Abstract
Recent work implicates regulation of neurogenesis as a form of plasticity in the adult rat hippocampus. Given the known effects of opiates such as morphine and heroin on hippocampal function, we examined opiate regulation of neurogenesis in this brain region. Chronic administration of morphine decreased neurogenesis by 42% in the adult rat hippocampal granule cell layer. A similar effect was seen in rats after chronic self-administration of heroin. Opiate regulation of neurogenesis was not mediated by changes in circulating levels of glucocorticoids, because similar effects were seen in rats that received adrenalectomy and corticosterone replacement. These findings suggest that opiate regulation of neurogenesis in the adult rat hippocampus may be one mechanism by which drug exposure influences hippocampal function.
Opiates are among the most commonly abused illegal drugs in the United States (1, 2). Several reports suggest that chronic exposure to opiates, such as morphine and heroin, can result in cognitive deficits (3–5). For example, heroin users have poorer performance on attention, verbal fluency, and memory tasks than controls (3), and rats chronically exposed to morphine show impaired acquisition of reference memory (5). Such findings suggest that long-term opiate use may produce maladaptive plasticity in brain structures involved in learning and memory, such as the hippocampus.
One aspect of the mammalian hippocampus that recently has received considerable attention is the birth of new neurons that occurs in the dentate gyrus throughout the lifetime of the animal (6–8). This phenomenon has been described in rodents, nonhuman primates, and, most recently, humans (9–12). Research suggests that cells are born in the subgranular zone of the dentate gyrus, migrate into the granule cell layer and express neuronal markers (8, 13, 14), extend processes to CA3 pyramidal neurons (15, 16), receive synaptic connections (10, 13, 16), and demonstrate long-term potentiation (17). Although a growing number of pharmacological and environmental manipulations have been shown to influence adult neurogenesis, the functional implication of the newly born neurons remains poorly understood (see Discussion). It has been proposed that the thousands of new neurons born each day in the adult rodent hippocampus may contribute to a variety of hippocampal-related functions, including learning and memory (6, 7).
Drugs of abuse, including opiates, can significantly alter the birth of neural progenitors during early stages of development (18–20), yet it remains unclear what effect drug exposure has on the birth of neural progenitors in the mature brain. Here we examine the consequence of long-term opiate exposure on the birth of new neurons in the adult rat hippocampus.
Materials and Methods
Animals and Drug Treatment.
Adult, male Sprague–Dawley rats (initial weight 275–300 g; Charles River Breeding Laboratories) were used for all experiments. For chronic morphine treatment, rats were given sham surgery (n = 10) or a morphine pellet (75 mg/pellet s.c.; n = 16) under light halothane anesthesia once a day for 5 days. On day 6, rats were given BrdUrd (100 mg/kg i.p.; Boehringer Mannheim) to label dividing cells. To evaluate the effect of morphine on cell proliferation, some rats (sham, n = 6; morphine, n = 9) were perfused 2 h after BrdUrd injection. To evaluate the effect of morphine on survival of newly born cells, the remaining rats (sham, n = 4; morphine, n = 7) were perfused 4 weeks after BrdUrd injection. For acute morphine treatment, rats were given 0.9% NaCl (1 ml/kg i.p.) for 3 days to habituate them to injection. On day 4, rats received either 0.9% NaCl (n = 3) or morphine (10 mg/kg; n = 7) at time t = 0, BrdUrd at t = 2 h, and were perfused at t = 4 h. The 2-h delay between morphine and BrdUrd injection corresponds to the peak neurochemical response and morphine-induced increase in locomotion. To examine drug effects on general activity, locomotor activity was measured on a separate group of chronic morphine (n = 6) and sham rats (n = 6) (21). Morphine and control rats did not differ in locomotor activity in circular chambers measured for 6 h the day after the last morphine or sham treatment (data not shown). To evaluate the ability of an opioid receptor antagonist to attenuate morphine-induced changes in cytogenesis, some sham and chronic morphine-treated rats were treated concurrently with naltrexone (5 days of 50 mg/kg i.p. and 50 mg/kg s.c. emulsion; Naltrex–Sham, n = 4; Naltrex–Morph, n = 5) or saline (Sal–Sham, n = 6; Sal–Morph, n = 4) (22).
Self-Administration Paradigm.
To facilitate acquisition of i.v. drug self-administration, rats were first food-restricted and trained to press one of two levers for food pellets. Once correct active lever-press behavior had been established, rats were fed ad libitum for 2 days before surgical implantation of a jugular catheter (23). After 5 days of recovery, rats were returned to the operant chamber and allowed to self-administer saline or heroin on a FR1 schedule of reinforcement in a daily 6-h session. Heroin self-administering rats (n = 5) received 60 μg/kg per injection in 0.1-ml volume per active-lever press. Saline self-administering rats (n = 4) received 0.1 ml saline per active-lever press. Active-lever presses resulted in infusion of heroin or saline over a 5-sec period, followed by a 10-sec timeout. Inactive-lever presses were recorded as a measure of general activity. The two groups were tested simultaneously for 6 h/day during their dark cycle, 7 days/week for 26 days. During the three sessions before the final session (days 23–25), rats received 0.9% NaCl (10 ml/kg, i.p.) at the fourth h of the 6-h session to habituate them to injection. During the final session (day 26), rats received BrdUrd 4 h into the session and were perfused at the conclusion of the 6-h session.
