Abstract
Pure-tone song is a common and widespread phenomenon in birds. The mechanistic origin of this type of phonation has been the subject of long-standing discussion. Currently, there are three hypotheses. (i) A vibrating valve in the avian vocal organ, the syrinx, generates a multifrequency harmonic source sound, which is filtered to a pure tone by a vocal tract filter (“source-filter” model, analogous to human speech production). (ii) Vocal tract resonances couple with a vibrating valve source, suppressing the normal production of harmonic overtones at this source (“soprano” model, analogous to human soprano singing). (iii) Pure-tone sound is produced as such by a sound-generating mechanism that is fundamentally different from a vibrating valve. Here we present direct evidence of a source-filter mechanism in the production of pure-tone birdsong. Using tracheal thermistors and air sac pressure cannulae, we recorded sound signals close to the syringeal sound source during spontaneous, pure-tone vocalizations of two species of turtledove. The results show that pure-tone dove vocalizations originate through filtering of a multifrequency harmonic sound source.
There is a long history of discussion regarding the manner in which birds produce “whistled” vocalizations (e.g., refs. 1–6). Normally, voiced sound production in tetrapods involves some kind of vibrating valve that modulates expiratory airflow in a pulsatile fashion (7). Sound generated this way is characterized by a multifrequency harmonic spectrum, consisting of a fundamental frequency and a series of strong harmonic overtones that are integer multiples of the fundamental frequency. Pure-tone sound as found in whistled birdsong, however, is characterized by a simple harmonic frequency spectrum, in which essentially all acoustic energy is concentrated at a single frequency.
The apparent qualitative difference between whistled song and the sound that vibrating valves normally generate has led to the suggestion that birds produce pure-tone signals in a fundamentally different way. The fact that the structure of the avian vocal organ, the syrinx, differs in many ways from the typical tetrapod vocal organ, the larynx, offers some support for this idea. Various mechanisms have been hypothesized to be the acoustic source in whistled song. Among these are a hole-tone whistle (4, 8, 9), membranes vibrating in a string-like mode (4), and air-jet excited membrane oscillations (5). So far, only the involvement of an aerodynamic whistle has been subjected to experimental tests. The results of such experimentation, however, do not support this idea for the species investigated (10–12).
An alternative explanation for the absence of harmonic overtones in whistled birdsong maintains that a multifrequency harmonic source sound is filtered to a pure tone by vocal tract resonances (10). This idea of a source-filter mechanism is derived from models of human speech production (13). If such a model also proves correct in birds, then this would extend the already known parallels between human speech and birdsong (for a review see ref. 14) to the level of acoustic production. Moreover, it would obviate the need to invoke a sound generator that is radically different from a vibrating valve. Nowicki (10) provided experimental evidence to support the idea of a source-filter mechanism in birdsong by recording nine species of songbirds in a helium-enriched atmosphere. This light gas caused harmonic overtones to appear in vocalizations that were normally pure-tonal. Such overtones are expected when a vocal tract band-pass filter, normally centered on the fundamental frequency, shifts upward because of the increased speed of sound.
The appearance of harmonic overtones in helium, however, could also be explained by assuming a different model for pure-tone sound production. In this alternative model, which is derived from human soprano singing, an overlap between vocal tract resonance and valve vibration frequencies results in nonlinear feedback that suppresses production of harmonic overtones at the source (6, 15, 16). In helium, the resonance frequency of the vocal tract shifts upward, breaking the coupling between vocal tract resonance and sound source, which results in the production of harmonic overtones.
Although the source-filter and soprano models differ in their prediction of whether the sound source produces harmonic overtones, both predict that it is the presence of specific vocal tract resonances that makes birdsong pure-tonal. Reports of helium studies in two other bird species, however, weaken the idea of vocal tract resonances as a general explanation for pure-tone birdsong. Brittan-Powell et al. (11) concluded that in budgerigar contact calls the vocal tract filter has only a slight effect on the spectral content of the vocalization, which suggests that its narrow band frequency spectrum is produced as such by the syringeal source. Furthermore, Ballintijn and ten Cate (12) concluded that the pure-tonality of collared dove coos is also due to syringeal source mechanisms, as coo vocalizations remain pure-tonal in helium.
