Abstract
To investigate the possible consequences of brood-temperature regulation in honey bee colonies on the quality of behavioral performance of adults, we placed honey bee pupae in incubators and allowed them to develop at temperatures held constant at 32°C, 34.5°C, and 36°C. This temperature range occurs naturally within hives. On emergence, the young adult bees were marked and introduced into foster colonies housed in normal and observation hives and allowed to live out their lives. No obvious difference in within-hive behavior was noted between the temperature-treated bees and the foster-colony bees. However, when the temperature-treated bees became foragers and were trained to visit a feeder 200 m from the hive, they exhibited clear differences in dance performance that could be correlated with the temperatures at which they had been raised: bees raised at 32°C completed only ≈20% of the dance circuits when compared with bees of the higher-temperature group. Also, the variance in the duration of the waggle phase is larger in 32°C-raised bees compared with 36°C-raised bees. All other parameters compared across all groups were not significantly different. One-trial learning and memory consolidation in the bees raised at different temperatures was investigated 1 and 10 min after conditioning the proboscis-extension reflex. Bees raised at 36°C performed as expected for bees typically classified as “good learners,” whereas bees raised at 32°C and 34.5°C performed significantly less well. We propose that the temperature at which pupae are raised will influence their behavioral performance as adults and may determine the tasks they carry out best inside and outside the hive.
Collective control of brood temperature is an essential aspect of the behavior of honey bees, and air temperatures measured close to the brood combs are, although never constant, always within a range of 33–36°C (1–7). High temperatures outside the hive are compensated by bringing water into the hive and evaporating this by wing fanning (8). Low temperatures inside the hive are compensated by the production of heat through thoracic muscle activity in individual bees, which then is transferred to the brood (9–11). Extended deviations from an optimal temperature window during development are known to result in morphological deficits (2, 12–14), so, by stabilizing the brood temperature, bees are able to control the influence of this environmental variable on the development of their offspring.
Temperature regimes during the development of individual pupae, however, are dynamic and more complex than is implied by a single average air temperature (15). Measurements of the temperature within individual pupal cells, for example, revealed values that ranged from 32.6°C for the coldest pupa to 35.9°C for the warmest pupa. Taken over a 3-h period, the mean temperatures for these pupae were 33.7°C for the coldest and 35.0°C for the warmest pupa, but no pupae raised in the combs experienced a completely constant temperature (M. Kleinhenz, B. Bujok, S. Fuchs, and J.T., unpublished results).
During the first weeks of life, bees do not leave the hive. Instead, they perform indoor activities that do not appear to demand the same degree of behavioral plasticity needed by foragers that leave the hive, find food sources, remember their locations, return to the hive, and pass on information about the nature, quality, and location of the food sources to nest mates. The foragers achieve this by means of executing a complex dance containing a number of variables that the follower bees are able to read. An important aspect of the foraging behavior for this study is that it has been very well described and, unlike many of the behaviors performed within the nest, is quantifiable. The foraging behavior therefore provides us with an opportunity to investigate possible consequences of brood temperature on a behavior that has many aspects, ranging from orientation to learning, and so could be a highly sensitive indication of the influence of temperature on development of the nervous system.
Two features of nectar foraging lend themselves to analysis. The first is the waggle dance communication process, and the second is the food source learning process. Waggle dances can be broken down into several components, the duration or repetition of which can be recorded accurately. The nonassociative (sensitization) and associative learning abilities and memory consolidation of individual bees can be tested by using the proboscis-extension reflex, in which sugar water stimulation of the antennae leads to the extension of the proboscis and that, if adequately rewarded, provides a learning paradigm. In the natural situation, the presence of nectar increases the exploratory behavior for flowers, which, coupled with an associative learning process, establishes a memory for particular flower characters. Hence, the learning process is central to successful foraging.
In this study, we have explored the effect of raising pupae at different constant temperatures. We tested their performance as foragers by using the waggle dance and as learners by using the proboscis-extension reflex. We found that subjecting honey bees during their pupal development to constant temperatures representing the lowest, highest, and mean temperatures that naturally occur in the hive has a significant effect on their foraging and learning abilities.