Adrenalectomy (ADX) and Corticosterone Replacement.
Rats underwent either bilateral ADX (n = 16) or sham surgery (n = 16) between 8 a.m. and 10 a.m. as described (24). ADX rats were given 0.9% NaCl after ADX to compensate for loss of salt. Some sham and ADX rats (n = 5 each) were examined 5 days after surgery to verify that ADX suppressed plasma corticosterone levels and increased the number of newly born cells in the subgranular zone of hippocampus as reported (25). All remaining ADX rats (n = 11) received a corticosterone replacement treatment that mimics the circadian rhythm of circulating adrenal steroids (ADX/Cort). This treatment consisted of (i) s.c. implantation of a corticosterone pellet (40 mg, adjusted to 100 mg with cholesterol) to mimic the basal level of corticosterone in the diurnal cycle (26) and (ii) corticosterone in their drinking water at night to mimic the nocturnal rise in corticosterone (25 μg/ml corticosterone in 0.9% NaCl with 0.15% ethanol). This corticosterone replacement treatment has been shown to normalize circulating levels of corticosterone, neurogenesis in the adult hippocampus, and the morphine-induced increase in locomotor activity (24, 27). Sham and ADX/Cort rats began morphine or sham treatment 4 days after surgery (Sham–Sham, n = 5; Sham–Morph, n = 6; ADX/Cort–Sham, n = 6; ADX/Cort–Morph, n = 5). Tail-vein blood from sham and ADX/Cort rats was collected for analysis of baseline (day 4) and postdrug (day 9) levels of plasma corticosterone via RIA (ICN).
Immunocytochemistry.
All rats were killed via intracardial perfusion with 4% paraformaldehyde. A freezing microtome was used to collect serial coronal 30-μm sections through striatum and hippocampus. Every ninth section was slide-mounted and coded before processing for immunocytochemistry to ensure objectivity. BrdUrd labeling requires the following pretreatment steps: DNA denaturation (0.01 M citric acid, pH 6.0, 100°C, 10 min), membrane permeabilization (0.1% trypsin, 10 min), and acidification (2 M HCl, 30 min). Primary antibody concentrations were as follows: mouse anti-BrdUrd (Becton Dickinson, 1:100), rat anti-BrdUrd (Accurate Chemicals, 1:100), rabbit anti-glial fibrillary acidic protein (GFAP) (Dako; 1:2,000), and mouse anti-NeuN (Chemicon; 1:50). Single-labeling immuno-cytochemistry for BrdUrd cell counts was completed by using the avidin-biotin/diaminobenzidine visualization method (Vector Laboratories; Pierce) followed by counterstaining with Fast Red or cresyl violet. Double-labeling for phenotypic markers and triple-labeling for BrdUrd, GFAP, and NeuN used simultaneous incubation in combinations of the following fluorescent secondaries (Jackson ImmunoResearch): anti-rat CY2 (1:200), anti-mouse CY3 (1:200), anti-rabbit CY5 (1:500), and anti-mouse rhodamine RX (1:300). For all immunocytochemistry, omittance of the primary antibodies served as a negative control. In addition, whereas most research suggests that BrdUrd is only significantly incorporated into cells that are undergoing DNA synthesis (28), some research suggests that BrdUrd can be incorporated during RNA synthesis as well (29). To address this possibility, adjacent sections from similar rostral/caudal levels received BrdUrd pretreatment, treatment with either RNase A (20 μg/ml; 30 min at 45°), DNase (1 unit/μl; 30 min at 37°), RNase buffer (0.5 M NaCl/10 mM Tris, pH 8.0/1 mM EDTA; 30 min at 45°), or DNase buffer (40 mM Tris⋅Cl, pH 7.4/6 mM MgCl2/2 mM CaCl2; 30 min at 37°) alone, and then were placed in primary antibody for BrdUrd and processed for avidin-biotin/diaminobenzidine immunocytochemistry. Activity of the RNase A enzyme was verified by its ability to abolish mRNA signal from in situ hybridization with a riboprobe (30). Pretreatment with RNase resulted in a similar number of BrdUrd-positive cells, whereas pretreatment with DNase resulted in no BrdUrd-positive cells, supporting the utility of BrdUrd as a marker of newly synthesized DNA. Three techniques were used to assess cellular damage in sections adjacent to those processed for BrdUrd immunocytochemistry. First, apoptotic nuclei were detected by using both morphological indicators and fragment end labeling via terminal deoxynucleotidyl transferase (FragEL kit, Oncogene) (31). Second, Fluoro-Jade labeling was used to screen for degenerating neurons as described (32). Finally, pyknotic cells were counted in sections stained for cresyl violet. Pyknotic cells were identified by dark staining, condensed chromatin, and pale or absent cytoplasm (33). Positive control tissue for all three techniques was generated by treating rats with kainic acid (15 mg/kg, i.p.) and analyzing brain tissue at various time points.
BrdUrd Quantification and Histological Examination.
