Abstract
Mice deficient in the epidermal water/glycerol transporter aquaporin-3 (AQP3) have reduced stratum corneum (SC) hydration and skin elasticity, and impaired barrier recovery after SC removal. SC glycerol content is reduced 3-fold in AQP3 null mice, whereas SC structure, protein/lipid composition, and ion/osmolyte content are not changed. We show here that glycerol replacement corrects each of the defects in AQP3 null mice. SC water content, measured by skin conductance and 3H2O accumulation, was 3-fold lower in AQP3 null vs. wild-type mice, but became similar after topical or systemic administration of glycerol in quantities that normalized SC glycerol content. SC water content was not corrected by glycerol-like osmolytes such as xylitol, erythritol, and propanediol. Orally administered glycerol fully corrected the reduced skin elasticity in AQP3 null mice as measured by the kinetics of skin displacement after suction, and the delayed barrier recovery as measured by transepidermal water loss after tape-stripping. Analysis of [14C]glycerol kinetics indicated reduced blood-to-SC transport of glycerol in AQP3 null mice, resulting in slowed lipid biosynthesis. These data provide functional evidence for a physiological role of glycerol transport by an aquaglyceroporin, and indicate that glycerol is a major determinant of SC water retention, and mechanical and biosynthetic functions. Our findings establish a scientific basis for the >200-yr-old empirical practice of including glycerol in cosmetic and medicinal skin formulations.
Keywords: water transport, stratum corneum
The most superficial layer of skin is the stratum corneum (SC), which contains flattened dead epidermal cells (corneocytes) embedded in a lipid-rich matrix. The underlying epidermis consists of a viable keratinocyte multilayer that carries out the biosynthesis of lipids and proteins to be incorporated into the SC. Hydration of the SC depends on multiple factors, including its structure and composition, the external humidity, and the barrier and biosynthetic functions of the epidermal layer.
The basal layer of epidermal keratinocytes contains aquaporin-3 (AQP3), a small integral membrane protein that functions as a facilitated transporter of water and glycerol (1–3). AQP3 has thus been called an “aquaglyceroporin.” We reported that mice deficient in AQP3 had ≈3-fold reduced SC water content compared with wild-type littermates (4), and tested the most likely explanation involving defective AQP3-facilitated water transport from the dermis into the SC. Contrary to expectations, the decreased SC hydration in AQP3 null mice was not corrected by a humidified atmosphere or surface occlusion, suggesting an intrinsic defect in SC water-holding capacity rather than reduced epidermal water permeability. In a follow-up study, we found reduced skin elasticity in AQP3 null mice and delayed SC barrier recovery after tape stripping (5). A systematic analysis of SC structure and composition revealed as the only difference a >2-fold reduced glycerol content in SC and epidermis of AQP3 null mice, without altered serum or dermal glycerol concentrations.
In this study we test the hypothesis that glycerol transport by AQP3 is responsible for the functional defects in skin of AQP3-deficient mice. We found that glycerol, but not glycerol analogs, corrected the SC hydration defect when administered topically or systemically. Glycerol also corrected the reduced skin elasticity and delayed barrier recovery in AQP3 null mice. Analysis of [14C]glycerol dynamics indicated reduced glycerol transport in AQP3-deficient mice without altered metabolism. These data provide functional evidence for a key role of AQP3-mediated glycerol transport in epidermal physiology, establishing a scientific basis for the common practice of including glycerol in cosmetics and other skin formulations.
Experimental Procedures
Mice. AQP3 null mice, originally generated in a CD1 genetic background (6), were back-crossed into the SKH1 hairless genetic background. Six- to 10-week-old mice were used. The mice were maintained in air-filtered cages in the University of California at San Francisco Animal Care facility. All procedures were approved by the University of California at San Francisco Committee on Animal Research. Measurements were done on mice under normal conditions (external temperature 22 ± 2°C, humidity 40 ± 3%) or after exposure to high (90%) external humidity.
Glycerol Administration. Glycerol was administered to wild-type and AQP3 null mice by topical, i.p., and oral routes. For topical administration, 2-M water solutions of glycerol (or glycerol analogs) were applied to the skin for 1 h under occlusion. For i.p. administration, mice were given a single injection of glycerol (2 M, 10 μl/g). For oral administration, mice were provided ad libitum with water containing glycerol (up to 10% for wild-type mice and 2% for AQP3 null mice) along with standard solid mouse chow.
