences in the repertoire of adhesins expressed, but also in the specific sequences of the individual adhesins.
The current finding that the repertoire of GAG-binding adhesins is a determinant of the spectrum of mammalian cell types recognized in vitro provides added importance to the identification of the complete set of GAG-binding adhesins of B. burgdorferi. Neither DbpA nor DbpB were capable of mediating bacterial attachment to EA-Hy926 endothelial cells, which express heparan sulfate GAGs that are recognized by B. burgdorferi strain N40. Presumably, another adhesin is required for endothelial cell attachment, likely an adhesin with specificity for heparan sulfate (19). Indirect evidence suggests that Bgp may contribute to endothelial cell binding by strain N40 (31). In addition, an unidentified heparan sulfate-binding adhesin that is expressed by host-adapted strain N40 apparently promotes efficient bacterial attachment to cultured endothelial cells (25). The approach of using a shuttle vector to express individual proteins on the surface of an otherwise nonadherent strain could theoretically be adapted to identify and analyze any other adhesin of B. burgdorferi. Indeed, any bacterial protein that is likely to be expressed on the surface of B. burgdorferi might be tested for a role in mammalian cell attachment. Given that signals that govern the localization of proteins may be conserved among diverse spirochetes, this strategy might be adapted to spirochetal pathogens, such as Treponema pallidum, for which in vitro culture and genetic manipulation are currently impossible.
Acknowledgments
We thank P. Rosa and J. Bono for providing pBSV2, and T. Schwan and A. Barbour (University of California, Irvine) for providing strain B314. We thank J. Benach for the anti-flagellin monoclonal antibody CB1 and Jenifer Coburn for critical review of the manuscript. This work was supported by National Institutes of Health Grant RO1-AI37601 to J.M.L., who was an Established Investigator of the American Heart Association.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GAG, glycosaminoglycan; Dbp, decorin-binding protein.
References
- 1.Steere, A. C. (1989) N. Engl. J. Med. 321, 586-596. [DOI] [PubMed] [Google Scholar]
- 2.Wang, G., Ojaimi, C., Wu, H., Saksenberg, V., Iyer, R., Liveris, D., McClain, S. A., Wormser, G. P. & Schwartz, I. (2002) J. Infect. Dis. 186, 782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, G., van Dam, A. P., Schwartz, I. & Dankert, J. (1999) Clin. Microbiol. Rev. 12, 633-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seinost, G., Dykhuizen, D. E., Dattwyler, R. J., Golde, W. T., Dunn, J. J., Wang, I. N., Wormser, G. P., Schriefer, M. E. & Luft, B. J. (1999) Infect. Immun. 67, 3518-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hovius, K. E., Stark, L. A., Bleumink-Pluym, N. M., van de Pol, I., Verbeek-de Kruif, N., Rijpkema, S. G., Schouls, L. M. & Houwers, D. J. (1999) Vet. Q. 21, 54-58. [DOI] [PubMed] [Google Scholar]
- 6.Beachey, E. H. (1981) J. Infect. Dis. 143, 325-345. [DOI] [PubMed] [Google Scholar]
- 7.Bloch, C. A. & Orndorff, P. E. (1990) Infect. Immun. 58, 275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas, D. D. & Comstock, L. E. (1989) Infect. Immun. 57, 1324-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szczepanski, A., Furie, M. B., Benach, J. L., Lane, B. P. & Fleit, H. B. (1990) J. Clin. Invest. 85, 1637-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comstock, L. E. & Thomas, D. D. (1989) Infect. Immun. 57, 1626-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorward, D. W., Fischer, E. R. & Brooks, D. M. (1997) Clin. Infect. Dis. 25, Suppl. 1, S2-S8. [DOI] [PubMed] [Google Scholar]
- 12.Galbe, J. L., Guy, E., Zapatero, J. M., Peerschke, E. I. & Benach, J. L. (1993) Microb. Pathog. 14, 187-201. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Monco, J. C., Fernandez-Villar, B. & Benach, J. L. (1989) J. Infect. Dis. 160, 497-506. [DOI] [PubMed] [Google Scholar]
- 14.Guo, B. P., Norris, S. J., Rosenberg, L. C. & Hook, M. (1995) Infect. Immun. 63, 3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaacs, R. D. (1994) J. Clin. Invest. 93, 809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong, J. M., Morrissey, P. E., Ortega-Barria, E., Pereira, M. E. & Coburn, J. (1995) Infect. Immun. 63, 874-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjellen, L. & Lindahl, U. (1991) Annu. Rev. Biochem. 60, 443-475. [DOI] [PubMed] [Google Scholar]
- 18.Leong, J. M., Wang, H., Magoun, L., Field, J. A., Morrissey, P. E., Robbins, D., Tatro, J. B., Coburn, J. & Parveen, N. (1998) Infect. Immun. 66, 994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parveen, N., Robbins, D. & Leong, J. M. (1999) Infect. Immun. 67, 1743-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magoun, L., Zuckert, W. R., Robbins, D., Parveen, N., Alugupalli, K. R., Schwan, T. G., Barbour, A. G. & Leong, J. M. (2000) Mol. Microbiol. 36, 886-897. [DOI] [PubMed] [Google Scholar]