Materials and Methods
Temperature Treatment of the Pupae. Honey bee queens (Apis mellifera carnica) in their hives were allowed access only to empty combs. They laid their eggs in these, thus providing us with brood combs in which all the larvae would pupate almost simultaneously. The queens were transferred to a new, empty comb every day. The combs with the eggs were left in the colony for ≈8 days until most of the brood cells had been capped. These combs then were taken out of the hive and placed in incubators (Rumed 1000-72039, Laatzen, Germany, and Bachofer 400 HY-E, Reutlingen, Germany) set to 32°C, 34.5°C, and 36°C. The temperature of the brood area was monitored continuously during the entire incubation periods by using thermoprobes on the brood combs (Almemo 2290-8 V5, Holzkirchen, Germany; precision, ±0.15°C). Deviations from the preset temperatures were minimal, ranging from 31.9°C to 32.1°C, 34.2°C to 34.8°C, and 35.6°C to 36.4°C.
Immediately after emerging from the cells, ≈300 young adult bees of each temperature group were color-marked. Eighty individuals from each temperature group were transferred to a foster colony established in an observation hive in which the in-hive behavior and foraging activities of the temperature-treated bees could be observed and recorded. The remaining temperature-treated bees were transferred to a foster colony housed in a standard hive. Given the time-consuming nature of these experiments, the waggle dance experiments were restricted to bees that had been temperature-treated at 32°C and 36°C.
Waggle Dance Performance. The experiments started ≈3 weeks after the temperature-treated bees had been introduced into the foster colony in the observation hive. By this time, the temperature-treated bees all had progressed to the stage of forager. There are three ways in which forager bees can be trained to regularly visit a feeder. (i) The feeder is placed in front of the hive, and, after bees start feeding, the feeder is moved gradually further and further from the hive. (ii) Individual bees are carried to the feeder already placed at some distance from the hive, and, after feeding, they fly back to the hive. (iii) Bees are recruited by foragers trained by using method 1 or 2 and then are allowed to return to the hive. In all cases, the trained foragers are marked with colored paint spots on their abdomens as they feed and so can be identified both at the feeder and in the observation hive.
In the experiments reported here, we used only bees that had been recruited by foragers (method 3) because we found that none of the bees raised at 32°C could be trained to visit the feeder regularly when using the other two training methods. Even so, only four of the 32°C-raised bees were recruited to the feeder and could be used for the analysis of their dance performances.
Each of the recruited bees was videotaped at 25 frames per s after it had performed five round trips to the feeder (the feeder contained 2.4 mol nonscented sugar solution and was placed 200 m from the hive). Each bee started dancing, at the latest, after the third visit to the feeder. The dances were analyzed from video recordings. The criteria used for scoring the waggle dance performance included how often the foragers danced when they returned to the hive after visiting the feeder, the number of dance circuits (waggle phase plus return run) per visit, and the duration of each waggle phase.
Learning and Memory Consolidation. Bees of all three temperature groups were tested for nonassociative and associative learning performance and memory consolidation 7 days after emergence (16). The bees used for these experiments were taken from the standard-hive foster colony after 1 week, that is, before they had reached the stage at which they normally would become foragers. On the day before the experiments, bees were collected from the combs of the foster hive, chilled on ice, and harnessed in metal tubes (17). Before the learning experiment, bees were tested for their proboscis-extension response with sugar solution concentrations of 0.1%, 1%, 10%, and 30% (wt/vol) (18). Only bees that responded at a concentration of 0.1% or 1% were used for the experiments. This threshold test was used to ensure that only bees with similar states of motivation were compared for learning performance.
For proboscis-extension reflex conditioning, bees were exposed to a constant air flow for 45 s. Citronella scent (1:100 in mineral oil) was then pulsed into the constant air flow for 2 s as the conditioned stimulus. Immediately after odor stimulation, bees were rewarded with a 30% sugar solution (unconditioned stimulus), which was presented to the antennae and the proboscis.
In the learning experiment, each bee was given one conditioning trial, i.e., one conditioned stimulus/unconditioned stimulus pairing, and was tested for response to the conditioned stimulus after either 1 min or 10 min. Training and testing after 1 min was carried out on 55 bees raised at 32°C, 43 bees raised at 34.5°C, and 40 bees raised at 36°C. Training and testing after 10 min was carried out on 58 bees raised at 32°C, 54 bees raised at 34.5°C, and 23 bees raised at 36°C.