Skin Water Content and Elasticity Measurements. SC hydration (water content) was measured by high-frequency surface electrical conductance by using a Skicon-200 (IBS, Hamamatsu, Japan) and by 3H2O accumulation as described (5). Skin elasticity was measured by using a Cutometer SEM474 (Courage & Khazaka, Cologne, Germany) in which immediate distention (Ue), final distention (Uf), and immediate retraction (Ur) were calculated from the distension kinetics (7).
Barrier Recovery After Tape Stripping. Barrier recovery was assessed before and 1 day after oral glycerol administration from measurements of transepidermal water loss (TEWL) by using a Meeco moisture analyzer (Meeco, Warrington, PA). The SC barrier was disrupted by 9–10 strippings with cellophane tape until the TEWL increased by 10-fold above baseline. TEWL was measured at 2, 6, and 24 h (8).
Glycerol Assay. Glycerol was assayed in SC, epidermis, dermis, and serum by using a Glycerol Assay kit based on enzymatic analysis (Boehringer). The SC was collected by nine tape-strippings, and the tapes were soaked in PBS to dissolve the glycerol. To separate epidermis from dermis, full-thickness skin was heat-split at 60°C for 10 s, and then homogenized in PBS.
Morphology. For electron microscopy, skin samples were fixed in 2% glutaraldehyde and 0.2% ruthenium tetroxide (RuO4) in 0.1 M sodium cacodylate buffer. After fixation, samples were dehydrated in graded ethanol solutions, and embedded in an Epon-epoxy mixture. Ultra-thin sections were examined in an electron microscope (JEOL JEM 100S).
Glycerol Metabolism. Mice were injected with [14C]glycerol i.p. [0.1 μCi/g of body weight (1 Ci = 37 GBq), 100 μCi/ml; Perkin–Elmer]. Skin and blood were sampled at specified times after injection. Epidermal sheets were separated from dermis by heat-split. [14C]radioactivity was measured in blood and homogenates of dermis and epidermis. An in vitro assay was also done in which flank skin was removed and 18-mm diameter punch samples were floated on 1 ml of keratinocyte growth medium (Clonetics, San Diego) containing [14C]glycerol (1 μCi/ml). After incubation at 37°C/5% CO2 for 6–24 h, tissue samples were rinsed three times with PBS, epidermal sheets were separated from dermis by heat-split, and 14C radioactivity was measured. To resolve lipid vs. aqueous-phase 14C radioactivity, epidermis was soaked in CHCl3:MeOH:water (1:2:0.8) for extraction of lipid and aqueous phases by using standard procedures (9). Aliquots of the lipid and aqueous phase were resolved high-performance thin layer chromatography (HPTLC), and 14C radioactivity was measured in each fraction (10).
Results
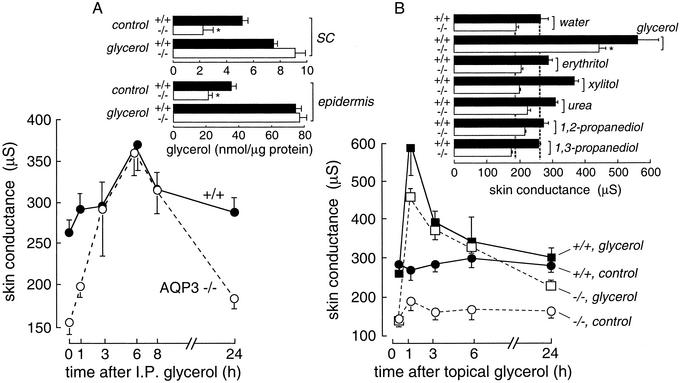
Fig. 1A shows the time course of increased high-frequency superficial skin conductance after a single i.p. injection of glycerol. Skin conductance was much lower in AQP3 null mice than in wild-type mice before glycerol administration, but became similar at 3–8 h when the conductances were maximal. The glycerol content in the SC and epidermis was similar in wild-type and AQP3 null mice when measured at a single 3-h time point (Fig. 1 A Inset). Fig. 1B shows that skin surface conductance was also increased by topical application of glycerol. However, topical application of water, glycerol-like polyols (erythritol, xylitol), propanediols, and urea did not increase skin conductance at the same concentration/time in which glycerol produced a substantial increase (Fig. 1B Inset).