Available evidence, therefore, does not allow one to distinguish between the three hypotheses put forth to explain pure-tone birdsong production. (i) A vibrating valve produces a multifrequency harmonic sound, which is filtered to a pure tone by a vocal tract resonance filter (“source-filter” model). (ii) Vocal tract resonances are coupled to the syrinx, causing a valve structure to vibrate sinusoidally and produce pure-tone sound (“soprano” model). (iii) A different, as-yet-unknown source mechanism produces pure-tone sound (e.g., a whistle).
In the present study, we examined the first of these hypotheses, the source-filter mechanism, as an explanation for the origin of pure-tone coo vocalizations in two species of turtledove, Streptopelia risoria and Streptopelia decaocto. If pure-tonality is caused by filtering, then a multifrequency source might reveal itself by the presence of harmonic overtones in its immediate vicinity. During normal and spontaneous vocalization, we recorded acoustic signals by using pressure-sensitive transducers attached to cannulae inserted into the interclavicular air sac (ICAS), a cavity in which the syrinx is located, and by using airflow-sensitive thermistors, implanted in the tracheal lumen just above the syrinx. In addition, we recorded sound pressure in the cranial thoracic air sac (CTAS), which is not in direct contact with the syrinx, but is connected to the ICAS and other air sacs through secondary bronchi. The results provide, to our knowledge, the first direct evidence that supports the hypothesis of a multifrequency harmonic source sound in pure-tone birdsong.
Materials and Methods
Subjects. We used five adult male ring doves (S. risoria) and two adult Eurasian collared doves (S. decaocto) as subjects. Ring doves were obtained commercially, whereas the collared doves had originally been captured from the wild in The Netherlands, 2 years before the experiment.
Surgical Procedures and Recording of Data. The procedure to record air sac pressure and tracheal flow signals is described in detail in Suthers et al. (17), so we will give only a summary here.
Birds were anesthetized with isoflurane (Abbott). A midline incision was made in the skin between the clavicles to expose the trachea as it entered the ICAS membrane. Airflow associated with near-field sound was measured with a microbead thermistor probe (Thermometrics, Edison, NJ, BB05JA202N) inserted into the tracheal lumen, just rostrally to the interclavicular membrane and about 1.7 cm (4 cm in two birds) craniad from the lateral tympaniform membranes of the syrinx (the trachea is about 7 cm long in ring doves). Thermistor wires were routed s.c. to connectors on a backpack that the birds wore. The flow velocity in the trachea was measured by a feedback circuit in which the current necessary to maintain the heated thermistor at a constant temperature was proportional to the rate of airflow (Hector Engineering, Elletsville, IN). Fluctuations in air pressure associated with sound production were monitored by a piezoresistive silicone diaphragm pressure transducer (Fujikura FPM-02PG, Marietta, GA) attached to an air sac cannula consisting of a flexible silastic tube (Dow Corning, i.d. = 1.02 mm, wall thickness = 0.57 mm). A cannula 18.5 cm long was inserted into the ICAS through a small hole in the interclavicular membrane, from which it was routed s.c. to a backpack carrying the pressure transducer. The CTAS was cannulated by a similar tube 14 cm long inserted into the air sac through the abdominal wall just posterior to the last rib and a few millimeters lateral to the ventral midline. In each case, the cannula extended 13 mm into the air sac, and tissue adhesive was used to ensure an air-tight seal around the cannula.
Vocalizations were recorded on a condenser microphone [Audio-technica (Stow, OH) AT835b or Sennheiser (Old Lyme, CT) MKH 40] placed 0.5–1 m in front of the cage. All signals (emitted vocalization on microphone, ICAS pressure or tracheal flow velocity, and CTAS pressure) were recorded digitally (20,000 samples per s) on a rotary storage recorder (Metrum Information Storage, Littleton, CO, model RSR 512) or on a DAT data recorder (TEAC, Montebello, CA, model RD135T). The low-frequency components of signals in the trachea and air sacs, related to respiratory ventilation or phonatory motor patterns, were removed with an analogue 100-Hz high-pass filter (Krohn-Hite, Avon, MA, model 3550, or Princeton Applied Research, model 113) before recording, or by digital filtering after recording (see Data Analysis). We transferred the recorded signals from tape to a microcomputer by resampling (20,000 samples per s), by using a Data Translation (Marlboro, MA) DT-2821G board and a TTE (St. Pete Beach, FL) J87 antialiasing filter (high cut-off at 8 kHz, stopband attenuation 60 dB per one-third octave).