Statistical Analysis. For each bee, we calculated the mean number of dance circuits, the mean duration of the waggle phase, and the probability of dancing on return from the feeder. Using these mean values for individuals, we then calculated the mean for each treatment. Differences between means were tested for significance (P < 0.01 significant, two-tailed) by using Student's t test. The proboscis-extension reflex results were analyzed for significance (P < 0.05) by using the χ2 test.
Results
Emergence Probability and In-Hive Behavior. We found no significant differences in the emergence probability among the three temperature groups (32°C, 100%; 34.5°C, 99%; 36°C, 98%). On emergence, none of the young adult bees exhibited obvious morphological defects or slow, uncoordinated movement of the type that have been described in bees raised at more extreme temperatures (2, 12–14). All of the bees raised at 32°C or 36°C introduced into the foster colony in the observation hive behaved apparently normally and started foraging after ≈2 weeks, just as their untreated control nest mates did. However, although the number of 36°C-treated bees did not decrease noticeably, fewer and fewer of those raised at 32°C were seen in the hive as time passed. Individuals treated at 32°C were seen to exit the hive on their normal orientation flights but then disappear. Their bodies were not found within the hive nor at the entrance, suggesting that they had suffered some subtle motor deficiency that prevented them from flying or feeding.
Waggle Dance Performance. Three groups of bees were compared. The first group consisted of five individuals from the foster colony in the observation hive that had been raised under natural conditions. These constituted the control group. The second group consisted of five individuals of the 36°C-treated bees. The third group consisted of individuals of the 32°C-treated bees.
After the bees had made five visits to the feeder, we assumed that they had sufficient experience in terms of the orientation of the feeder and to the hive to convey information through the dance to dance followers.
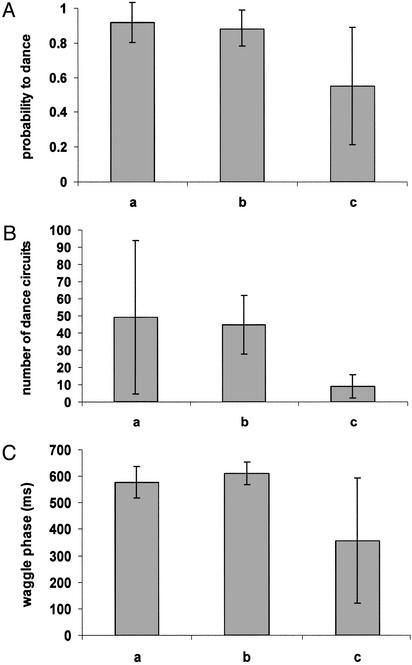
The three criteria of the dance that were analyzed from the video recordings were the probability to perform waggle dances after a return from the feeder (Fig. 1A), the number of dance circuits (Fig. 1B), and the duration of the waggle phase (Fig. 1C). The three columns in each of the figures depict the performances of the five control bees that were raised under normal conditions (column a), the five bees raised at 36°C (column b), and the four bees that were raised at 32°C (column c), respectively. We could not discern any obvious differences in the behavior of the bees of the three groups in terms of moving in the hive, leaving the hive, or arriving and feeding at the feeders.
Fig. 1.
(A) Probability (mean ± SD) that bees would dance after a visit to the feeder. Column a, probability for bees raised naturally in a standard hive (25 visits from five foragers); column b, probability for bees that were treated as pupae at 36°C (25 visits from five foragers); column c, those treated at 32°C (20 visits from four foragers). There is no significant difference among the three groups. (B) Number of dance circuits (mean ± SD) performed by the bees after each visit to the feeder. Columns represent the same three groups as described in A. Column a, 23 dances from five foragers; b, 22 dances from five foragers; c, 11 dances from four foragers. There is a significant difference between the bees raised at 32°C (c) and those raised at 36°C (b). (C) Duration of the waggle phase (mean ± SD, ms) in the dances performed by the bees. Columns represent the same three groups as described in A. Column a, 1,127 dance circuits from five foragers; b, 1,224 dance circuits from five foragers; c, 176 dance circuits from four foragers.
In 20 visits by the four foragers raised at 32°C, the probability that they would dance on their return to the hive was ≈60% in comparison with ≈90% in the other two groups. However, these differences were not significant because of a large variance in the 32°C bees, which was significantly larger in the 32°C group compared with the other two groups.