Fig. 1.
Glycerol corrects the SC hydration defect in AQP3 null mice. (A) Time course of high-frequency surface skin conductance after i.p. administration of glycerol (2 M, 10 μl/g) in wild-type (filled circles) and AQP3 null (open circles) mice (mean ± SE, five mice per group). (Inset) Glycerol content in SC and epidermis before and 3 h after i.p. glycerol administration (SE, five mice per group); *, P < 0.01. (B) Time course of skin conductance after superficial glycerol application (2 M in water) for 1 h by topical occlusion (SE, five mice per group). (Inset) Skin conductance at 1 h after topical application of glycerol analogs (2 M; SE, five mice per group).
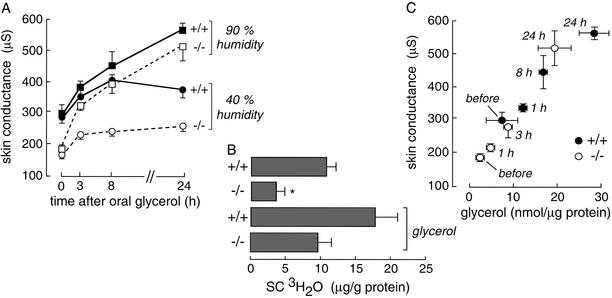
Fig. 2A shows the time course of increasing SC water content after oral glycerol administration. Mice were kept in a 40% or 90% humidity atmosphere as indicated. For these studies, the mice were provided a glycerol/water solution as their only fluid source. To correct for the polydipsia/poluria in AQP3 null mice, the fluid provided to wild-type and AQP3 null mice contained 10% and 2% glycerol, respectively. Because of potential concerns in interpreting high-frequency skin conductance in terms of SC hydration when the content of glycerol, a small polar solute, varied, the SC water content was measured directly by a 3H2O water accumulation method. Fig. 2B shows reduced 3H2O content in SC of AQP3 null mice under control conditions at 40% humidity, with increased 3H2O content in SC of wild-type and AQP3 null mice after glycerol administration for 24 h. These results support the interpretation of high frequency skin conductance in terms of SC water content.
Fig. 2.
Increased SC water and glycerol content after oral glycerol administration. (A) Time course of skin conductance after oral glycerol administration for mice maintained in a 90% or 40% humidity environment (SE, five mice per group). Mice were offered glycerol in water ad libitum as their only fluid source (10% for wild-type and 2% for AQP3 null mice). (B) 3H2O content in SC without or after oral glycerol administration for 24 h in mice maintained in a 40% humidity atmosphere. 3H2O radioactivity in SC was measured at 1 h after i.p. 3H2O injection (SE, five mice per group); *, P < 0.01. (C) Correlation between SC glycerol content and skin conductance for wild-type (filled circles) and AQP3 null (open circles) mice in a 90% atmosphere. Glycerol was administered orally as in A.
To examine the relationship between SC hydration and glycerol content, conductance measurements and SC glycerol content were assayed before and at specified times after oral glycerol administration. Mice were exposed to a 90% humidity atmosphere to maximize the range of skin conductances and to minimize water gradients in the SC by preventing evaporative water loss. Fig. 2C shows an excellent correlation between SC glycerol content and skin conductance (water content) for measurements done at different times in wild-type and AQP3 null mice. These results provide evidence for glycerol as a water-retaining humectant in the SC.