Coded slides were examined for BrdUrd-positive cells in the dentate gyrus, which were quantified by using the optical fractionator method in which every ninth section through the rostral/caudal extent of the hippocampus was examined (bregma −1.4 mm to −7.60 mm) (34). The code was not broken until analysis for an individual experiment was complete. All BrdUrd-positive cells within the granule cell layer and hilus of the dentate gyrus were counted regardless of size or shape. To enable counting of cell clusters, cells were examined under ×400 and ×1,000 magnification. BrdUrd-positive cells within the subgranular zone that were within two cell body widths of the granule cell layer were considered part of the granule cell layer (35). The total numbers of BrdUrd-positive cells in the dentate gyrus (granule cell layer + hilus), or the granule cell layer or hilus alone, were multiplied by 9 and are reported as total number of cells per region (mean and SE). Raw data for cell counts were subjected to one-way ANOVA followed by Dunnett's post hoc comparisons. To control for possible differences in bioavailability of BrdUrd between treatment groups, BrdUrd-positive cells within the subependymal zone and corpus callosum were quantified in a similar manner. For immunofluorescent analysis, BrdUrd-positive cells were first examined with fluorescent microscopy (Olympus, New Hyde Park, NY) for double-labeling with NeuN or GFAP. Confirmation of double-labeling was performed on a confocal microscope (Zeiss LSM 510) using a ×65 objective. Confocal analysis was restricted to the top 15 μm of the section where penetration of all three antibodies was reliable. Twenty-five BrdUrd-positive cells per rat were subjected to confocal analysis for verification of colocalization within the granule cell layer. Sections were optically sliced in the Z plane by using 1.0-μm intervals. Triple-labeled images presented here were taken from one optical slice and imported into photoshop (Adobe Systems, Mountain View, CA) for composition purposes. To determine the cross-sectional area of the dentate gyrus, sections from the same rostral/caudal level were analyzed by using an image analysis system (bioquant). Briefly, the counterstained granule cell layer at bregma −3.60 to −3.80 was outlined at a magnification of ×400. At least four sections (a total of eight sides) were examined for each rat (36), and average area was reported as μm2.
Results
Chronic morphine-treated and control rats were given BrdUrd to label dividing cells and killed 2 h later. BrdUrd-positive cells were identified within the dentate gyrus granule cell layer and hilus (Figs. 1 and 2). Morphine-treated rats showed 28% fewer BrdUrd-positive cells in the granule layer of the dentate gyrus relative to control rats (Figs. 1a and 2; dentate gyrus: −28%, F1,14 = 5.71, P < 0.05; granule cell layer: −28%, F1,14 = 5.56, P < 0.05; hilus: F1,14 = 4.22, P = 0.06). The inhibitory effect of morphine on cell birth in the dentate gyrus depended on the duration of drug exposure, because rats given an acute injection of morphine showed no difference from control in the number of BrdUrd-positive cells in the granule cell layer or hilus (Fig. 1b; dentate gyrus: F1,8 = 0.03, P > 0.05; granule cell layer: F1,8 = 0.09, P > 0.05; hilus: F1,8 = 0.00, P > 0.05). In contrast to the decrease of newly born cells in the dentate gyrus, chronic morphine-treated and control rats showed equivalent numbers of BrdUrd-positive cells in the subependymal zone and corpus callosum, two brain regions where cell division occurs in the adult brain (data not shown; P > 0.05). This finding suggests that the decrease in BrdUrd-positive cells in the dentate gyrus seen in chronic morphine-treated rats is not caused by decreased bioavailability of BrdUrd. Concurrent treatment with the opioid receptor antagonist naltrexone attenuated the morphine-induced decrease in the number of BrdUrd-positive cells in the granule cell layer (granule cell layer: F3,15 = 5.7, P < 0.001; Saline–Morph rats 33% fewer BrdUrd-positive cells than Saline–Sham rats, post hoc P < 0.05; Naltrex–Morph rats post hoc P > 0.05). In sum, these data indicate that chronic, but not acute, morphine treatment results in fewer newly born cells in the dentate gyrus granule cell layer relative to control rats.
Figure 1.
(a and b) Effect of chronic or acute morphine on cell proliferation in the adult hippocampus. Means (SE) of BrdUrd-labeled cells in dentate gyrus 2 h after BrdUrd administration. Filled bars = sham; empty bars = morphine. (a) Five days of morphine exposure decreases the number of proliferating cells in the granule cell layer relative to control rats. (b) Acute morphine has no effect on the number of proliferating cells in the dentate gyrus. *, P < 0.05. (c) Effect of chronic morphine on the survival of new cells in the adult hippocampus. Means (SE) of BrdUrd-labeled cells in dentate gyrus 4 weeks after BrdUrd administration. Chronic morphine decreases the number of surviving cells in the granule cell layer relative to control rats. *, P < 0.05.
Figure 2.