Data Analysis. We considered recordings suitable for analysis if they had a good signal-to-noise-ratio and did not contain any recording artifacts, such as clipping. Tracheal sound signals were analyzed only if they were recorded no later than 1 week after thermistor implantation, because mucus and other material gradually accumulates on the thermistor, degrading its time constant and lowering the high-frequency cut-off. Signals were analyzed by using the software program PRAAT (available from Paul Boersma and David Weenink, www.praat.org), version 3.9.14 for Linux. All signals were filtered with a digital high-pass filter at 200 Hz, by using a built-in function of the PRAAT program (frequency domain filter, Hann-like shaped band, 100-Hz smoothing).
We visually screened all signals for the occurrence of harmonic overtones by using printed spectrograms, calculated by a short-time Fourier transform (frame length 30 ms, Gaussian window, time step 1 ms, frequency step 10 Hz, 40-dB dynamic range). In a selection of maximally 20 vocalizations for each combination of recorded variables, we then quantified the degree of pure-tonality of signals by measuring the intensity of the second (2f0) and third (3f0) harmonics, relative to that of the fundamental frequency (f0). In pure-tone signals, the intensity of 2f0 and 3f0 should be very low. In the case of thermistor signals, we selected the first 20 recordings, because recording quality deteriorates over time. In the case of pressure signals, we selected 20 recordings on the basis of recording quality. First, we determined the dominant frequency in the power spectrum of the emitted coo sound. Using the spectrogram (see settings above), we then selected a portion of the coo where this frequency occurred with little frequency modulation. We calculated the mean fundamental frequency of a 200-ms time frame around this time point, by using PRAAT's autocorrelation algorithm. The 200-ms time frame of the emitted coo and concurrent time frames of the available ICAS, CTAS, or tracheal signals were then transformed to the frequency domain (8,192-point Fast Fourier Transform). The resulting spectra were divided up into 20-Hz bins. The amplitudes of f0, 2f0, and 3f0 were determined by measuring the bin with the highest average amplitude in a one-eighth-octave band around the expected harmonic frequencies, based on the (independent) calculation of mean fundamental frequency in the time domain.
Transducer Calibration. Microbead thermistor probes, and to a much lesser degree piezoresistive pressure transducers, have nonlinear response characteristics, which cause artificial introduction of some signal energy at harmonic overtone frequencies. We determined the extent of this effect by calibrating the transducer systems (including the same signal conditioning electronics used during the experiments) in a large anechoic (>100-Hz) room [ISO 3745-1977 (E) and ISO 8253-2 1992] at the Netherlands Central Organization for Applied Scientific Research Institute at Soesterberg, The Netherlands. We played four pure tones (397, 500, 630, and 794 Hz) spanning the frequency range of f0 in our subjects, by using an Aardvark Direct (Ann Arbor, MI) Pro 24/96 soundcard and a JBL (Northridge, CA) Studio Monitor speaker (model 4425). The generated sound was recorded 50 cm in front of the speaker with both transducer systems, and a reference microphone (B&K, Edmonds, WA, precision integrating sound level meter type 2236, with 4188 B&K microphone). The thermistor tip, the end of the pressure cannula, and the head of the reference microphone were located at the same position (±<5 mm) in the anechoic room. The intensity level was adjusted to approximate the peak-to-peak voltage output of the complete transducer system as measured in the experimental subjects.
The results showed that the intensity level of artificially introduced harmonic overtones is lower than -48 ± 6 dB (mean ± SD) and -48 ± 5 dB for 2f0 and 3f0, respectively, in the pressure transducer system, and -24 ± 3 dB and -35 ± 8 dB in the thermistor system (all dB values relative to the intensity of f0).
We also determined the frequency response of our transducer systems by recording speaker-generated pink noise in the same calibration setup. Variance in the frequency response of the pressure transducer system was mainly attributable to resonances in the cannula probe, which introduced deviations of +2.3 ± 0.6 dB (mean ± SD) and +3.7 ± 0.5 dB in the intensities of 2f0 and 3f0, respectively, for the f0 values used in the quantitative analyses of our dove coo recordings. The frequency response of the thermistor system decreases with increasing frequency, which introduces deviations of -4.0 ± 1.9 dB and -4.6 ± 2.2 dB in the intensities of 2f0 and 3f0 for the frequencies in the analyses.