We found a significantly smaller mean number of dance circuits by the bees raised at 32°C compared with those raised at 36°C. Also, the variance in the duration of the waggle phase was larger in 32°C-raised bees compared with 36°C-raised bees.
Learning and Memory Consolidation. Because testing the learning and memory consolidation is far less time consuming than measuring the dancing behavior, we were able to include bees from all three temperature-treated groups, namely those raised at 32°C, 34.5°C, and 36°C. The learning behavior of bees raised under natural conditions has been studied extensively (19), so it was not considered necessary to repeat the experiments here with a group of control animals.
Each bee from each of the three temperature regimes was given one conditioning trial and then was tested for the conditioned response either 1 or 10 min after the conditioning trial. Both time intervals test for nonassociative (sensitization) or for associative learning and for the ability to consolidate memory.
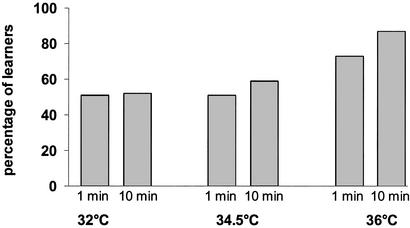
The results of the learning and memory consolidation tests are shown in Fig. 2, and the results of the statistical analysis of these data are given in Table 1. We found that bees raised at 36°C performed significantly better at both test intervals than bees raised at either 32°C or 34.5°C (Fig. 2). The differences in response level between the 36°C group and the two other groups are similar to those found for what have been termed “good” and “bad” learners (ranges given in ref. 20: “bad” learners, 55–70% for 1 min and 50–68% for 10-min interval; “good” learners, 50–70% for 1 min and 72–80% for 10-min intervals). In the 1-min test, bees raised at 36°C showed a response level of 70%, and in the 10-min test, the response level increased to 85%, a performance that is close to the maximum expected in the conditioning of the proboscis extension. There were no significant differences in the response level between the bees reared at 32°C or 34.5°C at both time intervals tested.
Fig. 2.
Proboscis-extension reflex response 1 or 10 min after one-trial odor conditioning in bees, the pupae of which were reared at 32°C, 34.5°C, and 36°C. Columns represent the percentage of responders after 1 min (55 bees raised at 32°C, 43 raised at 34.5°C, and 40 raised at 36°C) or 10 min (58 bees raised at 32°C, 54 raised at 34.5°C, and 23 raised at 36°C).
Table 1. Statistical χ2 test on the data presented in Fig. 2.
| Temperature, °C | 1 min | 10 min | 1 min vs. 10 min |
|---|---|---|---|
| 32 | P = 0.93 | ||
| 32 vs. 34.5 | P = 0.98 | P = 0.42 | |
| 34.5 | P = 0.42 | ||
| 34.5 vs. 36 | P = 0.04 | P = 0.01 | |
| 36 | P = 0.18 | ||
| 32 vs. 36 | P = 0.04 | P = 0.014 |
P < 0.05, significant
Discussion
Brood Warming and Hive Energy Partition. The members of many social insect colonies cooperate with one another to regulate the aspects of the environment within their nests (21), but the honey bees appear to be the only group that have achieved a high degree of homeothermy in their nests, keeping the brood combs at temperatures which, although never constant, vary within a relatively narrow window of ≈3°C, despite outside temperatures that range from below freezing to above the melting point of the wax of the combs. The results of the experiments that we have reported in this paper show that the temperature at which the pupae are reared influences their dance communication and odor-learning abilities, the consequences of which are explored below.
The energy required to maintain the relatively stabile brood temperature is obtained from the sugar contained in the nectar brought into the hive by the foragers. “Heater” bees transform the energy in the nectar to heat by rapidly contracting the antagonistic flight muscles in the thorax (6, 7, 22) and then transmitting this warmth to the pupal cells (ref. 23; M. Kleinhenz, B. Bujok, S. Fuchs, and J.T., unpublished data).
The partition of energy within a bee hive can be estimated from the following information, which has been taken from publications. (i) The number of foragers produced by one colony (based on the egg-laying rate of a queen) per year is ≈200,000 (3). (ii) The number of foraging days per bee is ≈10 (3, 4). (iii) The mean number of trips per forager per day is around five (24, 25). (iv) The average energy brought back to the hive per trip is ≈500 J (4, 26). Thus, the total energy collected in a summer season per honey bee colony is ≈5,000,000 kJ.