Effect of chronic morphine on the morphology and distribution of proliferating cells in the adult hippocampus. (a) Schematic of coronal section of rat hippocampal dentate gyrus indicating regions examined for BrdUrd-positive cells. (b) BrdUrd-positive cells in the granule cell layer of a rat after 5 days of sham surgery. Note small, dark cells on border between granule cell layer and hilus. (c) BrdUrd-positive cells in the granule cell layer of a morphine-treated rat after 5 days of morphine. As quantified in Fig. 1, chronic morphine treatment results in fewer BrdUrd-positive cells in the dentate gyrus relative to control rats. (d–l) Examples of proliferating cells in the rat dentate gyrus 2 h after BrdUrd administration. Dark, irregular shaped images are the BrdUrd-positive nuclei; faint pink images indicate counterstained cells within the granule cell layer. Many proliferating cells occur in clusters near the granule cell layer (f–j) and within the hilus (l). Mitotic-like figures were evident in every rat (e.g., k). GCL, granule cell layer; H, hilus. [Scale bar: (b and c) 100 μm; (d–l) 10 μm.]
The morphology of proliferating cells is depicted in Fig. 2. In both morphine-treated and control rats, BrdUrd-labeled cells were darkly stained and irregularly shaped, appeared frequently in clusters of two or more, and were localized within the subgranular zone (the border between the granule cell layer and the hilus; Fig. 2) and throughout the hilus (e.g., Fig. 2 k and l). The irregular shape and small size of the BrdUrd-labeled cells is characteristic of newly born cells and provides evidence that BrdUrd was not labeling mature cells undergoing DNA repair (13). Mitotic-like figures were evident in every rat (e.g., Fig. 2k), which further supports the identification of newly born cells by this method. BrdUrd-positive cells in morphine-treated and control rats did not differ in terms of their gross morphology, the number of cells per cluster, the number of mitotic-like figures, or the location of labeled cells within the subgranular zone or hilus. In addition, morphine-treated and control rats did not differ in terms of the number of pyknotic cells, the number of apoptotic cells as detected by fragment-end labeling, or the number of Fluoro-Jade-positive cells evident within the dentate gyrus.
Newly born cells in the dentate gyrus have different fates. Some die, whereas others differentiate into neurons or glia (8, 14, 37). It was of interest to determine whether the morphine-induced decrease in proliferation corresponded to a decrease in the number of labeled cells that differentiate into neurons and survive many weeks after BrdUrd administration. To study the effect of morphine on the fate of newly born cells in the dentate gyrus, cells labeled with BrdUrd were allowed to mature for 4 weeks. We determined the phenotype of the mature BrdUrd cells by examining their morphology and location within the dentate gyrus and the colocalization of BrdUrd-positive cells with neuronal and glial markers. Morphine-treated rats killed 4 weeks after BrdUrd administration showed 47% fewer BrdUrd-positive cells in the granule layer of the dentate gyrus relative to control rats (Fig. 1c; dentate gyrus: −42%, F1,9 = 7.54, P < 0.05; granule cell layer: −47%, F1,9 = 6.51, P < 0.05; hilus: F1,9 = 4.00, P = 0.08). Microscopic analysis showed that all BrdUrd-positive cells in the granule cell layer 4 weeks after BrdUrd administration were round and large, with a pale, BrdUrd-negative cytoplasm (Fig. 3 a–g). In contrast, BrdUrd-positive cells in the hilus were smaller and irregularly shaped (Fig. 3k). BrdUrd-positive cells in morphine-treated and control rats did not obviously differ in terms of their morphology or location and depth within the granule cell layer. In addition, morphine-treated and control rats did not differ in terms of the number of pyknotic cells, the number of apoptotic cells as detected by fragment-end labeling, or the number of Fluoro-Jade-positive cells evident within the dentate gyrus. The cross-sectional area of the granule cell layer was not significantly different between control and chronic morphine-treated rats (control: 2.75 × 105 μm2 ± 1.81 × 104; morphine-treated: 2.69 × 105 μm2 ± 0.84 × 104). To determine the phenotype of mature BrdUrd-positive cells, sections were triple-labeled for BrdUrd, the mature neuronal marker NeuN, and the glial marker GFAP. Confocal analysis of triple-labeled sections showed that the large majority of BrdUrd-positive cells in the granule cell layer colocalized with NeuN (mean of 90% or 23/25 for controls, n = 4 rats; mean of 88% or 22/25 for morphine-treated rats, n = 7 rats; Fig. 3 h–m) (14). For both control and morphine-treated rats, no BrdUrd-positive cells in the granule cell layer colocalized with GFAP, whereas many BrdUrd-positive cells remaining in the hilus were GFAP-positive. In sum, these data indicate that most mature, BrdUrd-labeled cells in the granule cell layer of morphine and control rats are neurons.
Figure 3.

Effect of chronic morphine on the morphology, distribution, and phenotype of surviving cells in the adult hippocampus. (a–g) BrdUrd-positive cells in the granule cell layer examined via bright-field microscopy show neuronal morphology: large, round nucleus, pale cytoplasm, and localization within the granule cell layer. BrdUrd-labeled nuclei show both dark, uniform labeling (a, b, e, and f) and sparse, multipunctate labeling (c–e and g). (h–m) Confocal images of triple-labeled cells from a sham surgery rat (h–k) and two morphine-treated rats (l and m). Cells are labeled for the mitotic marker BrdUrd (green; h), the neuronal marker NeuN (red; i), and the glial marker GFAP (blue; j). Arrows indicate cells double-labeled with BrdUrd and NeuN but not with GFAP. Pointed line indicates cell double-labeled with BrdUrd and GFAP but not with NeuN. Merged images of the three labels (k–m) show that all BrdUrd-positive cells in the granule cell layer are NeuN-positive (yellow cells; k–m), whereas hilar cells are NeuN-negative (green cell; k). Note that in contrast to the neuronal morphology of BrdUrd-labeled cells in the granule cell layer, BrdUrd-labeled cells in the hilus are small and irregularly shaped (h and k). Morphine-treated and control rats did not differ in depth of BrdUrd-positive cells within the granule cell layer. GCL, granule cell layer; H, hilus. (Scale bar: a–g, 10 μm.)