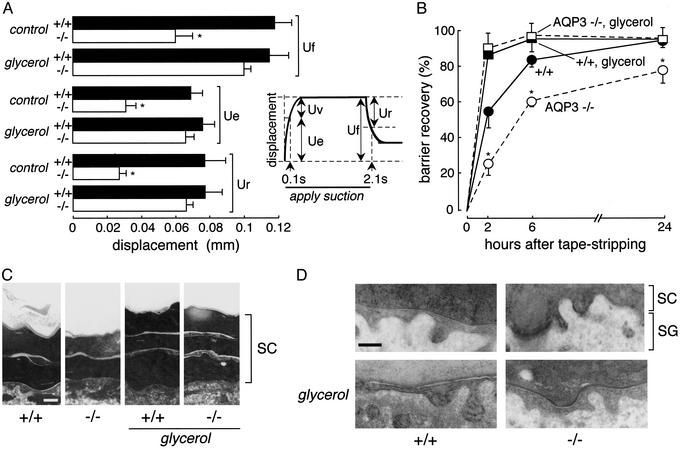
We tested the hypothesis that replacement of SC glycerol in AQP3 null mice could correct skin elasticity and barrier recovery abnormalities in AQP3 null mice. From the studies in Fig. 2, mice were given glycerol orally for 24 h. Skin elasticity was measured by cutometry, in which displacement of the skin surface was measured in response to application of a mild suction force for 2 s followed by release (Fig. 3A Inset). Fig. 3A shows that the elasticity parameters Uf, Ue, and Ur were remarkably reduced in AQP3 null vs. wild-type mice under control conditions, but became similar after glycerol. Barrier recovery was examined by using a standard protocol in which the time course of TEWL was measured after SC removal by tape-stripping. Fig. 3B shows the percentage barrier recovery where TEWL was normalized to 100% before tape stripping and 0% just after tape stripping. Barrier recovery was significantly delayed at 2 and 6 h in AQP3 null mice. Oral glycerol administration for 24 h before tape stripping increased to comparable rates the recovery of SC barrier recovery in wild-type and AQP3 null mice. Electron micrographs at 2 h after tape stripping (Fig. 3C) showed accelerated recovery of SC structure after 24 h of oral glycerol (average number SC layers without glycerol: wild-type, 2.2 ± 0.4; AQP3 null, 1.8 ± 0.2; with glycerol: wild-type, 3.6 ± 0.5; AQP3 null, 3.3 ± 0.5). Fig. 3D shows increased secretion of lamellar bodies at the interface between SC and the stratum granulosum in AQP3 null mice after glycerol administration, indicating improved biosynthetic function.
Fig. 3.
Glycerol corrects defective skin elasticity and delayed barrier recovery in AQP3 null mice. (A) Elasticity in dorsal skin measured from the kinetics of skin surface displacement by using a 2-mm diameter suction probe to produce a 50-mbar pressure transient for 2 s. Skin elasticity parameters (final distention, Uf; immediate distention, Ue; immediate retraction, Ur; see Inset) were measured before (control) and at 24 h after oral glycerol administration (SE, five mice per group); *, P < 0.01. (B) Barrier recovery measured from the kinetics of TEWL after SC removal by tape stripping. Results as percentage change from TEWL at time 0 (SE, four to six mice per group). Where indicated, mice received oral glycerol for 24 h before (and after) tape stripping. *, P < 0.01 comparing +/+ to -/-. (C) Lipid lamellar structure of SC at 2 h after tape stripping as examined by electron microscopy with RuO4 postfixation. Where indicated, mice were given glycerol orally for 24 h before tape stripping. Scale bar = 0.1 μm. (D) Thin section electron micrographs showing lipid lamellar bodies at the interface between stratum granulosum (SG) and SC at 2 h after tape stripping as in C. Scale bar = 1 μm.