Microbead thermistors have been successfully used to record sound in or near the syrinx in previous studies (e.g., ref. 17), and the ex situ calibrations described above confirm that thermistor measurements are usable for our purposes. The physical mechanism that underlies sound recording by thermistors, however, remains as yet unknown. Therefore it cannot be completely excluded that in situ factors affect thermistor measurements in unanticipated ways. We verified our in situ tracheal thermistor recordings by recording tracheal sound with the pressure transducer system in one dove. All procedures and measurements in this control experiment, including the length of the cannula, were the same as described above for measuring the ICAS pressure, except that the pressure cannula was attached to a 5 mm length of stainless steel tubing (o.d. = 1.83 mm, i.d. = 1.53 mm) inserted through the wall of the trachea, halfway between the syrinx and glottis.
Results
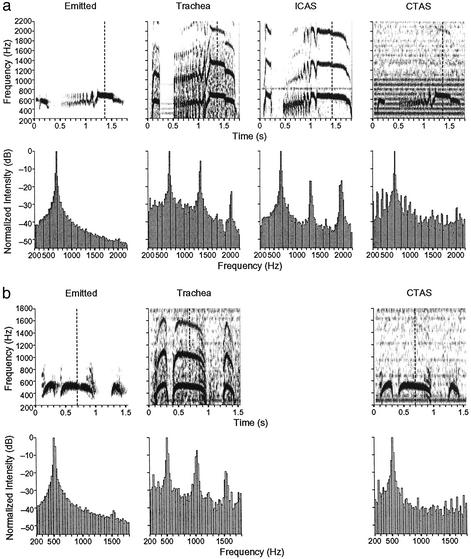
Table 1 gives an overview of which signal combinations were obtained for each bird, as well as the number of recorded coos. Visual inspection of the spectrograms of these signals (Fig. 1) shows that both ICAS and tracheal sound signals always have a multifrequency harmonic spectrum, with harmonic overtones at both even and odd multiples of the fundamental frequency. In low-noise ICAS recordings, one can even see the harmonic series reach the 11th harmonic, if the spectrogram's dynamic range is increased appropriately. Concurrent recordings of the emitted coos and CTAS signals never have a multifrequency spectrum, but consist only of the fundamental frequency of the tracheal and ICAS spectra (Fig. 1).
Table 1. Recorded combinations of sound signals.
| Sound signal
|
|||||
|---|---|---|---|---|---|
| Dove | Emitted | Trachea | ICAS | CTAS | n |
| CD1 | + | + | 392 | ||
| CD2 | + | + | + | 2 | |
| RD1 | + | + | 86 | ||
| RD2 | + | + | + | 9 | |
| RD2 | + | + | + | 208 | |
| RD3 | + | + | 88 | ||
| RD4 | + | + | + | 39 | |
Plus symbols in the same row indicate that signals have been recorded concurrently. Eurasian collared doves are designated as ”CD” and ring doves as ”RD,” and different numbers refer to different individuals
Fig. 1.
Sound signals in different compartments of respiratory and vocal system in a ring dove (a) and Eurasian collared dove (b). Emitted, tracheal, and CTAS signals are from the same coo vocalization. The ICAS signal is from a different coo from the same individual. Shown are spectrograms (Upper) and power spectra (Lower; in averaged 20-Hz bins) of a 100-ms segment, centered on the time indicated with a dashed line in the spectrogram above. The intensity values in power spectra are relative to the intensity of f0. The smaller peaks at integer multiples of 100 Hz in the CTAS signal of the ring dove (a) are an artifact attributable to electronic noise.
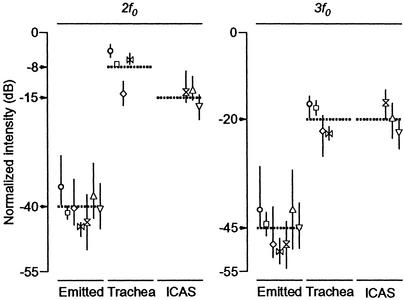
A quantitative comparison of the intensity differences between f0 and 2f0 or 3f0 confirms our qualitative observations. The intensities of 2f0 and 3f0, relative to that of f0, are much higher in the trachea and ICAS than in the emitted coo (Fig. 2). The intensity of 2f0 and 3f0 in CTAS is always below noise level (i.e., <-30 ± 4 dB, mean ± SE, and -29 ± 4 dB at the expected frequencies of 2f0 and 3f0, respectively). CTAS signals can therefore be regarded as being pure-tonal.