Comparison of BrdUrd-positive cells in the granule cell layer of rats killed at the proliferation time point (2 h after BrdUrd injection on day 6; Fig. 1a) versus those killed at the survival time point (4 weeks after BrdUrd injection on day 6; Fig. 1c) reveals that 64% of BrdUrd-labeled cells survive out to 4 weeks in control rats but only 50% of BrdUrd-labeled cells survive out to 4 weeks in morphine-treated rats (P < 0.05). Interestingly, there is no difference in the survival of BrdUrd-labeled cells in the hilus, as both control and morphine rats show 64% of BrdUrd-labeled hilus cells surviving at 4 weeks. These findings suggest that the survival of BrdUrd-labeled cells in the granule cell layer, but not in the hilus, may be diminished by chronic exposure to morphine.
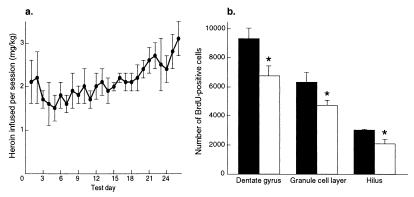
Having demonstrated that forced administration of opiates decreases neurogenesis in the hippocampal dentate gyrus, it was of interest to determine whether a similar effect occurs under conditions more relevant to addiction. To study this possibility, we examined the effect of i.v. self-administration of heroin on BrdUrd-positive cells in the adult rat dentate gyrus. Daily i.v. heroin intake is depicted in Fig. 4a (cumulative mean = 2.1 mg/kg ± 0.1). Heroin self-administering rats and control rats did not differ in inactive-lever presses (mean/session: control = 19.5 ± 2.1, heroin = 18.1 ± 6), indicating no difference in overall motor activity. Rats that self-administered heroin 6 h/day for 26 days and were killed 2 h after BrdUrd administration showed 27% fewer BrdUrd-positive cells in the granule cell layer and hilus relative to control rats (Fig. 4b; dentate gyrus: −27%; F1,7 = 7.091, P < 0.05; granule cell layer: −26%, F1,7 = 5.46, P < 0.05; hilus: −31%, F1,7 = 5.41, P < 0.05). Taken together, these findings show that volitional self-administration of opiates in daily patterns that resemble human drug intake decreases the birth of new cells in the dentate gyrus of adult rats.
Figure 4.
Effect of chronic self-administration of heroin on cell proliferation in the adult hippocampus. (a) Means (SE) of daily i.v. heroin (mg/kg) self-administered over 26 days. (b) Means (SE) of BrdUrd-labeled cells in dentate gyrus 2 h after BrdUrd administration. Filled bars, saline; empty bars, heroin. Rats that self-administer heroin for 26 days show fewer BrdUrd-positive cells relative to control rats. *, P < 0.05.
Circulating levels of gluococorticoids are known to regulate neurogenesis in the adult rat dentate gyrus. For example, stress or administration of corticosterone decreases, and ADX increases, the number of newly born cells in the adult dentate gyrus (11, 25, 38). Morphine and heroin are known to activate the hypothalamic-pituitary-adrenal axis, which causes an increase in serum levels of corticosterone (39–42). Therefore, we addressed the hypothesis that morphine mediates its inhibition of neurogenesis via an increase in circulating corticosteroids. One group of rats underwent either sham surgery or bilateral ADX. All ADX rats showed suppression of serum corticosterone (less than 1 ng/ml) and an increased number of BrdUrd-positive cells relative to sham rats, confirming the effective removal of adrenal glands (43). To normalize the ADX-induced decrease in corticosterone and the ADX-induced increase in cytogenesis, some bilateral ADX rats received a corticosterone replacement treatment (ADX/Cort) to mimic the circadian cycle of corticosteroids and normalize both cytogenesis and the morphine-induced increase in locomotor activity (24, 27).