This energy is used for (i) basic metabolism and heating of the winter cluster during winter (1,600,000 kJ; calculation based on refs. 27–29) and (ii) basic metabolism of all bees, energy for comb building, walking in the hive, and all outdoor trips (but not brood heating) during summer (1,500,000 kJ; calculation based on refs. 30 and 31); leaving (iii) ≈2,000,000 kJ per year for heating of the brood nest, which is ≈40% of the total energy brought back into the nest in 1 year.
Two aspects are relevant for the present study. First, the 40% investment in maintaining the brood temperature is a significant portion of the energy budget and attests to the importance of this activity. Second, the total energy required for the colony is collected with surprisingly little effort. On average, a forager will make only five trips in a day and will forage only for 10 days; hence, it will make a total of only 50 trips (25). Given that some bees have been recorded to make many more than five foraging trips per day, it would seem that others are working far less hard than popularly supposed.
It has been known for many years that honey bees hatch and progress through a number of stages within the hive during which they will carry out various tasks ranging through cell cleaning, nursing, comb building, entrance guarding, and foraging. It is also known that although cell cleaning and foraging are the specialties of the youngest and the oldest bees, the bees between these extremes carry out a number of different tasks and will frequently switch from one task to another in a short space of time (3). Different individuals also will spend different amounts of time on in-hive activities compared with outside activities whereas others may never forage at all (4). The reason for these differences between bees has been the subject of speculation, and we would suggest here that the temperature regime to which they were subjected as pupae may play an important role.
Bees raised artificially at constant temperatures on the lower end of the naturally occurring brood nest temperature range perform less well in dance communication and scent learning. Under natural conditions, this would lead to “bad dancers,” as von Frisch termed forager bees with a low probability to dance and those performing only few dance circuits compared with “good dancers” (unpublished protocols by K. von Frisch, available from J.T.). Such behavior will lead to less nectar inflow into the colony (32, 33) because no dances or short dances with only few circuits will attract no or few recruits (3). Also, a highly variable waggle phase should guide recruited bees to areas scattered around the goal because the feeder distance is coded by the duration of the waggle phase (34).
We have qualitative evidence that exposing the pupae of bees to a temperature of 32°C may have a more far-reaching effect than producing poor dancers. Of the 80 bees that were treated at 32°C and introduced to the observation hive, only very few remained at the end of the 2-week period. The others apparently left the hive and never returned. One could speculate that these animals, seemingly perfectly able to carry out the in-hive nest duties, had trouble finding their way back to the hive after undertaking their orientation flights, and, indeed, some of these bees were found in hives in the neighborhood of their own. The learning and memory consolidation tests (see below) confirm that not only are the 32°C-raised bees poor dancers, they also are bad learners. Learning and memory consolidation, therefore, do appear to be critically important for foraging.
A characteristic of bee-training experiments is that only a small number of bees can be trained to the feeder compared with the large number of individuals that will fly past a feeder placed next to the entrance to a hive. Although there is no reason not to select the best bees in such experiments for the study of the foraging process, the broader picture of a diversity of foraging ability, or even the ability to forage at all, has been obscured. Our results indicate that the existence of different brood-temperature regimes creates diversity in the abilities of the bees that emerge and even a limitation of the numbers of bees that forage successfully. This suggests that there will be an effect on the balance between the bees that carry out in-hive activities and those that forage. We propose that when outside temperatures are high, fewer heater bees will be needed and a larger number of successful foragers will be produced by the higher temperature in the brood. A large amount of nectar, potential energy, will be stored in the combs. However, during colder weather, fewer foragers will be produced, more heater bees will be available to raise the brood temperature, and so the number of successful foragers required for the next round of warmer weather will rise.
The bees that were selected arbitrarily from the colony and for which we did not know the raising conditions showed a significantly larger variance in the number of dance circuits. The dance circuit number was significantly different between the “low-” and “high-temperature” bee groups; this makes sense because it can be expected that bees from a certain range of raising conditions were summed under “normal bees.”