Fig. 2.
Intensities of second (2f0) and third (3f0) harmonics in emitted, tracheal, and ICAS signals, relative to that of the fundamental frequency (f0). Symbols indicate means (with ranges specified by vertical lines), calculated from 20 recordings, except for the two cases in which <20 recordings were available (see Table 1). Identical symbols indicate that measurements are from concurrent recordings. Horizontal dashed lines indicate means per anatomical compartment, calculated from the mean values of individual birds. The values of these means are indicated through arrows on the vertical axis. Circle, CD1; square, CD2; diamond, RD1; horizontal hourglass, RD2; hourglass, RD2; triangle, RD3; inverted triangle, RD4.
Tracheal pressure measurements with the piezoresistive transducer in one control dove confirmed the presence of strong harmonic overtones in the trachea as recorded by thermistors (-9 ± 1 dB, mean ± SE, and -25 ± 2 dB for 2f0 and 3f0, respectively).
Discussion
Our results demonstrate that the source sound of pure-tone song in ring doves and Eurasian collared doves has a multifrequency harmonic spectrum. The coo vocalization that is emitted by the doves consists only of the fundamental frequency part of this spectrum. We conclude therefore that the pure-tone quality of turtledove song originates by a source-filtering mechanism. Although we did not record source vibrations directly, but rather sound near the syrinx, the harmonic overtones in tracheal and air sac signals must be generated by the sound source, whatever its nature. Resonant structures can play an important role in sound production, but in sustained sounds they can only respond to frequencies at which they are excited; they cannot generate their own frequencies (18).
In both the trachea and ICAS the spectral pattern of the first three harmonics is similar: the f0 has the highest intensity, whereas the 2f0 and 3f0 show considerable, but decreasing, amounts of energy. It is interesting to note that this spectral pattern resembles that of human glottal flow waveforms during normal speech. Modal human speech source spectra decay according to f -2 (19); for 2f0 and 3f0, this gives intensities of -12 and -19 dB, respectively. In our doves these intensities are, on average, -11 and -20 dB. It should be noted, however, that the accuracy of our intensity measurements is affected by three shortcomings: First, the thermistors used to record tracheal signals attenuate higher frequencies. This attenuation leads to a systematic underestimation of the intensity of 2f0 and 3f0, relative to f0. We calibrated our thermistor system for the relevant frequencies, but in situ recordings may be affected by gradual mucus deposition on the thermistor tip, which will change the filtering properties of the thermistor system. Second, thermistors introduce energy at harmonic overtone frequencies into recorded waveforms due to their nonlinear responses. This energy leads to an overestimation of the intensity of 2f0 and 3f0. Calibrations showed the levels of signal energy introduced this way to be considerably below those obtained in our experimental recordings. However, these calibrations were necessarily performed ex situ, in conditions different from those inside a vocalizing dove, thus the real introduced error may deviate from that found in the calibration. Nevertheless, our control experiment in which we recorded tracheal sound with a pressure transducer confirmed the presence of harmonic overtones with similar intensities in the trachea. Third, our measurements are based on signals recorded in the trachea and ICAS. These locations are close to the syringeal source, but not at the source. As a result, the measured signals are inevitably affected by filtering through resonances of tracheal and ICAS air cavities. Furthermore, it is likely that the sound present in the ICAS is generated in the trachea and is transmitted through the extremely thin dorsal membrane and medial tympaniform membranes (20) that are present in the tracheal and bronchial walls, respectively. This structure adds another opportunity for filtering.
Despite this uncertainty regarding the exact level of harmonics at the syringeal source, we believe the presence of high-intensity harmonic overtones close to the syrinx provides convincing evidence for the multifrequency, harmonic nature of this sound source. We cannot, however, directly extend our findings to pure-tone birdsong production in general, because we have tested only two closely related nonoscine species. It is possible that other species use different types of sound sources to generate pure-tone sound. Nevertheless, the results do show that it is not necessary to invoke source mechanisms radically different from valves, vibrating largely independently from resonant cavities, to explain pure-tonality. The idea of a source-filter production mechanism as originally proposed by Nowicki (15) can adequately account for pure-tone birdsong.