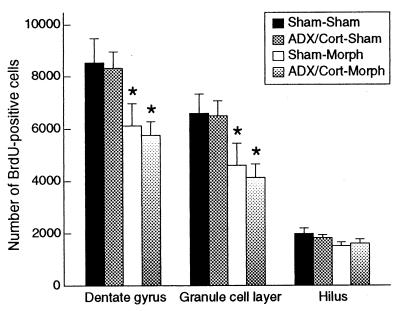
After confirming similar serum levels of corticosterone in Sham and ADX/Cort rats, we then examined the effect of chronic morphine on ADX/Cort rats. Four groups of rats were examined for the effect of pretreatment (Sham or ADX/Cort) and subsequent drug treatment (Sham or Morph): Sham–Sham, Sham–Morph, ADX/Cort–Sham, and ADX/Cort–Morph. Sham–Morph rats showed elevated corticosterone levels after morphine treatment relative to levels before morphine treatment (+150%) and relative to Sham–Sham rats (+196%). ADX/Cort–Morph rats did not show elevated corticosterone levels relative to levels before morphine treatment rats, confirming the removal of the adrenals and the efficacy of the corticosterone replacement treatment (24, 27). Sham–Morph rats killed 2 h after BrdUrd administration showed 30% fewer BrdUrd-positive cells in the granule cell layer of the dentate gyrus relative to Sham–Sham rats (Fig. 5; dentate gyrus: −28%, F3,18 = 4.37, P < 0.05, post hoc P < 0.05; granule cell layer: −30%, F3,18 = 4.16, P < 0.05, post hoc P < 0.05; hilus: F3,18 = 1.77, P > 0.05). ADX/Cort–Morph rats showed a 37% decrease in BrdUrd-positive cells in the granule cell layer relative to Sham–Sham rats (Fig. 5; dentate gyrus: −33%, post hoc P < 0.05; granule cell layer: −37%, post hoc P < 0.05; hilus: F3,18 = 1.77, P > 0.05). These results show that a morphine-induced increase in corticosterone levels cannot account for the decrease in BrdUrd-labeled cells in the dentate gyrus.
Figure 5.
Effect of ADX and corticosterone replacement on morphine regulation of cell proliferation in the adult hippocampus. Means (SE) of BrdUrd-labeled cells in dentate gyrus 2 h after BrdUrd administration. Sham–Sham: sham ADX and subsequent sham surgery; ADX/Cort–Sham: bilateral ADX, corticosterone replacement, and subsequent sham surgery; Sham–Morph: sham ADX and subsequent morphine treatment; ADX/Cort–Morph: bilateral ADX, corticosterone replacement, and subsequent morphine treatment. Sham–Morph and ADX/Cort–Morph rats had fewer BrdUrd-positive cells in the dentate gyrus relative to Sham–Sham and ADX/Cort–Sham rats. *, P < 0.05.
Discussion
Here we found that long-term exposure to opiates inhibits neurogenesis in the adult rat hippocampus. We demonstrate that chronic, but not acute, morphine decreases the number of BrdUrd-positive cells in the subgranular zone of the dentate gyrus. In addition, we found that chronic morphine decreases the number of newly born neurons evident in the dentate gyrus 4 weeks after BrdUrd administration. Opiate regulation of cytogenesis had been observed only in vitro or in a developmental context. In vitro, opiates have predominantly inhibitory effects on cell proliferation (44). In vivo, opiates have pronounced effects on cell proliferation and DNA synthesis in the developing brain, although the direction of the effect depends on the age, length of drug exposure, and brain region examined (18, 45, 46). The present work constitutes evidence that chronic opiate exposure can decrease the proliferation and survival of new neurons in the mature, adult brain.
Forced, chronic administration of opiates, as in the morphine pellet implantation protocol used here, is complicated by the stress that is associated with nonvolitional exposure to the drug. Volitional self-administration of opiates, as in daily “binge-like” patterns, more accurately models the dose and duration of opiate exposure experienced by heroin addicts (42). As presented here, 6 h/day of self-regulated heroin exposure also decreases the number of BrdUrd-positive cells in the dentate gyrus. It is interesting to note that two distinct paradigms of chronic opiate exposure—morphine pellet implantation and heroin self-administration—both result in ≈30% decrease in the number of BrdUrd-positive cells in the dentate gyrus. The similar magnitude may suggest a limit to the inhibitory effects of opiates. However, given the differences in the pharmacokinetics and routes of administration of morphine and heroin used here, additional studies are needed to evaluate this potential saturation effect.
Stress and circulating corticosteroids have potent inhibitory effects on adult neurogenesis (11, 25, 43). Like stressful experiences, morphine and heroin are powerful activators of the hypothalamic-pituitary-adrenal axis (39–42). We addressed the possibility that a morphine-induced surge of corticosterone might be responsible for the decrease in BrdUrd-positive cells by removing the adrenal glands before morphine treatment. ADX itself is known to increase neurogenesis and decrease drug-induced behaviors (24, 43). Therefore, adrenalectomized rats were given exogenous corticosterone to mimic the circadian cycle and normalize both cytogenesis and the morphine-induced increase in locmotor activity (24, 27). Adrenalectomized rats given corticosterone replacement could not mount a morphine-induced increase in corticosterone, yet they still showed fewer newly born cells in the dentate gyrus relative to control rats. This experiment demonstrates that increased levels of corticosterone are not responsible for the opiate-induced decrease in cell birth seen in the adult rat hippocampus.
Voluntary locomotor activity has been shown to increase neurogenesis in the adult hippocampus (6). However, the opiate-induced decrease in cell birth found here cannot be attributed to opiate effects on general motor activity. Rats exposed chronically to morphine or heroin showed equivalent levels of activity compared with control rats (see Materials and Methods).