Learning and Memory Consolidation. Although it is possible that the facilities of learning and memory are necessary for bees to carry out in-hive activities, there is little doubt of the need for these in foraging bees, and we find that bees raised at different temperatures during pupal development show differences in learning performance. The measurement of memory consolidation, first established by Pelz et al. (35), allows different, important features of learning and memory formation to be tested. This test is a condensed version of the original experiment on memory dynamics in honey bees that demonstrated that, after one learning trial, bees exhibit a biphasic memory curve (36, 37). In a series of experiments, Menzel and coworkers (19, 37) revealed that the biphasic memory dynamic is a result of two different and separate processes. The high response level to an odor stimulus within the first minute after the conditioning trial is a result of a nonassociative sensitization induced by the sugar-water stimulation. The associative response to the conditioned odor, also induced by sugar-water stimulation, develops with time. After 10 min, the learned response reaches its maximum response level (38, 39).
In our experiments, the bees reared at 36°C during pupal development performed significantly better than those reared at 32°C and 34.5°C, and this was the case at both test intervals, 1 and 10 min after the conditioning trial. The higher response level must be a result of an increased sensitization and reward function of the sugar stimulus and, to a lesser extent, to an increased odor-stimulus strength (36, 38, 39).
Regarding behavioral consequences of the biphasic memory dynamic, it was suggested that both time intervals, (i) <1 min and (ii) in the range of up to 10 min after the learning experience, are important time windows for the foraging honey bee. Immediately after the flower visit, an increased arousal state will heighten the perception and recognition of any important flower feature (odor, color, and shape). About 10 min pass before the bee returns from the hive to the feeding place, and, to recognize the flower from which it had successfully collected nectar, the associative memory should be high. Both processes increase the bee's ability to respond appropriately and efficiently to flower signals and, thus, reduce searching as well as handling time. Reduction of searching and handling time should have direct consequences for the colony's food collecting.
Temperature and the Development of the Nervous System. In holometabolous insects like the honey bee, the larval nervous system becomes completely remodeled during pupal metamorphosis to accommodate the changes in sensory equipment and motor systems, which are associated with the change from the worm-like larva to the adult insect (40). Because of these extensive changes in the CNS taking place during the pupal phase, this period may be expected to be very sensitive to environmental changes such as temperature. In the moth Manduca sexta, for example, temperature can be used to desynchronize axonal or dendritic outgrowth, or both, and related defects during the development of a functional circuitry in the olfactory system (41).
The differences that we found in waggle dance performance and odor-learning abilities in temperature-treated honey bees may well be caused by temperature-mediated influences on anatomical and physiological characters of the developing CNS, the hormonal system, or both. An investigation of these effects presently is underway in our laboratories.
Acknowledgments
Dirk Ahrens helped in supplying and keeping the honey bees. We thank David Sandeman for his extensive comments on and discussion of an earlier version of this paper and Tom Seeley for enlightening discussions. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 554 and GK 200).
References
- 1.Hess, W. R. (1926) Z. Vgl. Physiol. 4, 465-487. [Google Scholar]
- 2.Himmer, A. (1927) Erlanger Jb. Bienenkunde 5, 1-32. [Google Scholar]
- 3.Seeley, T. D. (1985) Honeybee Ecology, A Study of Adaptation in Social Life (Princeton Univ. Press, Princeton).
- 4.Seeley, T. D. (1995) The Wisdom of the Hive (Harvard Univ. Press, Cambridge, MA).
- 5.Southwick, E. E. & Heldmaier, G. (1987) Bioscience 37, 395-399. [Google Scholar]
- 6.Heinrich, B. (1979) Science 205, 1269-1271. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich, B. (1993) The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation (Springer, Berlin).