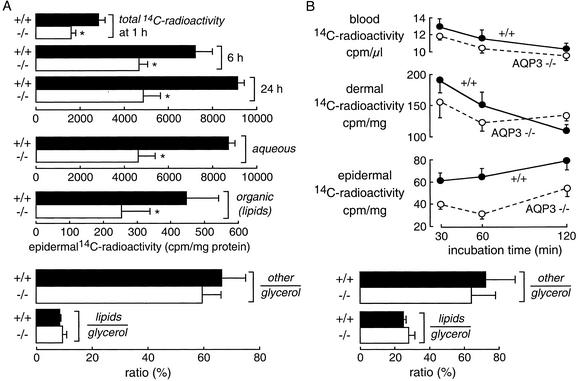
To study the mechanism of reduced epidermal glycerol content in AQP3 null mice, the kinetics of [14C]glycerol accumulation and metabolism were analyzed in excised full-thickness skin and in vivo. For excised skin measurements, the skin was floated on keratinocyte growth medium containing [14C]glycerol and the epidermis was isolated at 1, 6, and 24 h. Fig. 4A shows significantly reduced total 14C-radioactivity in epidermis of skin derived from AQP3 null mice. 14C-radioactivity (at 24 h) was reduced in both aqueous and organic (lipid) phases obtained from epidermal homogenates (Fig. 4A Middle). The aqueous phase was further resolved into glycerol and non-glycerol (“other”) fractions. Fig. 4A Bottom shows that the ratio of aqueous-phase 14C-containing glycerol metabolites to epidermal glycerol was not affected by AQP3 deletion, nor was the ratio of 14C-containing lipids to epidermal glycerol. Thus, AQP3 deletion reduces the absolute epidermal content of glycerol and its metabolites, but not the rate of glycerol metabolism when corrected for glycerol content. Further studies were done to confirm this conclusion in mice in vivo. After i.p. injection of [14C]glycerol, blood and skin samples were obtained at 30, 60, and 120 min for assay of 14C-radioactivity. Fig. 4B Top shows similar 14C-radioactivity in blood and dermis, but substantially reduced 14C-radioactivity in epidermis, as expected. Resolution of epidermal 14C-radioactivity as above indicated reduced absolute epidermal content of glycerol and its metabolites, but not the relative (corrected for glycerol content) rate of glycerol conversion to metabolites.
Fig. 4.
Glycerol accumulation and metabolism in epidermis. (A) In vitro measurements. Full-thickness skin was floated on physiological saline containing [14C]glycerol as described in Experimental Procedures. (Top) Total epidermis-associated 14C-radioactivity measured at 6 and 24 h. (Middle) Epidermal 14C-radioactivity at 24 h was resolved into aqueous and organic (lipid) fractions. (Bottom) Aqueous-phase 14C-radioactivity was further resolved into non-glycerol (other) and glycerol fractions, and expressed as ratio (%) other or lipids to glycerol (SE, five skin samples per group from different mice); *, P < 0.01. (B) In vivo measurements. Mice were given [14C]glycerol (0.1 μCi/g of body weight) by i.p. injection. (Upper) Time course of 14C-radioactivity in blood, dermis, and epidermis (SE, four mice per group). (Lower) Epidermal 14C-radioactivity was resolved into aqueous glycerol and non-glycerol phases and an organic lipid phase as in A.
Discussion
The principal finding here was that glycerol replacement corrected the reduced hydration and skin elasticity in AQP3 null mice, as well as the delayed restoration of SC barrier function after tape stripping. Structural analogs of glycerol applied topically did not correct the reduced SC hydration under conditions where glycerol fully corrected the defect. Our data suggest a simple mechanism for the skin phenotype abnormalities in AQP3 null mice: impaired glycerol transport into the epidermis and SC through the relatively glycerol impermeable basal keratinocyte layer resulting in reduced epidermal and SC glycerol content. One consequence of reduced SC glycerol content that relates to its water-retaining (humectant) properties is decreased SC hydration. Reduced skin elasticity results directly from reduced SC hydration. Another consequence of reduced epidermal cell glycerol content is impaired lipid biosynthetic function, resulting in delayed restoration of the SC after disruption. Our results thus indicate that the glycerol-transporting function of AQP3 is important in skin physiology, providing functional evidence for a physiological role of an aquaglyceroporin.
The determinants of SC water content are believed to include the water permeability of the epidermis, the water-retaining properties of the SC, and the rate of evaporative loss from the skin surface. We found previously that, although AQP3 was functional as a water channel in epidermal keratinocytes, the reduced SC hydration in AQP3-deficient mice could not be corrected by skin occlusion or placement in a humidified atmosphere (4), indicating that water transport through AQP3 is not a rate-limiting factor in SC hydration. However, the intrinsic “water-holding capacity” of the SC, as assessed from surface conductance after brief exposure to water, was reduced in AQP3 null mice. We found here that glycerol replacement by topical or systemic routes increased SC water content, with excellent correlation between SC water and glycerol content in wild-type and AQP3 null mice. The determinants of the intrinsic water-holding capacity of the SC have been controversial, with indirect evidence for potentially important roles of natural moisturizing factors (NMFs), including free amino acids, ions, small osmolytes (11, 12), SC lipids (13), and the rate of cell turnover (14). It has been reported that glycerol is incorporated into lipid lamellae when added to SC lipids in vitro, resulting in water absorption and inhibition of the transition of lipid lamellar structure from liquid to solid crystal, preventing water loss (15). Pure glycerol absorbs its own weight in water over 3 days (16). Thus, on theoretical grounds, and from the data presented here, glycerol probably enhances SC water absorption and retention. When expressed in millimolar, the measured SC glycerol content of 7.2 mM in wild-type mice is equivalent to ≈40 mM after correction for the 15–20% vol/vol water in the SC. Glycerol is thus a key small molecule in skin physiology in terms of its primary humectant and biosynthetic functions, and the secondary effects of increased SC hydration.