If the inhibitory effect of cytogenesis in the hippocampus is not secondary to activation of the hypothalamic-pituitary-adrenal axis or to decreased locomotion, what is the mechanism underlying this action? One possibility is that opiates act directly on the progenitor population to decrease proliferation. Morphine's ability to decrease proliferation of certain cultured cells appears to be mediated through its direct action at the mu opioid receptor (47). Although hippocampal progenitor cells have been examined for a variety of receptors [e.g., N-methyl-d-aspartate (NMDA), adrenal steroid (36), growth factors (37)], no study has yet examined these cells for the presence of opioid receptors, although opioid receptor mRNA and protein are present within the subgranular zone of the adult dentate gyrus (48–50). An alternative possibility is that opiates may act indirectly on the progenitor population to decrease proliferation. For example, opioid receptors on granule cell layer interneurons could mediate local release of growth factors (51). Clearly, resolution of this question will require further investigation.
The present results further elucidate the neurobiological factors that influence neurogenesis in the adult rat hippocampus (51, 52). The birth of new cells in the dentate gyrus is decreased by stress (11) and age (35, 53) and is increased by hippocampal-dependent learning (7), environmental enrichment (54), and voluntary exercise (6). Although the importance of identifying factors that regulate adult neurogenesis is clear, there is still significant debate about the functional implications of this phenomenon. It has been proposed that neurogenesis plays a role in cognitive aspects of hippocampal functioning (6, 7, 55, 56). For example, evidence suggests that the survival of newly born cells in the adult dentate gyrus is positively influenced by hippocampal-dependent learning (7, 55).
This hypothesis remains controversial, however, in that it has not yet been established that newly born neurons are essential to normal cognitive functioning in the adult. Nevertheless, it is tempting to speculate that the cognitive deficits seen after chronic opiate use may be partly related to the decrease in neurogenesis demonstrated here. Clinical research has shown that opiate addicts demonstrate deficits in memory, attention, verbal fluency, and general cognitive performance relative to controls (3, 4). Although the central depressant effects of opiates complicate the interpretation of studies of opiates on cognition, evidence from basic research suggests that opiates interfere with cognition independent of performance effects. For example, chronic morphine treatment impairs the acquisition of radial maze and Y-maze choice escape tasks, but does not alter performance of the task if learned before drug exposure (5).
In sum, the decrease in neurogenesis documented in the present study represents one mechanism by which opiates may exert long-lasting effects on the neural circuitry involved with learning, memory, and cognition. Although the functional implications of opiate-induced inhibition of neurogenesis remain unclear, the present data establish the importance of considering opiate-induced inhibition of hippocampal neurogenesis, and the possibility of associated cognitive deficits, when considering the long-term consequences of opiate addiction.
Acknowledgments
We thank Heather Cameron and David Kornack for helpful discussions and Ifeoma Okafor and Ariel Otero for excellent technical assistance. This work was supported by the National Institute on Drug Abuse and the Abraham Ribicoff Research Facilities of the Connecticut Mental Health Center, State of Connecticut Department of Mental Health and Addiction Services. M.B. is supported by a long-term fellowship from the Human Frontier Science Program Organization. C.A.S. was supported by a National Institute on Drug Abuse National Research Service Award.
Abbreviations
- ADX
adrenalectomy
- GFAP
glial fibrillary acidic protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120552597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120552597
References
- 1.Kreek M J. Pharmacol Biochem Behav. 1997;57:551–569. doi: 10.1016/s0091-3057(96)00440-6. [DOI] [PubMed] [Google Scholar]
- 2.Hughes P H, Rieche O. Epidemiol Rev. 1995;17:66–73. doi: 10.1093/oxfordjournals.epirev.a036186. [DOI] [PubMed] [Google Scholar]
- 3.Guerra D, Sole A, Cami J, Tobena A. Drug Alcohol Depend. 1987;20:261–270. doi: 10.1016/0376-8716(87)90036-6. [DOI] [PubMed] [Google Scholar]
- 4.Ciopolli C, Galliani I. Psychol Rep. 1987;60:1099–1105. doi: 10.1177/0033294187060003-216.1. [DOI] [PubMed] [Google Scholar]
- 5.Spain J W, Newsom G C. Psychopharmacology. 1991;105:101–106. doi: 10.1007/BF02316870. [DOI] [PubMed] [Google Scholar]