- 21.Guo, B. P., Brown, E. L., Dorward, D. W., Rosenberg, L. C. & Hook, M. (1998) Mol. Microbiol. 30, 711-723. [DOI] [PubMed] [Google Scholar]
- 22.Scott, J. E. & Orford, C. R. (1981) Biochem. J. 197, 213-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole, A. R., Webber, C., Pidoux, I., Choi, H. & Rosenberg, L. C. (1986) J. Histochem. Cytochem. 34, 619-625. [DOI] [PubMed] [Google Scholar]
- 24.Fisher, L. W., Termine, J. D. & Young, M. F. (1989) J. Biol. Chem. 264, 4571-4576. [PubMed] [Google Scholar]
- 25.Parveen, N. M. C., Radolf, J. D. & Leong, J. M. (2003) Mol. Microbiol. 47, 1433-1444. [DOI] [PubMed] [Google Scholar]
- 26.Brown, E. L., Wooten, R. M., Johnson, B. J., Iozzo, R. V., Smith, A., Dolan, M. C., Guo, B. P., Weis, J. J. & Hook, M. (2001) J. Clin. Invest. 107, 845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng, S., Hodzic, E., Stevenson, B. & Barthold, S. W. (1998) Infect. Immun. 66, 2827-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadziene, A., Wilske, B., Ferdows, M. S. & Barbour, A. G. (1993) Infect. Immun. 61, 2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escudero, R., Halluska, M. L., Backenson, P. B., Coleman, J. L. & Benach, J. L. (1997) Infect. Immun. 65, 1908-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassatt, D. R., Patel, N. K., Ulbrandt, N. D. & Hanson, M. S. (1998) Infect. Immun. 66, 5379-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parveen, N. & Leong, J. M. (2000) Mol. Microbiol. 35, 1220-1234. [DOI] [PubMed] [Google Scholar]
- 32.Coburn, J., Chege, W., Magoun, L., Bodary, S. C. & Leong, J. M. (1999) Mol. Microbiol. 34, 926-940. [DOI] [PubMed] [Google Scholar]
- 33.Probert, W. S. & Johnson, B. J. (1998) Mol. Microbiol. 30, 1003-1015. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh, A., Bandyopadhyay, K., Kole, L. & Das, P. K. (1999) Biochem. J. 337, 551-558. [PMC free article] [PubMed] [Google Scholar]
- 35.Deibel, C., Dersch, P. & Ebel, F. (2001) Int. J. Med. Microbiol. 290, 683-691. [DOI] [PubMed] [Google Scholar]
- 36.Stewart, P. E., Thalken, R., Bono, J. L. & Rosa, P. (2001) Mol. Microbiol. 39, 714-721. [DOI] [PubMed] [Google Scholar]
- 37.Moody, K. D., Barthold, S. W. & Terwilliger, G. A. (1990) Am. J. Trop. Med. Hyg. 43, 87-92. [DOI] [PubMed] [Google Scholar]
- 38.Barbour, A. G. (1988) J. Clin. Microbiol. 26, 475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson, R. C., Marek, N. & Kodner, C. (1984) J. Clin. Microbiol. 20, 1099-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norris, S. J., Howell, J. K., Garza, S. A., Ferdows, M. S. & Barbour, A. G. (1995) Infect. Immun. 63, 2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson, W. J., Garon, C. F. & Schwan, T. G. (1990) Microb. Pathog. 8, 109-118. [DOI] [PubMed] [Google Scholar]
- 42.Labandeira-Rey, M. & Skare, J. T. (2001) Infect. Immun. 69, 446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purser, J. E. & Norris, S. J. (2000) Proc. Natl. Acad. Sci. USA 97, 13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coburn, J., Barthold, S. W. & Leong, J. M. (1994) Infect. Immun. 62, 5559-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadziene, A., Thomas, D. D. & Barbour, A. G. (1995) Infect. Immun. 63, 1573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuels, D. S. (1995) Methods Mol. Biol. 47, 253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser, C. M., Casjens, S., Huang, W. M., Sutton, G. G., Clayton, R., Lathigra, R., White, O., Ketchum, K. A., Dodson, R., Hickey, E. K., et. al. (1997) Nature 390, 580-586. [DOI] [PubMed] [Google Scholar]
- 48.Coburn, J., Leong, J. M. & Erban, J. K. (1993) Proc. Natl. Acad. Sci. USA 90, 7059-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leong, J. M., Robbins, D., Rosenfeld, L., Lahiri, B. & Parveen, N. (1998) Infect. Immun. 66, 6045-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poblacion, C. A. & Michelacci, Y. M. (1986) Carbohydr. Res. 147, 87-100. [DOI] [PubMed] [Google Scholar]
- 51.Wang, F. Z., Akula, S. M., Pramod, N. P., Zeng, L. & Chandran, B. (2001) J. Virol. 75, 7517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen, T., Belland, R. J., Wilson, J. & Swanson, J. (1995) J. Exp. Med. 182, 511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pradel, G., Garapaty, S. & Frevert, U. (2002) Mol. Microbiol. 45, 637-651. [DOI] [PubMed] [Google Scholar]
- 54.Hagman, K. E., Lahdenne, P., Popova, T. G., Porcella, S. F., Akins, D. R., Radolf, J. D. & Norgard, M. V. (1998) Infect. Immun. 66, 2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown, E. L., Guo, B. P., O'Neal, P. & Hook, M. (1999) J. Biol. Chem. 274, 26272-26278. [DOI] [PubMed] [Google Scholar]