- 8.Lindauer, M. (1954) Z. Vgl. Physiol. 36, 391-432. [Google Scholar]
- 9.Kronenberg, F. & Heller, H. C. (1982) J. Comp. Physiol. 148, 65-76. [Google Scholar]
- 10.Harrison, J. M. (1987) J. Exp. Biol. 129, 53-61. [DOI] [PubMed] [Google Scholar]
- 11.Schmarantzer, S. & Stabentheiner, S. (1987) Thermology 2, 563-572. [Google Scholar]
- 12.Himmer, A. (1932) Biol. Rev. 7, 224-253. [Google Scholar]
- 13.Muralevskij, B. M. (1933) USSR Arch. Bienenkunde 14, 146-152. [Google Scholar]
- 14.Weiss, K. (1962) Zeitschrift Bienenforschung 6, 104-114. [Google Scholar]
- 15.Milum, V. G. (1930) J. Econ. Entomol. 23, 441-447. [Google Scholar]
- 16.Laloi, D., Gallois, M., Roger, B. & Pham-Delegue, M. H. (2001) Apidologie 32, 231-242. [Google Scholar]
- 17.Bitterman, M. E., Menzel, R., Fietz, A. & Schafer, S. (1983) J. Comp. Physiol. 97, 107-119. [PubMed] [Google Scholar]
- 18.Malun, D. (1998) Learn. Mem. 5, 90-101. [PMC free article] [PubMed] [Google Scholar]
- 19.Menzel, R. & Muller, U. (1996) Annu. Rev. Neurosci. 19, 379-404. [DOI] [PubMed] [Google Scholar]
- 20.Brandes, C., Frisch, B. & Menzel, R. (1988) Anim. Behav. 36, 981-985. [Google Scholar]
- 21.Seeley, T. D. & Heinrich, B. (1981) in Insect Thermoregulation, ed. Heinrich, B. (Wiley, New York), pp. 160-234.
- 22.Esch, H., Goller, F. & Heinrich, B. (1991) Naturwissenschaften 78, 325-328. [DOI] [PubMed] [Google Scholar]
- 23.Bujok, B., Kleinhenz, M., Fuchs, S. & Tautz, J. (2002) Naturwissenschaften 89, 299-301. [DOI] [PubMed] [Google Scholar]
- 24.Weippl, T. (1928) Arch. Bienenk. 9, 70-79. [Google Scholar]
- 25.Thom, C., Seeley, T. & Tautz, J. (2000) Apidologie 31, 737-738. [Google Scholar]
- 26.Nunez, J. (1977) J. Insect Physiol. 23, 265-275. [Google Scholar]
- 27.Moritz, R. F. A. & Southwick, E. E. (1992) Bees as Superorganisms: An Evolutionary Reality (Springer, Heidelberg).
- 28.Michel, C., Fuchs, S. & Heldmeier, G. (1995) Verh. Dtsch. Zool. Ges. 88, 67. [Google Scholar]
- 29.Fahrenholz, L., Lamprecht, I. & Schricker, B. (1989) J. Comp. Physiol. B 159, 551-560. [Google Scholar]
- 30.Rosov, S. A. (1944) Bee World 25, 94-95. [Google Scholar]
- 31.Crailsheim, K., Stabentheiner, N., Hrassnigg, B. & Leonhard, B. (1995) Verh. Dtsch. Zool. Ges. 88, 59. [Google Scholar]
- 32.Sherman, G. & Visscher, P. K. (2002) Nature 419, 920-922. [DOI] [PubMed] [Google Scholar]
- 33.von Frisch, K. (1967) The Dance Language and Orientation of Bees (Harvard Univ. Press, Cambridge, MA).
- 34.Esch, H., Zhang, S., Srinivasan, M. & Tautz, J. (2001) Nature 411, 581-583. [DOI] [PubMed] [Google Scholar]
- 35.Pelz, C., Gerber, B. & Menzel, R. (1997) J. Exp. Biol. 200, 837-847. [DOI] [PubMed] [Google Scholar]
- 36.Mercer, A. R. & Menzel, R. (1982) J. Comp. Physiol. 145, 363-368. [Google Scholar]
- 37.Menzel, R., Heyne, A., Kinzel, C., Gerber, B. & Fiala, A. (1999) Behav. Neurosci. 113, 744-754. [PubMed] [Google Scholar]
- 38.Scheiner, R., Erber, J. & Page, R. E. (1999) J. Comp. Physiol. 185, 1-10. [DOI] [PubMed] [Google Scholar]
- 39.Scheiner, R., Page, R. E. & Erber, J. (2001) Behav. Brain Res. 120, 67-73. [DOI] [PubMed] [Google Scholar]
- 40.Levine, R. B., Morton, D. B. & Restifo, L. L. (1995) Curr. Opin. Neurobiol. 5, 28-35. [DOI] [PubMed] [Google Scholar]
- 41.Rössler, W., Tolbert, L. P. & Hildebrand, J. G. (2000) J. Comp. Neurol. 425, 233-243. [PubMed] [Google Scholar]