Ballintijn and ten Cate (12) concluded, on the basis of helium studies, that the pure-tone coo vocalizations of Eurasian collared doves are produced as such at the syringeal source. Our current study, however, shows this is not the case. Perhaps the interpretation of helium experiments depends too heavily on the assumption that vocal tract filtering can be modeled as the resonances of a simple tube. In reality, the avian vocal system includes a complex system of interconnected tubes and air sacs, partially separated by thin membranes. The resonant behavior of such a system is not easily predicted (21).
Larsen and Goller (22) recorded vibrations of lateral tympaniform membranes in the trachea concurrently with the emitted sound during brain stimulation-induced phonation in anesthetized pigeons (Columba livia). Membrane vibrations had waveforms that were highly similar to that of the emitted sound, and were pure-tonal (f0 exceeded 2f0 by at least 20 dB). This quality contrasts to the relatively strong tracheal harmonic overtones found in the pure-tone coos of our doves. It seems unlikely that the syringeal source spectrum in pigeons is fundamentally different from that in the dove species we studied. Because both doves and pigeons are in the family Columbidae and share a basically similar syringeal and vocal tract anatomy, the discrepancy between our data and those of Larsen and Goller (22) may be due to the fact that they short-circuited part of the vocal tract by opening the trachea for laser vibrometry measurements, or due to the fact that they elicited vocalizations from anesthetized birds by using brain stimulation.
The extent of filtering needed to transform the dove's multifrequency source signal into a pure tone is remarkable. The relative intensity of 2f0 measured in the trachea is on average 32 dB higher than in the emitted coo. It has been suggested that the resonance characteristics of the trachea and oral cavity are the basis for vocal filtering (6, 10, 15, 16). If only tracheal resonance filtering were responsible for the generation of pure tones from a multifrequency harmonic source, then we would expect to find only a pure tone inside the trachea during phonation, because a simple tube like the trachea is expected to act as a high-pass filter. Our finding of harmonic overtones inside the trachea suggests, therefore, that in doves the trachea is not the only structure involved in filtering. The idea of simple tracheal filtering in these doves can also be rejected on theoretical grounds, because the lowest resonant frequencies of a simple tube of 7.7 cm, which is the average length of the trachea in Eurasian collared doves (23), is 1,149 Hz if only one end is open, or 2,299 Hz if both ends are open (ref. 18; assuming the speed of sound is 354 m/s). The f0 of coo vocalizations in Eurasian collared doves is ≈500 Hz, less than one-half or one-fourth of these expected frequencies.
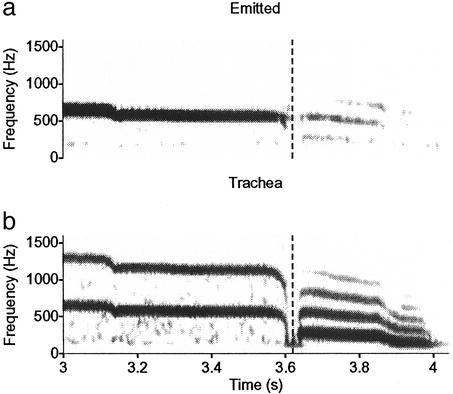
Because ring doves (8), and most likely also collared doves, coo with their beaks and nares closed, one alternative explanation could be that the source sound is simply low-pass filtered below 2f0 when it passes through soft tissues as it radiates from the body. However, this hypothesis is inconsistent with the spectral structure of the wah sound that is produced by both dove species during inhalation immediately following the end of a coo. This sound has multiple harmonics above a f0 of 150–250 Hz. Although the intensity of f0 is high in the trachea, it is almost completely absent in the emitted vocalization (Fig. 3). Simple low-pass filtering by transmission through soft tissue cannot account for this, because the frequency range is well below that of the coo. We therefore suggest that the filtering mechanism that makes coos pure-tonal involves a single-peak resonator that is sympathetic to the f0 of the multifrequency harmonic source. This resonance would also explain the finding that coo intensity is strongly reduced when doves vocalize in helium (12). The air sac system may be involved as the cavity part of such a resonator. Alternatively, or perhaps additionally, the air cavity of the inflated esophagus or crop in cooing doves (8) may serve as part of an acoustic resonator. Whatever the nature of the filter mechanism involved in pure-tone birdsong, this study demonstrates its presence and raises the intriguing question of why birds produce pure-tone song at all.
Fig. 3.