The greater importance of the glycerol vs. water transporting function of AQP3 is understandable from a biophysical perspective. AQP3 is expressed in the basal layer of keratinocytes, where net movement of water from dermis to superficial layers of epidermis and SC is driven by small osmotic gradients produced by slow evaporative water loss at the SC surface. Transepidermal water loss measurements indicate a water flow of ≈10 nl/min/cm2 skin under basal conditions that can increase to >100 nl/min/cm2 after SC removal (17, 18). These flow rates are remarkably lower than those of >5 × 1010 nl/min/cm2 in kidney tubules and exocrine glands such as salivary gland where aquaporin-mediated water transport is important (19). Indeed, aquaporin deletion does not have functional consequences in other tissues where fluid transport rates are slower, such as the lung alveolar barrier (20). In addition, water movement through the complex multilayer structure of the epidermis is predicted to be unstirred layer-limited, so that the rate of transepidermal water transport becomes independent of the intrinsic water permeability of AQP3-expressing epidermal cells. In contrast, glycerol is transported into the epidermis very slowly and thus its transport rate is sensitive to the intrinsic glycerol permeability of the basal keratinocyte layer.
Our findings provide a scientific basis for the long-standing practice of including glycerol in skin cosmetic and medicinal formulations, as well as for a body of empirical observations about improved skin properties after glycerol exposure. Indeed, there are reports of topical glycerol application in humans improving skin moisture (21), elasticity (22), and barrier properties (23). Since its discovery in 1779, glycerol has been used extensively in topical skin preparations, with 160,000 tons of glycerol used annually in the U.S. Also, large quantities of glycerol (4–70 g) have been administered systemically to humans to reduce brain swelling after injury (24), for treatment of endolymphatic hydrops (25, 26), and in studies of its nutritional efficacy in exercise physiology (27). Our data support the use of glycerol in skin preparations as a humectant to increase SC water content and elasticity, and as a precursor to increase epidermal biosynthesis.
Acknowledgments
We thank Liman Qain for mouse breeding and genotype analysis. This work was supported by Grants DK35124, EB00415, EY13574, HL73856, and HL59198 from the National Institutes of Health; a grant from the Cystic Fibrosis Foundation; and a gift from Kanebo Ltd. of Japan.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SC, stratum corneum; AQP3, aquaporin-3; Ue, immediate distention; Ur, immediate retraction; Uf, final distention; TEWL, transepidermal water loss.