- 6.Van Praag H, Kempermann G, Gage F H. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 7.Gould E, Beylin A, Tanapat P, Reeves A, Shors T J. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 8.Kornack D R, Rakic P. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman J, Das G D. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan M S, Hinds J W. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 11.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ericksson P S, Perfilieva E, Bjork-Eriksson T, Alborn A-M, Nordborg C, Peterson D A, Gage F H. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan M S, Bell D H. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron H A, Woolley C S, McEwen B S, Gould E. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 15.Stanfield B B, Trice J E. Exp Brain Res. 1987;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 16.Markakis E A, Gage F H. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 17.Wang S, Scott B W, Wojtowicz J M. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 18.Zagon I S, McLaughlin P J. Pharmacology. 1977;15:276–282. doi: 10.1159/000136699. [DOI] [PubMed] [Google Scholar]
- 19.Hammer R P J, Hauser K F. In: Development of the CNS: Effects Alcohol and Opiates. Miller M, editor. New York: Wiley–Liss; 1992. pp. 319–339. [Google Scholar]
- 20.Reznikov K, Hauser K F, Nazarevskaja G, Trunova Y, Derjabin V, Bakalkin G. Eur J Neurosci. 1999;11:2711–2719. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- 21.Miserendino M J D, Guitart X, Terwilliger R Z, Chi S, Nestler E J. Mol Cell Neurosci. 1993;4:440–448. doi: 10.1006/mcne.1993.1055. [DOI] [PubMed] [Google Scholar]
- 22.Beitner-Johnson D, Guitart X, Nestler E J. J Neurochem. 1993;61:1766–1773. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 23.Self D W, McClenahan A W, Beitner-Johnson D, Terwilliger R Z, Nestler E J. Synapse. 1995;21:312–318. doi: 10.1002/syn.890210405. [DOI] [PubMed] [Google Scholar]
- 24.Marinelli M, Piazza P V, Deroche V, Maccari S, Le Moal M, Simon H. J Neurosci. 1994;14:2724–2731. doi: 10.1523/JNEUROSCI.14-05-02724.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron H A, Gould E. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 26.Meyer J S, Micco D J, Stephenson B S, Krey L C, McEwen B S. Physiol Behav. 1979;22:867–870. doi: 10.1016/0031-9384(79)90330-5. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez J J, Montaron M F, Petry K G, Aurousseau C, Marinelli M, Premier S, Rougon G, Le Moal M, Abrous D N. Eur J Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 28.Miller M W, Nowakowski R S. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 29.Jensen P O, Larsen J, Christiansen J, Larsen J K. Cytometry. 1993;14:455–458. doi: 10.1002/cyto.990140416. [DOI] [PubMed] [Google Scholar]
- 30.Gold S J, Ni Y G, Dohlman H G, Nestler E J. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao D, Bullock R. J Neurosurg. 1996;85:655–661. doi: 10.3171/jns.1996.85.4.0655. [DOI] [PubMed] [Google Scholar]
- 32.Eisch A J, Schmued L C, Marshall J F. Synapse. 1998;30:329–333. doi: 10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Gould E, Woolley C S, McEwen B S. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- 34.West M J, Slomianka L, Gundersen H J G. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron H A, Woolley C S, Gould E. Brain Res. 1993;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- 37.Okano H J, Pfaff D W, Gibbs R B. Dev Neurosci. 1996;15:199–209. doi: 10.1159/000111408. [DOI] [PubMed] [Google Scholar]
- 38.Cameron H A, Gould E. J Comp Neurol. 1996;369:56–63. doi: 10.1002/(SICI)1096-9861(19960520)369:1<56::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Bryant H U, Bernton E W, Kenner J R, Holaday J W. Endocrinology. 1991;128:3253–3258. doi: 10.1210/endo-128-6-3253. [DOI] [PubMed] [Google Scholar]
- 40.Nock B, Wich M, Cicero T J. J Pharmacol Exp Ther. 1997;282:1262–1268. [PubMed] [Google Scholar]
- 41.Pechnick R N. Annu Rev Pharmacol Toxicol. 1993;32:353–382. doi: 10.1146/annurev.pa.33.040193.002033. [DOI] [PubMed] [Google Scholar]
- 42.Kreek M J. Mol Psychiatry. 1996;1:232–254. [PubMed] [Google Scholar]
- 43.Gould E, Cameron H A, Daniels D C, Woolley C S, McEwen B S. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauser K F, Steine-Martin A. In: The Neurobiology of Opiates. Hammer R P, editor. Boca Raton, FL: CRC; 1993. pp. 23–62. [Google Scholar]
- 45.Dodge Miller C R, O'Steen W K, Deadwyler S A. J Comp Neurol. 1982;208:209–214. doi: 10.1002/cne.902080209. [DOI] [PubMed] [Google Scholar]
- 46.Kornblum H I, Loughlin S E, Leslie F M. Dev Brain Res. 1987;31:45–52. doi: 10.1016/0165-3806(87)90081-2. [DOI] [PubMed] [Google Scholar]
- 47.Hauser K F, Stiene-Martin A, Mattson M P, Elde R P, Ryan S E, Godleske C C. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLean S, Rothman R B, Jacobson A E, Rice K C, Herkenham M. J Comp Neurol. 1987;255:497–510. doi: 10.1002/cne.902550403. [DOI] [PubMed] [Google Scholar]
- 49.Delfs J M, Kong H, Mestek A, Chen Y, Yu L, Reisine T, Chesselet M F. J Comp Neurol. 1994;345:46–68. doi: 10.1002/cne.903450104. [DOI] [PubMed] [Google Scholar]
- 50.Mansour A, Fox C A, Akil H, Watson S J. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 51.Cameron H A, Hazel T G, McKay R D G. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 52.Gage F H, Kempermann G, Palmer T O, Peterson D A, Ray J. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Cameron H A, McKay R D G. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 54.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez-Buylla A, Kirn J R, Nottebohm F. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- 56.Ciaroni S, Cuppini R, Cecchini T, Ferri P, Ambrogini P, Cuppini C, Del Grande P. J Comp Neurol. 1999;411:495–502. [PubMed] [Google Scholar]