Spectrograms of emitted sound (a) and concurrent tracheal sound (b) at the end of a ring dove coo. The vertical dashed lines indicate where the coo (produced with the beak and nares shut) stops and the inhalatory “wah” begins. Both signals have been high-pass filtered at 100 Hz.
Acknowledgments
We are grateful to Johan Bolhuis, Coen Elemans, and three anonymous referees for comments on the manuscript, and to Sandra Ronan for assistance with the experiments. We thank Adelbert Bronkhorst at the Netherlands Central Organization for Applied Scientific Research (TNO) Human Factors Institute for use of their facilities. This study was supported by a travel grant from the Netherlands Organization for Scientific Research (NWO) to G.J.L.B. and by National Institutes of Health Grant NS29467 to R.A.S.
Abbreviations: ICAS, interclavicular air sac; CTAS, cranial thoracic air sac.
References
- 1.Cuvier, G. & Duvernoy, G. L. (1846) Leçons d'Anatomie Compareé (Fortin & Masson, Paris), Part 18, 2nd Ed.
- 2.Rüppell, W. (1933) J. Ornithol. 81, 433-542. [Google Scholar]
- 3.Greenwalt, C. H. (1968) Bird Song: Acoustics and Physiology (Smithsonian Inst. Press, Washington, DC).
- 4.Casey, R. M. & Gaunt, A. S. (1985) J. Theor. Biol. 116, 45-64. [Google Scholar]
- 5.Fletcher, N. H. (1989) Comments Theor. Biol. 1, 237-251. [Google Scholar]
- 6.Gaunt, A. S. & Nowicki, S. (1998) in Animal Acoustic Communication, eds. Hopp, S. L., Owren, M. J. & Evans, C. S. (Springer, Berlin), pp. 291-321.
- 7.Stein, R. C. (1973) Am. Zool. 13, 1249-1255. [Google Scholar]
- 8.Gaunt, A. S., Gaunt, S. L. L. & Casey, R. M. (1982) Auk 99, 474-494. [Google Scholar]
- 9.Nottebohm, F. (1971) J. Comp. Physiol. 108A, 157-170. [Google Scholar]
- 10.Nowicki, S. (1987) Nature 235, 53-55. [Google Scholar]
- 11.Brittan-Powell, E. F., Dooling, R. J., Larsen, O. N. & Heaton, J. T. (1997) J. Acoust. Soc. Am. 101, 578-589. [DOI] [PubMed] [Google Scholar]
- 12.Ballintijn, M. R. & ten Cate, C. (1998) J. Exp. Biol. 201, 1637-1649. [DOI] [PubMed] [Google Scholar]
- 13.Fant, G. (1960) Acoustic Theory of Speech Production (Mouton, The Hague, The Netherlands).
- 14.Doupe, A. J. & Kuhl, P. K. (1999) Annu. Rev. Neurosci. 22, 567-631. [DOI] [PubMed] [Google Scholar]
- 15.Nowicki, S. & Marler, P. (1988) Music Percep. 5, 391-426. [Google Scholar]
- 16.Hoese, W. J., Podos, J., Boetticher, N. C. & Nowicki, S. (2000) J. Exp. Biol. 203, 1845-1855. [DOI] [PubMed] [Google Scholar]
- 17.Suthers, R. A., Goller, F. & Hartley, R. S. (1994) J. Neurobiol. 25, 917-936. [DOI] [PubMed] [Google Scholar]
- 18.Kinsler, L. E., Frey, A. R., Coppens, A. B. & Sanders, J. V. (2000) Fundamentals of Acoustics (Wiley, New York), 4th Ed.
- 19.Flanagan, J. L. (1972) Speech Analysis, Synthesis and Perception (Springer, Berlin), 4th Ed.
- 20.Ballintijn, M. R., ten Cate, C., Nuijens, F. W. & Berkhoudt, H. (1995) Neth. J. Zool. 45, 455-479. [Google Scholar]
- 21.Fletcher, N. H. & Tarnopolsky, A. (1999) J. Acoust. Soc. Am. 105, 35-49. [DOI] [PubMed] [Google Scholar]
- 22.Larsen, O. L. & Goller, F. (1999) Proc. R. Soc. London Ser. B 266, 1609-1615. [Google Scholar]
- 23.Ballintijn, M. R. & ten Cate, C. (1997) Auk 114, 22-39. [Google Scholar]