References
- 1.Sougrat, R., Morand, M., Gondran, C., Barre, P., Gobin R., Bonte, F., Dumas, M. & Verbavatz, J. M. (2002) J. Invest. Dermatol. 118, 678-685. [DOI] [PubMed] [Google Scholar]
- 2.Frigeri, A., Gropper, M. A., Umenishi, F., Kawashima, M., Brown, D. & Verkman, A. S. (1995) J. Cell Sci. 108, 2993-3002. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki, T., Suzuki, T., Koyama, H., Tanaka, S. & Takata, K. (1999) J. Histochem. Cytochem. 47, 1275-1286. [DOI] [PubMed] [Google Scholar]
- 4.Ma, T., Hara, M., Sougrat, R., Verbavatz, J. M. & Verkman, A. S. (2002) J. Biol. Chem. 277, 17147-17153. [DOI] [PubMed] [Google Scholar]
- 5.Hara, M., Ma, T. & Verkman, A. S. (2002) J. Biol. Chem. 277, 46616-46621. [DOI] [PubMed] [Google Scholar]
- 6.Ma, T., Song, Y., Yang, B., Gillespie, A., Carlson, E. J., Epstein, C. J. & Verkman, A. S. (2000) Proc. Natl. Acad. Sci. USA 97, 4386-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agache, P. G., Monneur, C., Leveque, J. L. & De Rigal, J. (1980) Arch. Dermatol. Res. 269, 221-232. [DOI] [PubMed] [Google Scholar]
- 8.Taljebini, M., Warren, R., Mao-Oiang, M., Lane, E., Elias, P. M. & Feingold, K. R. (1996) Skin Pharmacol. 9, 111-119. [DOI] [PubMed] [Google Scholar]
- 9.Bligh, E. G. & Dyer, W. J. (1959) Can. J. Biochem. Physiol. 37, 911-919. [DOI] [PubMed] [Google Scholar]
- 10.Tanno, O., Ota, Y., Kitamura, N., Katsube, T. & Inoue, S. (2000) Br. J. Dermatol. 143, 524-531. [DOI] [PubMed] [Google Scholar]
- 11.Denda, M., Hori, J., Koyama, J., Yoshida, S., Nanba, R., Takahashi, M., Horii, I. & Yamamoto, A. (1992) Arch. Dermatol. Res. 284, 363-367. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson, T. M., Yuksel, K. U., Geesin, J. C., Gordon, J. S., Lane, A. T. & Gracy, R. W. (1990) J. Invest. Dermatol. 95, 296-300. [DOI] [PubMed] [Google Scholar]
- 13.Imokawa, G., Kuno, H. & Kawai, M. (1991) J. Invest. Dermatol. 96, 845-851. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe, M., Tagami, H., Horii, I., Takahashi, M. & Kligman, A. M. (1991) Arch. Dermatol. 127, 1689-1692. [PubMed] [Google Scholar]
- 15.Froebe, C. L., Simon, F. A., Ohlmeyer, H., Rhein, L. D., Mattai, J., Cagan, R. H. & Friberg, S. E. (1990) J. Soc. Cosmet. Chem. 41, 51-65. [Google Scholar]
- 16.Rieger, M. M. & Deem, D. E. (1974) J. Soc. Cosmet. Chem. 25, 253-262. [Google Scholar]
- 17.Ghadially, R., Brown, B. E., Hanley, K., Reed, J. T., Feingold, K. R. & Elias, P. M. (1996) J. Invest. Dermatol. 106, 1064-1069. [DOI] [PubMed] [Google Scholar]
- 18.Haratake, A., Uchida, Y., Mimura, K., Elias, P. M. & Holleran, W. M. (1997) J. Invest. Dermatol. 108, 319-323. [DOI] [PubMed] [Google Scholar]
- 19.Schnermann, J., Chou, C. L., Ma, T., Traynor, T., Knepper, M. A. & Verkman, A. S. (1998) Proc. Natl. Acad. Sci. USA 95, 9660-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma, T., Fukuda, N., Song, Y., Matthay, M. A. & Verkman, A. S. (2000) J. Clin. Invest. 105, 93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissett, D. L. & McBride, J. F. (1984) J. Soc. Cosmet. Chem. 35, 345-350. [Google Scholar]
- 22.Olsen, L. O. & Jemec, G. B. E. (1993) Acta Dermatol. Venereol. 73, 404-406. [DOI] [PubMed] [Google Scholar]
- 23.Fluhr, J. W., Gloor, M., Lehmann, L., Lazzerini, S., Distante, F. & Berardesca, E. (1999) Acta Dermatol. Venereol. 79, 418-421. [DOI] [PubMed] [Google Scholar]
- 24.Wald, S. L. & McLaurin, R. L. (1982) J. Neurosurg. 56, 323-331. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, C. C. & Zou, L. D. (1986) Chin. Med. J. (Engl.) 99, 727-730. [PubMed] [Google Scholar]
- 26.Manak, J., Knotova, M. & Reiterova, M. (1985) Cesk. Otolaryngol. 34, 19-23. [PubMed] [Google Scholar]
- 27.Robergs, R. A. & Griffin, S. E. (1998) Sports Med. 26, 145-167. [DOI] [PubMed] [Google Scholar]